Abstract
The clustered protocadherin genes encode a diverse collection of neuronal cell surface receptors. These genes have been proposed to play roles in axon targeting, synaptic development and neuronal survival, although their specific cellular roles remain poorly defined. In zebrafish there are four clustered protocadherin genes, 2 pcdh clusters and 2 pcdh clusters, that give rise to over 100 distinct proteins, each with a distinct ectodomain. The zebrafish is an excellent model in which to address the function of protocadherins during neural development, as the embryos are transparent, develop rapidly, and are amenable to experimental manipulation. As a first step to investigating the clustered protocadherins during zebrafish development, we have generated antibodies against the common cytodomains of zebrafish Pcdh. We compare the distribution of Pcdh with Pcdh and find a similar pan-neuronal pattern, with strong labeling of neurons within all major regions of the central nervous system. Pcdh and Pcdh are particularly enriched in the developing visual system, with strong labeling found in the synaptic layers of the retina, as well as the optic tectum. Consistent with studies in mouse, we find that Pcdh and Pcdh are present in a complex, as they can be co-immunoprecipitated from zebrafish larval extracts. This interaction is direct and occurs through the ectodomains of these proteins. Using standard bead aggregation assays, we find no evidence for intrinsic adhesive ability by either Pcdh or Pcdh, suggesting that they do not function as cell adhesion molecules.
Keywords: protocadherin, zebrafish, central nervous system, synapse
Introduction
The cadherins were first discovered on the basis of their ability to mediate calcium-dependent cell adhesion (Yoshida and Takeichi, 1982; Yoshida-Noro et al., 1984). It is now known that the cadherin superfamily is a large, diverse collection of genes that encode proteins with multiple repeats of a ~110 residue cadherin motif (Hulpiau and Van Roy, 2009; Nollet et al., 2000). The protocadherins are the largest subgroup within the cadherin superfamily and can be divided into the clustered protocadherins and the non-clustered protocadherins (Hulpiau and Van Roy, 2009; Hulpiau and van Roy, 2011). The clustered protocadherin genes, pcdh and pcdh, are characterized by an unusual genomic organization in which multiple, variable first exons are arranged in tandem upstream from three constant exons (Wu and Maniatis, 1999). Each of the variable exons encodes a signal peptide, an ectodomain comprised of 6 cadherin motifs, a transmembrane domain and a short cytoplasmic region. The constant exons encode a conserved, common cytodomain (Wu and Maniatis, 1999). Thus, each gene encodes a diverse array of cell surface proteins that can signal through common cytodomains.
Several studies have shown that individual neurons express only a subset of the clustered protocadherins (Esumi et al., 2005; Frank et al., 2005; Kohmura et al., 1998; Wang et al., 2002). The neuronal expression of the protocadherins and the diversity of their ectodomains motivated the hypothesis that the protocadherins contribute both to establishing neuronal identity and specifying correct patterns of synaptic connections through homophilic adhesion (Shapiro and Colman, 1999; Takeichi, 2007; Yagi, 2003; Yagi, 2008). It has been demonstrated that Pcdh (Triana-Baltzer and Blank, 2006) and Pcdh (Fernández-Monreal et al., 2009; Frank et al., 2005; Schreiner and Weiner, 2010) can mediate weak, homophilic interactions. Protocadherins are recruited to cell-cell junctions in an isoform-specific manner and can induce weak cell aggregation (Fernández-Monreal et al., 2009; Frank et al., 2005). In addition, a quantitative enzymatic assay demonstrated homophilic interaction of individual Pcdh isoforms, and by cis-heteromeric, Pcdh -Pcdh complexes (Schreiner and Weiner, 2010). However, while purified ectodomains of classical cadherins are capable of mediating robust adhesion, similar approaches using the ectodomains of the clustered protocadherins have failed to show evidence for adhesion (Morishita et al., 2006; Schreiner and Weiner, 2010). Thus, neither the involvement of the clustered protocadherins in adhesion, nor their specific roles in cell-cell interactions, has been established.
In the zebrafish, the pcdh and pcdh genes have been duplicated, resulting in 45 and 65 distinct protein products, respectively (Noonan, 2004; Tada et al., 2004; Wu, 2005). Each of these genes is expressed broadly in the developing nervous system (Bass et al., 2007; Emond and Jontes, 2008). We previously showed that depletion of Pcdh using antisense morpholino oligonucleotides causes neuronal cell death (Emond and Jontes, 2008). This phenotype differs from targeted deletion of mouse pcdh (Hasegawa et al., 2008; Katori et al., 2009), but is similar to the phenotype of neuronal death occurring in mice lacking pcdh (Wang et al., 2002). A relationship between Pcdh and Pcdh has been suggested by reports that they associate both in vitro and in vivo, and by the observation that they both associate with Pyk2 (Chen et al., 2009). To better understand the role of the clustered protocadherins in zebrafish, we generated polyclonal antibodies against the constant regions of the Pcdh and Pcdh cytodomains. We previously provided an initial characterization of Pcdh distribution in the developing zebrafish (Emond and Jontes, 2008). Here, we characterize Pcdh and compare it with Pcdh. Immunocytochemistry for Pcdh and Pcdh reveals remarkably similar distributions, labeling all major brain regions and axon tracts. Labeling was particularly strong in the visual system, as the synaptic layers of the retina were enriched for both protocadherins, as was the synaptic neuropil of the optic tectum. Immuoprecipitation of zebrafish larval extracts showed that Pcdh and Pcdh are found in a complex, consistent with observations in mouse and rat brain. Moreover, experiments with secreted forms of Pcdh and Pcdh show that this interaction is direct and mediated by the ectodomains. Using Pcdh and Pcdh ectodomains fused to the Fc region of human IgG or to a 6xHis tag, we show that the clustered protocadherins do not mediate adhesion in bead aggregation assays. These data show that, in contrast to classical cadherins, any homophilic adhesive activity of Pcdh or Pcdh is so weak as to be undetectable by standard bead aggregation assays.
Materials and Methods
Fish Maintenance
Adult zebrafish (Danio rerio) and embryos of the Tübingen longfin and AB strains were maintained at ~ 28.5°C and staged according to The Zebrafish Book (Westerfield, 1995). Embryos were raised in E3 embryo medium (Westerfield, 1995) with 0.003% phenylthiourea (Sigma-Aldrich, St. Louis, MO, USA) to inhibit pigment formation. Larvae were anaesthetized in 0.016% ethyl 3-amino benzoate methanesulfonate (Sigma-Aldrich, St. Louis, MO, USA). All procedures were carried out in accordance with IACUC approved protocols.
Protein sequence alignment and analysis
Alignment of the clustered protocadherin cytodomain sequences was performed using ClustalW (http://www.genome.jp/tools/clustalw/). For analysis of disorder, we used IUPred (http://iupred.enzim.hu/).
