Abstract
Interleukin-23 (IL-23) plays an essential role in the mucosal immune system. It has been suggested that IL-23 is able to induce carcinogenesis as well as inflammation and a recent study revealed that IL-23R is expressed in colorectal carcinoma cells. However, neither the differences in the IL-23R expression among the patients nor the concrete functions of IL-23 in colorectal carcinoma cells have been revealed. The aim of the present study was to examine the characteristics of IL-23R expression in colorectal carcinoma and the direct effects of IL-23 on colorectal cancer cells. We examined the IL-23R expression in human colorectal cancer tissue samples by immunohistochemistry. Cell proliferation and invasion assays under IL-23 stimulation were performed using cultured cells derived from colorectal cancer. ELISA and real-time PCR were used to evaluate the transforming growth factor (TGF)-β production due to IL-23 stimulation. All of the TNM stage IV patients were positive for IL-23R. IL-23R expression in the carcinoma tissue was also relatively high at the deepest point of invasion in certain cases. The proliferative and invasive activities and/or TGF-β production of DLD-1 cells increased by IL-23 stimulation, whereas no change was observed in the activities of MIP101 and KM12c cells. IL-23 directly enhanced the malignancy of the colon carcinoma cells. An autocrine mechanism via TGF-β production may underlie these effects. IL-23 is therefore a potential target for cancer immunotherapy. However, the homogeneity in IL-23R expression and the effects of IL-23 on colorectal carcinoma cells should be considered.
Keywords: interleukin-23, interleukin-23 receptor, colorectal carcinoma, transforming growth factor-β
Introduction
Interleukin-23 (IL-23), a heterodimeric type 1 cytokine composed of the specific p19 subunit and the IL12/p40 subunit, is predominantly secreted by activated dendritic cells, monocytes and macrophages and is crucial in the mucosal immune system (1–5). The IL-23 receptor is also a heterodimer composed of the specific IL-23R subunit and the IL-12Rβ1 subunit. Various studies support the contribution of IL-23 to the expansion and stabilization of T helper type 17 (TH17) cells, a subset of T cells characterized by IL-17 secretion and the expression of the transcription factor retinoic acid-related orphan receptor (ROR) γT and IL-23R (6). IL-23R is also expressed on other immune cells (e.g., dendritic cells, macrophages and eosinophils) (7–9) and is considered to modulate inflammation via these cells.
Results of previous studies showed that IL-23 is associated with carcinogenesis as well as inflammation. High levels of IL-23 were detected in human squamous carcinoma (10). In a cutaneous carcinogenesis model, IL-23a knockout rodents demonstrated decreased skin tumor formation (11). The antibody blockade of IL-23R in vivo decreased the growth rates of certain transplanted tumors (12). Although the precise mechanisms are unclear, IL-23 is considered to affect tumor cells through T-cell responses via signal transducer and activator of transcription 3 (STAT3) signaling, since IL-23 increases the activity of TH17 and regulatory T cells (Tregs), which positively affects the activity of STAT3 in tumor cells and promotes tumor growth (12,13).
Findings of another study showed that IL-23R is expressed in colorectal carcinoma cells (14). The expression of IL-23R increased progressively from normal tissue to colorectal carcinoma tissue, and the IL-23R protein was also detected in the human colorectal carcinoma cell line SW-480. The study suggested that IL-23 directly regulated tumor growth and enhanced the malignant change from adenoma to adenocarcinoma. However, important issues such as the differences in IL-23R expression among patients or cell lines have yet to be elucidated. Moreover, no study has directly investigated the effects of IL-23 on colorectal carcinoma cells.
The aim of this study was to examine the characteristics of IL-23R expression in human colorectal carcinoma tissues and the direct effect of IL-23 on colorectal cancer cells.
Materials and methods
Cell culture
The human colon carcinoma cell lines (MIP101, DLD-1 and KM12c) were provided by Tohoku University (Sendai, Japan). The cells were grown in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich) and antibiotic antimycotic solution (Sigma-Aldrich) under an atmosphere of 95% air and 5% CO2 at 37°C. In some experiments, the cultured cells were stimulated with 10 μg/ml of recombinant human IL-23 (HumanZyme, Chicago, IL, USA).
