Abstract
BACKGROUND AND PURPOSE
The present study was designed to determine how diabetes in pregnancy affects vascular function in their offspring, the influence of age and whether COX activation is involved in this effect.
EXPERIMENTAL APPROACH
Relaxation responses to ACh were analysed in mesenteric resistance arteries from the offspring of control rats (O-CR) and those of diabetic rats (O-DR) at 3, 6 and 12 months of age. TxB2, PGE2 and PGF2α release were determined by enzyme immunoassay. COX-1 and COX-2 expression were measured by Western blot analysis.
KEY RESULTS
O-DR developed hypertension from 6 months of age compared with O-CR. In O-DR, relaxation responses to ACh were impaired in all ages studied and were restored by COX-2 inhibition. TP receptor blockade (SQ29548) restored ACh relaxation in arteries from 3-month-old O-DR while TP and EP receptor blockade (SQ29548 + AH6809) was required to restore it in 6-month-old O-DR. In 12-month-old O-DR, ACh relaxation was restored when TP, EP and FP receptors were blocked (SQ29548 + AH6809 + AL8810). ACh-stimulated TxB2 was higher in all O-DR. ACh-stimulated PGE2 release was increased in arteries from 6- and 12-month-old O-DR, whereas PGF2α was increased only in 12-month-old O-DR. COX-2, but not COX-1, expression was higher in O-DR than O-CR.
CONCLUSIONS AND IMPLICATIONS
The results indicate an age-dependent up-regulation of COX-2 coupled to an enhanced formation of vasoconstrictor prostanoids in resistance arteries from O-DR. This effect plays a key role in the pathogenesis of endothelial dysfunction, which in turn could contribute to the progression of vascular dysfunction in these rats.
Keywords: diabetes, hypertension, fetal programming, endothelial dysfunction, cyclooxygenase, prostanoids, insulin resistance
Introduction
In the past few decades, the incidence of cardiovascular diseases has been increasing worldwide. In the early 20th century, it was responsible for less than 10% of deaths around the entire world, while at the beginning of the 21st century it accounted for almost 50% and 25% of deaths in developed and developing countries, respectively [World Health Organization (WHO), 2002]. In most countries, the increased incidence of cardiovascular diseases has been attributed in part to environmental factors such as diet, smoking and reduced physical exercise (WHO, 2002). On the other hand, the susceptibility to cardiovascular diseases may also be acquired in utero through changes in the uterine environment. Fetal programming refers to the observations that disturbances during the critical period of development can cause lifelong changes in the structure and function of the organism leading to diseases in later life (Barker, 2004). This concept comes from epidemiological studies by Barker and colleagues who obtained evidenced for an inverse relationship between low weight at birth and development of cardiovascular diseases in adulthood (Barker, 1995; 2004; Barker et al., 2002). However, other maternal conditions that produce an adverse environment to fetal development, including chronic hyperglycaemia, also increase the risk of metabolic and cardiovascular diseases in the offspring (Simeoni and Barker, 2009).
Fetal exposure to maternal hyperglycaemia can result in insulin resistance, changes in glucose metabolism and increased risk of hypertension in adult life (Nehiri et al., 2008; Segar et al., 2009; Simeoni and Barker, 2009; Chen et al., 2010; Blondeau et al., 2011). This hypertension has been partially attributed to renal mechanisms. Amri et al. (1999) demonstrated that exposure to maternal diabetes impairs the offspring's nephrogenesis, reducing the number of nephron, which could be a risk factor for the development of chronic renal disease and hypertension in adulthood. In addition, baroreflex dysfunction and increased activity of the renin-angiotensin system have also been reported in adult offspring of diabetic mothers (Wichi et al., 2005).
Other studies have shown that maternal diabetes promotes alterations in vascular reactivity in their offspring. Rocha et al. (2005) demonstrated that offspring of streptozotocin-induced diabetic rats had reduced endothelium-dependent relaxation and hypertension in adulthood. Likewise, Holemans et al. (1999) observed impaired endothelial function in resistance arteries from offspring of diabetic rats, but without changes in BP. The reason for this discrepancy with regard to BP is not fully known, but the age of animals used may have influenced the results. Rocha et al. (2005) used 12-month-old rats while Holemans et al. (1999) used animals that were only 3 months old, which suggest that age may influence the development of hypertension in these animals. The results of Nehiri et al. (2008) confirm this hypothesis; these authors demonstrated an increased BP in the offspring of diabetic rats when they were only 6 months-old. The contribution of the vascular system to these changes in BP as well as the mechanisms involved remain to be elucidated.
Several studies have demonstrated that the balance between vasodilator and vasoconstrictor prostanoids is modified in hypertension (Alvarez et al., 2005; Virdis et al., 2009; Félétou et al., 2011) and diabetes (Bagi et al., 2005; Félétou et al., 2011), and may involve the inducible form of the enzyme, COX-2. In healthy arteries, most prostanoids are produced by the constitutive isoform of COX (COX-1). However, these products may also be synthesized by the COX-2 that is usually expressed at undetectable levels in the vascular wall but can be up-regulated by inflammatory and physical stimuli (Félétou et al., 2011). COX-2-derived prostanoids are associated with the development of vascular complications under conditions of insulin resistance and cardiovascular risk (Helmersson et al., 2004; Bagi et al., 2005; Elmarakby and Imig, 2010; Retailleau et al., 2010). On the basis of these findings, we hypothesized that endothelial dysfunction in resistance arteries from the offspring of insulin-resistant diabetic rats is the result of enhanced COX-2 expression/metabolism, which increases the formation of contractile prostanoids.
In the present study, we tested our hypothesis and specifically investigated the effects of COX inhibitors on ACh-induced relaxation of mesenteric resistance arteries from the offspring of control and diabetic rats. Secondly, we identified the prostanoids released by ACh in arteries from the offspring of diabetic rats and compared their concentrations with those produced in arteries from the offspring of control rats. The influence of age on these parameters was also investigated.
Methods
Experimental animals
Male and female Wistar rats (250–300 g) were obtained from colonies maintained in the animal quarters of the Universidade Federal de Pernambuco (UFPE). Rats were housed at a constant room temperature, humidity and light cycle (12 h light/ dark) and had free access to tap water and standard rat chow ad libitum. This study was approved by the Institutional Animal Care and Use Committee of UFPE (approval reference number: 23076.015755/2008-96), and conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23, revised 1996). The results of all studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
On the 7th day of pregnancy, diabetes was induced by a single injection of streptozotocin (50 mg·kg−1; i.p.). The diabetes was confirmed by measuring plasma glucose concentrations (ACCU-CHEK®, Roche Diagnostics, Mannheim, Germany). After birth, each litter was reduced to six pups and restricted to male offspring only. The unwanted pups were killed by CO2 inhalation followed by cervical dislocation. When the number of males was not enough to complete six, females were used but discarded at weaning. The offspring were divided into two groups: (i) O-CR – offspring of control rats and (ii) O-DR – offspring of diabetic rats. In this study, we used O-CR and O-DR at 3, 6 and 12 months of age.