Co-immunoprecipitation and Western blotting
Zebrafish larvae were collected at 3 dpf, homogenized on ice in cell lysis buffer (CLB: 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1 mM PMSF, Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN, USA)) and microcentrifuged at 4°C for 10 minutes. Supernatants were incubated with 2 g anti-Pcdh1 or anti-Pcdh1 primary antibody for 2 hours at 4°C prior to the addition of protein A sepharose (GE Healthcare, Piscataway, NJ, USA), and the co-immunoprecipitation was allowed to incubate overnight at 4°C. The beads were washed 5 times in CLB, resuspended in loading buffer, and boiled for 5 minutes. Samples were loaded onto 10% Bis-Tris NuPAGE gels (Life Technologies, Carlsbad, CA, USA) and subjected to electrophoresis. Proteins were then transferred to nitrocellulose (GE Healthcare), blocked with 5% nonfat milk in TBST, and incubated overnight with primary antibody (1:100). HRP-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) were used at 1:5000 and the chemiluminescent signal was amplified using Western Lightning Ultra (Perkin Elmer, Waltham, MA, USA). Blots were imaged on an Omega 12iC Molecular Imaging System (UltraLum, Inc., Claremont, CA, USA).
Generation of polyclonal Pcdh1 antibody
Antibodies were produced against the entire constant cytodomain of the zebrafish Pcdh1 cluster. Rabbits were immunized against a GST-fusion of the Pcdh1 cytodomain and affinity purified using an MBP-fusion of the cytodomain (Covance Research Products, Princeton, NJ).
HEK293 cell culture, transfection, and immunocytochemistry
Human embryonic kidney (HEK293) cells were maintained in DMEM supplemented with 10% FBS and penicillin-streptomycin at 37°C with 5% CO2. HEK293 cells were transfected with plasmids encoding CD4-1 CP-GFP or CD4-2 CP-GFP using Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions. Cells were fixed in 4% paraformaldehyde in PBS, permeabilized in PBS + 0.25% Triton X-100, and blocked in PBS + 2% NGS and 3% BSA. The Pcdh1 polyclonal antibody was used at a dilution of 1:200 in block solution and an anti-rabbit rhodamine secondary antibody was used at 1:500. DAPI was included with the secondary antibody at a concentration of 300 nM. Coverslips were mounted in Fluoromount G (Electron Microscopy Sciences, Hatfield, PA, USA) and imaged on a Zeiss Axiostar microscope (Carl Zeiss Microimaging, Thornwood, NY, USA).
Whole-mount Immunocytochemistry
Embryos and larvae were fixed overnight in 4% paraformaldehyde, permeabilized in 1% Triton X-100 in PBS for 1 hour and blocked for 1 hour in PBS containing 1% dimethylsulfoxide, 2 mg/ml BSA, 0.2% Triton X-100, and 5% normal goat serum. Anti-Pcdh1 Antibody was added at a dilution of 1:100 and incubated overnight at 4°C. Rhodamine-conjugated secondary antibodies (Jackson ImmunoResearch) were added at a dilution of 1:500. Larvae were embedded in 1.5% agarose and imaged on a custom built two-photon microscope. Image stacks were taken by collecting optical sections 2 m apart. Image processing was performed using ImageJ (http://rsb.info.nih.gov/ij).
Cryosectioning
Larvae were equilibrated in 30% sucrose in PBS overnight. They were then embedded in OCT (Ted Pella, Inc., Redding, CA, USA), sectioned on a cryostat at 16-20 m, and placed on gelatin-coated glass slides. Immunocytochemistry was performed as described for cultured HEK293 cells.
Bead Aggregation Assays
Bead aggregations were performed essentially as described previously (Biswas et al., 2010; Emond et al., 2011; Sivasankar et al., 2009). Briefly, Pcdh1 EC or Pcdh1 EC fusion proteins (tagged with either -Fc or -6xHis) were transfected into HEK293 cells using FuGENE HD (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer's instructions. After 24 h, the growth medium was replaced with serum-free medium. After an additional 48 h, the culture medium containing the secreted fusion proteins was filtered and concentrated. Protein A-Dynabeads (Life Technologies) were added to the concentrated media containing the Fc-fusion proteins to isolate the protein complexes. The beads were washed extensively, split into two tubes, and either 2 mM EDTA or 2 mM CaCl2 (final concentrations) was added to each tube. Beads were allowed to aggregate with gentle rocking for 1 h. To document bead aggregation, 50-75 l drops were immediately deposited in glass depression slides and images were collected on a Zeiss Axiostar microscope (Thornwood, NY, USA) using a 10X objective. Expression of each fusion protein was verified by Western blotting using standard techniques. Briefly, beads were collected following aggregation assays, resuspended in 2X loading buffer (Life Technologies), boiled and loaded onto 4–12% NuPAGE gels (Life Technologies), and transferred to PVDF membranes (GE Healthcare, Piscataway, NJ, USA). An anti-human antibody was used for immunodetection of Fc-fusion proteins (1:200; Jackson ImmunoResearch). Chemiluminescent detection was performed using an HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Western Lightning substrate (Perkin Elmer, Waltham, MA, USA).
mRNA Injections
The cytodomains of both Pcdh1 and Pcdh1 were fused to Myc and inserted into the pCS2+ plasmid to generate mRNA (pCS2+: CD-Myc and pCS2+: CD-Myc). Synthesis of mRNA was performed using mMessage mMachine (Life Technologies, Grand Island, NY) according to manufacturer's instructions. Embryos were injected with 200 pg of mRNA at the 1-cell stage, and then processed as described for Western blotting and whole-mount immunocytochemistry (anti-myc was used at 1:100 (Sigma-Aldrich, St. Louis, MO)).
