Abstract
BACKGROUND AND PURPOSE
The renin-angiotensin system (RAS) is critical for the control of blood pressure by the CNS. Recently, direct renin inhibitors were approved as antihypertensive agents. However, the signalling mechanism of renin, which regulates blood pressure in the nucleus tractus solitarii (NTS) remains unclear. Here we have investigated the signalling pathways involved in renin-mediated blood pressure regulation, at the NTS.
EXPERIMENTAL APPROACH
Depressor responses to renin microinjected into the NTS of Wistar-Kyoto rats were elicited in the absence and presence of the endothelial nitric oxide synthase (eNOS)-specific inhibitor, N(5)-(-iminoethyl)-L-ornithine, Akt inhibitor IV and LY294002, a PI3K inhibitor and GP antagonist-2A [Gq inhibitor]. Lisinopril (angiotensin converting enzyme inhibitor), losartan, valsartan (angiotensin AT1 receptor antagonists), D-Ala7-Ang-(1-7) (angiotensin-(1-7) receptor antagonist) were used to study the involvement of RAS on renin-induced depressor effects.
KEY RESULTS
Microinjection of renin into the NTS produced a prominent depressor effect and increased NO production. Pretreatment with Gq-PI3K-Akt-eNOS pathway-specific inhibitors significantly attenuated the depressor response evoked by renin. Immunoblotting and immunohistochemical studies further showed that inhibition of PI3K significantly blocked renin-induced eNOS-Ser117 and Akt-Ser473 phosphorylation in situ. In addition, pre-treatment of the NTS with RAS inhibitors attenuated the vasodepressor effects evoked by renin. Microinjection of renin also increased Ras activation in the NTS.
CONCLUSIONS AND IMPLICATIONS
Taken together, these results suggest renin modulated blood pressure at the NTS by AT1 and Mas receptor-mediated activation of Gq and Ras to evoke PI3K-Akt-eNOS signalling.
Keywords: renin, nucleus tractus solitarii, nitric oxide, angiotensin type-1 receptor, Mas receptor
Introduction
Renin is a critical component of the renin-angiotensin system (RAS) and catalyzes the rate-limiting step of converting angiotensinogen to angiotensin I. Angiotensin I is converted to angiotensin II (Ang II) by angiotensin converting enzyme (ACE). Dzau and his colleagues have demonstrated the expression of renin gene in mouse and rat brains. (Dzau et al., 1986) Local synthesis of angiotensin, independent of angiotensin generation in the circulation, is now widely accepted, indicating that renin can function in tissue sites, including the brain. (Dampney, 1994; Davisson et al., 2000) Taken together, these studies indicate that renin is expressed locally in the brain and may functionally regulate brain RAS through the generation of angiotensin peptides.
The RAS plays an important role in blood pressure regulation and cardiovascular disease risk profile. ACE inhibitors and antagonists of the angiotensin AT1 receptor (receptor nomenclature follows Alexander et al., 2011) are current antihypertensive therapies. In 2007, direct renin inhibitors were released as a new class of antihypertensive agents (Brown, 2008). Direct renin inhibitors can reduce renin activity, even in the presence of elevated renin activity induced by ACE inhibitors or AT1 receptor antagonists. Direct renin inhibitors not only lower blood pressure but also have renoprotective effects and reduce the risk of heart failure (McMurray et al., 2008; Parving et al., 2008). These findings suggest that renin plays a critical role in blood pressure regulation. However, the role of renin itself and its mechanism of action in the brainstem remain unclear.
The nucleus tractus solitarii (NTS), which is located in the dorsal medulla of the brain stem, participates in the central control of blood pressure and sympathetic nerve activity. Our previous studies demonstrated that several neuromodulators are involved in cardiovascular control by the NTS, including Ang II (Mosqueda-Garcia et al., 1990; Cheng et al., 2010) and NO (Tseng et al., 1996).
NO is synthesized by nitric oxide synthase (NOS) and there are three different types of NOS: neuronal nitric oxide synthase (nNOS), endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS; Calabrese et al., 2007) In vivo, eNOS gene transfer experiments in the NTS caused hypotension and bradycardia (Sakai et al., 2000; Hirooka et al., 2003; Tai et al., 2004) and eNOS and nNOS both participated in central cardiovascular regulation by the NTS. (Ho et al., 2008; Chiang et al., 2009; Cheng et al., 2010; 2011) Nevertheless, the relationship between renin, NO synthase activity and the corresponding modulation of blood pressure by the NTS has not been established.
In this study, we investigated whether microinjection of renin into the NTS might regulate blood pressure, and we determined which form of NOS could be activated by renin administration. In addition, we investigated which receptors and downstream signalling pathways were involved in renin-induced effects in the NTS. Our results suggest that the Gq- PI3K-Akt-eNOS signalling pathway was involved in the modulation of blood pressure by activation of AT1 and Mas receptors in the NTS.
Methods
Animals
All animal care and experimental research protocols had been approved by the Research Animal Facility Committee of Kaohsiung Veterans General Hospital. Male Wistar-Kyoto (WKY) rats of 250 to 300 g were obtained from National Science Council Animal Facility and housed in the animal room of Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan). The rats were kept in individual cages in a room in which lighting was controlled (12 h on/12 h off), and the temperature was maintained at 23 to 24°C. The rats were allowed to acclimatise to the housing conditions for 1 week.
Intra-NTS microinjection
The preparation of animals for intra-NTS microinjection and the methods used to locate the NTS have been described previously (Tseng et al., 1996). In the present study, each injection volume in the NTS was restricted to 60 nL. Rats were anaesthetized with urethane (1.0 g·kg−1 i.p., supplemented with 300 mg·kg−1 i.v. as necessary). A polyethylene catheter was placed in the femoral vein for drug administration. blood pressure was measured directly through a catheter placed in the femoral artery and connected to a pressure transducer (P23 ID, Gould Electronics, Eichstetten, Germany) and polygraph (RS3800, Gould Electronics). Heart rate (HR) was monitored continuously by a tachograph preamplifier (13-4615-65, Gould Electronics). Tracheostomy was performed to maintain airway patency during the experiment. For microinjection into brain stem nuclei, the rats were placed in a stereotaxic instrument (Kopf), with the head flexed downward at a 45° angle. The dorsal surface of the medulla was exposed by limited craniotomy, and the rats were rested for at least 1 h before experiments. Single-barrel glass catheter were prepared (0.031-inch OD, 0.006 inch ID; Richland Glass Co, Vineland, NJ, USA) that had external tip diameters of 40 µm. The volume of microinjection (60 nL) was directly measured by the displacement of fluid meniscus in the micropipette barrel and confirmed visually under a microscope with a fine reticule (Tseng et al., 1989; Dhar et al., 2000). To verify that the needle tip of the glass electrode was exactly in the NTS, L-glutamate (0.154 nmol/60 nL) was microinjected. This would induce a characteristically abrupt fall in blood pressure (ΔBP ≥−35 mmHg) and HR (ΔHR ≥−50 bpm) if the needle tip was located precisely in the medial site of the intermediate one third of the NTS with the coordinates of anteroposterior, 0.0 mm; mediolateral, 0.5 mm; and vertical, 0.4 mm with the obex as reference (Tseng et al., 1996).