Glucose tolerance and insulin sensitivity
The oral glucose tolerance test was performed according to a standard protocol. After a 10 h fast, a single oral dose (2 g·kg−1 of body weight) of glucose was delivered. Blood glucose was then measured from the tail vein just before, and 30, 60, 90 and 120 min after glucose injection, using test strips and reader (ACCU-CHEK®, Roche Diagnostics). After 48 h, the animals were subjected to a new 10 h fast for assessment of insulin sensitivity by insulin tolerance test. For this, regular insulin was administered i.p. at the dose of 1.5 U·kg−1 body weight. Blood glucose was determined before and 15, 30, 45 and 60 min after insulin administration.
Arterial BP measurement
Rats were anaesthetized with a mixture of ketamine, xylazine and acetopromazin (64.9, 3.2 and 0.78 mg·kg−1, respectively, i.p.). The right carotid artery was cannulated with a polyethylene catheter (PE-50) that was exteriorized in the mid scapular region. Adequacy of anaesthesia was assessed by monitoring withdrawal reflexes. After 24 h, arterial pressure was measured in conscious, freely moving rats. The arterial cannula was connected to a transducer and pressure signals were recorded for a 60 min period using an interface and software for computer data acquisition (ADInstruments Pty Ltd, Castle Hill, New South Wales, Australia).
Vessel preparation
Rats were anaesthetized with ketamine, xylazine and acetopromazin mixture (64.9, 3.2 and 0.78 mg·kg−1, respectively, i.p.) and killed by exsanguination. The mesenteric vascular bed was removed and placed in cold (4°C) Krebs-Henseleit solution (KHS; in mM: 115 NaCl, 2.5 CaCl2, 4.6 KCl, 1.2 KH2PO4, 1.2, MgSO4.7H2O, 25 NaHCO3, 11.1 glucose and 0.03 EDTA). For reactivity experiments, the third-order branch of the mesenteric arcade was dissected and cut into segments of approximately 2 mm in length. Segments of mesenteric resistance arteries were mounted in a small vessel chamber myograph (Danish Myo Technology A/S, Aarhus, Denmark) to measure isometric tension according to the method described by Mulvany and Halpern (1977).
Experimental protocols
After a 45 min equilibration period, each arterial segment was exposed to KCl (120 mM) to assess its maximum contractility. After a washout period, the presence of the vascular endothelium was confirmed by the ability of 1 µM ACh to relax segments precontracted with noradrenaline at a concentration that produced approximately 50–70% of the contraction induced by KCl. The segments were rinsed with KHS for 1 h and then a cumulative ACh concentration-response curve (0.1 nM to 3 µM) was obtained in the noradrenaline-precontracted segments. Endothelium-independent relaxation was studied by evaluating relaxation to sodium nitroprusside (1 nM–10 µM) in arteries previously contracted with noradrenaline. The possible role of COX-derived metabolites was investigated in segments from O-CR and O-DR rats. Arteries were pre-incubated with either indomethacin (a COX-1 and COX-2 inhibitor, 10 µM), SC-560 (a COX-1 inhibitor, 1 µM), NS-398 (COX-2 inhibitor, 10 µM), SQ29548 [TxA2 receptor (TP) antagonist, 1 µM], AH6809 [PGE2 receptor (EP1, EP2 and EP3) antagonist, 30 µM] or AL8810 [PGF2α receptor (FP) antagonist, 10 µM], before generating concentration-response curves to ACh. All drugs were added 30 min before the concentration-response curve to ACh.
In another set of experiments, the vasoactive responses to the TP receptor agonist U46619 (1 nM–10 µM), PGE2 (10 nM–10 µM) or PGF2α (1 nM to 3 µM), were analysed in quiescent arteries from all groups.
Prostanoid production
To measure the release of TxA2, PGE2, PGF2α and PGI2, we used specific enzyme immunoassay kits (Cayman Chemical Company, Ann Arbor, MI, USA) for each prostanoid. The second, third and fourth branches of mesenteric artery were pre-incubated for 45 min in 200 µL of gassed KHS at 37°C. Afterwards, three washout periods of 10 min in a bath of 200 µL of KHS were run before incubation with ACh (0.1 nM–3 µM). The different assays were performed following the manufacturer's instructions. Results are expressed as pg·mL−1 mg−1 wet tissue.
Western blot analysis of COX-1 and COX-2
Mesenteric resistance arteries from O-CR and O-DR rats were homogenized; proteins were electrophoretically separated (30 µg per lane) and transferred to polyvinyl difluoride membranes (GE Healthcare do Brasil Ltda, São Paulo, SP, Brazil). Membranes were incubated with antibodies (Cayman Chemical Company) against COX-1 (1:1000 dilution), COX-2 (1:500 dilution) or α-actin (1:3000 dilution) proteins and individual horseradish peroxidase-conjugated secondary antibodies (GE Healthcare do Brasil Ltda) in blocking buffer. Immunoreactive proteins were detected by chemiluminescence with ECL Plus (GE Healthcare do Brasil Ltda) and subjected to autoradiography (Hyperfilm ECL, GE Healthcare do Brasil Ltda). Signals on the immunoblot were quantified using a computer programme (NIH Image V1.56, Bethesda, MD, USA). Ram seminal vesicle microsomes and mouse macrophage microsomes protein (Cayman Chemical Company) were used as positive control for COX-1 and COX-2, respectively.
Drugs
Drugs used were noradrenaline hydrochloride, ACh chloride, sodium nitroprusside, indomethacin (Sigma, St. Louis, MO, USA), NS-398, SQ29548, AH6809, AL8810, PGE2, PGF2α (Cayman Chemical Company) and U46619 (Calbiochem-Novabiochem GmbH, Darmstadt, Germany). Stock solutions of ACh and sodium nitroprusside were made in distilled water; noradrenaline was dissolved in an NaCl (0.9%)–ascorbic acid (0.01% w v−1) solution; indomethacin, SQ29548, PGE2 and PGF2α were dissolved in ethanol; and AH6809, AL8810 and U46619 were dissolved in dimethyl sulfoxide. These solutions were kept at −20°C and appropriate dilutions were made on the day of the experiment. Drug/molecular target nomenclature conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2011).
