Abstract
BACKGROUND AND PURPOSE
Under conditions of increased oxidative stress, such as pre-eclampsia and diabetes, overstimulation of PARP leads to endothelial dysfunction. Inhibition of PARP has been demonstrated to reverse the vascular dysfunction associated with diabetes in vivo. The present study was carried out to investigate the role of PARP in mediating the endothelial dysfunction associated with pre-eclampsia.
EXPERIMENTAL APPROACH
Uteroplacental perfusion was surgically reduced in pregnant rats to produce the reduced uterine perfusion pressure (RUPP) rat model of pre-eclampsia and the PARP inhibitor, PJ34, was administered either before or after surgery. Mean arterial BP and vascular function were measured in normal pregnant (NP) and both control and PJ34-treated RUPP rats. Mesenteric vessels from NP rats were incubated with either 3% RUPP or NP plasma alone or in combination with PJ34. Finally, immunohistochemical staining was carried out to measure nitrotyrosine (byproduct of peroxynitrite) immunoreactivity.
KEY RESULTS
RUPP rats were characterized by hypertension, fetal growth restriction and endothelial dysfunction when compared with NP rats. PJ34 administered in vivo before, but not after, surgery prevented the development of both endothelial dysfunction and hypertension. RUPP plasma-induced impaired vasorelaxation was prevented following co-incubation with PJ34 in vitro. Furthermore, the protective effect of PARP inhibition in vivo was accompanied by a reduction in nitrotyrosine immunoreactivity.
CONCLUSIONS AND IMPLICATIONS
PJ34 prevented the development of both endothelial dysfunction and hypertension and reduced vascular nitrotyrosine immunoreactivity, thus suggesting a role for oxidative–nitrosative stress/PARP activation in the aberration in both vascular and haemodynamic function in this rat model of pre-eclampsia.
Keywords: PARP, peroxynitrite, pre-eclampsia, reduced uterine perfusion pressure, endothelial dysfunction
Introduction
Pre-eclampsia is a multisystemic disorder of pregnancy that affects more than eight million pregnancies worldwide annually (Lewis, 2001). The underlying aetiology is incompletely understood, but current thinking suggests that poor trophoblast invasion early in pregnancy and the development of a relatively hypoperfused placenta stimulates the maternal response, which involves alterations in the maternal vascular endothelium (Hayman et al., 1999). Impaired endothelium-dependent responses have been reported in systemic vessels isolated from both women with pre-eclampsia(Pascoal et al., 1998; Svedas et al., 2002) and animal models of this condition, including the reduced uterine perfusion pressure (RUPP) rat (Crews et al., 2000; Turgut et al., 2008; Walsh et al., 2009; McCarthy et al., 2011). There is extensive evidence to suggest that the vascular dysfunction associated with pre-eclampsia is induced by one or more circulating mediators (Hayman et al., 2000; Crocker et al., 2005). Furthermore, we have recently demonstrated that endothelial dysfunction observed in the RUPP rat is mediated by circulating plasma factors (Walsh et al., 2009).
Pregnancies complicated by pre-eclampsia are associated with elevated blood and tissue levels of lipid peroxidation products, thus implicating increased oxidative stress in the aetiology of this condition (Kaur et al., 2008). Furthermore, studies have shown that increased levels of peroxynitrite (a free radical produced via the combination of NO and superoxide) are localized to the maternal vasculature (Beckman and Koppenol, 1996), and that this free radical actively disrupts the maternal endothelium (Roggensack et al., 1999). Peroxynitrite acts as a key pathophysiological trigger of DNA single-strand breakage, an event that elicits a restorative response by PARP, an abundant eukaryotic cell nuclear enzyme (Schraufstatter et al., 1986; Virag and Szabo, 2002). PARP repairs DNA strand breakage by catalysing the covalent post-translation modification of nuclear proteins with poly(ADP-ribose) using nicotinamide adenine dinucleotide as a precursor (Virag and Szabo, 2002). Under normal physiological conditions, PARP is cytoprotective, but under conditions of increased oxidative stress, overstimulation of this enzyme reduces the rate of glycolysis and mitochondrial respiration leading to cellular dysfunction (Szabo, 2009). Furthermore, the overactivity of this enzyme has been implicated in the pathogenesis of stroke, autoimmune β-cell destruction, shock, inflammation and diabetes (Pacher and Szabo, 2008). Accumulating evidence has demonstrated a correlation between the overstimulation of PARP and endothelial cell dysfunction induced by plasma from pre-eclamptic patients (Crocker et al., 2005) and more recently the vasculoprotective effects of PARP inhibition in rodent models of both diabetes (Pacher et al., 2002a) and hypertension (Pacher et al., 2002b). However, whether or not PARP plays a role in mediating the endothelial dysfunction documented in pre-eclampsia has yet to be examined. Therefore, the present study was carried out using the RUPP rat model of pre-eclampsia (produced via chronic surgical reduction of uteroplacental perfusion), which is characterized by hypertension, reduced glomerular filtration rate, proteinuria, fetal growth restriction and endothelial dysfunction (Khalil and Granger, 2002) to investigate this.