The injected renin (2.4 fg, 24 fg, 240 fg and 2400 fg) was dissolved in artificial cerebrospinal fluid (aCSF; 142 mmol·L−1 NaCl, 5 mmol·L−1 KCl, 10 mmol·L−1 glucose and 10 mmol·L−1 HEPES, pH 7.4). Other compounds – L-NAME (33 nmol; non-selective NOS inhibitor, Huang et al., 2004); L-NIO (6 nmol; inhibitor of eNOS, Ho et al., 2008); vinyl-L-NIO (600 pmol; selective nNOS inhibitor Chiang et al., 2009); lisinopril (2.4 fmol; ACE inhibitor); losartan (4 nmol; AT1 receptor antagonist); D-Ala7-Ang-(1-7) (144 fmol; angiotensin-(1-7) (Ang-(1-7)) antagonist) and GPA-2A (1.98 pmol, G protein q subunit (Gq) inhibitor) were dissolved in aCSF. PD98059 (6 pmol; MEK inhibitor, Ho et al., 2008); LY294002 (6 pmol; PI3K inhibitor, Huang et al., 2004); valsartan (7.5 pmol; AT1 receptor antagonist); gallein (240 fmol; G protein βγ subunit (Gβγ) inhibitor); 7-NI (66 pmol; nNOS inhibitor) and Akt inhibitor IV (375 fmol) were dissolved in DMSO, then diluted with aCSF.
Determination of NO in NTS
Renin and aCSF groups of rats (6 rats per group) were enrolled in the experiment. Rats were anesthetized with urethane (1.0 g kg−1 i.p., supplemented with 300 mg kg−1 i.v., if necessary).The NTS was dissected by micropunch (1-mm inner diameter) from a 1-mm thick brainstem slice at the level of the obex under a microscope. Total protein was prepared by homogenizing (BBX24 Bullet Blender homogenizer, Next Advance, Inc., Averill Park, NY, USA) the NTS tissue in lysis buffer (CelLytic MT Cell Lysis Reagent, Sigma-Aldrich, St. Louis, MO, USA), and incubating for 1 hour at 4 oC. Samples were then deproteinized using Microcon YM-30 centrifugal filter units (Millipore, Bedford, MA, USA). The amount of total NO (as nitrite and nitrate) in the samples was determined by a modification of the procedure based on the purge system of Sievers Nitric Oxide Analyzer (NOA 280i; Sievers Instruments, Boulder, CO, USA), which involves the use of chemiluminescence. Samples (10 µL) were injected into a reflux column containing 0.1 mol·L−1 VCl3 in 1 mol·L−1 HCl at 90°C to reduce any nitrates and nitrites into NO. The NO was then combined with the O3 produced by the analyzer to form NO2. The resulting emission from the excited NO2 was detected by a photomultiplier tube and recorded digitally (mV). The values were then interpolated to a standard curve of NaNO3 concentrations determined concurrently. The measurements were made in triplicate for each sample. The levels of NO were expressed as concentrations of nitrate in each sample, corrected for the total protein in the NTS samples from each rat (as µM nitrate per µg protein).
Immunoblotting analysis
The NTS of rats were removed after injection of renin or vehicle. Total protein was prepared by homogenizing the NTS for 1 h at 4°C in lysis buffer and proteinase inhibitor cocktail. Protein extracts (20 µg per sample assessed by bicinchoninic acid protein assay,. Pierce Chemical Co., IL, USA) were subjected to 6% to 12.5% SDS-Tris glycine gel electrophoresis and transferred to a polyvinylidene fluoride membrane (GE Healthcare, Buckingamshire, UK). The membranes were blocked and incubated at 4°C overnight with the appropriate antibody: rabbit anti-P-ERK1/2-Thr202/Tyr204 (1:1000; Cell Signaling Technology, Denvers, MS, USA), rabbit anti-ERK1/2 (1:1000; Cell Signaling Technology), rabbit anti-P-Akt-Ser473 (1:1000; Cell Signaling Technology), rabbit anti-Akt (1:1000; Cell Signaling Technology), mouse anti-P-eNOS-Ser1177 (1:1000; BD Biosciences, San Jose, CA, USA) and mouse anti-eNOS (1:1000; BD Biosciences). Also, mouse anti-actin (1:10 000; Millipore), anti-P-nNOS-Ser1416 (1:1000; Abcam, Cambridge, UK), anti-nNOS (1:2000; Millipore), and anti-iNOS (1:1000; Millipore) were diluted in PBST with 5% bovine serum albumin. Horseradish peroxidase (HRP)-conjugated anti-mouse (1:10 000) or anti-rabbit antibody (1:10 000) was used as the secondary antibody at room temperature for 1 h.
Immunohistochemistry analysis
After perfusion with 0.9% normal saline, the rat brainstems were fixed immediately in 4% formaldehyde overnight and embedded in paraffin. The brainstems were sectioned coronally at a 5 µm thickness. The sections were deparaffinized, quenched in 3% H2O2/methanol, heated (microwave) in citrate buffer (10 mmol·L−1, pH 6.0), blocked in 5% goat serum and incubated with an anti-P-eNOS-Ser1177 antibody overnight at 4°C. Next, the sections were incubated with biotinylated secondary antibodies (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 h and in AB complex (1:100) for 30 min at room temperature. The sections were visualized with a DAB substrate kit (Vector Laboratories) and counterstained with haematoxylin. The sections were then photographed with a microscope mounted with a charge-coupled device camera.
Ras activation assay
Ras activation was measured using a Ras Activation elisa Assay Kit (Millipore) following the manufacturer's instructions. The assay was initiated by the addition of recombinant Ras-binding domain of Raf-1 to the glutathione-coated elisa plate via a glutathione-S-transferase/glutathione interaction, thus capturing the active Ras and allowing the inactive/GDP-bound Ras to be washed away. The captured, active Ras was detected and measured quantitatively through the addition of a monoclonal anti-Ras antibody that detects K-, H-, N-Ras isoforms from rat. A HRP-conjugated secondary antibody was then added for the detection. Following the addition of the chemiluminescent substrate, signals can be measured using a luminometer (Promega, Madison, WI, USA).
Data analysis
A paired Student's t-test was used for comparing blood pressure measurements and counting cells expressing P-eNOS-Ser1177. One-way anova with Scheffe's post hoc test were applied to compare group differences. Differences with P < 0.05 were considered significant. All data are expressed as the means ± SEM.
Materials
Experimental drugs, such as urethane, Triton-X100, L-glutamate, heparin, human renin, lisinopril, losartan, N-nitro-L-arginine methyl ester (L-NAME), 7-nitroindazole (7-NI), LY294002, PD98059 and D-Ala7-Ang-(1-7), were obtained from Sigma-Aldrich (St. Louis, MO, USA). N(5)-(-iminoethyl)-L-ornithine (L-NIO), GP antagonist-2A (GPA-2A) and Akt inhibitor IV were obtained from Calbiochem (Darmstadt, Germany). N(5)-(1-imino-3-butenyl)-ornithine (vinyl-L-NIO) was obtained from ALEXIS (Lausen, Switzerland). Gallein and valsartan were obtained from Tocris (Bristol, UK).
Results
Renin induces systemic vasodepressor effect and NO release in the NTS
In this study, we utilized urethane-anaesthetized WKY rats to investigate the cardiovascular effects of microinjection of renin into the NTS. Tachyphylaxis was observed after repeated administration of similar doses of renin in the same site of the NTS under these experimental conditions (data not shown). Different concentrations of renin were then injected into the NTS of several rats. All the doses subsequently used (2.4 fg, 24 fg, 240 fg and 2400 fg) decreased mean blood pressure and heart rate (Supporting Information Figure S1). The response to renin (240 fg) unilaterally injected into the NTS was a fall in blood pressure (Figure 1A; mean values in Figure 1B, n= 6). aCSF was used as the drug vehicle and for control experiments. Interestingly, NO production in the NTS was significantly increased after the administration of renin.
Figure 1.