Statistical analysis
Relaxation responses to ACh and sodium nitroprusside are expressed as a percentage of the maximum contractile response induced by noradrenaline. U46619, PGE2 and PGF2α contractile responses are expressed as a percentage of the maximum response produced by KCl.
All values are expressed as means ± SEM of the number of animals used in each experiment. Results were analysed using Student's t-test or by one-way or two-way anova for comparison between groups. When anova showed a significant treatment effect, Bonferroni's post hoc test was used to compare individual means. Differences were considered statistically significant at P < 0.05.
Results
Dams injected with streptozotocin had severe hyperglycaemia on gestational days 14 and 21 compared with control dams (control 852 ± 24 vs. diabetic 4820 ± 225 mg·L−1, t-test: P < 0.05). Gestation occurred normally, and the rats delivered spontaneously at term (21 days of gestation). Diabetic dams gave birth to fewer pups than the controls (control: 10 ± 1 vs. diabetic 6 ± 2 pups per litter; t-test: P < 0.05). As shown in Table 1, mean body weight was significantly lesser in O-DR than O-CR. Blood glucose levels were similar in O-CR and O-DR (Table 1).
Table 1.
Body weights (BW) and blood glucose (BG) at the times of vascular testing
| 3 months | 6 months | 12 months | ||||
|---|---|---|---|---|---|---|
| BW (g) | BG (mg·L−1) | BW (g) | BG (mg·L−1) | BW (g) | BG (mg·L−1) | |
| O-CR | 318 ± 7.23 | 957 ± 34 | 422 ± 6.18 | 952 ± 27 | 467 ± 0.40 | 942 ± 41 |
| O-DR | 295 ± 9.40* | 994 ± 13 | 375 ± 11.7* | 925 ± 30 | 443 ± 2.50* | 908 ± 21 |
Values are means ± SEM. anova:
P < 0.05 compared with O-CR at the same time point.
O-CR, offspring of control rats; O-DR, offspring of diabetic rats.
Oral glucose tolerance test was performed at 3 and 12 months of age. Blood glucose levels were higher in both 3 and 12-month-old O-DR at 30 min compared with O-CR rats (results not shown) and remained increased until the time of 120 min (3-month-old rats: O-CR, 1050 ± 49 vs. O-DR, 1280 ± 45 mg·L−1, t-test: P < 0.05; 12-month-old rats: O-CR, 1060± 32 vs. O-DR, 1420 ± 23 mg·L−1, t-test: P < 0.05). Results from the insulin tolerance test demonstrated significant insulin resistance among the O-DR rats, as they presented a higher blood glucose from 15 min to 60 min after an insulin injection (blood glucose 60 min after the insulin injection; 3-month-old rats: O-CR, 350 ± 40 vs. O-DR, 470 ± 23 mg·L−1, t-test: P < 0.05; 12-month-old rats: O-CR, 330 ± 15 vs. O-DR, 572 ± 32 mg·L−1, t-test: P < 0.05).
O-DR presented higher BP in adulthood. Although the mean arterial pressure of 3-month-old rats was similar in both groups (O-CR: 97.5 ± 2.54 vs. O-DR: 104 ± 8.40 mmHg, t-test, P > 0.05), it was significantly increased in O-DR at both 6 (O-CR: 105 ± 4.70 vs. O-DR: 132 ± 5.30 mmHg, t-test, P > 0.05) and 12 months (O-CR: 102 ± 5.10 vs. O-DR: 149 ± 3.70 mmHg, t-test, P > 0.05) compared with O-CR. The heart rate was similar in all O-DR groups compared with their respective age-matched O-CR (results not shown).
Vascular function in adult diabetic offspring
KCl (120 mM) evoked similar contractions in vessels from both diabetic and age-matched control offspring rats (3-month-old rats, O-CR: 3.15 ± 0.04 vs. O-DR: 3.19 ± 0.07 mN·mm−1; 6-month-old rats, O-CR: 3.28 ± 0.12 vs. O-DR: 3.19 ± 0.09 mN·mm−1; 12-month-old rats, O-CR: 3.21 ± 0.02 vs. O-DR: 3.25 ± 0.13 mN·mm−1; anova, P > 0.05).
ACh induced cumulative concentration- and endothelium-dependent relaxations of noradrenaline-contracted arteries from 3-, 6- and 12-month-old O-CR (Figure 1). However, in mesenteric arteries from O-DR, ACh induced a biphasic response, characterized by a relaxing effect at concentrations equal or below 0.1 µM, which was lower than that in age-matched O-CR, and by a contractile response at concentrations above 0.3 µM that was absent in arteries from O-CR (Figure 1A). Further deterioration of this relaxation was noted with aging (compare Figure 1A–C). Mesenteric resistance arteries from 3-month-old O-DR relaxed 86% to ACh while 6- and 12-month-old O-DR showed 59% and 37% relaxation, respectively (anova: P < 0.05). In arteries from O-CR, relaxation to ACh did not change with age (Figure 1). In all groups, relaxation induced by sodium nitroprusside was comparable (Figure 2).
Figure 1.
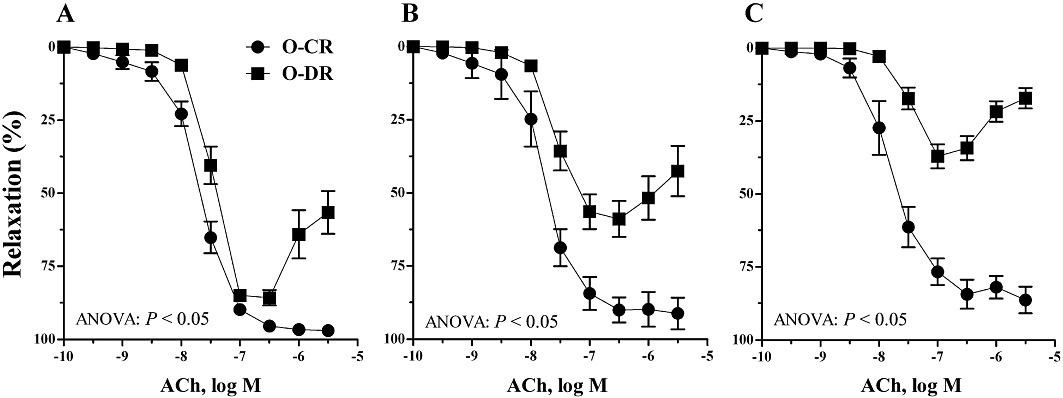
Endothelium-dependent relaxation induced by ACh in mesenteric resistance arteries from (A) 3-, (B) 6- and (C) 12-month-old offspring of control (O-CR) and diabetic (O-DR) rats. Each point represents the mean of 7–8 experiments ± SEM.