Methods
Animals
Pregnant Sprague–Dawley rats (12 weeks) were supplied and maintained by the Biological Services Unit at University College Cork. Animals were maintained at a temperature of 21 ± 2°C, with a 12 h light/dark cycle and with free access to food and tap water. All procedures were performed in accordance with national guidelines and the European Community Directive 86/609/EC and approved by the University College Cork Local Animal Experimentation Ethics Committee. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010).
Protocol for RUPP procedure
On day 14 of pregnancy, animals destined for the RUPP experimental groups were anaesthetized via inhalation with isoflurane (2–5%) supplemented with oxygen, and using an aseptic technique, the abdominal cavity opened via a midline incision to expose the lower abdominal aorta. A silver clip (0.203 mm ID) was placed around the aorta (above the iliac bifurcation) to reduce uterine perfusion pressure by ∼40% (Eder and McDonald, 1987). Since compensation of blood flow to the placenta occurs via an adaptive response of the uterine arteries (Nienartowicz et al., 1989), silver clips (0.10 mm ID) were also placed on the main uterine branches of both right and left uterine arteries. Following closure of the abdominal muscle, 200 µL of lidocaine (10 mg·mL−1) was flushed underneath the skin before closure to reduce pain post surgery. On day 18 of pregnancy, all animals were anaesthetized with isoflurane (2–5% inhalation) and chronically instrumented with a carotid catheter (0.58 mm ID × 0.99 mm OD) for subsequent mean arterial blood pressure (MABP) measurements. On day 19, animals were placed in restraints, and following acclimatization, MABP was recorded for 1 h in a quiet room. Following completion of MABP measurements, animals were anaesthetized with isoflurane (2–5% inhalation), blood was collected via the abdominal aorta into pre-cooled heparin-containing vacutainers and the animal was killed via an overdose of anaesthetic and removal of the heart. The mesenteric arterial arcade was excised and placed in ice-cold physiological salt solution (PSS), all pups and placentas were removed and weighed, and litter size was noted. Any animals in which the clipping procedure had resulted in total re-absorption of fetuses were excluded from the study.
Plasma preparation
Blood collected into pre-cooled heparin-containing vacutainers was centrifuged at 2400× g for 10 min at 4°C. The plasma was then removed and stored in 250 µL aliquots at −80°C. Before commencement of the in vitro studies, equal volumes of stored plasma were mixed to produce both normal pregnant (NP) and RUPP plasma pools (n= 7–9).
Isometric myography
In all groups, third-order mesenteric arteries were dissected out and mounted onto a four-channel wire myograph [Model 610 M, Danish Myo Technology (DMT), Aarhus, Denmark] containing oxygenated (95% O2 and 5% CO2) PSS at 37°C. Vessels were normalized to achieve a transmural pressure of 100 mmHg using the DMT Normalization software. Isometric tension was recorded and displayed using a Powerlab and Chart Software (both from ADInstruments, Oxford, UK). The viability of the smooth muscle was tested via the addition of potassium PSS (KPSS) containing 123 mM KCl. Following two PSS washes, cumulative concentration-responses were carried out with the thromboxane mimetic, U46619 (10−9–3 × 10−7 M) and bradykinin (BK; 10−9–10−6 M).
In vivo experimental protocols
Both normal pregnant rats (n= 9) and those in the control RUPP group (n= 9; pregnant animals subjected to chronic reduction of uteroplacental perfusion) were allowed free access to water. In the first PJ34-treated group (PJ34-Pre; n= 9), pregnant rats were administered PJ34 in drinking water (at a concentration sufficient to achieve a daily dose of 10 mg·kg−1·day−1 based on previous observations relating to daily intake of water by pregnant rats) on days 11–13 of pregnancy before chronic reduction of uteroplacental perfusion on day 14. In the second PJ34-treated group (PJ34-Post; n= 9), RUPP rats were administered PJ34 (10 mg·kg−1·day−1) in drinking water on days 16–18 of pregnancy (post RUPP surgery). The dose of PJ34 used in the present study was chosen based on previously published data demonstrating a vasculoprotective effect of PJ34 in vivo via the inhibition of PARP activity (Soriano et al., 2001; Pacher et al., 2002a,b).
In vitro experimental protocols
As we have previously found that treatment of mesenteric vessels from normal pregnant (NP) rats with RUPP plasma induced endothelial dysfunction (Walsh et al., 2009), a separate series of experiments were carried out to investigate the role of PARP in mediating this plasma-induced vascular dysfunction. Pregnant rats (gestational day 19) were killed via CO2 asphyxiation; third-order mesenteric arteries were dissected out and incubated overnight in the following treatment groups.