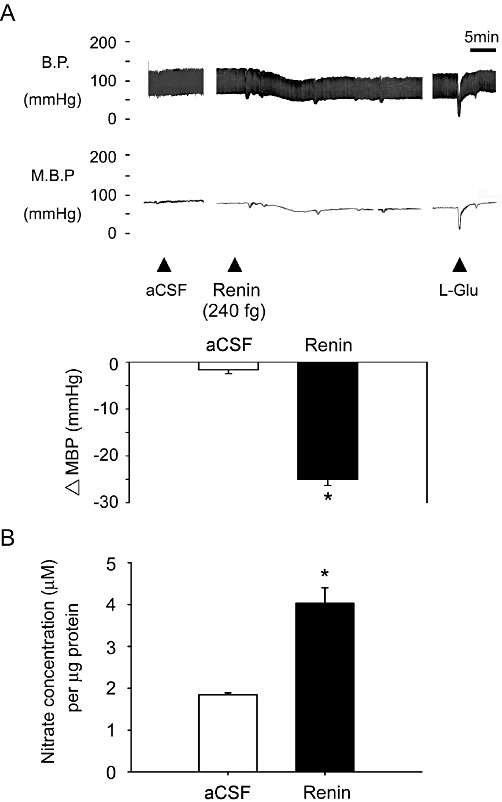
Systemic blood pressure (BP) and NO production in the NTS after administration of renin to the NTS. (A) Representative tracing show the depressor effect after unilateral microinjection of renin (240 fg) into the NTS. Artificial cerebrospinal fluid (aCSF) as the drug vehicle did not change blood pressure. Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the aCSF group. (B) Levels of NO in samples of the NTS after microinjection of renin. The bar graph shows the NO concentration (as µM nitrate per µg of total protein). Pretreatment with renin significantly elevated NO levels in the NTS compared with aCSF. *P < 0.05, significantly different from the aCSF group.
Renin induces eNOS phosphorylation in the NTS
We further investigated which NOS isoform contributes to depressor effects and NO release induced by renin in the NTS of WKY rats. Pretreatment with the non-selective NOS inhibitor, L-NAME, attenuated the depressor effect of renin (Figure 2A, n= 6). Prior treatment with the selective eNOS inhibitor, L-NIO, also significantly attenuated the depressor effect of renin (Figure 2B, n= 6). In contrast, administration of the specific nNOS inhibitor, vinyl-L-NIO or 7-NI, did not diminish the depressor effect of renin in the NTS (Figure S2A and 2B, n= 6).
Figure 2.
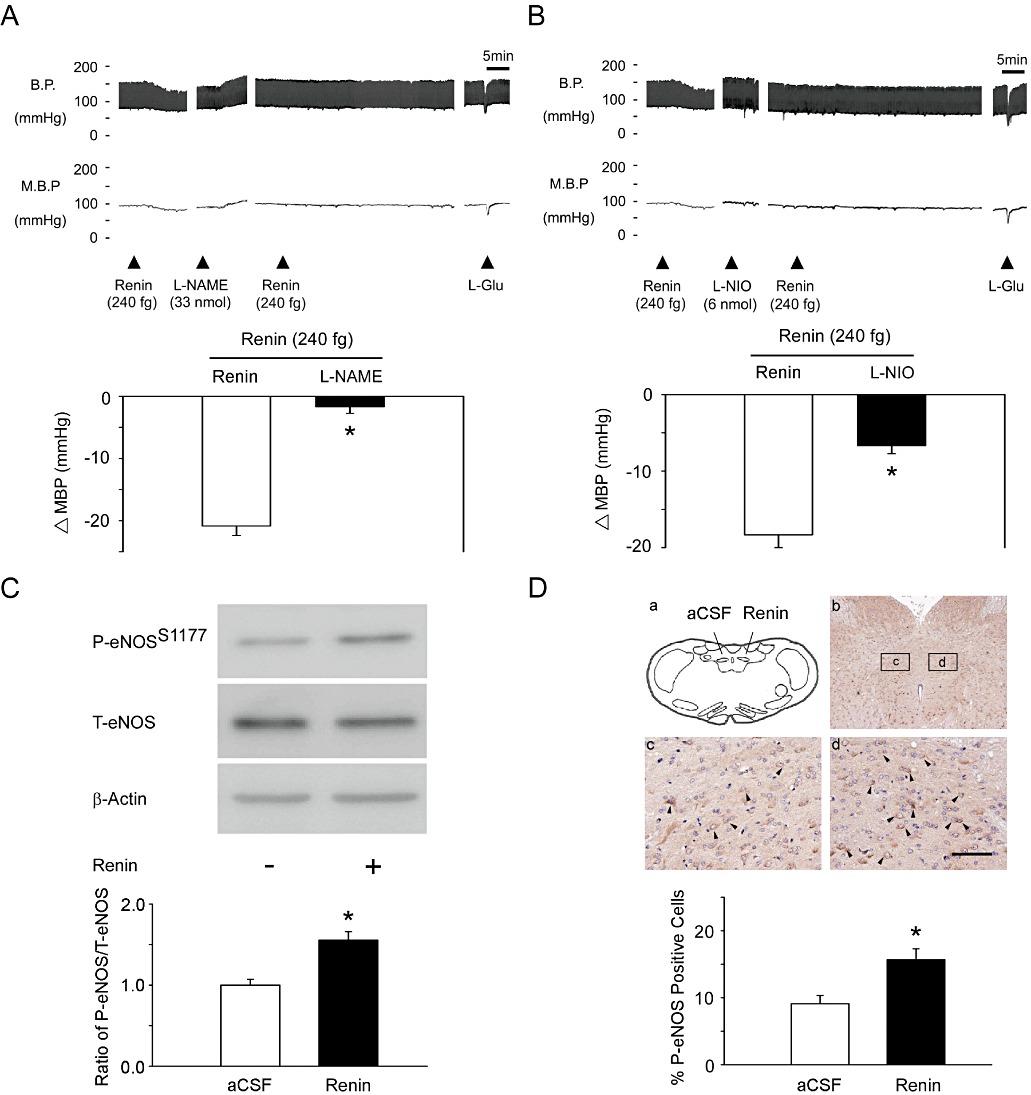
Microinjection of renin induces eNOS-Ser1177 phosphorylation in the NTS. (A) Representative tracing demonstrates that the depressor effect of renin was significantly attenuated by a non-selective NOS inhibitor, L-NAME (33 nmol). Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05 significantly different from the renin group. (B) Representative tracing demonstrates that the depressor effect of renin was significantly attenuated by the specific eNOS inhibitor, L-NIO (6 nmol). Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the renin group. (C) The quantitative immunoblotting analysis demonstrates that renin treatment increased the level of P-eNOS-Ser1177 protein in the NTS. Densitometric analysis of P-eNOS-Ser1177 protein levels (means ± SEM, n= 6) after administration of aCSF or renin. *P < 0.05, significantly different from the aCSF group. (D) Immunohistochemical staining of the brainstem for P-eNOS-Ser1177 showed that injection of renin into the NTS induced P-eNOS-Ser1177 (c vs. d). Arrows indicate P-eNOS-Ser1177-positive cells. The scale bar represents 200 µm. Summary data (means ± SEM, n= 6) are shown in the graph. The percentage of P-eNOS-Ser1177-positive cells was determined by counting P-eNOS-Ser1177-expressing cells in each hemisphere of the NTS at 200 × magnification. These counts were divided by all of the cells in the same paraffin section. *P < 0.05, significantly different from the a CSF group.