Figure 2.
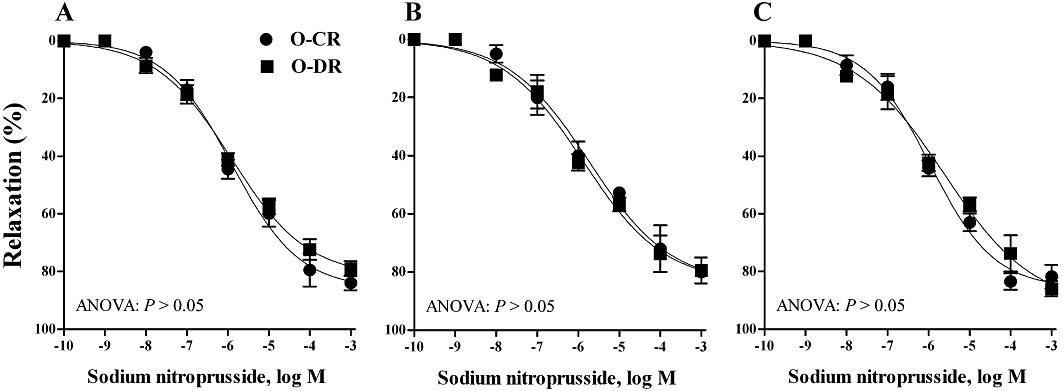
Endothelium-independent relaxation induced by sodium nitroprusside in mesenteric resistance arteries from (A) 3-, (B) 6- and(C) 12-month-old offspring of control (O-CR) and diabetic (O-DR) rats. Each point represents the mean of 7–8 experiments ± SEM.
Effects of COX-1 and COX-2 inhibition and TP, EP and FP receptor antagonism on endothelium-dependent relaxation
In 3-, 6- and 12-month-old O-CR, ACh-induced vasodilatation was not modified by indomethacin or NS-398 (Figure 3A,C,E). In contrast, the altered response to ACh in arteries from 3-, 6- and 12-month-old O-DR was normalized by either indomethacin or NS-398, but not by SC-560, (Figure 3B,D,F).
Figure 3.
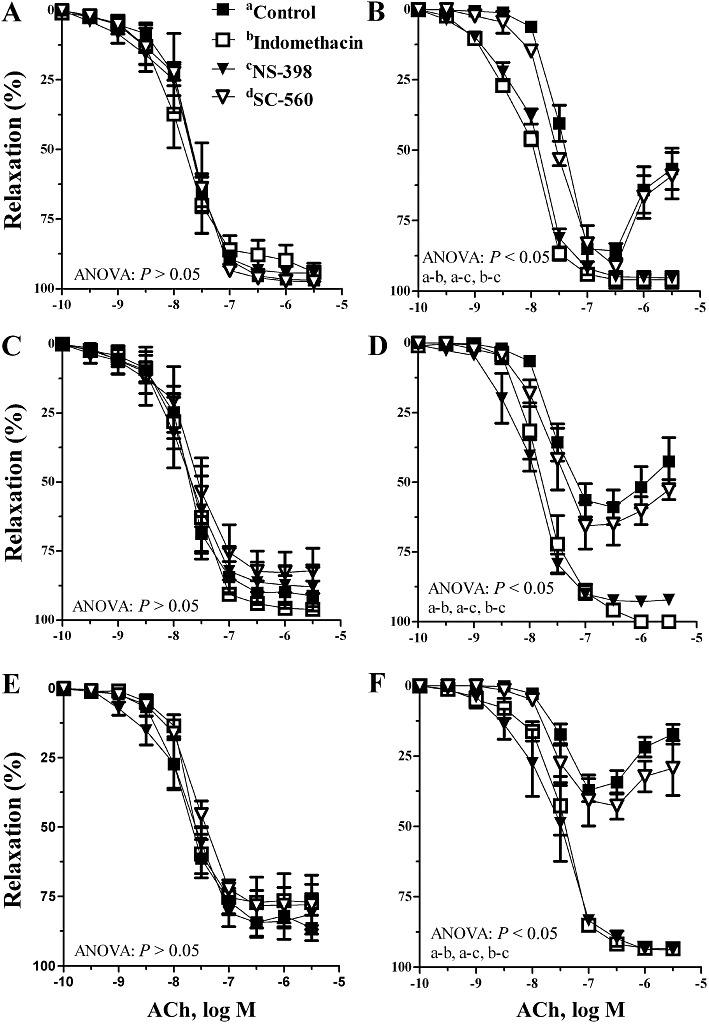
Effect of indomethacin (10 µM) or NS-398 (10 µM) on the concentration-dependent relaxation to ACh in segments of mesenteric resistance arteries from (A) 3-month-old O-CR, (B) 3-month-old ODR, (C) 6-month-old O-CR, (D) 6-month-old ODR, (E) 12-month-old O-CR and (F) 12-month-old ODR. Results are expressed as mean ± SEM; n= 7–8 animals in each group.
In vessels from 3-month-old O-DR, the response to ACh was also enhanced by SQ29548 to similar values to those obtained with COX-2 blockade (Figure 4A). When applied concomitantly with SQ29548, AH6809 or AH6809 plus AL8810 failed to further increase ACh-induced relaxation (Figure 4A). In 6-month-old O-DR, SQ29548 increased the relaxation to ACh, but its effect was reduced compared to that obtained with NS-398 (Figure 4B). When co-incubated with SQ29548, AH6809 increased the response to ACh to similar values to those obtained with NS-398. AL8810 failed to enhance the effect of SQ29548 plus AH6809 (Figure 4B). In arteries from 12-month-old O-DR, SQ29548 partly increased the response to ACh (Figure 4C). In arteries from this group, although co-incubation with AH6809 increased the effect of SQ29548, the normalization of the ACh response was achieved only when arteries were treated with SQ29548 in combination with AH6809 and AL8810 (Figure 4C).
Figure 4.