I Comparison of vascular function following incubation of mesenteric vessels from NP rats in either NP or RUPP plasma overnight
Mesenteric vessels were incubated overnight at 4°C in 3% NP plasma (3% NPP; n= 12) or 3% RUPP plasma (3% RP; n= 15) solutions made up with PSS. This plasma concentration was chosen as it induced endothelial dysfunction in mesenteric vessels in our previous study (Walsh et al., 2009). Following two subsequent washes with PSS, concentration–response curves to U46619 and BK were carried out as previously described.
II Role of PARP in RUPP plasma-induced vascular dysfunction in normal pregnant rat mesenteric vessels
Mesenteric vessels (n= 7–19) were incubated overnight at 4°C in 3% NPP or 3% RP with the PARP inhibitor, PJ34 (3 µM). This concentration of PJ34 was chosen on the basis that previously published data demonstrated a vasculoprotective effect of PJ34 at 3 µM (Soriano et al., 2001; Crocker et al., 2005). In addition, data from an activity assay for PARP (HT Universal Colorimetric PARP Assay Kit, Trevigen Inc., Gaithersburg, MD; used as per manufacturer's instructions), carried out before the vascular studies, demonstrated an IC50 of 25 nM for PJ34 and ∼99% inhibition of PARP enzyme activity at 3 µM PJ34 (data not shown). Following subsequent washes with PSS, concentration–response curves to U46619 and BK were carried out as previously described.
Immunohistochemical staining for nitrotyrosine
Immunohistochemical staining for nitrotyrosine was performed in fixed thoracic aortic sections. Thoracic aorta samples were embedded in paraffin wax, and 5 µm transverse sections were cut and mounted on polysine slides. Following dehydration and deparaffinization, sections were permeabilized with 1% (w v-1) Triton X-100 to unmask the antigens. Sections were blocked and incubated with the primary antibody (murine monoclonal anti-nitrotyrosine antibody (Abcam, Cambridgeshire, UK) at a dilution of 1:500 in a humidifying chamber overnight at 4°C. Sections were incubated with the secondary antibody (anti-mouse IgG), which was coupled to horseradish peroxidase for 30 min at room temperature. Sections were incubated with Vector VIP substrate (Vector Labs, Peterborough, UK) until the desired stain intensity was achieved (3–5 min) and then mounted with Immu-mount (Fisher Scientific, Loughborough, UK) and analysed to detect positive staining for nitrotyrosine (as indicated by intense localized purple staining). Each staining run contained a negative control slide in which the primary antibody was replaced with an IgG-negative antibody control. Protein expression was examined by image analysis using Image Pro-Plus (version 6.3, Media Cybernetics, Silver Spring, MD) introduced by Xavier et al. (2005) and further validated by Wang et al. (2009). For each measurement, four digital images (×400 magnification) of adjacent sections of each tissue sample were captured with the use of a Leica DMI4000 B camera (Leica Microsystems, Bucks, UK) in conjunction with the Leica Application Suite software (Leica Microsystems). Standard optical density calibration was first performed before an area of interest (AOI) was assigned from each slide. Each AOI encompassed all tissue except outer surfaces, which often had irregular staining. Optical density was first set on the negative control using hue (red 0–180, blue 0–255, green 0–255) saturation (0–255) and intensity (0–255). This signal was subsequently applied to all images. Before measurements of mean density were recorded, each AOI was converted to a single object, thus averaging intensity per pixel across the whole tissue. Mean values for background were first defined on non-immunoreactivity areas prior to subtraction and the process expedited using a macro. All densitometric analysis was carried out by a ‘blinded’ observer.
Statistical analysis
For haemodynamic, weight and immunohistochemical data a one-way anova and Bonferroni's post hoc test were used, in which all groups were compared with each other. Concentration–response curves for all vascular data were generated using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). Concentration–responses between groups were compared via a repeated-measures anova and Bonferroni's post hoc test. Rmax values (maximal relaxation as a percentage of induced tone) were compared using a one-way anova and Dunnett's post hoc test. All data are expressed as the mean ± SEM, and significance was determined as P < 0.05.
Solutions and chemicals
All chemicals were purchased from Sigma-Aldrich (Dorset, UK). The composition of PSS was as follows (in mM): NaCl 119, KCl 4.7, MgSO4·7H2O 1.17, KH2PO4 1.18, CaCl2·2H2O 2.5, NaHCO3 25, EDTA 0.03 and glucose 5.5 (pH 7.4 when gassed continuously with 95% O2 and 5% CO2). KPSS was made as per PSS with the absence of NaCl and the inclusion of KCl 123 mM. All drug/molecular target nomenclature (e.g. receptors, ion channels, etc.) conforms to British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2011).