Immunohistochemistry
Colorectal carcinoma tissue and normal adjacent tissue samples were obtained from 15 patients (two cecum, two ascending colon, two transverse colon, two descending colon, five sigmoid colon and two rectum) undergoing colectomy or anterior resection at Tohoku University Hospital. Informed consent was obtained from all patients prior to the operation. The tissues were fixed in 10% formalin, and embedded in paraffin for the histological analysis. Immunohistochemical staining for IL-23R was performed using the streptavidin-biotin-peroxidase complex (SAB-PO) method, using a Histofine SAB-PO kit (Nichirei Co., Tokyo, Japan) according to the manufacturer’s instructions. The rabbit anti-human polyclonal antibody against IL-23R (LifeSpan Biosciences, Seattle, WA, USA) was diluted at 1:100. The goat anti-human polyclonal antibody against IL-23R (AbD Serotec, Oxford, UK) was diluted at 1:125. Counterstaining was carried out with hematoxylin. This study was approved by the ethics review board of Tohoku University Graduate School of Medicine.
Protein extraction and western blot analysis
Protein was extracted from cells with the M-PER mammalian protein extraction reagent (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The protein concentration was determined using the Bradford method. Total protein (20 μg) was extracted and resolved by SDS-PAGE. The proteins in the gels were transferred electrophoretically to PVDF membranes at a constant voltage of 30 V for 1 h. The PVDF membrane was blocked with 5% skim milk for 1 h, incubated with the primary antibody at 4°C overnight, and incubated in secondary antibody at room temperature for 1 h. The rabbit anti-human polyclonal antibody against IL-23R (LifeSpan Biosciences) was diluted at 1:1000. The mouse anti-human monoclonal antibody against β-actin (Applied Biological Materials, Richmond, Canada) was diluted at 1:2000. The HRP-linked goat anti-rabbit and goat anti-mouse IgG secondary antibodies were purchased from Epitomics (Burlingame, CA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively.
Cell proliferation assay
Cell proliferation was assessed by a tetrazolium salt (WST-8)-based colorimetric assay using the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). Briefly, the cultured cells were seeded onto 96-well plates at an initial density of 1×104 cells/ml with or without recombinant human IL-23 (10 μg/ml). The plates were incubated for 24 h, and CCK-8 solution was added to each well. Following a 4-h incubation, cell proliferation was determined by scanning the plates with a microplate reader at a wavelength of 450 nm.
Invasion assay
The cell invasion assay was performed using BD BioCoat Matrigel Invasion Chambers (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, the cultured cells (2×103 cells/ml in 0.5 ml serum-free medium) were added in suspension to each insert and medium (0.8 ml, supplemented with 5% FBS) was added to each bottom well. Recombinant human IL-23 (10 μg/ml) was added to the upper wells. Following a 24-h incubation, invasive cells on the under surface of the membrane were stained with Diff-Quik (Sysmex Corporation, Kobe, Japan) and counted microscopically.
RNA extraction, reverse transcription and real-time PCR
The mRNA expression of TGF-β1 was examined by real-time PCR. Total RNA from cultured cells was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol, and washed using an RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA, USA). Total RNA (2 μl) was reverse-transcribed into complementary DNA (cDNA) using the First-strand cDNA synthesis system for the quantitative RT-PCR kit (Marligen Biosciences, Ijamsville, MD, USA). Real-time PCR was performed using an Applied Biosystems 7300 Sequence Detection system (Applied Biosystems, Foster City, CA, USA). The 20 μl PCR reaction included 1 μl of cDNA, 10 μl of 2× TaqMan gene expression master mix and 1 μl of TaqMan gene expression assays reagent (Applied Biosystems). The reactions were incubated in 96-well optical plates at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The Ct values for the reference gene (β-actin) and target gene (TGF-β1) were determined using default threshold settings, and relative quantification was performed according to the 2−ΔΔCt method.