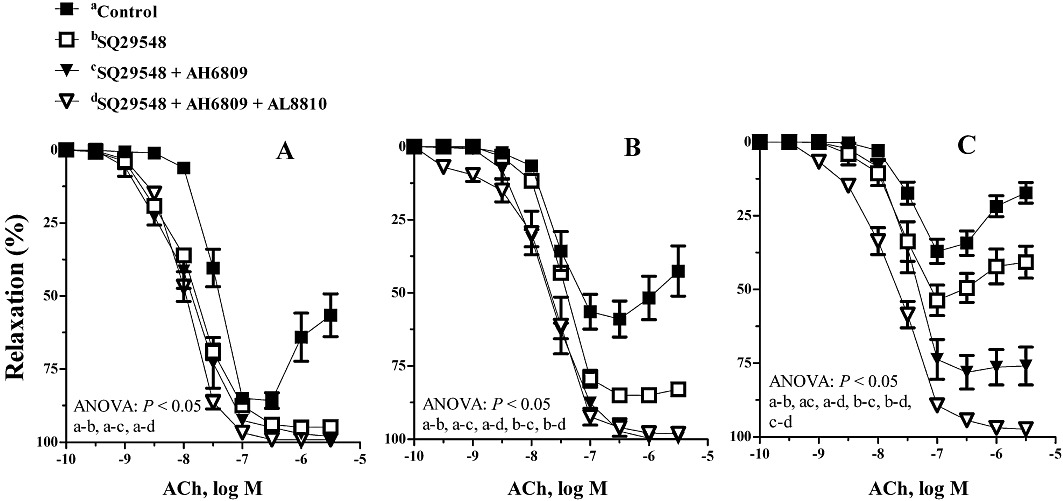
Effect of SQ29548 (1 µM) alone or in association with AH6809 (30 µM) or AH6809 plus AL8810 (10 µM) on the concentration-dependent relaxation to ACh in segments of mesenteric resistance arteries from (A) 3-, (B) 6- and (C) 12-month-old offspring of diabetic (O-DR) rats. Results are expressed as mean ± SEM; n= 7–8 animals in each group.
Vascular response to U46619, PGE2 and PGF2α
U46619, PGE2 and PGF2α caused cumulative concentration-dependent contractions in quiescent arteries. These contractions were not significantly different (anova: P > 0.05) between preparations of O-CR and O-DR (results not shown).
Prostanoids production
In mesenteric resistance arteries from all groups, ACh increased the production of TxB2, PGE2 and PGF2α (Figure 5). The ACh-stimulated TxB2 levels were higher in arteries from both 3-, 6- and 12-month-old O-DR compared with their respective age-matched O-CR (Figure 5A). In arteries from 6- and 12-month-old O-DR, the ACh-stimulated levels of PGE2 were higher, while in 3-month-old O-DR they were not modified (Figure 5B). The ACh-stimulated PGF2α levels were higher only in arteries from 12-month-old O-DR (Figure 5C).
Figure 5.
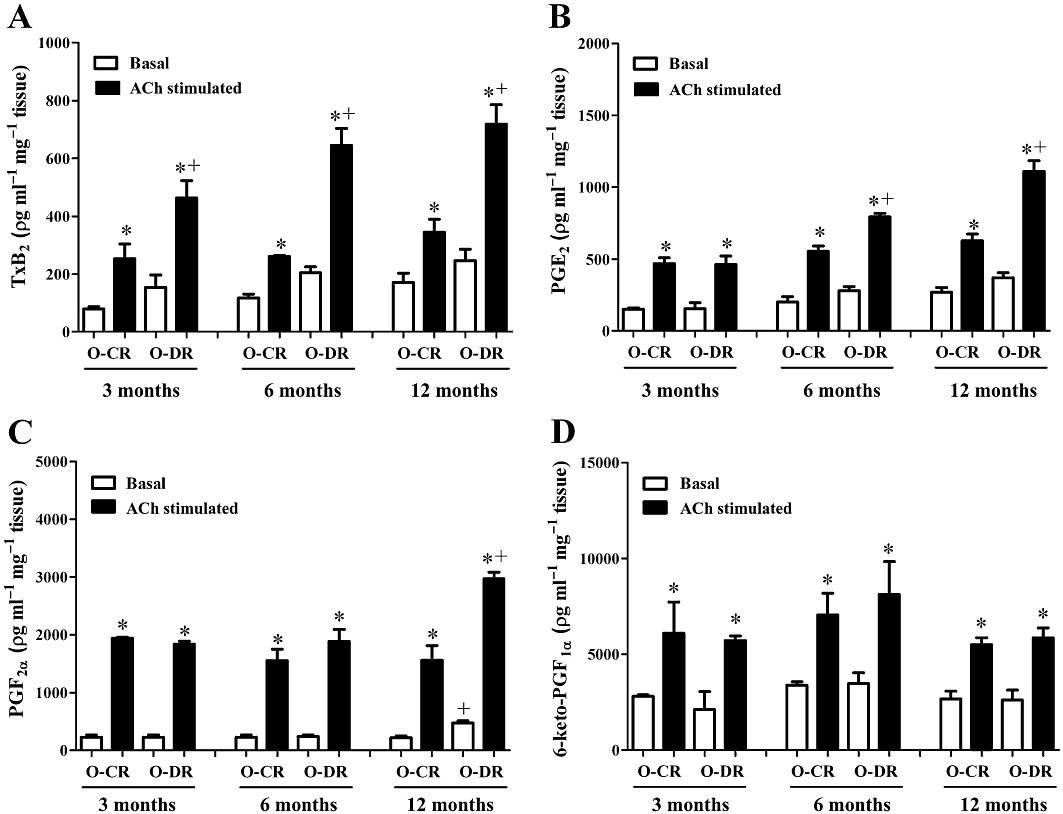
Basal and ACh-stimulated production of TxB2 (A), PGE2 (B), PGF2α (C) and prostacyclin (6-keto-PGF1α, D) in mesenteric resistance segments from 3-, 6- and 12-month-old offspring of control (O-CR) or diabetic (O-DR) rats. n= 6–7 animals in each group. anova: *P < 0.05 ACh-stimulated vs. basal; +P < 0.05 O-DR vs. age-matched O-CR.
COX-1 and COX-2 protein expression
Western blot analysis showed a basal protein expression of COX-2 in mesenteric arteries from O-CR and O-DR (Figure 6). This expression was higher in arteries from O-DR and increased with age, reaching higher levels in 12-month-old O-DR (Figure 6). COX-1 expression was similar in all groups (Figure 6).
Figure 6.