Results
Effect of PJ34 administration on RUPP-mediated effects in pregnant rats
The surgical reduction of uteroplacental perfusion in day 14 pregnant (RUPP) rats resulted in a significant increase in MABP when compared with normal pregnant rats on day 19 of pregnancy (127 ± 5 vs. 99 ± 3 mmHg; P < 0.001; Figure 1). Treatment with the PARP inhibitor, PJ34, before uteroplacental perfusion reduction significantly decreased MABP when compared with untreated RUPP rats [104 ± 5 (PJ34-Pre) vs. 127 ± 5 mmHg; P < 0.01; Figure 1]. In contrast, administration of PJ34 post surgery did not significantly alter MABP when compared with vehicle-treated RUPP rats [118 ± 4 (PJ34-Post) vs. 127 ± 5 mmHg; not significant (ns); Figure 1]. RUPP rats were also characterized by significantly reduced pup weight when compared with the offspring from normal pregnant rats (2.2 ± 0.1 vs. 3.2 ± 0.1 g; P < 0.001; Figure 2A). PARP inhibition did not significantly affect the RUPP-induced reduction in fetal weight [2.2 ± 0.1 (PJ34-Pre) and 2.1 ± 0.1 (PJ34-Post) vs. 2.2 ± 0.1 g; ns; Figure 2A]. Placental weights from RUPP rats were also significantly reduced compared with normal pregnant rats (0.33 ± 0.01 vs. 0.43 ± 0.01 g; P < 0.001; Figure 2B). However, treatment with PJ34 did not significantly affect the reduced placental weight documented in RUPP rats [0.36 ± 0.01 (PJ34-Pre) and 0.36 ± 0.01 (PJ34-Post) vs. 0.33 ± 0.01 g; ns; Figure 2B]. Furthermore, RUPP rats were associated with increased fetal re-absorption (resulting in fewer live pups on day 19 of pregnancy) compared with normal pregnant rats (4 ± 0.9 vs. 13 ± 0.5; P < 0.001; data not shown), which was not significantly affected by PJ34 administration [3 ± 0.6 (PJ34-Pre) and 5 ± 0.8 (PJ34-Post) vs. 4 ± 0.9; ns; data not shown].
Figure 1.

Effect of PARP inhibition on reduced uterine perfusion pressure-induced hypertension in pregnant rats. MABP in NP rats, rats with RUPP and RUPP rats treated with the PARP inhibitor, PJ34 (10 mg·kg−1·day−1), either before (PJ34-Pre; gestational days 11–13) or post (PJ34-Post; gestational days 16–18) surgical reduction in uterine perfusion pressure (gestational day 14). Data are expressed as mean ± SEM (n= 7–9) *P < 0.001 versus NP rats and †P < 0.01 versus RUPP control rats.
Figure 2.
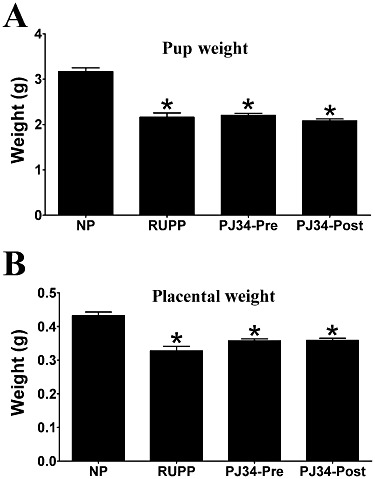
Effect of PARP inhibition on reduced uterine perfusion pressure-mediated restricted fetal growth and reduced placental weight in pregnant rats. Pup weight (A) and placental weight (B) in NP rats, rats with RUPP and RUPP rats treated with the PARP inhibitor, PJ34 (10 mg·kg−1·day−1), either before (PJ34-Pre) or post (PJ34-Post) surgical reduction in uterine perfusion pressure. Data are expressed as mean ± SEM (n= 7–9) *P < 0.001 versus NP rats.
Role of PARP in RUPP induced vascular dysfunction in the pregnant rat
Third-order mesenteric vessels from healthy pregnant rats relaxed by approximately 60% (maximal contraction of 100% induced by U46619) in response to the maximum concentration of BK (Figure 3). Mesenteric arteries from RUPP rats displayed a significantly impaired maximum relaxation to BK when compared with control vessels (Rmax 40 ± 3 vs. 60 ± 2%; P < 0.01; Figure 3). Treatment with PJ34 (10 mg·kg−1·day−1) before the surgical reduction of uteroplacental perfusion prevented the development of the endothelial dysfunction documented in untreated RUPP rats (Rmax 62 ± 4 vs. 40 ± 3%; P < 0.001; Figure 3). However, administration of PJ34 (10 mg·kg−1·day−1) post RUPP surgery did not significantly affect the RUPP-induced impaired vasorelaxation documented in control RUPP rats (Rmax 43 ± 4 vs. 40 ± 3%; ns; Figure 3). While U46619 induced a concentration-dependent increase in active wall tension (represents degree of contraction) in all groups, the maximum contraction to the vasoconstrictor did not differ significantly between the groups (data not shown).
Figure 3.