In addition, immunoblotting analyses of proteins extracted from the NTS demonstrated that treatment with renin did not increase nNOS-Ser1416 phosphorylation (Figure S2C, n= 6) or iNOS protein expression (Figure S2D, P > 0.05, n= 6) in the NTS. However, treatment with renin significantly increased the level of eNOS-Ser1177 phosphorylation (Figure 2C, n= 6) and (Figure 2D, n= 6) the proportion of P- eNOS-Ser1177-positive cells in the NTS, compared with the corresponding values in the group treated with aCSF.
PI3K inhibitor attenuated renin-induced eNOS phosphorylation in the NTS
Our previous studies demonstrated that Akt and ERK could regulate NOS activity in the NTS (Huang et al., 2004; Ho et al., 2008). To test whether Akt or ERK may induce eNOS phosphorylation, the phosphorylation of Akt was determined by immunoblotting analyses with an antibody against P-Akt-Ser473. After administration of renin into the NTS, the phosphorylation level of Akt in the NTS was significantly elevated, compared with that after aCSF (Figure 3A, n= 6). However, there were no differences in ERK phosphorylation levels between the renin-treated and aCSF groups (Figure S3A, n= 6). Furthermore, pretreatment with the PI3K inhibitor, LY294002, attenuated renin-induced eNOS-Ser1177 phosphorylation in the NTS (Figure 3D, lane 2 and 3, n= 6).
Figure 3.
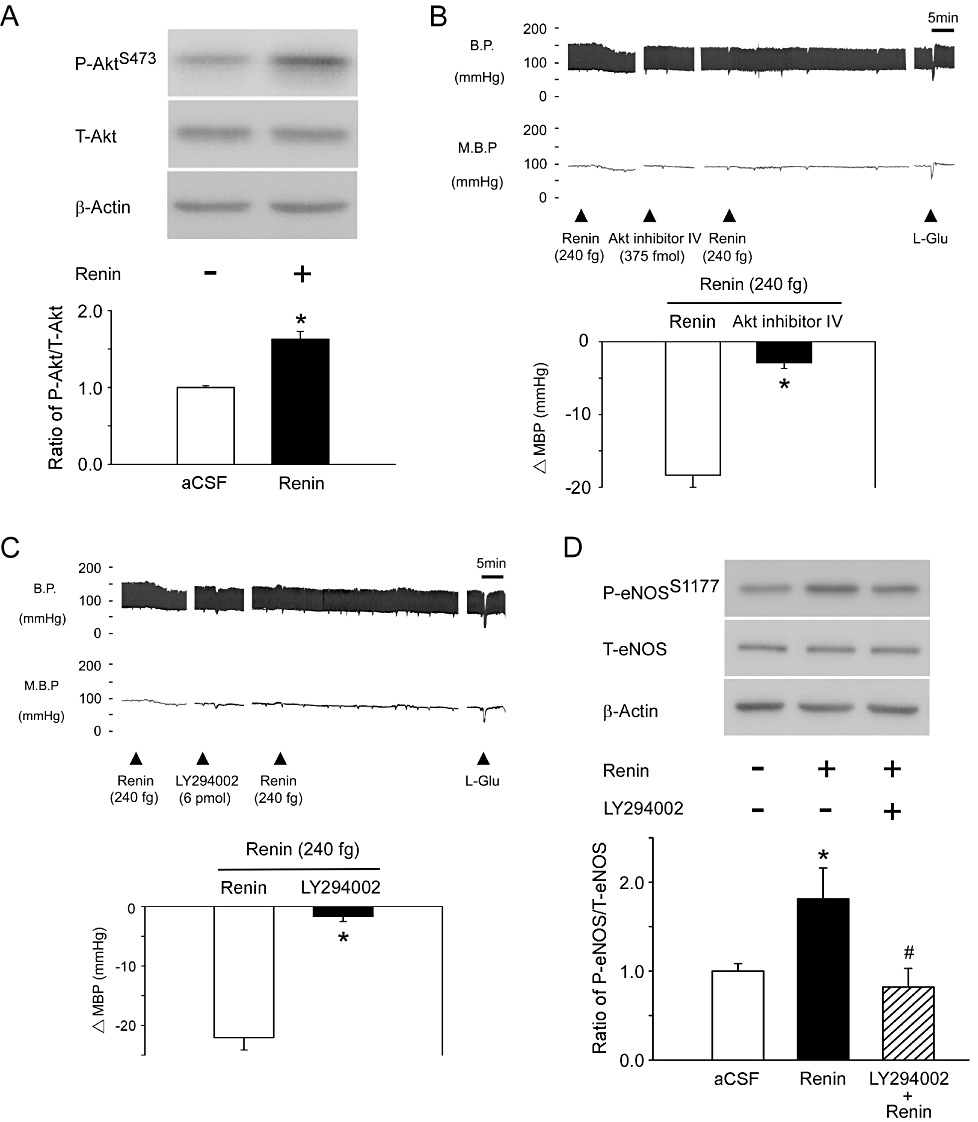
PI3K and Akt participate in renin-mediated eNOS-Ser1177 phosphorylation in the NTS. (A) The quantitative immunoblotting analysis demonstrates that renin treatment increased the level of P-Akt-Ser473 protein in the NTS. Densitometric analysis of P-Akt-Ser473 protein levels (means ± SEM, n= 6) after administration of aCSF or renin. *P < 0.05, significantly different from the aCSF group. (B) Representative tracings reveal the effects of BP by microinjection renin (240 fg) into the NTS pretreated with Akt inhibitor, Akt inhibitor IV (375 fmol). *P < 0.05, significantly different from the renin group. (C) The blood pressure of renin (240 fg) injection into the NTS after administration of the PI3K inhibitor, LY294002 (6 pmol). Representative tracings demonstrate that the depressor effect of renin was significantly attenuated by LY294002. Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the renin group. (D) Immunoblotting analysis reveals that the P-eNOS-Ser1177 protein level was increased after renin administration in the NTS. Phosphorylation of eNOS-Ser1177 was reduced by pretreatment with LY294002. Densitometric analysis of P-eNOS-Ser1177 protein levels (means ± SEM, n= 6) after treatment with aCSF, renin or LY294002. *P < 0.05, significantly different from the aCSF group; #P < 0.05, significantly different from the renin group.
We then used specific pharmacological inhibitors to confirm these results. Prior microinjection of the Akt-specific inhibitor, Akt inhibitor IV, significantly diminished the vasodepressor response evoked by renin (Figure 3B, n= 6). Pretreatment with LY294002 similarly attenuated the response to renin (Figure 3C, n= 6). In contrast, pretreatment of the NTS with the MEK inhibitor, PD98059, did not attenuate the depressor effect of renin (Figure S3B, n= 6).
Central administration of renin may regulate blood pressure through AT1 and Mas receptors in the NTS
To assess the connection between renin and the downstream PI3K-Akt-eNOS signalling cascade in the NTS, we tested the effects of lisinopril (ACE inhibitor), losartan, valsartan (AT1 receptor antagonists) and D-Ala7-Ang(1-7) [Ang-(1-7) antagonist] on blood pressure responses to renin, in the NTS. Pre-treatment with lisinopril, losartan or valsartan all attenuated the depressor effect of renin (Figures 4A, 4B and 4C, n= 6). In addition, Figure 4D shows that injection of the Mas receptor antagonist, D-Ala7-Ang-(1-7) also attenuated the depressor effect of renin (n= 6).
Figure 4.