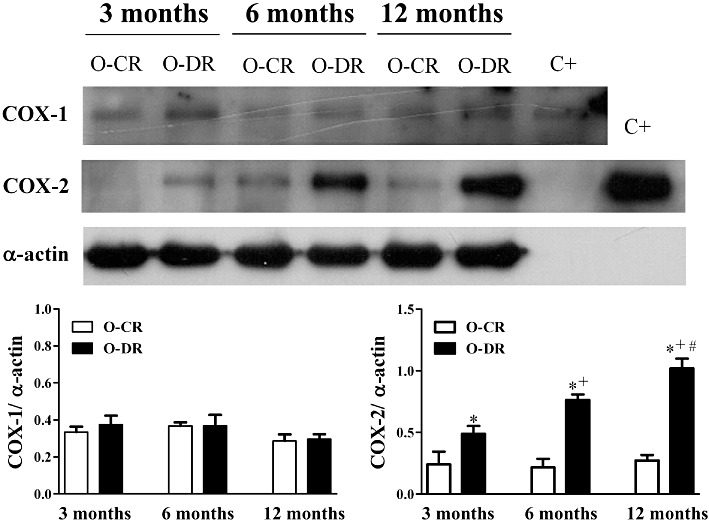
Representative Western blot for COX-1, COX-2, α-actin protein expression and the positive control (C+) for COX-1 and COX-2 in mesenteric resistance arteries from 3-, 6- and 12-month-old offspring of control (O-CR) or diabetic (O-DR) rats (upper panel). Graph shows densitometric analysis of the Western blot for COX-1 and COX-2. Results (means ± SEM) are expressed as the ratio between the signal for the COX-1 or COX-2 protein and the signal for α-actin. n= 6 animals in each group. anova: *P < 0.05 O-DR versus age-matched O-CR; +P < 0.05 6-month-old O-DR versus 3-month-old O-DR; #P < 0.05 12-month-old O-DR versus 6-month-old O-DR.
Discussion and conclusions
In the present study, we investigated the long-term vascular consequences of in utero exposure to maternal hyperglycaemia in rats. Our major novel finding is that this exposure is associated with an age-dependent COX-2 up-regulation, coupled with an enhanced formation of contracting prostanoids that contribute to the impairment of endothelial function in mesenteric resistance arteries from adult offspring of diabetic rats.
Hyperglycaemia in pregnancy leads to insulin resistance in the offspring (Segar et al., 2009; Blondeau et al., 2011), leading to vasoconstriction, inflammation and thrombosis, which occasionally produces hypertension (Chahwala and Arora, 2009). In the current study, although no offspring group developed pre-diabetes or diabetes during the follow-up period, O-DR showed insulin resistance and glucose intolerance at 3 months of age and a further impairment of these parameters at 12 months of age. This effect did not seem to be related to a toxic effect of streptozotocin on the fetal endocrine pancreas as: (i) streptozotocin has a very short (30 min) half-life (Schein and Loftus, 1968); (ii) beta-cell development in the rat occurs mainly during the last week of fetal growth (Blondeau and Breant, 2005); (iii) streptozotocin presented no cytotoxic effect on fetal pro-islets (Liu et al., 1994); and (iv) insulin resistance in O-DR occurs irrespective of the time of streptozotocin administration.
The mechanisms of the hyperglycaemia-programmed hypertension are complex and involve renal, neural and vascular effects (Amri et al., 1999; Holemans et al., 1999; Rocha et al., 2005; Wichi et al., 2005; Nehiri et al., 2008; Segar et al., 2009; Chen et al., 2010). Previous studies have demonstrated a reduced endothelium-dependent relaxation response to dilators in both conductance (Segar et al., 2009; Porto et al., 2010) and resistance (Holemans et al., 1999; Rocha et al., 2005) arteries from O-DR compared with O-CR. This response has been accompanied, or not, by hypertension. Holemans et al. (1999) demonstrated impaired relaxation to ACh and bradykinin in arteries from 3-month-old offspring of diabetic dams, but without changes in BP. On the other hand, Rocha et al. (2005) demonstrated increased BP in 12-month-old diabetic offspring associated with impaired endothelial and renal functions. Therefore, it is possible to postulate that the phenotype of the offspring of diabetic rats tends to vary according to age at the time of analysis.
The results obtained here show that although the BP in 3-month-old rats was similar in both groups, it was increased in the 6- and 12-month-old rats in O-DR compared with those in the O-CR group, as previously reported by Nehiri et al. (2008). Our results also demonstrate that in mesenteric resistance arteries from 3-, 6- and 12-month-old O-DR, the relaxation to ACh, but not to sodium nitroprusside, was impaired compared with that observed in O-CR. In O-DR arteries, this relaxation was progressively impaired with age while in O-CR it remained unmodified. The importance of abnormal endothelium-dependent dilation, as observed in O-DR, lies in the possible pathological consequences. Reduced endothelial function may contribute to coagulation, inflammation and atherosclerosis, as well as to increased peripheral resistance and hypertension (Félétou and Vanhoutte, 2006). Our results also revealed that the endothelial dysfunction in O-DR precedes hypertension, suggesting that this mechanism could be involved in the genesis of hypertension in these animals. Furthermore, a greater impairment of the endothelium-dependent relaxation in arteries from 6- and 12-month-old O-DR was associated with a higher BP in these rats. The mechanism by which maternal diabetes impairs the endothelial function is not fully known. Insulin resistance, perhaps as a result of raised plasma cholesterol and triglycerides, is implicated in endothelial dysfunction (Johnstone et al., 1993). Transient hyperglycaemia (Holemans et al., 1997), oxidative stress (Siman and Eriksson, 1997) or the synthesis of advanced glycosylation end-products (Vlassara et al., 1992) in utero have also been suggested as possible mechanisms involved in decreased endothelium-dependent vasodilatation in the adult offspring.
Insulin resistance is associated with a pro-inflammatory state of the vascular wall leading to vascular remodelling and endothelial dysfunction in systemic arteries (Chahwala and Arora, 2009). Various studies have shown that COX-2 is induced during some inflammatory process and that prostanoids produced by this COX isoform are responsible for many inflammatory symptoms (Parente and Perretti, 2003). In this sense, COX-2-derived prostanoids have been shown to be associated with the development of vascular complications under conditions of insulin resistance and cardiovascular risk (Helmersson et al., 2004; Bagi et al., 2005; Virdis et al., 2009; Elmarakby and Imig, 2010; Retailleau et al., 2010, Félétou et al., 2011), which in turn could contribute to elevation in BP (Tian et al., 2011). The O-DRs used in the present study were insulin resistant and presented endothelial dysfunction in adulthood. To investigate the participation of COX-derived products in the ACh responses, we used the non-selective COX inhibitor indomethacin, the selective COX-1 inhibitor SC-560 and the selective COX-2 inhibitor NS-398. In arteries from 3-, 6- and 12-month-old O-DR, indomethacin or NS-398, but not SC-560, restored the ACh-induced relaxation to similar levels to that in O-CR, indicating that contractile prostanoids from COX-2, but not from COX-1, contribute to the endothelial dysfunction in these rats. In keeping with these functional results, Western blot analysis revealed an up-regulation of COX-2, but not COX-1, in arteries from all O-DR, reaching higher levels at 12 months of age. In O-CR arteries, inhibition of COX-1 or COX-2 did not affect the response to ACh, suggesting that in these rats COX isoenzymes do not have a significant effect on the ACh-induced relaxation, as previously reported (Xavier et al., 2008).