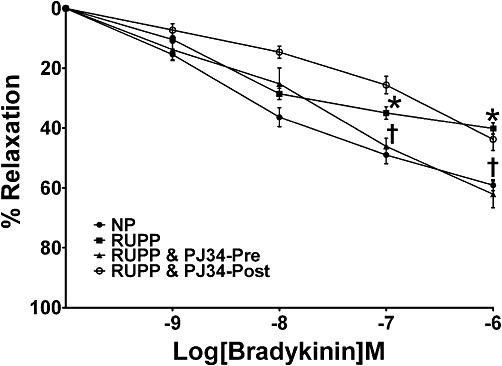
Effect of PARP inhibition on RUPP-induced endothelial dysfunction in mesenteric vessels. Third-order mesenteric vessels from NP rats, RUPP control rats and RUPP rats administered PJ34 (PARP inhibitor; 10 mg·kg−1·day−1) either before (PJ34-Pre) or post (PJ34-Post) surgical reduction of uterine perfusion pressure were assessed using isometric myography, and their vascular responses to the endothelium-dependent vasodilator, BK, were measured. Relaxation is calculated as a percentage of the maximum contraction and expressed as mean ± SEM (n= 7–11) *P < 0.01 versus NP rats and †P < 0.001 versus RUPP rats.
Role of PARP in RUPP plasma-mediated endothelial dysfunction in mesenteric vessels from pregnant rats
Mesenteric vessels from healthy pregnant rats treated with 3% NPP overnight at 4°C produced a maximum relaxation of 54% (Rmax) in response to the highest concentration of BK (Figure 4). Co-incubation of vessels with 3% NPP and PJ34 (3 µM) did not significantly affect the maximum vasorelaxation to BK (Rmax 52 ± 6 vs. 54 ± 5%; ns; Figure 4) compared with vessels incubated in 3% NPP alone. Following overnight incubation in 3% RP, an impaired relaxation was detected in mesenteric vessels in response to BK (Rmax 31 ± 4 vs. 54 ± 5%; P < 0.01; Figure 4) when compared with vessels incubated in 3% NPP. Concomitant treatment with PJ34 (3 µM) inhibited the RUPP plasma-induced impairment of the vasorelaxation to BK (Rmax 54 ± 6 vs. 31 ± 4%; P < 0.001; Figure 4). Although, U46619 induced a concentration-dependent increase in active wall tension (represents degree of contraction) in all groups, the maximum contraction to the vasoconstrictor did not differ significantly between the groups (not shown).
Figure 4.
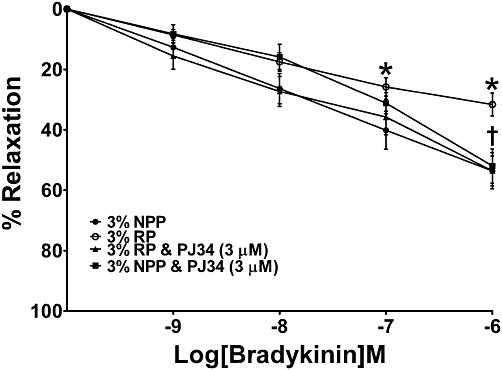
Effect of PARP inhibition on RUPP plasma-induced endothelial dysfunction in mesenteric vessels. Third-order mesenteric vessels from NP rats were incubated in either 3% NPP or 3% RP overnight at 4°C with or without PJ34 (3 µM), and their vascular responses to BK were assessed. Relaxation is calculated as a percentage of the maximum contraction and expressed as mean ± SEM (n= 9–15) *P < 0.001 versus 3% NPP and †P < 0.001 versus 3% RP.
Effect of PJ34 administration on vascular nitrotyrosine immunoreactivity
The surgical reduction of uteroplacental perfusion in day 14 pregnant (RUPP) rats resulted in a significant increase in nitrotyrosine (byproduct of peroxynitrite) immunoreactivity within the vasculature when compared with normal pregnant rats (0.075 ± 0.01 vs. 0.150 ± 0.02 mean optical density/pixel; P < 0.05; Figure 5). Treatment with the PARP inhibitor, PJ34, before uteroplacental perfusion reduction significantly reduced the intensity of staining of nitrotyrosine within the vasculature when compared with untreated RUPP rats (0.067 ± 0.01 vs. 0.150 ± 0.02 Mean optical density/pixel; P < 0.05; Figure 5). In contrast, administration of PJ34 post surgery did not significantly alter nitrotyrosine immunoreactivity when compared with vehicle-treated RUPP rats (0.151 ± 0.02 vs. 0.150 ± 0.02 mean optical density/pixel; ns; Figure 5).
Figure 5.