Results
Production of antibodies against zebrafish Pcdh
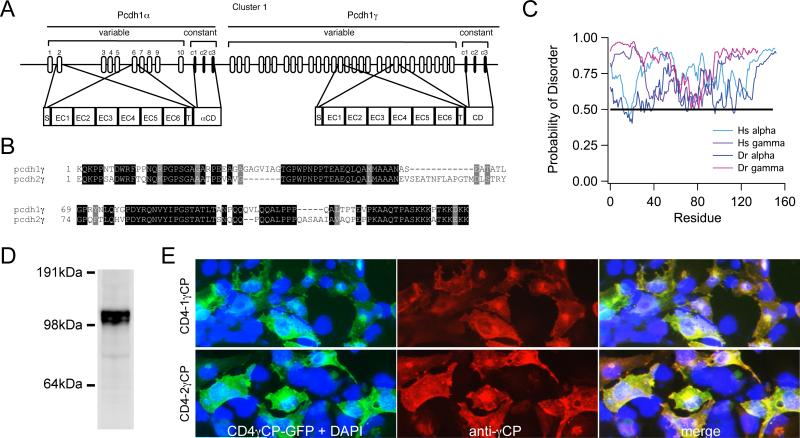
The zebrafish clustered protocadherins are arrayed as a pair of genes, pcdh and pcdh (Fig. 1a). The clustered protocadherins are notable for the diversity of the ectodomains. Each of the tandemly-arrayed variable exons encodes an entire ectodomain and a single-pass transmembrane domain, which are spliced onto 3 constant exons that encode a common cytodomain. In contrast to mammals, the zebrafish genome harbors two arrays of clustered protocadherins: pcdh1 and pcdh1 on chromosome 10 and pcdh2 and pcdh2 on chromosome 14. The common cytodomains encoded by the two pcdh genes are highly homologous (Fig. 1b). Interestingly, sequence analysis predicts that the cytodomains of Pcdh and Pcdh are largely disordered (Fig. 1c). Intrinsically Disordered Proteins (IDPs) lack defined secondary or tertiary structure, and is increasingly recognized as a class of proteins involved in signaling (Babu et al., 2011; Uversky, 2011). Here, we used a GST-fusion protein to produce affinity-purified polyclonal antisera against the Pcdh1 cytodomain (Fig. 1). Western blots of protein extracts from 3 dpf zebrafish larvae showed that our antibody recognizes a doublet ~110-120kDa, consistent with the expected size of Pcdh proteins (Fig. 1d). Transfection of fusions of human CD4 to either the Pcdh1 or Pcdh2 cytodomains reveals that the antibody recognizes the protein products of both genes (Fig. 1e).
Figure 1. Characterization of the Pcdh1 cytodomain.
(A) Zebrafish Pcdh1 and Pcdh1 genes are arranged in tandem on chromosome 10. Both Pcdh1 and Pcdh1 genes consist of an array of 5’ variable exons, each encoding the entire ectodomain, the transmembrane domain and the variable part of the cytodomain. The three 3’ constant domains are spliced onto any one of the variable exons and encode the rest of the cytoplasmic region of the proteins.
(B) Protein sequence alignment of the cytoplasmic region of the two Pcdh clusters shows the regions of homology.
(C) The cytodomains of both human (Hs) and zebrafish (Dr) Pcdh1 and Pcdh1 genes are predicted to be disordered.
(D) Extracts from 3 dpf larvae were probed with affinity–purified antibody against the cytodomain of Pcdh1. A strong doublet of bands of ~110-120 kDa, coincident with the predicted mass of Pcdh1 protein, was detected.
(E) HEK293 cells were transfected with constructs encoding either the Pcdh1 or Pcdh2 cytodomains fused to human CD4 at the N-terminus and GFP at the C-terminus (CD4-1 CP-GFP or CD4-2 CP-GFP). The cells were fixed and immunostained using the Pcdh1 antibody. Immunolabeling with the Pcdh1 antibody (red) overlaps with the GFP signal (green) for both CD4-1 CP-GFP and CD4-2 CP-GFP in the transfected cells, but not in the untransfected cells. DAPI staining labels the nuclei (blue). Scale bar = 10 m.
Distribution of Pcdh and Pcdh in larval zebrafish
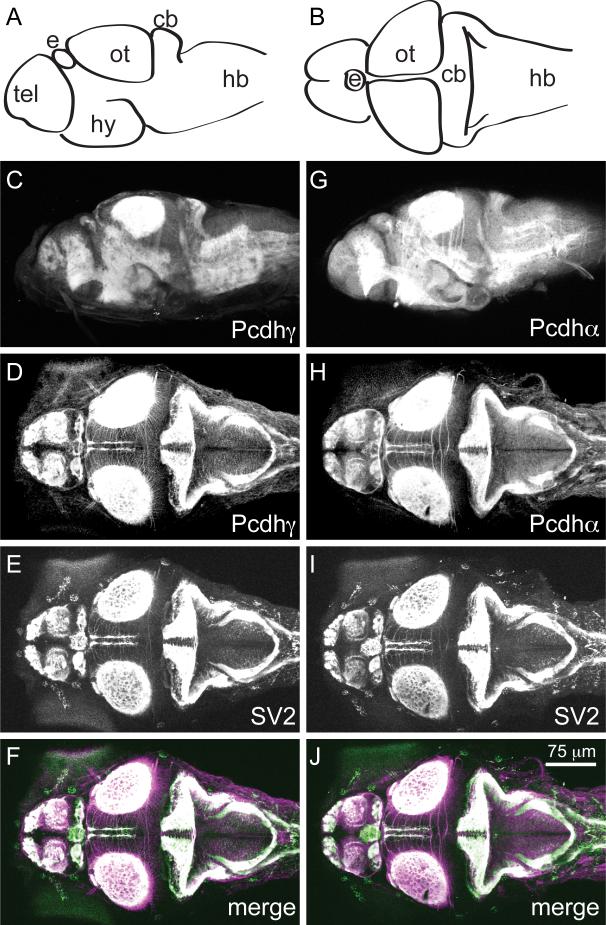
To show the distribution of Pcdh protein in the developing zebrafish, we performed whole-mount immunocytochemistry in 5 days post-fertilization (dpf) larvae (Fig. 2). For orientation, diagrams of a lateral view (Fig. 2a) and a dorsal view (Fig. 2b) of the zebrafish brain are shown. Pcdh is present throughout the larval nervous system and is localized both to neuropil and to axon tracts (Fig. 2c, d). However, Pcdh is particularly strong in neuropil, including the telencephalon and the optic tectum (Fig. 2c, d). By comparison, Pcdh exhibits a similar distribution, but appears to label axon tracts more strongly than Pcdh (Fig. 2g, h). The distribution of both Pcdh and Pcdh were compared to the synaptic vesicle marker, SV2 (Fig. 2e, i). SV2 labels regions of high synaptic density and overlaps extensively with both Pcdh and Pcdh (Fig. 2f, j).
Figure 2. Pcdh and Pcdh expression colocalizes with the synaptic vesicle marker SV2 in 5 dpf larvae.
(A and B) Schematic diagram showing the lateral view (A) and dorsal view (B) of the 5 dpf embryo. The major subdivisions are highlighted. Abbreviations: tel, telencephalon; e, epiphysis; hy, hypothalamus; ot, optic tectum; cb, cerebellum; hb, hindbrain.
(C and D) Whole-mount immunostaining was performed on 5 dpf larvae using the Pcdh antibody. Shown here are lateral (C) and dorsal (D) views of the larvae.
(E and F) Staining for SV2 (E) overlaps with that of Pcdh (D) as shown in the overlay (F). SV2 is shown in green; Pcdh is shown in magenta.
(G and H) Whole-mount immunostaining was performed on 5 dpf larvae using the Pcdh.