Enzyme-linked immunosorbent assay (ELISA)
The cultured cells were grown to confluence in RPMI-1640 medium supplemented with 10% FBS. The medium was then replaced with FBS-free RPMI-1640 medium with or without recombinant human IL-23 (10 μg/ml). Following 48-h incubation, the levels of TGF-β1 in the supernatant were analyzed using the Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s protocol. The color reaction was measured by scanning the plates with a microplate reader at a wavelength of 450 nm. The concentration of TGF-β1 was determined via a standard curve that was obtained using the kit’s standards.
Statistical analyses
The effects of IL-23 on the cell invasion, proliferation and TGF-β1 production of the cultured cells were evaluated by t-tests.
Results
IL-23R expression in colorectal carcinoma tissue
First, we performed an immunohistochemical analysis and were able to detect the expression of IL-23R in the colorectal carcinoma tissues in 9 out of 15 patients (Fig. 1A, Table I). All of the TNM stage IV patients were positive for IL-23R, but the other TNM stages and tissue types of cancer had little correlation with the IL-23R expression. In normal sections of the colon or rectum, a few epithelial cells expressed IL-23R, especially at the crypt bases (Fig. 1B). We also found that the IL-23R expression of the carcinoma tissue was relatively high at the deepest point of invasion in certain cases (Fig. 1C and D). We used two different anti-IL23R antibodies (rabbit and goat), and obtained similar results (data not shown).
Figure 1.
The immunohistochemical analysis of IL-23R using a rabbit anti-human IL-23R antibody (magnification, ×100). (A) One of the IL-23R-positive colorectal cancer tissue specimens is shown. (B) A few cells in the normal colon tissue were IL-23R-positive. The black arrows indicate the positive cells in the normal section of the sigmoid colon. (C) Some cases indicated that there was IL-23R expression at the deepest point of invasion and (D) that this expression was higher than that present in the surface lesion.
Table I.
Patient characteristics.
| Age | Gender | Portion | TNM-Stage | Tissue type | IL-23R expression |
|---|---|---|---|---|---|
| 56 | M | S | I | Tub2 | Positive |
| 62 | M | A | I | Tub2 | Positive |
| 68 | M | R | II | Tub2 | Positive |
| 73 | F | D | IIIA | Tub2+muc | Positive |
| 70 | M | R | IIIB | Tub2 | Positive |
| 64 | F | D | IIIB | Tub2+por1 | Positive |
| 72 | M | S | IV | Tub2 | Positive |
| 76 | M | S | IV | Tub2 | Positive |
| 82 | M | T | IV | Tub2 | Positive |
| 41 | F | T | I | Tub2 | Negative |
| 68 | M | S | I | Tub2 | Negative |
| 35 | M | C | II | Tub2 | Negative |
| 66 | M | C | IIIA | Por1 | Negative |
| 75 | M | A | IIIB | Tub2 | Negative |
| 26 | F | S | IIIB | Tub2 | Negative |
IL-23R expression was evaluated using an immunohistochemical analysis. C, cecum; A, ascending colon; T, transverse colon; D, descending colon; S, sigmoid colon; R, rectum; tub, tubular adenocarcinoma; por, poorly differentiated adenocarcinoma; muc, mucinous adenocarcinoma.
IL-23R expression in colon carcinoma cell lines
The expression of IL-23R in three types of cultured cells derived from colon carcinoma, MIP101, DLD-1 and KM12c was investigated. Western blot analysis revealed that all of the cell lines express the IL-23R protein (Fig. 2). However, the signal intensity differed among the cell lines. We also confirmed the expression of IL-23R mRNA in the cell lines by RT-PCR (data not shown).
Figure 2.