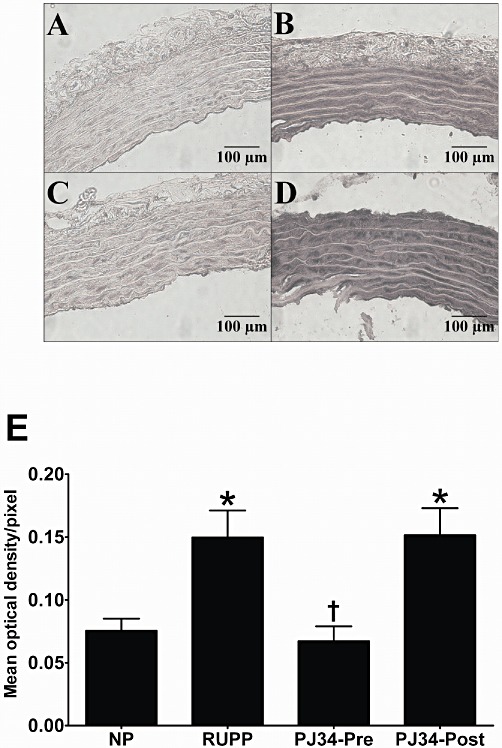
Effect of PARP inhibition on nitrotyrosine immunoreactivity in thoracic aorta samples from pregnant rats. Representative photomicrographs (×400 magnification) of immunohistochemical staining of nitrotyrosine (byproduct of peroxynitrite; indicated by intense dark staining) in thoracic aortic sections (5 µm) from normal pregnant rats (A), vehicle-treated RUPP rats (B), RUPP rats administered PJ34 (10 mg·kg−1·day−1) before (C) and post (D) surgical reduction of uterine perfusion pressure. Effect of PARP inhibition on optical density measurements for nitrotyrosine in thoracic aortic samples from NP rats, control RUPP rats and rats treated with PJ34 either before or post surgery (E). Immunoreactivity is calculated as mean optical density per pixel and expressed as mean ± SEM (n= 7–9) *P < 0.05 versus NP and †P < 0.05 versus RUPP.
Discussion
Pre-eclampsia is characterized by increased oxidative stress, and there is extensive evidence that reactive oxygen species (ROS), in conjunction with other mediators, are released from a compromised placenta and target the vascular endothelium leading to the clinical syndrome (Cooke and Davidge, 2003; Gilbert et al., 2008). Recently published data have suggested that excessive stimulation of the enzyme, PARP by ROS (and in particular peroxynitrite), may be responsible for mediating vascular dysfunction in many conditions (Pacher and Szabo, 2008). We have previously demonstrated, using human samples, a correlation between increased peroxynitrite levels and the overstimulation of PARP in endothelial cells treated with plasma from pre-eclamptic women (Crocker et al., 2005), which may suggest a role for this enzyme in pre-eclampsia-associated vascular dysfunction.
While recent findings have demonstrated that the RUPP rat is associated with increased oxidative stress and administration of the superoxide dismutase mimetic, tempol, ameliorates the pregnancy-induced hypertension in this model (Sedeek et al., 2008), the present study demonstrates for the first time the involvement of PARP in mediating several of the pathophysiological events associated with this model. This animal model of pre-eclampsia is characterized by both hypertension and endothelial dysfunction (Crews et al., 2000; Barron et al., 2001; Anderson et al., 2005; Gilbert et al., 2007; Walsh et al., 2009; McCarthy et al., 2011), and our data demonstrate that administration of the PARP inhibitor, PJ34, prevents the development of both. Our findings are in agreement with those from numerous studies that have demonstrated a protective effect of PARP inhibitors in several other pathophysiological states, including diabetes (Soriano et al., 2001) and age-associated cardiac dysfunction (Radovits et al., 2007). In the present study, PJ34 was only beneficial when administered before, and not post, RUPP surgery and the development of endothelial dysfunction and hypertension. In contrast, a study by Soriano et al. (2001) demonstrated that PJ34 both prevented and reversed endothelial dysfunction in a murine model of diabetes. Possible reasons for this disparity may simply be that the underlying mechanisms responsible for mediating vascular dysfunction in both diabetes and pre-eclampsia involve different triggers/mediators, or that the duration of PJ34 treatment post surgery was not sufficient to reverse established vascular dysfunction in the RUPP rat. Previous data have shown that PJ34-mediated protection persists for weeks beyond the completion of the treatment protocol (Soriano et al., 2001), which may suggest that the vasculoprotective effects of PARP inhibition involve chronic mechanisms. This is supported by the ex vivo vasculoprotective effect observed during the isolated vessel studies almost a week post termination of PJ34 administration in the pre-surgery treatment group. In contrast, animals in the post-surgery treatment group were only exposed to PJ34 for a brief period (3 days) before vascular studies were undertaken, which may explain the lack of a protective effect of PJ34.