(I and J) Larvae were immunostained for the synaptic vesicle marker, SV2 (I), which overlaps substantially with Pcdh immunostaining (J). SV2 is shown in green; Pcdh is shown in magenta.
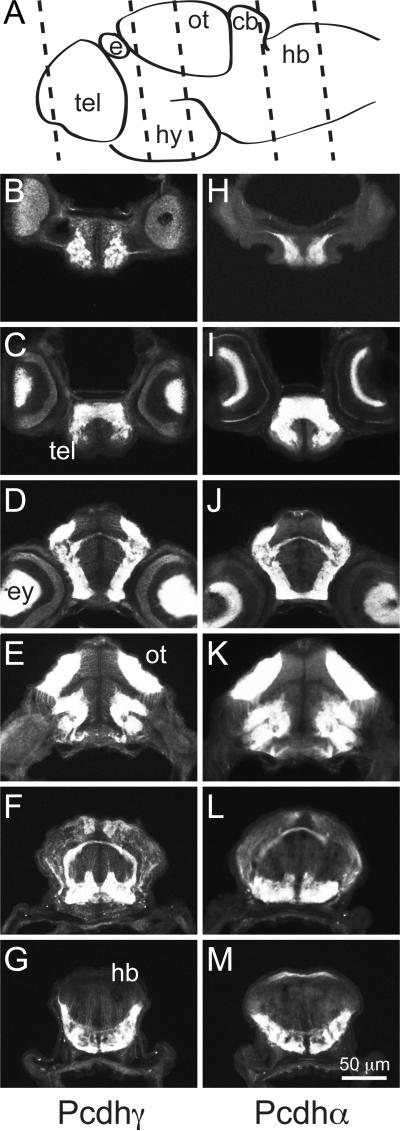
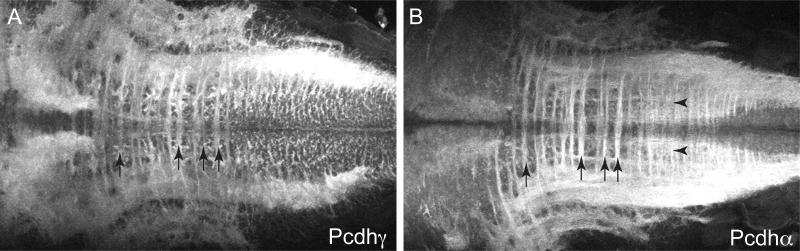
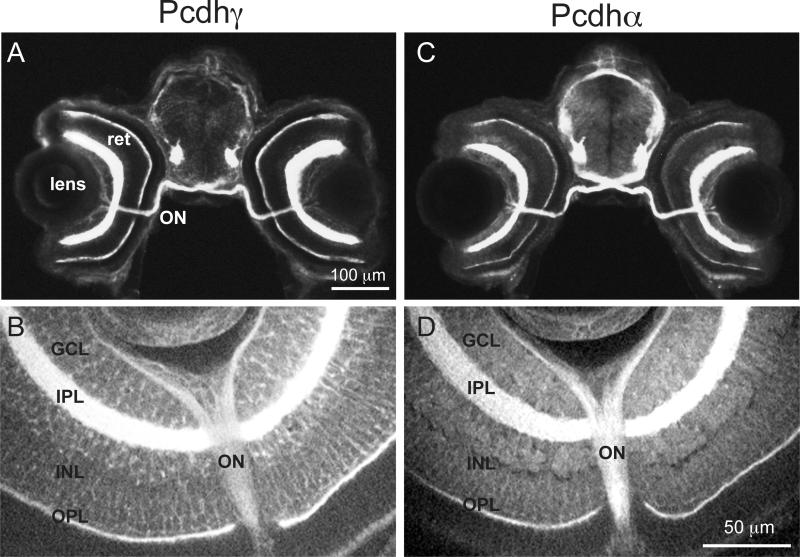
To compare the distribution of Pcdh and Pcdh in more detail, 5 dpf brains were sectioned and immunostained for Pcdh or Pcdh (Fig. 3). While there is an overall similarity in the distribution of Pcdh and Pcdh, careful inspection suggested some subtle differences. Previous work has shown that Pcdh localizes more strongly to axons and presynaptic terminals (Blank et al., 2004). We collected images of the ventral hindbrain, which is organized as a ladder-like array of neurons with lateral longitudinal axon tracts and an array of commissures. Comparison of Pcdh and Pcdh staining in the hindbrain reveals a differential distribution, with Pcdh more strongly labeling axons and Pcdh more strongly labeling cell bodies (Fig. 4). In the retina, Pcdh and Pcdh are largely confined to the synaptic layers (Fig. 5). Sections through the retina showed strong labeling of retinal ganglion cells and the optic nerve (Fig. 5). In the case of Pcdh, some sparse labeling of neurons was seen in the ganglion cell layer and the inner nuclear layer, but most of the immunolabeling was confined to the inner and outer plexiform layers (Fig. 5e).
Figure 3. Distribution of Pcdh and Pcdh in sectioned 5 dpf larvae.
(A) Schematic diagram showing the planes of section through the brain of a 5 dpf larva.
Abbreviations: tel, telencephalon; e, epiphysis; hy, hypothalamus; ot, optic tectum; cb, cerebellum; hb, hindbrain.
(B-G) Pcdh is expressed in all the major subdivisions of the brain, including telencephalon (B and C), optic tectum and hypothalamus (D and E), cerebellum (F), and hindbrain (G).
(H-M) Pcdh exhibits a similar distribution, labeling neuronal populations throughout the nervous system.
Figure 4. Pcdh1 and Pcdh1 distribution patterns in hindbrains of 5 dpf larvae.
(A and B) Dorsal views of maximum intensity projection images were obtained from the hindbrain region of immunolabeled larvae. The anterior of the larvae is located to the left. The Pcdh antibody (A) labels cell bodies of neurons in the ventral hindbrain, and to a lesser extent the commissural axonal tracts that are arranged in a ladder-like array across the two halves of the hindbrain. Pcdh immunolabeling (B) is strong in the commissural axonal tracts and lateral longitudinal axonal tracts that run orthogonal to the commissural tracts. Arrows indicate commissures and arrowheads indicate longitudinal tracts.
Figure 5. Expression patterns of Pcdh and Pcdh in the retina of 3 dpf larvae.
Cryosections through the eyes of 3 dpf larvae immunostained with Pcdh (A) or Pcdh (B) show strong labeling in the optic nerve (ON), the outer plexiform layer (OPL), and the inner plexiform layer (IPL) of the retina (ret). High magnification optical sections through the eye show additional strong Pcdh labeling (C) and diffuse Pcdh (D) labeling in the ganglion cell layer (GCL) and the inner nuclear layer (INL).