Activation of TP receptors allows vasoconstrictor prostanoids such as TxA2 to contribute to endothelial dysfunction in different cardiovascular disorders (Alvarez et al., 2005; Blanco-Rivero et al., 2005; Xavier et al., 2008). In the present study, blockade of the TP receptors (SQ29548) increased ACh-induced relaxation in mesenteric arteries from all O-DR. In the 3-month-old O-DR, this effect was similar to that produced by NS-398, suggesting that TxA2 is the COX-2-derived prostanoid involved in the endothelial dysfunction in this group. Indeed, in arteries from these rats, ACh-stimulated release of TxB2 (the TxA2 metabolite) was increased. On the other hand, it should be noted that ACh-induced relaxation in arteries from 6- and 12-month-old O-DR was not entirely restored by SQ29548. In vessels from 6-month-old O-DR, relaxation to ACh was completely restored by additional application of the EP receptor blocker, AH6809, while the combined treatment of SQ29548 plus AH6809 and AL8810 (an FP receptor antagonist) was required to fully restore this response in arteries from 12-month-old O-DR. This implies that in arteries from 6-month-old O-DR, TxA2 and PGE2 contribute to the COX-2-dependent endothelial dysfunction while in 12-month-old O-DR, in addition to these two prostanoids, PGF2α seems to play a role. In keeping with these functional findings, ACh-stimulated release of TxB2 and PGE2 was higher in arteries from 6-month-old O-DR. In addition to these two prostanoids, ACh-stimulated PGF2α was also increased in arteries from 12-month-old O-DR. These findings are in accordance with previous results showing that COX-2 induces TxA2, PGE2 and PGF2α production under inflammatory conditions (Kimura et al., 1994; Alvarez et al., 2005; Blanco-Rivero et al., 2005; Retailleau et al., 2010).
PGI2 is another prostanoid that could contribute to impaired endothelial function in arteries from O-DR. This prostanoid also produces vasoconstriction that is mediated by activation of TP and EP receptors (Gluais et al., 2005; Xavier et al., 2008; 2009). Thus, the improvement of relaxation to ACh in arteries from O-DR, obtained in the presence of SQ29548 or AH6809, could be also due to blockade of the vasoconstrictor action of PGI2. In a previous study, we reported that COX-2 overexpression is accompanied by increased PGI2 release and blunted ACh-induced relaxation in rat resistance vessels (Xavier et al., 2008). In the current study, the amount of ACh-stimulated 6-keto-PGF1α in arteries from O-DR was comparable with that observed in arteries from age-matched O-CR. Nevertheless, the involvement of PGI2 in the impaired ACh-induced relaxation in vessels from O-DR cannot be completely ruled out. Duong Van Huyen et al. (2010) reported that exposure to maternal diabetes is associated with decreased vascular expression of IP and impaired relaxation to a PGI2 analogue, which in turn could contribute to the impaired endothelium-dependent relaxation.
Taken together our findings provide the first demonstration of the participation of an age-dependent increased COX-2-derived TxA2, PGE2 and PGF2α in the changes in endothelial function in resistance vessels from adult offspring of diabetic rats, supporting the possible relevance of these prostanoids in cardiovascular changes induced by exposure in utero to hyperglycaemia. The fact that exogenously administered PGE2, PGF2α or the TP receptor agonist U46616 produced similar contractions in arteries from all groups studied eliminates the possibility that EP, FP or TP receptor initiated signalling mechanisms are altered in mesenteric resistance arteries from O-DR.
In summary, the present study demonstrates an age-dependent up-regulation of COX-2 coupled to an enhanced formation of vasoconstrictor prostanoids in resistance arteries from adult offspring of diabetic rats. This increased formation of vasoconstrictor prostanoids plays a key role in the pathogenesis of endothelial dysfunction, which in turn could contribute to progression of vascular dysfunction in these rats.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE). FER-A and DBdeQ were supported by a master's degree fellowship award from FACEPE. JS-R was supported by a scientific initiation fellowship award from PIBIC/UFPE. GPD and FEX are recipients of research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico. We are grateful to José Antonio de Albuquerque for his technical assistance.
Glossary
- AH6809
6-isopropoxy-9-oxoxanthene-2-carboxylic acid
- AL8810
9α,15R-dihydroxy-11β-fluoro-15-(2,3-dihydro-1H-inden-2-yl)-16,17,18,19,20-pentanor-prosta-5Z,13E-dien-1-oic acid
- EP
prostaglandin E2 receptors
- FP
prostaglandin F2α receptors
- KHS
Krebs-Henseleit solution
- NS-398
N-(2-cyclohexyloxy-4-nitrophenyl) methansulfonamide
- O-CR
offspring of control rats
- O-DR
offspring of diabetic rats
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- SC-560
5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole
- SQ29548
[1S-[1a,2a(Z),3a,4a]]-7-[3-[[2-(phenylamino) carbonyl]hydrazino]methyl]-7-oxabicyclo [2.2.1] hept-2-yl]-5-heptanoic acid
- TP
thromboxane A2 receptors
- TxA2
thromboxane A2
- U46619
(15)-hydroxy-11,9 -(epoxymethano)prosta-5,13-dienoic acid
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edn. Br J Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Y, Briones AM, Balfagón G, Alonso MJ, Salaices M. Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. J Hypertens. 2005;23:767–777. doi: 10.1097/01.hjh.0000163145.12707.63. [DOI] [PubMed] [Google Scholar]
- Amri K, Freund N, Vilar J, Merlet-Bénichou C, Lelièvre-Pégorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes. 1999;48:2240–2245. doi: 10.2337/diabetes.48.11.2240. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588–595. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forse'n T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Márquez-Rodas I, Salaices M, et al. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- Blondeau B, Breant B. Effect of nutrition on fetal development: a view on the pancreatic beta-cells. In: Djelmis J, Desoye G, Ivanisevic M, editors. Diabetology of Pregnancy. Basel: Karger; 2005. pp. 83–93. [Google Scholar]