It has been suggested that the widespread vascular dysfunction documented in pre-eclampsia occurs as a result of placental-derived mediators acting on the maternal endothelium as evidenced from numerous in vitro studies demonstrating plasma induced endothelial dysfunction in isolated human vessels (Hayman et al., 2000; VanWijk et al., 2002; Crocker et al., 2005). Our previous study demonstrated that incubation of mesenteric vessels from healthy pregnant rats with plasma from RUPP rats induced endothelial dysfunction, suggesting that one or more circulating factor(s) are responsible for mediating endothelial dysfunction in this animal model of pre-eclampsia (Walsh et al., 2009). In the present study, co-administration of the PARP inhibitor, PJ34, with RUPP plasma, prevented the development of endothelial dysfunction that would suggest that at least one of the targets for these circulating factors is the nuclear enzyme, PARP. The identity of the circulating trigger(s) responsible for mediating endothelial dysfunction in pre-eclampsia has yet to be determined, but a likely culprit may be peroxynitrite as evidence has been obtained demonstrating increased levels of this free radical in women with pre-eclampsia (Dordevic et al., 2008). The RUPP rat is characterized by elevated levels of superoxide (Sedeek et al., 2008) and reduced bioavailability of NO (Alexander et al., 2000), both of which coincide with increased peroxynitrite production (formed via a rapid reaction between the two aforementioned mediators), which was documented in the thoracic aortas of RUPP rats in the present study. Moreover, the increased vascular staining for nitrotyrosine (byproduct of peroxynitrite) documented in this study may explain earlier findings that demonstrated a lack of NO-mediated vasodilatation in both conduit and mesenteric vessels from this model (Crews et al., 2000; Anderson et al., 2005; Walsh et al., 2009), as NO bioavailability would be reduced as a consequence of increased peroxynitrite production.
In this study, the protective effects of PJ34 (in terms of preventing the development of endothelial dysfunction and hypertension) coincided with a reduction in nitrotyrosine expression in the thoracic aorta. While it is well established that peroxynitrite-induced DNA damage elicits a restorative response by PARP, which can lead to the overactivity of this enzyme (Virag and Szabo, 2002), the finding that the protective effects of PARP inhibition were associated with a reduction in peroxynitrite staining in the vasculature may suggest a bidirectional mechanism between oxidative–nitrosative stress and PARP activation. It has been demonstrated that PARP activation can enhance the expression of a number of pro-inflammatory mediators including inducible NOS (iNOS) (Oliver et al., 1999); thus, in the presence of PJ34, it may be possible that inhibition of PARP activity leads to a reduction in iNOS-related peroxynitrite production, both of which have been shown to be elevated in women with pre-eclampsia (Mazzanti et al., 2012). With this in mind, it may be tempting to suggest that inhibition of PARP not only prevents the depletion of cellular levels of ATP, which not only leads to cellular dysfunction (thus a possible limitation of the present study may be the lack of information regarding ATP levels in the vasculature) but may also reduce the amount of DNA damage induced by peroxynitrite and as a consequence the accompanying overactivity of this cytoprotective enzyme.
In conclusion, while the beneficial effects of PARP inhibitors have been previously demonstrated in conditions such as diabetes and myocardial ischaemia, the present study is the first to demonstrate a protective effect of a PARP inhibitor in a rat model of pre-eclampsia. PJ34 administration prevented the development of both hypertension and endothelial dysfunction, and reduced nitrotyrosine immunoreactivity in the maternal vasculature, suggesting a role for oxidative–nitrosative stress and PARP activation in mediating vascular dysfunction in this model of pre-eclampsia.
Glossary
- 3% NPP
3% normal pregnant plasma
- 3% RP
3% RUPP plasma
- AOI
area of interest
- BK
bradykinin
- KPSS
potassium physiological salt solution
- MABP
mean arterial blood pressure
- NP
normal pregnant
- PJ34
N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-N,N-dimethylacetamide.HCl
- PJ34-Post
PJ34 post-surgery
- PJ35-Pre
PJ34 pre-surgery
- PSS
physiological salt solution
- Rmax
maximal relaxation
- ROS
reactive oxygen species
- RUPP
reduced uterine perfusion pressure
- U46619
9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α
Conflicts of interest
None.
Sources of funding
This work was supported by Science Foundation Ireland grants SFI 07/RFP/BIMF796 and SFI 07/RFP/BIM/F796 UR08.