Co-immunoprecipitation of Pcdh and Pcdh
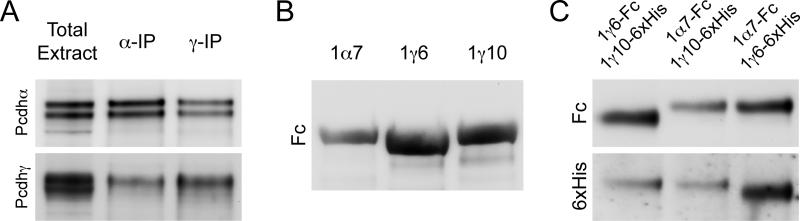
Previous reports indicate that Pcdh and Pcdh can exist in a multi-protein complex in (Bonn et al., 2007; Chen et al., 2009; Han et al., 2010; Murata et al., 2004; Schalm et al., 2010). Proteomic analysis of Pcdh IPs reveals the presence of Pcdh and Pcdh proteins, as well as the cadherin-catenin complex (Han et al., 2010). To determine whether a similar complex exists in zebrafish, we performed co-IPs using our antibodies against Pcdh and Pcdh. Protein extracts were prepared from 3 dpf zebrafish larvae and anti-Pcdh or anti-Pcdh antibodies were used in an immunoprecipitation. Subsequently, Western blots were probed for the presence of Pcdh and Pcdh. In both cases, immunoprecipitation of one clustered protocadherin pulled down the complementary clustered protocadherin (Fig. 6a).
Figure 6. Interaction of Pcdh and Pcdh ectodomains.
(A) Immunoprecipiation of endogenous Pcdh from 3 dpf embryos pulls down Pcdh and vice versa.
(B) Plasmids containing soluble extracellular domains of clustered Pcdhs (two isoforms of Pcdh1 : 1 6 and 1 10; one isoform of Pcdh1 : 1 7) were fused to the Fc region of human IgG. To verify the expression of these individual proteins, the constructs were transfected into HEK293 cells. The fusion proteins were then isolated on Protein A beads, run on a SDS-polyacrylamide gel and probed using an antibody against the Fc region.
(C) We constructed 6xHis-tagged versions of the extracellular domains of 1 6 and 1 10. HEK293 cells were cotransfected with various combinations of tagged (-Fc or -6xHis) isoforms of the clustered Pcdhs.
Recently, Pcdh isoforms were shown to associate in cis to form heteromeric complexes. To determine whether a comparable interaction occurs between the ectodomains of Pcdh and Pcdh, we performed pulldowns using epitope-tagged ectodomain fragments (1 7, 1 6, and 1 10). When expressed alone as Fc-fusions, each of the secreted proteins was isolated from the culture medium using Protein A magnetic beads and visualized by Western blot (Fig. 6b). We then co-transfected HEK293 cells to express two of these proteins, each tagged with either the Fc region of human IgG or with 6xHis. Secreted protein was pulled down, washed extensively and probed with antibodies against human IgG or 6xHis. In each case, the ectodomains were found to interact (Fig. 6c).
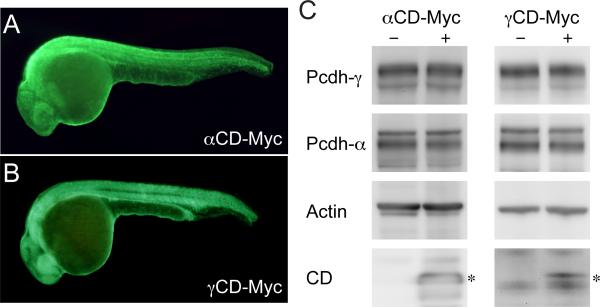
Previous work showed that both Pcdh and Pcdh undergo sequential proteolytic processing by matrix metalloproteases and by -secretase (Bonn et al., 2007; Haas et al., 2005; Hambsch et al., 2005). Cleavage by -secretase releases a soluble cytodomain (CD) fragment, which translocates to the nucleus and may regulate gene expression (Haas et al., 2005; Hambsch et al., 2005). We previously showed that the CD translocates to neuronal nuclei in zebrafish embryos (Emond and Jontes, 2008). To determine if either of the protocadherin cytodomains affects expression by the pcdh or pcdh loci, we injected embryos with mRNA encoding Myc-tagged CD or CD (Fig. 7a, b). Protein extracts from control or mRNA injected embryos were probed for levels of Pcdh and Pcdh by Western blot (Fig. 7c). Expression of CD-Myc did not alter the levels of either Pcdh or Pcdh (Fig. 7c). Similarly, levels of Pcdh or Pcdh were not affected by expression of CD-Myc (Fig. 7c). Thus, overall expression of Pcdh and Pcdh does not appear to be regulated by the levels of soluble CD or CD.
Figure 7. Effect of intracellular domains on protocadherin protein levels.
(A) Wholemount anti-Myc immunlabeling of embryos injected with mRNA encoding CD-Myc.
(B) Wholemount anti-Myc immunlabeling of embryos injected with mRNA encoding a CD-Myc.
(C) Protein extracts from control embryos and embryos expressing CD-Myc (left 2 lanes) or CD-Myc (right 2 lanes) were blotted with antibodies against Pcdh, Pcdh or Actin.
Adhesive properties of Pcdh and Pcdh
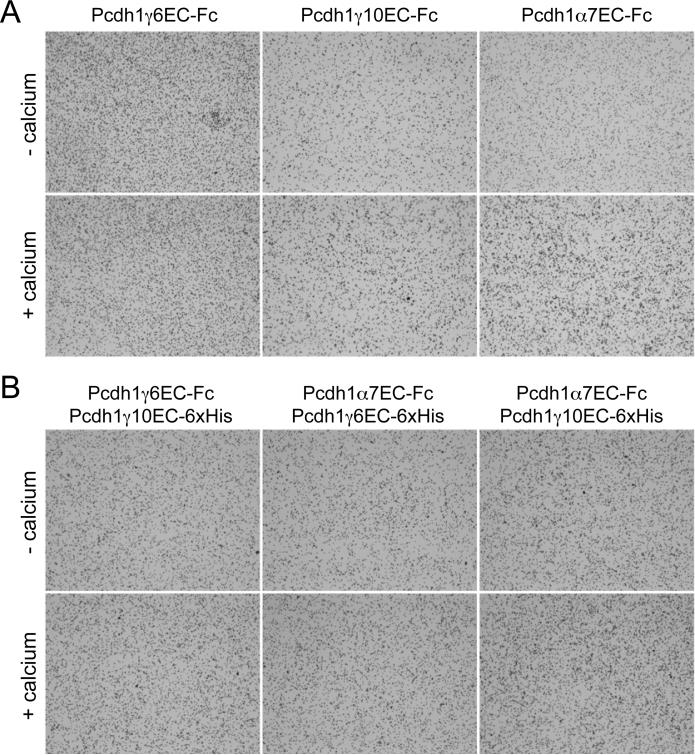
The clustered protocadherins are widely considered to be cell adhesion molecules, due largely to their homology to the classical cadherins. Though they have been shown to mediate weak, homophilic interactions, there is little evidence to suggest that the clustered protocadherins act as adhesion molecules. To investigate the intrinsic adhesive capacity of the zebrafish clustered protocadherins, we fused the ectodomains of zebrafish Pcdh1 6, Pcdh1 10 and Pcdh1 7 to the Fc region of human IgG. These constructs were transfected into either HEK293 or CHO cells and the secreted fusion-proteins were used in bead aggregation assays (Fig. 8). Consistent with previous studies, neither of the Pcdh isoforms (Pcdh1 6 or Pcdh1 10), nor Pcdh1 7 mediated calcium-dependent bead aggregation (Fig. 8a). While these proteins may be capable of mediating homophilic interactions, we find no evidence that they mediate adhesion. These results did not vary between the two cell types and support the conclusion that the clustered protocadherins should not be considered to be adhesion molecules.