- Blondeau B, Joly B, Perret C, Prince S, Bruneval P, Lelièvre-Pégorier M, et al. Exposure in utero to maternal diabetes leads to glucose intolerance and high blood pressure with no major effects on lipid metabolism. Diabetes Metab. 2011;37:245–241. doi: 10.1016/j.diabet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Chahwala V, Arora R. Cardiovascular manifestations of insulin resistance. Am J Ther. 2009;16:14–28. doi: 10.1097/MJT.0b013e3180a724b3. [DOI] [PubMed] [Google Scholar]
- Chen YW, Chenier I, Tran S, Scotcher M, Chang SY, Zhang SL. Maternal diabetes programs hypertension and kidney injury in offspring. Pediatr Nephrol. 2010;25:1319–1329. doi: 10.1007/s00467-010-1506-1. [DOI] [PubMed] [Google Scholar]
- Duong Van Huyen JP, Vessières E, Perret C, Troise A, Prince S, Guihot AL, et al. In utero exposure to maternal diabetes impairs vascular expression of prostacyclin receptor in rat offspring. Diabetes. 2010;59:2597–2602. doi: 10.2337/db10-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci (Lond) 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Félétou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011;164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmersson J, Vessby B, Larsson A, Basu S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation. 2004;109:1729–1734. doi: 10.1161/01.CIR.0000124718.99562.91. [DOI] [PubMed] [Google Scholar]
- Holemans K, Van Bree R, Verhaeghe J, Meurrens K, Van Assche FA. Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr. 1997;127:1371–1376. doi: 10.1093/jn/127.7.1371. [DOI] [PubMed] [Google Scholar]
- Holemans K, Gerber RT, Meurrens K, de Clerck F, Poston I, Van Assche FA. Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia. 1999;42:81–89. doi: 10.1007/s001250051117. [DOI] [PubMed] [Google Scholar]
- Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Hata Y, Islam MA, Kimura M. Diabetes mellitus-induced enhancement of prostaglandin F2 alpha-responses is inhibited by lipoxygenase- but not cyclooxygenase-inhibitors in mesenteric veins and arteries of mouse and rat. Jpn J Pharmacol. 1994;64:65–70. doi: 10.1254/jjp.64.65. [DOI] [PubMed] [Google Scholar]
- Liu X, Hering BJ, Brendel MD, Bretzel RG. The effect of streptozotocin on the function of fetal porcine and rat pancreatic (pro-)islets. Exp Clin Endocrinol. 1994;102:374–379. doi: 10.1055/s-0029-1211307. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nehiri T, Duong Van Huyen JP, Viltard M, Fassot C, Heudes D, Freund N, et al. Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes. 2008;57:2167–2175. doi: 10.2337/db07-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente L, Perretti M. Advances in the pathophysiology of constitutive and inducible cyclooxygenases: two enzymes in the spotlight. Biochem Pharmacol. 2003;65:153–159. doi: 10.1016/s0006-2952(02)01422-3. [DOI] [PubMed] [Google Scholar]
- Porto NP, Jucá DM, Lahlou S, Coelho-de-Souza AN, Duarte GP, Magalhães PJ. Effects of K+ channels inhibitors on the cholinergic relaxation of the isolated aorta of adult offspring rats exposed to maternal diabetes. Exp Clin Endocrinol Diabetes. 2010;118:360–363. doi: 10.1055/s-0029-1241824. [DOI] [PubMed] [Google Scholar]
- Retailleau K, Belin de Chantemèle EJ, Chanoine S, Guihot AL, Vessières E, Toutain B, et al. Reactive oxygen species and cyclooxygenase 2-derived thromboxane A2 reduce angiotensin II type 2 receptor vasorelaxation in diabetic rat resistance arteries. Hypertension. 2010;55:339–344. doi: 10.1161/HYPERTENSIONAHA.109.140236. [DOI] [PubMed] [Google Scholar]
- Rocha SO, Gomes GN, Forti AL, Franco MC, Fortes ZB, Cavanal MF, et al. Long-term effects of maternal diabetes on vascular reactivity and renal function in the rat male offspring. Pediatr Res. 2005;58:1274–1279. doi: 10.1203/01.pdr.0000188698.58021.ff. [DOI] [PubMed] [Google Scholar]
- Schein PS, Loftus S. Streptozotocin: depression of mouse liver pyridine nucleotides. Cancer Res. 1968;28:1501–1506. [PubMed] [Google Scholar]
- Segar EM, Norris AW, Yao J, Hu S, Koppenhafer SL, Roghair RD, et al. Programming of growth, insulin resistance and vascular dysfunction in offspring of late gestation diabetic rats. Clin Sci (Lond) 2009;117:129–138. doi: 10.1042/CS20080550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman CM, Eriksson UJ. Vitamin E decreases the occurrence of malformations in the offspring of diabetic rats. Diabetes. 1997;46:1054–1055. doi: 10.2337/diab.46.6.1054. [DOI] [PubMed] [Google Scholar]
- Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med. 2009;14:119–124. doi: 10.1016/j.siny.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Tian X, Wong WT, Leung FP, Zhang Y, Wang YX, Lee HK, et al. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin F2α impairs endothelial function in renovascular hypertensive rats. Antioxid Redox Signal. 2011;16:363–373. doi: 10.1089/ars.2010.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdis A, Colucci R, Versari D, Ghisu N, Fornai M, Antonioli L, et al. Atorvastatin prevents endothelial dysfunction in mesenteric arteries from spontaneously hypertensive rats: role of cyclooxygenase 2-derived contracting prostanoids. Hypertension. 2009;53:1008–1016. doi: 10.1161/HYPERTENSIONAHA.109.132258. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging. Proc Natl Acad Sci USA. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Integrated Management of Cardiovascular Risk. Geneva: WHO CVD Program; 2002. [Google Scholar]
- Wichi RB, Souza SB, Casarini DE, Morris M, Barreto-Chaves ML, Irigoyen MC. Fetal physiological programming increased blood pressure in the offspring of diabetic mothers. Am J Physiol Regul Integr Comp Physiol. 2005;288:1129–1133. doi: 10.1152/ajpregu.00366.2004. [DOI] [PubMed] [Google Scholar]
- Xavier FE, Aras-López R, Arroyo-Villa I, Campo LD, Salaices M, Rossoni LV, et al. Aldosterone induces endothelial dysfunction in resistance arteries from normotensive and hypertensive rats by increasing thromboxane A2 and prostacyclin. Br J Pharmacol. 2008;154:1225–1235. doi: 10.1038/bjp.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier FE, Blanco-Rivero J, Ferrer M, Balfagón G. Endothelium modulates vasoconstrictor response to prostaglandin I2 in rat mesenteric resistance arteries: interaction between EP1 and TP receptors. Br J Pharmacol. 2009;158:1787–1795. doi: 10.1111/j.1476-5381.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