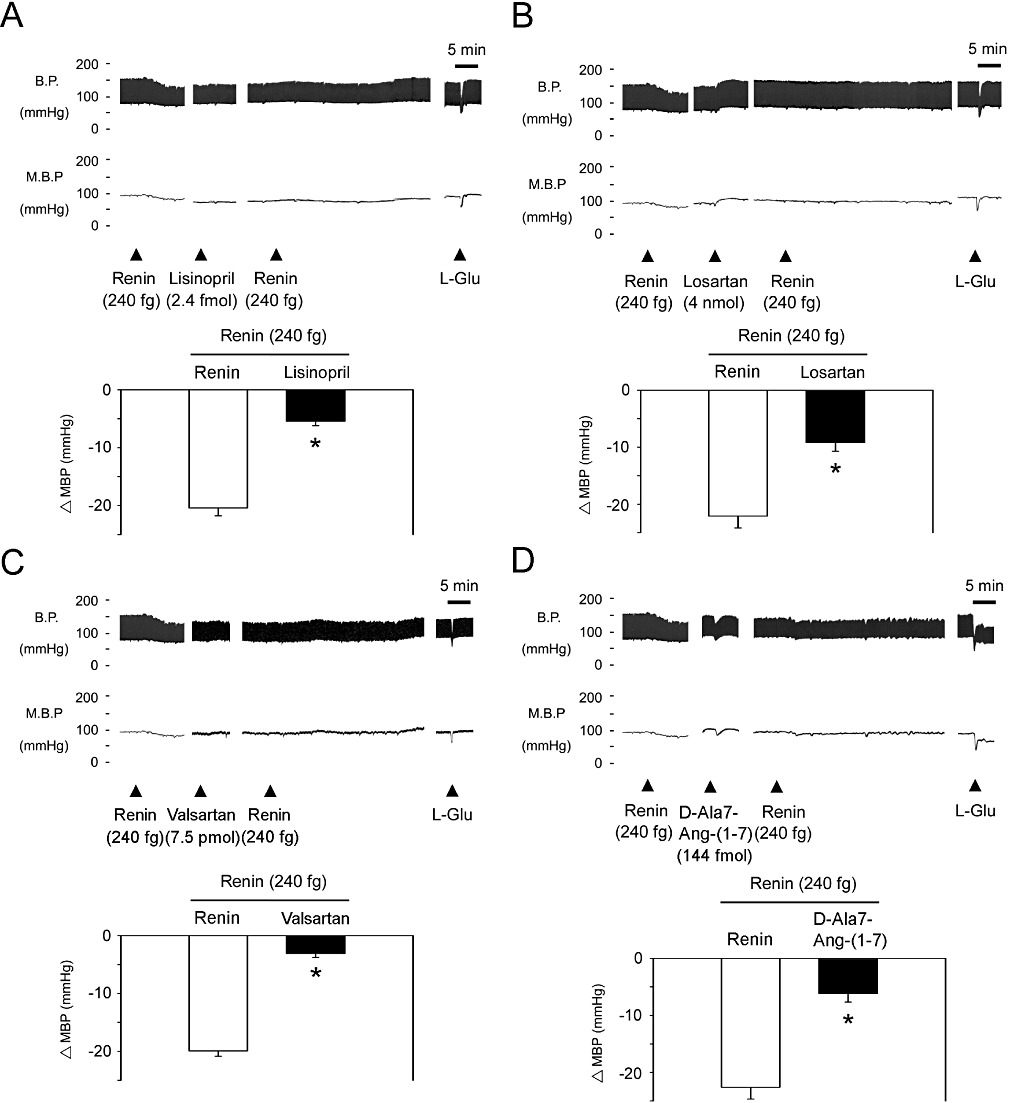
Angiotensin AT1 and Mas receptors participate in renin-mediated depressor effects at the NTS. (A) Blood pressure response to renin (240 fg) injection into the NTS after administration of the ACE inhibitor, lisinopril (2.4 fmol). Representative tracings demonstrate that the depressor effect of renin was significantly attenuated by lisinopril. Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the renin group. (B) Blood pressure response to renin (240 fg) injection of into the NTS after administration of losartan (4 nmol). Representative tracings demonstrate that the depressor effect of renin was significantly attenuated by losartan. Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the renin group. (C) Blood pressure response to renin (240 fg) injection of into the NTS after administration of valsartan (7.5 pmol). Representative tracings demonstrate that the depressor effect of renin was significantly attenuated by valsartan. Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the renin group. (D) Blood pressure response to renin (240 fg) injection of into the NTS after administration of the angiotensin-(1-7) antagonist, D-Ala7-Ang-(1-7) (144 fmol). Representative tracings demonstrate that the depressor effect of renin was significantly attenuated by D-Ala7-Ang-(1-7). Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the renin group.
Renin may activate the PI3K-Akt-eNOS cascade via Gαq and Ras signalling in the NTS
These results showed that renin could activate PI3K-Akt-eNOS signalling in the NTS. Previous studies have demonstrated that Ang II and Ang-(1-7) may activate PI3K and Akt through AT1 receptors coupled to Gβ (Quignard et al., 2001), AT1 receptors coupled to Gq (Dugourd et al., 2003) or Mas receptors (Sampaio et al., 2007) Therefore, we investigated the G-protein coupling involved in the effects of the PI3K-Akt-eNOS pathway within the NTS and its relevance to the response to renin. Pretreatment with the Gβγ inhibitor, gallein, did not diminish the depressor effect of renin in the NTS (Figure S4, n= 6). However, pre-treatment with the Gq inhibitor, GPA-2A, attenuated the depressor effect of renin at the NTS (Figure 5A, n= 6). We further assessed the involvement of Ras in the renin-induced depressor effect and found that administration of renin to the NTS enhanced Ras activation (Figure 5B, n= 6).
Figure 5.
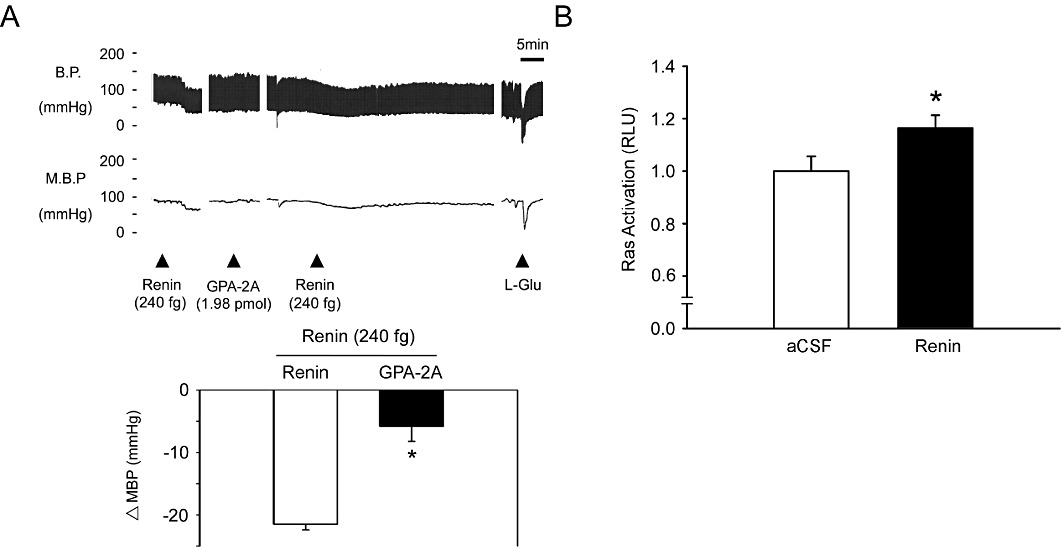
Microinjection of renin induces Gq-Ras signalling in the NTS. (A) Blood pressure response to renin (240 fg) injection into the NTS after administration of the Gq inhibitor, GPA-2A (1.98 pmol). Representative tracings demonstrate that the depressor effect of renin was significantly attenuated by GPA-2A. Summary data (means ± SEM, n= 6) are shown in the graph. *P < 0.05, significantly different from the renin group. (B) Bar graph showing the activation ratio of Ras after microinjection of renin into the NTS.. Note the significant increase in Ras activation after treatment with renin. *P < 0.05, significantly different from the aCSF group.