Figure 8. Bead aggregation assays using secreted Pcdh1 and Pcdh1 ectodomains.
(A) Fc-tagged extracellular domains of Pcdh1 6, Pcdh1 10 and Pcdh1 7 proteins were purified from HEK293 cells, bound to Protein A beads, and allowed to aggregate in the presence or absence of calcium.
(B) To test whether different pairs of Pcdh1 and Pcdh1 isoforms mediate bead aggregation, Fc-tagged and 6xHis-tagged versions of the Pcdh extracellular domains were isolated on Protein A beads and allowed to aggregate. Several heteromeric combinations were tested: Pcdh1 6-Fc/Pcdh1 10-6xHis; Pcdh1 7-Fc/Pcdh1 6-6xHis, and Pcdh1 7-Fc/Pcdh1 10-6xHis (left to right).
It was recently shown that different Pcdh isoforms assemble into heteromeric cis-complexes (Schreiner and Weiner, 2010). These heteromeric complexes interact with homophilic specificity, suggesting an increased combinatorial complement of potential interactions. The association of Pcdh and Pcdh suggests a further increase in combinatorial complexity. To determine whether Pcdh -Pcdh or Pcdh -Pcdh heteromers can mediate adhesion, we performed bead aggregation assays with Pcdh-Fc/Pcdh-6xHis hetero-complexes. Above, we showed that secreted forms of Fc- and 6xHis-tagged ectodomains can be isolated from culture medium as a complex when co-transfected into HEK293 cells (Fig. 6). When used in bead aggregation assays, we found that Pcdh1 6EC-Fc/Pcdh1 10EC-6xHis complexes did not exhibit calcium-dependent bead aggregation (Fig. 8b). Thus, the formation of a Pcdh /Pcdh heteromeric complex did not facilitate homophilic adhesion. In addition, complexes of Pcdh1 7EC-Fc with either Pcdh1 6EC-6xHis or Pcdh1 10EC-6xHis also failed to mediate calcium-dependent adhesion (Fig. 8b). Thus, our data are not consistent with a role for zebrafish clustered protocadherins mediating cell-cell adhesion, either as individual proteins or as Pcdh -Pcdh or Pcdh -Pcdh complexes.
Dominant-interfering Pcdh1 impairs clustering of synaptic vesicles
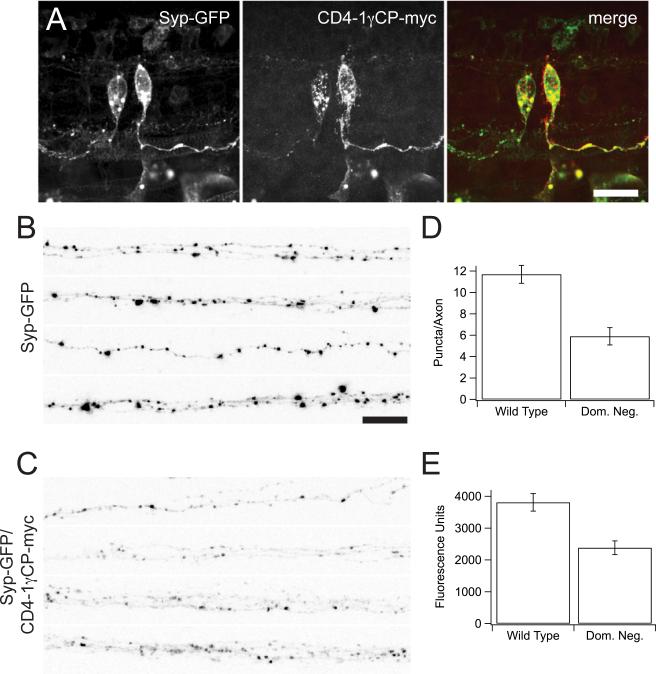
In order to determine the involvement of Pcdh in synaptic development in the zebrafish spinal cord, the common cytodomain of Pcdh1 was fused to the carboxy-terminus of human CD4 (CD4-1 CPmyc). These putative dominant-interfering constructs were co-expressed with the synaptic vesicle marker Synaptophysin-GFP (Syp-GFP; Fig. 9a). Spinal axons expressing Syp-GFP alone show large puncta, representing clusters of synaptic vesicles, giving the appearance of beads on a string (Fig. 9b, d). Overexpression of CD4-1 CPmyc disrupts synaptic vesicle clustering, as there are fewer Syp-GFP puncta and the remaining puncta are smaller and less regular (Fig. 9c, e).
Figure 9. Expression of the Pcdh1 cytodomain abrogates synaptic vesicle clustering in spinal neurons.
(A) The common cytodomain of the Pcdh1 cluster was fused to the human CD4 at the N-terminus and myc-tagged at the C-terminus (CD4-1 CP-myc). Synaptophysin-GFP (Syp-GFP) and CD4-1 CP-myc were co-expressed using a dual-expression construct (pDual:Syp-GFP/CD4-1 CP-myc). The left panel shows the punctate expression pattern of Syp-GFP in the cell bodies and axons of spinal neurons, whereas the middle panel shows the expected surface expression of CD4-1 CP-myc in cell bodies and axons of spinal neurons. The right panel shows the merged channels. Scale bar = 10 m.
(B-E) Embryos were injected with either Syp-GFP alone (B) or with the Pcdh1 dominant negative construct using pDual:Syp-GFP/CD4-1 CP-myc (C). Syp-GFP is concentrated in large, punctate accumulations all along the axons of the spinal neurons, representing regions of synaptic vesicle clustering (B). In the presence of the dominant negative Pcdh1, the puncta appear at a lower frequency along the length of the axon, and they appear to be smaller (C).
(D) Quantification of the density of Syp-GFP puncta.