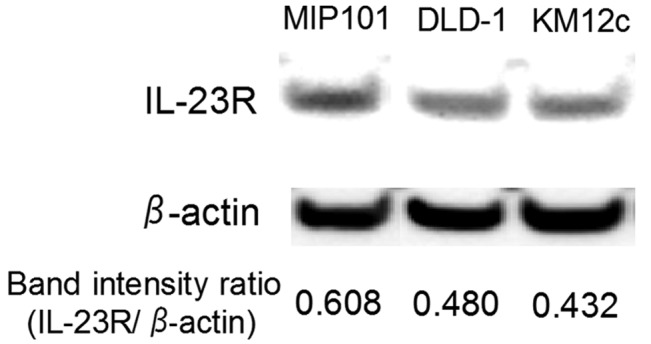
The results of the western blot analysis of IL-23R in the colon carcinoma cell lines. The band intensities of IL-23R were normalized to those of β-actin.
Cell proliferation and invasion assays
We investigated whether IL-23 directly contributes to the proliferation and invasive activity of the cultured colon carcinoma cells. Following stimulation with IL-23, the proliferative and invasive activities of DLD-1 cells were significantly increased by approximately 20%, whereas those of MIP101 and KM12c cells were not significantly altered (Fig. 3). The highest expression of IL-23R was observed in MIP101 cells, followed by DLD-1 and KM12c cells (Fig. 2). However, the changes in the proliferative and invasive activities did not reflect the IL-23R expression level.
Figure 3.
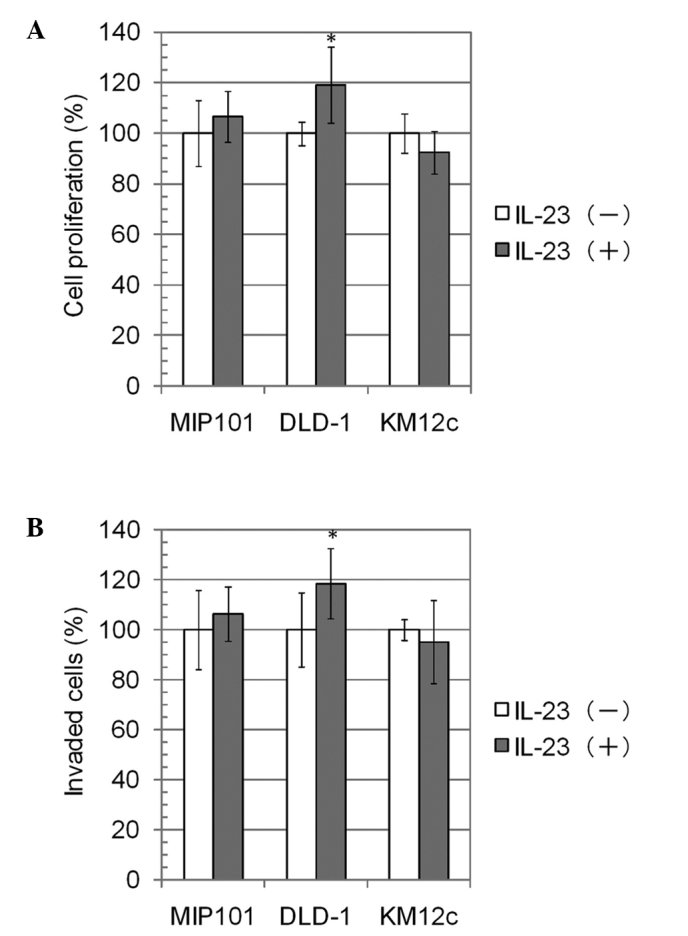
The results of the cell proliferation assay and invasion assays. The colon carcinoma cells were incubated for 24 h with or without recombinant human IL-23 (10 μg/ml) in the two assays. (A) In the cell proliferation assay, the OD450 value was measured, and the mean of the values without IL-23 was defined as 100%. The data are the means ± SD, n=7. (B) In the invasion assay, the number of invaded cells was counted and the mean of the numbers was defined as 100%. The data are the means ± SD, n=6. *p<0.05.