Discussion and conclusions
In the medulla of the brainstem, the nuclei have extensive connections to each other, and their major function is regulation of sympathetic nerve activity to modulate blood pressure. Ang II activates AT1 and AT2 receptors to modulate sympathetic nerve activity, in the brainstem but there is high expression of only AT1 receptors in the medial NTS (Lenkei et al., 1997). Our present data show that microinjection of renin into the NTS of WKY rats induced a depressor effect and increased NO production (Figure 1). This renin-mediated depressor response was similar to the effect induced by Ang II in the NTS (Mosqueda-Garcia et al., 1990; Fow et al., 1994; Tan et al., 2005). In addition, pre-treatment with an ACE inhibitor or AT1 receptor antagonists, separately attenuated the depressor effect of renin, suggesting that renin induced depressor responses through Ang II and AT1 receptors in the NTS (Figure 4). The Mas receptor is a G-protein-coupled receptor and is the endogenous receptor for Ang-(1-7) (Jackson et al., 1988). Mas receptors can antagonize AT1 receptor function by hetero-oligomeric interaction (Kostenis et al., 2005) and unilateral injections of Ang-(1-7) into the NTS caused depressor and bradycardic effects (Campagnole-Santos et al., 1989). In addition, the depressor effect evoked by Ang-(1-7) also involves a NO-mediated signalling (Alzamora et al., 2002). Moreover, Ang-(1-7) up-regulated the PI3K-Akt-eNOS pathway to increase NO production, through Mas receptors (Sampaio et al., 2007). However, we observed that pre-treatment with AT1 receptor antagonists and a Ang-(1-7) antagonist both attenuated the depressor effect produced by renin (Figure 4). Thus, we would propose that both AT1 and Mas receptors may contribute to the regulation of blood pressure by renin, at the NTS. The mechanisms connecting Mas receptors and Ras signalling in the NTS remain to be elucidated.
In the NTS, nNOS plays an important role in the modulation of blood pressure (Chiang et al., 2009; Cheng et al., 2010) and block of its expression in the NTS increased blood pressure (Maeda et al., 1999). Also, overexpression of eNOS induced a depressor response (Sakai et al., 2000; Hirooka et al., 2003; Tai et al., 2004). In Figure 2 and Figure S2, we found increased eNOS-Ser1177 phosphorylation levels in the NTS after administration of renin, whereas no activity or expression reductions were detected for nNOS or iNOS. Therefore, our results indicated that eNOS acts as a key factor in the production of NO in the NTS of WKY rats (Figure 1B). Because our previous study revealed that eNOS might play a key role in NTS cardiovascular function via the MAP kinase-ERK kinase (MEK)-ERK1/2 and eNOS signalling (Ho et al., 2008), we decided to further investigate this possibility. Our present data indicated that renin might regulate blood pressure through AT1 receptors. We propose that eNOS might act as a downstream target of the AT1 and Mas receptors and may be involved in renin-induced NO production in the NTS, thereby regulating blood pressure.
It has been reported that Akt, AMP-activated protein kinase, PKA, protein phosphatase 2A, protein kinase G, Ca2+/calmodulin-dependent protein kinases and ERK can all phosphorylate eNOS at Ser1177 (Mount et al., 2007; Ho et al., 2008). Previously, we revealed the crucial role of the PI3K-Akt-NOS pathway in regulating cardiovascular effects in the NTS (Huang et al., 2004). In the present study, LY294002 inhibited eNOS-Ser1177 phosphorylation induced by renin (Figure 3D) suggesting that renin may phosphorylate eNOS via a PI3K-Akt cascade in the NTS. We tested the effect of the Gq inhibitor, GPA-2A and the Gβγ inhibitor, gallein, on the renin-induced depressor effect in the NTS to provide further evidence that renin may produce Ang II, which in turn activates AT1 receptor-Gq signalling, as described previously (Wong et al., 2002). Ang II stimulated PI3K-Akt signalling via AT1 receptors in the brain (Yang and Raizada, 1999) and chronic blockade of PI3K in the NTS of spontaneously hypertensive rat (SHR) resulted in hypertension (Zubcevic et al., 2009). Furthermore, renin induced Ras activation in the NTS (Figure 5B) and similar results have been demonstrated in cardiac myocytes and vascular smooth muscle cells (Eguchi et al., 1996; Sadoshima and Izumo, 1996). Activation of the epidermal growth factor receptors (EGFR) leads to signalling mediated by the PI3K-Akt and Ras-MAPK pathways. The EGFRs also can be transactivated by AT1 receptors and lead to the activation of PI3K-Akt cascade or Ras-MAPK pathway (Eguchi et al., 2001; Kippenberger et al., 2005; Mifune et al., 2005). Thus, further studies are needed to elucidate this possible mechanism in the NTS. However, we did not observe MEK-ERK signalling in the renin-mediated depressor response (Figure S2). Our data support the hypothesis that, in the NTS, renin activated the PI3K-Akt-eNOS signalling pathway, and consequently, the Ras pathway.
The (pro)renin receptor (PRR) consists of a 350-amino-acid protein with a single transmembrane domain that has comparable affinity for (pro)renin and renin (Nguyen et al., 2002) The PRR is highly expressed in brain regions known to modulate the cardiovascular system, such as the cortex, hippocampus, subfornical organ and NTS (Shan et al., 2008; Contrepas et al., 2009). Levels of mRNA for PRR were 45% and 70% higher in the NTS and supraoptic nucleus of SHR, compared to WKY rats (Shan et al., 2010), indicating that PRR may contribute to central blood pressure regulation. In our study, inhibition of RAS activity attenuated renin-mediated depressor effects in the NTS (Figure 4 and Figure S3). Renin binds PRR with affinity in the nanomolar range (Nabi et al., 2006) and in the present study, we have microinjected renin into NTS in the picomolar range. However, further studies are needed to establish the mechanisms connecting PRRs and their downstream signalling cascade in the NTS.
The study herein represents a key step in understanding how renin may participate in regulating the cardiovascular system through effects exerted in the NTS. We have utilized SensoLyte® 520 Rat Renin Assay Kit (ANASPEC, San Jose, CA, USA) to determine the concentration of renin in the NTS of WKY rats as 56 pM (data not shown). In Figure 1A, we microinjected 240 fg of renin into the NTS of WKY rats, equivalent to a concentration of 100 pM. However, the levels of angiotensin I, Ang II and Ang-(1-7), after administration of renin to the NTS are not known and these values should be determined. Another limitation of this study derives from the use of pharmacological inhibitors and their specificity. For instance, administration of losartan at higher doses induced non-specific effects in the RVLM (Averill et al., 1994). Moreover, losartan may bind to non-Ang II binding sites and valsartan has approximately five-fold greater affinity than losartan, for the AT1 receptor (de Gasparo and Whitebread, 1995). We used both these AT1 receptor antagonists to assess the contribution of AT1 receptors to the modulation of renin-induced depressor effect in this study (Figure 4B and C). We intend to utilize shRNA-based gene delivery to validate the role of central cardiovascular effects more precisely, which may help us overcome the limitations imposed in animals.