(E) Quantification of the intensity of Syp-GFP puncta. Scale bar = 15 m.
Discussion
Assembly of synaptic junctions during vertebrate nervous system development requires linking cell-cell recognition and adhesion to signaling events that recruit and organize the synaptic machinery. The clustered protocadherins have long been proposed to participate in this process, but neither their role in adhesion, nor their role in synaptic development is well defined. As a first step in studying the function of protocadherins in synapse formation and development, we generated antibodies against the cytodomain of zebrafish Pcdh1. This antibody does not distinguish among the distinct Pcdh isoforms or between the protein products of the pcdh1 and pcdh2 genes, but provides a picture of the overall distribution of Pcdh. Pcdh exhibits an essentially pan-neural distribution, similar to what we observe for Pcdh. In addition to being expressed throughout the brain, both Pcdh and Pcdh are enriched in the synaptic layers of the retina. While the overall distribution of Pcdh and Pcdh is similar, Pcdh appears to be enriched in axons relative to Pcdh.
Previous studies have suggested that Pcdh and Pcdh can associate to form a complex (Bonn et al., 2007; Chen et al., 2009; Han et al., 2010; Murata et al., 2004; Schalm et al., 2010). Immunoprecipitation of Pcdh -GFP from knock-in mice pulls down Pcdh (Chen et al., 2009; Han et al., 2010), and Pcdh facilitates Pcdh trafficking to the plasma membrane when co-transfected into HEK293 cells (Murata et al., 2004). Here, we show that Pcdh and Pcdh co-immunoprecipitate from zebrafish embryo extracts. Moreover, we show that this association occurs through the ectodomains. These results suggest that the clustered protocadherins could function as a complex during neural development. Thus, the recent demonstration of heteromeric complexes of Pcdh isoforms (Schreiner and Weiner, 2010) could be extended to include Pcdh, as well. The association of Pcdh and Pcdh may partially define a larger macromolecular complex, as cadherins and catenins were identified in an earlier proteomic study of Pcdh (Han et al., 2010), and Pcdh19 was recently shown to form an adhesive complex with Ncad (Biswas et al., 2010; Emond et al., 2011).
When transfected into naïve cell lines, the clustered protocadherins are recruited to cell-cell junctions and induce weak cell aggregation (Fernandez-Monreal et al., 2009; Frank et al., 2005). In general, the observed aggregation is substantially weaker than that of classical cadherins. Studies of adhesion by classical cadherins increasingly rely on secreted cadherin ectodomains fused to human IgG or a 6xHis tag (Brieher et al., 1996; Emond et al., 2011; Niessen and Gumbiner, 2002; Sivasankar et al., 2009). The isolation of these fusion-proteins provides the means to directly assess the intrinsic adhesive capacity of these proteins using bead aggregation assays or more quantitative biophysical methods. Such direct methods have failed to detect calcium-dependent adhesion for either Pcdh (Morishita et al., 2006) or Pcdh (Schreiner and Weiner, 2010), raising doubts about the role of these molecules as bona fide adhesion molecules. Although evidence supports the idea that Pcdh proteins can interact homophilically, we do not observe any adhesive activity under conditions that support adhesion by classical cadherins. As it was recently observed that Pcdh isoforms can associate to form heteromeric cis-complexes (Schreiner and Weiner, 2010), we tested whether these complexes mediate adhesion in bead aggregation assays. Despite the fact that we can isolate cisheteromeric complexes of Pcdh ectodomains, we do not observe any evidence for adhesion. We observed similar results with Pcdh -Pcdh heteromers. We propose that any homophilic interactions of the clustered protocadherins is either too weak to be detected in these assays, or requires additional cellular co-factors, such as classical cadherins or non-clustered protocadherins.
Evidence suggests that Pcdh plays a role in synaptic development. Immunoelectron microscopy localizes Pcdh to synapses (Phillips et al., 2003) and hypomorphic alleles of pcdh result in reduced synaptic density in mouse spinal cord, as well as reduced synaptic currents (Weiner et al., 2005). In addition, over-expression of a Pcdh isoform or a truncated version, lacking the cytodomain, reduces synaptic density in cultured hippocampal neurons (Fernandez-Monreal et al., 2009). Here, we show that over-expressing the Pcdh cytodomain reduces the size and number of synaptic vesicle clusters in spinal axons in vivo. This reduction suggests that Pcdh plays a role in either the recruitment or retention of synaptic vesicle clusters. These results support the idea that Pcdh contributes to synapse assembly or development, although the detailed mechanisms remain to be determined.
Highlights.
-zebrafish Pcdh and Pcdh protein have similar distributions
-zebrafish Pcdh and Pcdh strongly label synaptic regions in the brain and retina
-Pcdh and Pcdh can be isolated as a complex in vivo
-neither Pcdh, nor Pcdh exhibit adhesive activity
-overexpression of the Pcdh cytodomain inhibits synaptic vesicle clustering in spinal axons
Acknowledgments
This work was supported by NSF/ARRA award (IOS 0920357) to J.D.J and a Neurosciences Core grant (P30 NS045758). We would like to thank Hao Le for technical assistance.