References
- Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2000;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Lopez F, Zhang HY, Pavlish K, Benoit JN. Reduced uteroplacental perfusion alters uterine arcuate artery function in the pregnant sprague-dawley rat. Biol Reprod. 2005;72:762–766. doi: 10.1095/biolreprod.104.036715. [DOI] [PubMed] [Google Scholar]
- Barron LA, Giardina JB, Granger JP, Khalil RA. High-salt diet enhances vascular reactivity in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension. 2001;38:730–735. doi: 10.1161/01.hyp.38.3.730. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Cooke CL, Davidge ST. Endothelial-dependent vasodilation is reduced in mesenteric arteries from superoxide dismutase knockout mice. Cardiovasc Res. 2003;60:635–642. doi: 10.1016/j.cardiores.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35:367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- Crocker IP, Kenny LC, Thornton WA, Szabo C, Baker PN. Excessive stimulation of poly(ADP-ribosyl)ation contributes to endothelial dysfunction in pre-eclampsia. Br J Pharmacol. 2005;144:772–780. doi: 10.1038/sj.bjp.0706055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordevic NZ, Babic GM, Markovic SD, Ognjanovic BI, Stajn AS, Zikic RV, et al. Oxidative stress and changes in antioxidative defense system in erythrocytes of preeclampsia in women. Reprod Toxicol. 2008;25:213–218. doi: 10.1016/j.reprotox.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Eder DJ, McDonald MT. A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats. Clin Exp Hypertens Preg. 1987;B6:431–451. [Google Scholar]
- Gilbert J, Dukes M, LaMarca B, Cockrell K, Babcock S, Granger JP. Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. Am J Hypertens. 2007;20:686–691. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:541–550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- Hayman R, Brockelsby J, Kenny L, Baker P. Preeclampsia: the endothelium, circulating factor(s) and vascular endothelial growth factor. J Soc Gynecol Investig. 1999;6:3–10. [PubMed] [Google Scholar]
- Hayman R, Warren A, Brockelsby J, Johnson I, Baker P. Plasma from women with pre-eclampsia induces an in vitro alteration in the endothelium-dependent behaviour of myometrial resistance arteries. BJOG. 2000;107:108–115. doi: 10.1111/j.1471-0528.2000.tb11586.x. [DOI] [PubMed] [Google Scholar]
- Kaur G, Mishra S, Sehgal A, Prasad R. Alterations in lipid peroxidation and antioxidant status in pregnancy with preeclampsia. Mol Cell Biochem. 2008;313:37–44. doi: 10.1007/s11010-008-9739-z. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. Why mothers die 1997–1999. Confidential enquiry into maternal deaths. London: Royal College of Obstetricians and Gynaecologists; 2001. [Google Scholar]
- Mazzanti L, Raffaelli F, Vignini A, Nanetti L, Vitali P, Boscarato V, et al. Nitric oxide and peroxynitrite platelet levels in gestational hypertension and preeclampsia. Platelets. 2012;23:26–35. doi: 10.3109/09537104.2011.589543. [DOI] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator activated receptor gamma as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011;58:280–286. doi: 10.1161/HYPERTENSIONAHA.111.172627. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienartowicz A, Link S, Moll W. Adaptation of the uterine arcade in rats during pregnancy. J Develop Physiol. 1989;21:101–108. [PubMed] [Google Scholar]
- Oliver FJ, Menissier-de MJ, Nacci C, Decker P, Andriantsitohaina R, Muller S, et al. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Szabo C. Role of peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002a;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002b;6:659–664. [PubMed] [Google Scholar]
- Pascoal IF, Lindheimer MD, Nalbantian-Brandt C, Umans JG. Preeclampsia selectively impairs endothelium-dependent relaxation and leads to oscillatory activity in small omental arteries. J Clin Invest. 1998;101:464–470. doi: 10.1172/JCI557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovits T, Seres L, Gero D, Berger I, Szabo C, Karck M, et al. Single dose treatment with PARP-inhibitor INO-1001 improves aging-associated cardiac and vascular dysfunction. Exp Gerontol. 2007;42:676–685. doi: 10.1016/j.exger.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggensack AM, Zhang Y, Davidge ST. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension. 1999;33:83–89. doi: 10.1161/01.hyp.33.1.83. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Hinshaw DB, Hyslop PA, Spragg RG, Cochrane CG. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986;77:1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, et al. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- Svedas E, Nisell H, VanWijk MJ, Nikas Y, Kublickiene KR. Endothelial dysfunction in uterine circulation in preeclampsia: can estrogens improve it? Am J Obstet Gynecol. 2002;187:1608–1616. doi: 10.1067/mob.2002.127378. [DOI] [PubMed] [Google Scholar]
- Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgut NH, Temiz TK, Bagcivan I, Turgut B, Gulturk S, Karadas B. The effect of sildenafil on the altered thoracic aorta smooth muscle responses in rat pre-eclampsia model. Eur J Pharmacol. 2008;589:180–187. doi: 10.1016/j.ejphar.2008.04.034. [DOI] [PubMed] [Google Scholar]
- VanWijk MJ, Svedas E, Boer K, Nieuwland R, VanBavel E, Kublickiene KR. Isolated microparticles, but not whole plasma, from women with preeclampsia impair endothelium-dependent relaxation in isolated myometrial arteries from healthy pregnant women. Am J Obstet Gynecol. 2002;187:1686–1693. doi: 10.1067/mob.2002.127905. [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Walsh SK, English FA, Edwards EJ, Kenny LC. Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization. Hypertension. 2009;54:345–351. doi: 10.1161/HYPERTENSIONAHA.109.132191. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li Y, Adell G, et al. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl Immunohistochem Mol Morphol. 2009;17:530–535. doi: 10.1097/PAI.0b013e3181a13bf2. [DOI] [PubMed] [Google Scholar]
- Xavier LL, Viola GG, Ferraz AC, Da Cunha C, Deonizio JM, Netto CA, et al. A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res Brain Res Protoc. 2005;16:58–64. doi: 10.1016/j.brainresprot.2005.10.002. [DOI] [PubMed] [Google Scholar]