In conclusion, we propose that renin could mediate central control of blood pressure at the NTS, through Ras-PI3K-Akt signalling-regulated phosphorylation of eNOS (Figure 6). The present data further show that renin may modulate blood pressure via centrally located AT1 and Mas receptors, and via Gq, to activate Ras, Akt and eNOS. The RAS modulates sympathetic function in the NTS, where integration of autonomic control of the cardiovascular system occurs. Direct renin inhibitors have been approved for clinical use, however, the mechanisms by which direct renin inhibitors regulate blood pressure in the CNS have not been clarified. Our findings provide new insights into the mechanism(s) of central regulation of blood pressure by renin and may be of help in further development of direct renin inhibitors in therapy.
Figure 6.
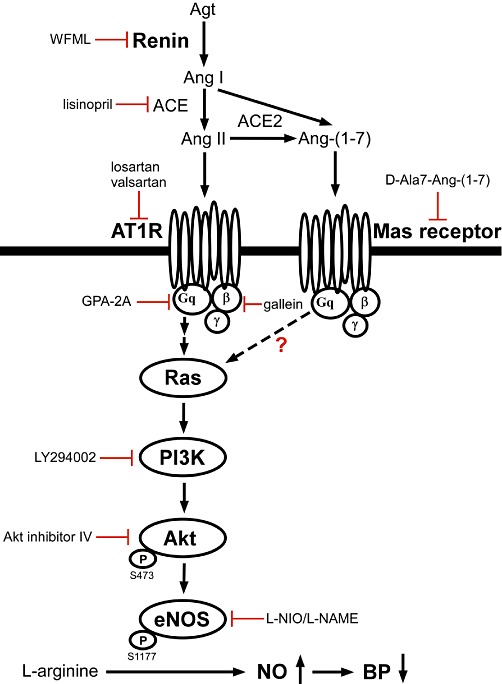
The proposed renin-induced signalling pathway in the NTS to regulate systemic blood pressure in WKY rats. Microinjection of renin stimulates the PI3K-Akt-eNOS cascade via activating AT1 and Mas receptors, ultimately leading to elevated NO concentrations in the NTS and decreased blood pressure. ACE2, angiotensin converting enzyme 2; Agt, angiotensinogen; Ang I, angiotensin I.
Acknowledgments
The authors gratefully acknowledge Ms Yi-Shan Wu for technical assistance and Mr Bo-Zone Chen for his invaluable input of this manuscript.
Glossary
- 7-NI
7-nitroindazole
- ACE
angiotensin converting enzyme
- aCSF
artificial cerebrospinal fluid
- Ang II
angiotensin II
- Ang-(1-7)
angiotensin-(1-7)
- DAB
diaminobenzidine
- EGFR
epidermal growth factor receptor
- eNOS
endothelium nitric oxide synthase
- GPA-2A
GP Antagonist-2A
- HR
heart rate
- iNOS
inducible nitric oxide synthase
- L-NAME
N-nitro-L-arginine methyl ester
- L-NIO
N(5)-(-iminoethyl)-L-ornithine
- MEK
MAP kinase-ERK kinase
- nNOS
neuronal nitric oxide synthase
- NTS
nucleus tractus solitarii
- PBST
phosphate-buffered saline with Tween 20
- PRR
(pro)renin receptor
- RAS
renin-angiotensin system
- RVLM
rostral ventrolateral medulla
- SDS
sodium dodecyl sulfate
- SFO
subfornical organ
- SHR
spontaneously hypertensive rats
- shRNA
short hairpin RNA
- vinyl-L-NIO
N(5)-(1-imino-3-butenyl)-ornithine
- WKY
Wistar-Kyoto rat
Sources of funding
This work was supported by funding from the National Science Council NSC98-2321-B-075B-002 (to Dr. C.-J. Tseng), NSC100-2320-B-283-001 (to Dr. W.-Y. Ho) and Kaohsiung Veterans General Hospital VGHKS97-080, VGHKS98-099. (to Dr. C.-J. Tseng)
Conflicts of interest
None.
Supporting information
Additional Supporting information may be found in the online version of this article:
Figure S1 The bar graphs reveal hypotensive and bradycardic effects of unilateral administration of different doses of renin in the nucleus tractus solitarii (NTS). Both the mean blood pressure (MBP) and heart rate (HR) effects were significantly changed by renin. *P < 0.05 versus the 0 group.
Figure S2 (A) The cardiovascular effect of injection of renin (240 fg) into the NTS after administration of the selective nNOS inhibitor, vinyl-L-NIO (600 pmol). Representative tracings demonstrate that vinyl-L-NIO did not diminish the depressor effect of renin in the NTS. Summary data (means ± SEM, n = 6) are shown in the graph (B) The cardiovascular effect of injection of renin (240 fg) into the NTS after administration of the potent nNOS inhibitor, 7-NI (66 pmol). Representative tracings demonstrate that 7-NI did not diminish the depressor effect of renin in the NTS. Summary data (means ±SEM, n = 6) are shown in the graph. (C) Immunoblot depicts the levels of P-nNOS-Ser1416 protein in the NTS. Densitometric analysis of P-nNOS-Ser1416 protein levels (means ± SEM, n = 6) before and after treatment with renin. Note that there are no significant differences in nNOS-Ser1416 phosphorylation levels in the NTS betweenthe aCSF and renin-treated groups. (D) Immunoblot shows iNOS protein levels (means ± SEM, n = 6) in the NTS. Note that there are no significant differences in iNOS protein levels in the NTS between aCSF and the renin-treated groups.
Figure S3 (A) Immunoblots depicting the levels of P-ERK1/2-Thr202/Tyr204 protein in the NTS. There were no significant differences in ERK1/2 phosphorylation levels in the NTS between the aCSF and renin-treated groups. Densitometric analysis shows P-ERK1/2-Thr202/Tyr204 protein levels (means ± SEM, n = 6) after treatment with renin. (B) The cardiovascular effect of injection of renin (240 fg) into the NTS after administration of the MEK inhibitor, PD98059 (10 pmol). Representative tracings demonstrate that PD98059 did not diminish the depressor effect of renin in the NTS. Summary data (means ± SEM, n = 6) are shown in the graph.
Figure S4 Blood pressure (BP) after injection of renin (240 fg) into the NTS following administration of the Gβγ inhibitor, gallein (240 fmol). Representative tracings demonstrate that the depressor effect of renin was not attenuated by gallein. Summary data (means ± SEM, n = 6) are shown in the graph.