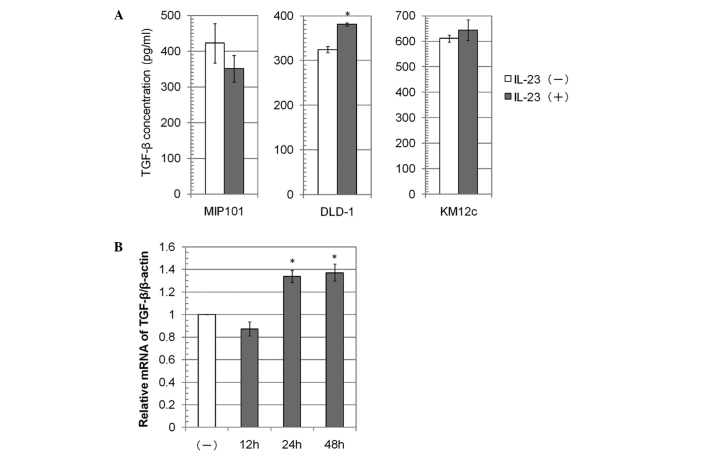
TGF-β secretion and mRNA expression by colon carcinoma cell lines
We investigated whether IL-23 contributes to TGF-β1 production by colon carcinoma cells. Following stimulation with IL-23, the TGF-β1 concentration in the medium increased approximately 20% in the DLD-1 cells, whereas it was not significantly altered in the MIP101 and KM12c cells (Fig. 4A). The relative mRNA expression of TGF-β1/β-actin in DLD-1 cells following stimulation with IL-23 for 24 or 48 h was also increased (Fig. 4B).
Figure 4.
The expression of TGF-β in the colon carcinoma cell lines. (A) The TGF-β1 concentration in the medium was measured by an ELISA following incubation of the cells for 48 h with or without recombinant human IL-23 (10 μg/ml). The mean of the values without IL-23 was defined as 100%. The data are the means ± SD, n=3. (B) The TGF-β1 mRNA level in DLD-1 cells was measured following culture of the cells with recombinant human IL-23 (10 μg/ml) for 12, 24 or 48 h. The data are the means ± SD, n=3. *p<0.05.
Discussion
Results of the present study revealed that IL-23R was expressed in the carcinoma tissue samples in 9 out of 15 cases, but not in the remaining 6 cases (Fig. 1A). The expression of IL-23R in colorectal carcinoma tissue was also reported in a recent study (14), but the differences among the patients were not described. All of the stage IV cases in the present study were positive for IL-23R. In addition, among the three colon carcinoma cell lines examined in the present study, the MIP101 cells demonstrated the highest expression of IL-23R. MIP101 cells were derived from a liver metastasis of colon adenocarcinoma, whereas DLD-1 and KM12c cells were derived from primary colon adenocarcinoma of Dukes’ stage B and C patients without distant metastasis, respectively. Therefore, the IL-23R expression in carcinoma may be associated with distant metastasis, although further studies are required to determine whether IL-23R expression is associated with the invasion and aggressiveness of the cancer.
Notably, the IL-23R expression was heterogeneous even within the same tumor (Fig. 1C and D). Our data revealed that IL-23R-positive and -negative (or very weakly positive) cells coexisted within the same tumor. Moreover, the IL-23R expression was relatively higher at the deepest point of invasion in certain cases, indicating that IL-23R expression may be correlated with the invasion of carcinoma.
Our results also indicated that there were a few IL-23R positive cells present in the normal colon tissue (Fig. 1B). Considering their localization, we expected that these IL-23R-positive cells were enterochromaffin cells, and we confirmed that these IL-23R-positive epithelial cells were positive for chromogranin A, a specific marker of enterochromaffin cells (data not shown). The effects of IL-23 on these IL-23R-positive enterochromaffin cells is currently under investigation.
Notably, our results demonstrated that the proliferative and invasive activities of DLD-1 cells increased when the cells were stimulated with IL-23, in spite of the absence of any immune cells, such as TH17 cells (Fig. 3). These findings indicate that IL-23 directly enhances the malignancy of the colon carcinoma cells. However, in the cases of MIP101 and KM12c cells, IL-23 did not change these activities in spite of their expression of IL-23R (Fig. 2). Therefore, the effects of IL-23 on colorectal carcinoma cells varies, and the effect is not in accordance with the intensity of the IL-23R expression. In TH17 cells, the increased STAT3 activity induced by IL-23 promotes the expression of cytokines such as IL-17 (13). Results of the western blot analysis showed whether STAT3 in the DLD-1 cells was phosphorylated by IL-23; however, only a minimal amount of phosphorylated STAT3 was detected (data not shown).