Abbreviations
- cDNA
complementary DNA
- cb
cerebellum
- dpf
days post-fertilization
- e
epiphysis
- EC
ectodomain
- ey
eye
- GCL
ganglion cell layer
- Hb
hindbrain
- hpf
hour post-fertilization
- hy
hypothalamus
- INL
inner nuclear layer
- IPL
inner plexiform layer
- mlf
medial longitudinal fasciculus
- mo
medulla oblongota
- Ncad
N-cadherin
- ON
optic nerve
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- ot
tectum
- pcdh
protocadherin
- ret
retina
- RGC
retinal ganglion cell
- RT-PCR
reverse transcription polymerase chain reaction
- tel
telencephalon
- th
thalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babu MM, van der Lee R, de Groot NS, Gsponer J. Intrinsically disordered proteins: regulation and disease. Current opinion in structural biology. 2011;21:432–440. doi: 10.1016/j.sbi.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Bass T, Ebert M, Hammerschmidt M, Frank M. Differential expression of four protocadherin alpha and gamma clusters in the developing and adult zebrafish: DrPcdh2Î3 but not DrPcdh1Î3 is expressed in neuronal precursor cells, ependymal cells and non-neural epithelia. Dev Genes Evol. 2007;217:337–351. doi: 10.1007/s00427-007-0145-4. [DOI] [PubMed] [Google Scholar]
- Biswas S, Emond MR, Jontes JD. Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. J Cell Biol. 2010;191:1029–1041. doi: 10.1083/jcb.201007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn S, Seeburg PH, Schwarz MK. Combinatorial expression of alpha- and gamma-protocadherins alters their presenilin-dependent processing. Mol Cell Biol. 2007;27:4121–4132. doi: 10.1128/MCB.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Triana-Baltzer G, Richards C, Berg D. Alpha-protocadherins are presynaptic and axonal in nicotinic pathways. Mol Cell Neurosci. 2004;26:530–543. doi: 10.1016/j.mcn.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lu Y, Meng S, Han MH, Lin C, Wang X. alpha- and gamma- Protocadherins negatively regulate PYK2. J Biol Chem. 2009;284:2880–2890. doi: 10.1074/jbc.M807417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond MR, Biswas S, Blevins CJ, Jontes JD. A complex of Protocadherin-19 and N-cadherin mediates a novel mechanism of cell adhesion. J Cell Biol. 2011;195:1115–1121. doi: 10.1083/jcb.201108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond MR, Jontes JD. Inhibition of protocadherin-alpha function results in neuronal death in the developing zebrafish. Dev Biol. 2008;321:175–187. doi: 10.1016/j.ydbio.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-α gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Kang S, Phillips GR. Gamma-protocadherin homophilic interaction and intracellular trafficking is controlled by the cytoplasmic domain in neurons. Mol Cell Neurosci. 2009;40:344–353. doi: 10.1016/j.mcn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Monreal M, Kang S, Phillips GR. Gamma-protocadherin homophilic interaction and intracellular trafficking is controlled by the cytoplasmic domain in neurons. Mol Cell Neurosci. 2009;40:344–353. doi: 10.1016/j.mcn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Ebert M, Shan W, Phillips GR, Arndt K, Colman DR, Kemler R. Differential expression of individual gamma-protocadherins during mouse brain development. Mol Cell Neurosci. 2005;29:603–616. doi: 10.1016/j.mcn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Haas IG, Frank M, Véron N, Kemler R. Presenilin-dependent processing and nuclear function of gamma-protocadherins. J Biol Chem. 2005;280:9313–9319. doi: 10.1074/jbc.M412909200. [DOI] [PubMed] [Google Scholar]
- Hambsch B, Grinevich V, Seeburg PH, Schwarz MK. {gamma}-Protocadherins, presenilin-mediated release of C-terminal fragment promotes locus expression. J Biol Chem. 2005;280:15888–15897. doi: 10.1074/jbc.M414359200. [DOI] [PubMed] [Google Scholar]
- Han MH, Lin C, Meng S, Wang X. Proteomics analysis reveals overlapping functions of clustered protocadherins. Mol Cell Proteom: MCP. 2010;9:71–83. doi: 10.1074/mcp.M900343-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S, Hamada S, Kumode Y, Esumi S, Katori S, Fukuda E, Uchiyama Y, Hirabayashi T, Mombaerts P, Yagi T. The protocadherin-alpha family is involved in axonal coalescence of olfactory sensory neurons into glomeruli of the olfactory bulb in mouse. Mol Cell Neurosci. 2008;38:66–79. doi: 10.1016/j.mcn.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Hulpiau P, Van Roy F. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol. 2009;41:349–369. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Hulpiau P, van Roy F. New insights into the evolution of metazoan cadherins. Mol Biol Evol. 2011;28:647–657. doi: 10.1093/molbev/msq233. [DOI] [PubMed] [Google Scholar]
- Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, Hasegawa S, Yagi T. Protocadherin- Family Is Required for Serotonergic Projections to Appropriately Innervate Target Brain Areas. J Neurosci. 2009;29:9137–9147. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Morishita H, Umitsu M, Murata Y, Shibata N, Udaka K, Higuchi Y, Akutsu H, Yamaguchi T, Yagi T, Ikegami T. Structure of the cadherin-related neuronal receptor/protocadherin-alpha first extracellular cadherin domain reveals diversity across cadherin families. J Biol Chem. 2006;281:33650–33663. doi: 10.1074/jbc.M603298200. [DOI] [PubMed] [Google Scholar]
- Murata Y, Hamada S, Morishita H, Mutoh T, Yagi T. Interaction with protocadherin-gamma regulates the cell surface expression of protocadherin-alpha. J Biol Chem. 2004;279:49508–49516. doi: 10.1074/jbc.M408771200. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156:389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet F, Kools P, Van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- Noonan JP. Gene Conversion and the Evolution of Protocadherin Gene Cluster Diversity. Gen Res. 2004;14:354–366. doi: 10.1101/gr.2133704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GR, Tanaka H, Frank M, Elste A, Fidler L, Benson DL, Colman DR. Gamma-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons. J Neurosci. 2003;23:5096–5104. doi: 10.1523/JNEUROSCI.23-12-05096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalm SS, Ballif BA, Buchanan SM, Phillips GR, Maniatis T. From the Cover: Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret. Proc Natl Acad Sci USA. 2010;107:13894–13899. doi: 10.1073/pnas.1007182107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between {gamma}-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Colman DR. The diversity of cadherins and implications for a synaptic adhesive code in the CNS. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- Sivasankar S, Zhang Y, Nelson WJ, Chu S. Characterizing the initial encounter complex in cadherin adhesion. Structure. 2009;17:1075–1081. doi: 10.1016/j.str.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada MN, Senzaki K, Tai Y, Morishita H, Tanaka YZ, Murata Y, Ishii Y, Asakawa S, Shimizu N, Sugino H, Yagi T. Genomic organization and transcripts of the zebrafish Protocadherin genes. Gene. 2004;340:197–211. doi: 10.1016/j.gene.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Blank M. Cytoplasmic domain of protocadherin-alpha enhances homophilic interactions and recognizes cytoskeletal elements. J Neurobiol. 2006;66:393–407. doi: 10.1002/neu.20228. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Intrinsically disordered proteins from A to Z. Int J Biochem Cell Biol. 2011;43:1090–1103. doi: 10.1016/j.biocel.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Weiner JA, Levi S, Craig AM, Bradley A, Sanes JR. Gamma protocadherins are required for survival of spinal interneurons. Neuron. 2002;36:843–854. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR: 1995. [Google Scholar]
- Wu Q. Comparative Genomics and Diversifying Selection of the Clustered Vertebrate Protocadherin Genes. Genetics. 2005;169:2179–2188. doi: 10.1534/genetics.104.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Yagi T. Diversity of the cadherin-related neuronal receptor/protocadherin family and possible DNA rearrangement in the brain. Genes Cells. 2003;8:1–8. doi: 10.1046/j.1365-2443.2003.00614.x. [DOI] [PubMed] [Google Scholar]
- Yagi T. Clustered protocadherin family. Dev Growth Differ. 2008;50(Suppl 1):S131–140. doi: 10.1111/j.1440-169X.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Yoshida C, Takeichi M. Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982;28:217–224. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]
- Yoshida-Noro C, Suzuki N, Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984;101:19–27. doi: 10.1016/0012-1606(84)90112-x. [DOI] [PubMed] [Google Scholar]