Table S1 Cardiovascular response to microinjection of renin (240 fg) into the NTS in rats before and after microinjection of the pharmacological inhibitors
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (5th Edition) 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzamora AC, Santos RAS, Campagnole-Santos MJ. Hypotensive effect of ANG II and ANG-(1-7) at the caudal ventrolateral medulla involves different mechanisms. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1187–R1195. doi: 10.1152/ajpregu.00580.2001. [DOI] [PubMed] [Google Scholar]
- Averill DB, Tsuchihashi T, Khosla MC, Ferrario CM. Losartan, nonpeptide angiotensin II-type 1 (AT1) receptor antagonist, attenuates pressor and sympathoexcitatory responses evoked by angiotensin II andL-glutamate in rostral ventrolateral medulla. Brain Res. 1994;665:245–252. doi: 10.1016/0006-8993(94)91344-7. [DOI] [PubMed] [Google Scholar]
- Brown MJ. Aliskiren. Circulation. 2008;118:773–784. doi: 10.1161/CIRCULATIONAHA.108.787630. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Diz DI, Santos RA, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1-7) injected into the dorsal medulla of rats. Am J Physiol Heart Circ Physiol. 1989;257:H324–H329. doi: 10.1152/ajpheart.1989.257.1.H324. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Lu PJ, Ho WY, Tung CS, Cheng PW, Hsiao M, et al. Angiotensin II inhibits neuronal nitric oxide synthase activation through the ERK1/2-RSK signaling pathway to modulate central control of blood pressure. Circ Res. 2010;106:788–795. doi: 10.1161/CIRCRESAHA.109.208439. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Lu PJ, Chen SR, Ho WY, Cheng WH, Hong LZ, et al. Central nicotinic acetylcholine receptor involved in Ca(2+) -calmodulin-endothelial nitric oxide synthase pathway modulated hypotensive effects. Br J Pharmacol. 2011;163:1203–1213. doi: 10.1111/j.1476-5381.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HT, Cheng WH, Lu PJ, Huang HN, Lo WC, Tseng YC, et al. Neuronal nitric oxide synthase activation is involved in insulin-mediated cardiovascular effects in the nucleus tractus solitarii of rats. Neuroscience. 2009;159:727–734. doi: 10.1016/j.neuroscience.2008.12.048. [DOI] [PubMed] [Google Scholar]
- Contrepas A, Walker J, Koulakoff A, Franek KJ, Qadri F, Giaume C, et al. A role of the (pro)renin receptor in neuronal cell differentiation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R250–R257. doi: 10.1152/ajpregu.90832.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S, Nagy F, McIntosh JM, Sapru HN. Receptor subtypes mediating depressor responses to microinjections of nicotine into medial NTS of the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R132–R140. doi: 10.1152/ajpregu.2000.279.1.R132. [DOI] [PubMed] [Google Scholar]
- Dugourd C, Gervais M, Corvol P, Monnot C. Akt is a major downstream target of PI3-kinase involved in angiotensin II-induced proliferation. Hypertension. 2003;41:882–890. doi: 10.1161/01.HYP.0000060821.62417.35. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension. 1986;8:544–548. doi: 10.1161/01.hyp.8.6.544. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Matsumoto T, Motley ED, Utsunomiya H, Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J Biol Chem. 1996;271:14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2001;276:7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- Fow JE, Averill DB, Barnes KL. Mechanisms of angiotensin-induced hypotension and bradycardia in the medial solitary tract nucleus. Am J Physiol. 1994;267:H259–H266. doi: 10.1152/ajpheart.1994.267.1.H259. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Whitebread S. Binding of valsartan to mammalian angiotensin AT1 receptors. Regul Pept. 1995;59:303–311. doi: 10.1016/0167-0115(95)00085-p. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Sakai K, Kishi T, Ito K, Shimokawa H, Takeshita A. Enhanced depressor response to endothelial nitric oxide synthase gene transfer into the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2003;26:325–331. doi: 10.1291/hypres.26.325. [DOI] [PubMed] [Google Scholar]
- Ho WY, Lu PJ, Hsiao M, Hwang HR, Tseng YC, Yen MH, et al. Adenosine modulates cardiovascular functions through activation of extracellular signal-regulated kinases 1 and 2 and endothelial nitric oxide synthase in the nucleus tractus solitarii of rats. Circulation. 2008;117:773–780. doi: 10.1161/CIRCULATIONAHA.107.746032. [DOI] [PubMed] [Google Scholar]
- Huang HN, Lu PJ, Lo WC, Lin CH, Hsiao M, Tseng CJ. In situ Akt phosphorylation in the nucleus tractus solitarii is involved in central control of blood pressure and heart rate. Circulation. 2004;110:2476–2483. doi: 10.1161/01.CIR.0000145116.75657.2D. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Blair LAC, Marshall J, Goedert M, Hanley MR. The mas oncogene encodes an angiotensin receptor. Nature. 1988;335:437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Kippenberger S, Loitsch S, Guschel M, Müller J, Knies Y, Kaufmann R, et al. Mechanical stretch stimulates protein kinase B/Akt phosphorylation in epidermal cells via angiotensin II type 1 receptor and epidermal growth factor receptor. J Biol Chem. 2005;280:3060–3067. doi: 10.1074/jbc.M409590200. [DOI] [PubMed] [Google Scholar]
- Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, et al. G-protein–coupled receptor mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- Maeda M, Hirano H, Kudo H, Doi Y, Higashi K, Fujimoto S. Injection of antisense oligos to nNOS into nucleus tractus solitarii increases blood pressure. Neuroreport. 1999;10:1957–1960. doi: 10.1097/00001756-199906230-00030. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Pitt B, Latini R, Maggioni AP, Solomon SD, Keefe DL, et al. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail. 2008;1:17–24. doi: 10.1161/CIRCHEARTFAILURE.107.740704. [DOI] [PubMed] [Google Scholar]
- Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, et al. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Tseng CJ, Appalsamy M, Robertson D. Cardiovascular effects of microinjection of angiotensin II in the brainstem of renal hypertensive rats. J Pharmacol Exp Ther. 1990;255:374–381. [PubMed] [Google Scholar]
- Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med. 2006;18:483–488. [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Mironneau J, Carricaburu V, Fournier B, Babich A, Nurnberg B, et al. Phosphoinositide 3-kinase gamma mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. J Biol Chem. 2001;276:32545–32551. doi: 10.1074/jbc.M102582200. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. The heterotrimeric G q protein-coupled angiotensin II receptor activates p21 ras via the tyrosine kinase-Shc-Grb2-Sos pathway in cardiac myocytes. EMBO J. 1996;15:775–787. [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hirooka Y, Matsuo I, Eshima K, Shigematsu H, Shimokawa H, et al. Overexpression of eNOS in NTS causes hypotension and bradycardia in vivo. Hypertension. 2000;36:1023–1028. doi: 10.1161/01.hyp.36.6.1023. [DOI] [PubMed] [Google Scholar]
- Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol. 2008;93:701–708. doi: 10.1113/expphysiol.2008.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, et al. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res. 2010;107:934–938. doi: 10.1161/CIRCRESAHA.110.226977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai MH, Hsiao M, Chan JY, Lo WC, Wang FS, Liu GS, et al. Gene delivery of endothelial nitric oxide synthase into nucleus tractus solitarii induces biphasic response in cardiovascular functions of hypertensive rats. Am J Hypertens. 2004;17:63–70. doi: 10.1016/j.amjhyper.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Tan PS, Potas JR, Killinger S, Horiuchi J, Goodchild AK, Pilowsky PM, et al. Angiotensin II evokes hypotension and renal sympathoinhibition from a highly restricted region in the nucleus tractus solitarii. Brain Res. 2005;1036:70–76. doi: 10.1016/j.brainres.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Tseng CJ, Mosqueda-Garcia R, Appalsamy M, Robertson D. Cardiovascular effects of neuropeptide Y in rat brainstem nuclei. Circ Res. 1989;64:55–61. doi: 10.1161/01.res.64.1.55. [DOI] [PubMed] [Google Scholar]
- Tseng CJ, Liu HY, Lin HC, Ger LP, Tung CS, Yen MH. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27:36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- Wong LF, Polson JW, Murphy D, Paton JF, Kasparov S. Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. FASEB J. 2002;16:1595–1601. doi: 10.1096/fj.02-0099com. [DOI] [PubMed] [Google Scholar]
- Yang H, Raizada MK. Role of phosphatidylinositol 3-kinase in angiotensin II regulation of norepinephrine neuromodulation in brain neurons of the spontaneously hypertensive rat. J Neurosci. 1999;19:2413–2423. doi: 10.1523/JNEUROSCI.19-07-02413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Waki H, Diez-Freire C, Gampel A, Raizada MK, Paton JF. Chronic blockade of phosphatidylinositol 3-kinase in the nucleus tractus solitarii is prohypertensive in the spontaneously hypertensive rat. Hypertension. 2009;53:97–103. doi: 10.1161/HYPERTENSIONAHA.108.122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