Another possible mechanism for the IL-23-induced proliferative and invasive activity of DLD-1 cells was suggested by our results. The TGF-β1 production by DLD-1 cells was significantly increased by IL-23 stimulation (Fig. 4), whereas IL-23 did not affect the TGF-β production in the MIP101 and KM12c cells. These results correlated with those of the cell proliferation and invasion assay (Fig. 3). Therefore, an autocrine mechanism via TGF-β production might underlie the IL-23-induced proliferative and invasive activity observed in the DLD-1 cells.
During the early stages of tumor development, the TGF-β/Smad pathway acts as a tumor suppressor (15,16). However, TGF-β also promotes the invasion or the proliferation of certain tumor cells (15–18). Epithelial-mesenchymal transition (EMT) is known to promote carcinoma progression through a variety of mechanisms such as endowing cells with migratory and invasive properties (19). TGF-β signaling is important in inducing the EMT (20). It is suggested that IL-23 stimulates DLD-1 cells to produce TGF-β and therefore promote malignancy via induction of the EMT. Moreover, Forkhead box P3 (FOXP3) production is elevated in colorectal carcinoma and the IL-23/IL-23R signaling pathway may affect the FOXP3-associated expression-repression and proliferation-suppression observed in tumor cells (14). The gene transcription of FOXP3 is regulated by TGF-β signaling, including the NFAT and Smad3 pathways, in regulatory T cells (21,22). Considering that IL-23 stimulated DLD-1 cells to produce TGF-β in the present study, our findings suggest that the IL-23/IL-23R signaling pathway may affect FOXP3 expression via TGF-β signaling.
In conclusion, this is the first study to demonstrate the direct effects of IL-23 on colorectal carcinoma cells and a correlation between IL-23/IL-23R and TGF-β in carcinoma cells. IL-23 is a potential target for colon cancer immunotherapy. However, the heterogeneity in the IL-23R expression and the effects of IL-23 on the colorectal carcinoma cells should be considered. Further characterization of IL-23R-positive colorectal carcinoma cells is required before IL-23-associated biotherapy becomes available in the clinic.
References
- 1.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarra M, Pallone F, Macdonald TT, et al. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 4.Monteleone I, Pallone F, Monteleone G. Interleukin-23 and Th17 cells in the control of gut inflammation. Mediators Inflamm. 2009;2009:297645. doi: 10.1155/2009/297645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 7.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 8.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 9.Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol. 2008;180:5625–5635. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M, Ehara M, Suzuki S, et al. IL-23 promotes growth and proliferation in human squamous cell carcinoma of the oral cavity. Int J Oncol. 2010;36:1355–1365. doi: 10.3892/ijo_00000620. [DOI] [PubMed] [Google Scholar]
- 11.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 12.Kortylewski M, Xin H, Kujawski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan F, Zhang L, Wu J, et al. IL-23/IL-23R: potential mediator of intestinal tumor progression from adenomatous polyps to colorectal carcinoma. Int J Colorectal Dis. 2011;26:1511–1518. doi: 10.1007/s00384-011-1232-6. [DOI] [PubMed] [Google Scholar]
- 15.Meulmeester E, Ten Dijke P. The dynamic roles of TGF-β in cancer. J Pathol. 2011;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 16.Ten Dijke P, Goumans MJ, Itoh F, et al. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 17.Tian M, Schiemann WP. The TGF-β paradox in human cancer: an update. Future Oncol. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruna A, Darken RS, Rojo F, et al. High TGFβ-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su J, Liu YC. Foxp3 positive regulatory T cells: a functional regulation by the E3 ubiquitin ligase Itch. Semin Immunopathol. 2010;32:149–156. doi: 10.1007/s00281-009-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-β-induced Foxp3 expression. Mucosal Immunol. 2010;3:230–238. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]