Abstract
Although obesity is implicated in numerous health complications leading to increased mortality, the relationship between obesity and outcomes for critically ill patients appears paradoxical. Recent studies have reported better outcomes and lower levels of inflammatory cytokines in obese patients with acute lung injury (ALI)/acute respiratory distress syndrome, suggesting that obesity may ameliorate the effects of this disease. We investigated the effects of obesity in leptin-resistant db/db obese and diet-induced obese mice using an inhaled LPS model of ALI. Obesity-associated effects on neutrophil chemoattractant response were examined in bone marrow neutrophils using chemotaxis and adoptive transfer; neutrophil surface levels of chemokine receptor CXCR2 were determined by flow cytometry. Airspace neutrophilia, capillary leak, and plasma IL-6 were all decreased in obese relative to lean mice in established lung injury (24 h). No difference in airspace inflammatory cytokine levels was found between obese and lean mice in both obesity models during the early phase of neutrophil recruitment (2–6 h), but early airspace neutrophilia was reduced in db/db obese mice. Neutrophils from uninjured obese mice demonstrated diminished chemotaxis to the chemokine keratinocyte cytokine compared with lean control mice, and adoptive transfer of obese mouse neutrophils into injured lean mice revealed a defect in airspace migration of these cells. Possibly contributing to this defect, neutrophil CXCR2 expression was significantly lower in obese db/db mice, and a similar but nonsignificant decrease was seen in diet-induced obese mice. ALI is attenuated in obese mice, and this blunted response is in part attributable to an obesity-associated abnormal neutrophil chemoattractant response.
Keywords: adult respiratory distress syndrome, chemotaxis, cytokines, innate immunity
Clinical Relevance
Obesity may be associated with improved outcomes from acute lung injury/acute respiratory distress syndrome. However, the effects of obesity on the pathogenesis of acute lung injury are poorly understood. This study demonstrates that obesity attenuates the development of acute lung injury in mouse models and implicates obesity-associated neutrophil chemotaxis dysfunction in this effect.
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are characterized by persistent, uncontrolled pulmonary inflammation that occurs in response to a wide range of insults, including pneumonia, sepsis, and trauma (1, 2). Alveolar recruitment of neutrophils is thought to be a central factor in the onset and progression of this syndrome (3, 4), and increases in airspace neutrophilia and plasma neutrophilic cytokine levels, including TNF-α, IL-1β, IL-6, and IL-8, are associated with increased morbidity and mortality from this disease (4–6). It is increasingly recognized that ALI pathogenesis and outcome are strongly influenced by host factors, including genetic polymorphisms and comorbid conditions (1, 2). Preliminary clinical evidence suggests that obesity may have an ameliorative effect on ALI outcome (7). Although ambiguity exists in smaller studies (8, 9), recent large cohort studies from our group and others, as well as several metaanalyses, have shown a reduction in mortality with rising body mass index in ALI and critical illness in general (7, 10–16). Such an association, though tentative, is surprising because obesity is believed to be an inflammatory state with mild baseline elevations in blood TNF-α, IL-1β, IL-6, and IL-8 (17) as well as increased blood neutrophil levels (18). Nevertheless, we have recently reported that in the context of established human ALI, plasma IL-6 and IL-8 fall with rising body mass index (19), suggesting that obesity may have an attenuating effect on inflammation in this disease.
Although animal studies examining the effects of obesity on ALI are scarce, recent reports demonstrate that spontaneously obese leptin-resistant (db/db) and leptin-deficient (ob/ob) mice (the most commonly used mouse models of obesity) have reduced lung injury and mortality from hyperoxic and ozone-induced lung injury (20–22). Only one report (22) has examined the effects of diet-induced obesity. Although these studies have implicated alterations in IL-6 (22, 23) and leptin (20, 21) signaling, mechanistic links between the obese state and the attenuation of ALI remain unclear. Most animal studies examining obesity-associated effects on pulmonary immunity have focused on models of asthma and pneumonia, and although airway inflammation appears to be amplified by obesity, the response to pneumonia is blunted (24, 25), suggesting that the inflammatory response in the alveoli (the site of ALI) is impaired. Although work in obese pneumonia models has highlighted alterations in macrophage function (26), little is known about obesity-associated effects on ALI pathogenesis, and even less is known of obesity’s effects on the hallmark effector cell of ALI, the neutrophil.
In this study we demonstrate that obesity attenuates inflammatory response in an inhaled LPS model of murine ALI, leading to a reduction in pulmonary neutrophilia and injury. Furthermore, we show that obesity is associated with defects in neutrophil chemoattractant response common to genetic and diet-induced models of obesity, indicating that disruption of neutrophil diapedesis into the lung contributes to the attenuated inflammatory response found in obese mice.
Materials and Methods
Mice
For the diet-induced obesity model, male C57BL/6 mice (Jackson Labs, Bar Harbor, ME) were fed high-fat (60% fat) versus normal-fat (10% fat) chow (Research Diets, New Brunswick, NJ) for 20 weeks. In a genetic model of obesity, male and female homozygous B6 db/db mice (leptin-resistant mice on a C57Bl/6 background that are spontaneously obese due to hyperphagia; Jackson Labs) and their lean heterozygous littermates were examined at 6 to 8 weeks of age. Experiments were performed in accordance with the Animal Welfare Act and the USPHS Policy on Humane Care and Use of Laboratory Animals after review by the Animal Care and Use Committee of the University of Vermont.
LPS-Induced Lung Injury
Mice were exposed to aerosolized Escherichia coli 0111:B4 LPS (Sigma, St. Louis, MO) (27, 28). The animals were killed 2, 6, or 24 hours later, and blood, bronchoalveolar lavage (BAL), and whole lungs were analyzed. Additional details are provided in the online supplement.
Determination of Cytokine, Total Protein, and Cholesterol Levels
IL-6, keratinocyte cytokine (KC), TNF-α, macrophage inflammatory protein (MIP)-2, and monocyte chemotactic protein (MCP)-1 levels in mouse plasma and BAL supernatants were assessed by Bio-Plex suspension-array system (Bio-Rad, Hercules, CA). BAL protein levels were measured by Bradford assay (Bio-Rad). Uninjured-mouse plasma LDL cholesterol levels were assayed by fast protein liquid chromatography. Additional details are provided in the online supplement.
Preparation of Morphologically Mature Murine Bone Marrow Neutrophils
Femurs/tibias of obese or lean mice were dissected, marrow flushed with HBSS, and layered on a three-step Percoll (GE Healthcare, Piscataway, NJ) gradient (72, 64, and 52%), which was centrifuged at 1,060 × g for 30 minutes as previously described (29, 30). Samples of the 72:64% interface revealed greater than 95% morphologically mature-appearing neutrophils.
Neutrophil Chemotaxis
Chemotaxis of marrow neutrophils was measured in response to KC (R&D Systems, Minneapolis, MN) using a 48-well modified Boyden Chamber with 5-μm pore polycarbonate membranes (NeuroProbe Inc., Gaithersburg, MD) as previously described (28).
Neutrophil Adoptive Transfer
Adoptive transfer of obese versus lean mouse neutrophils was performed as we have previously described (29–31). Briefly, 5 × 106 isolated bone marrow neutrophils per mouse were injected into lean mice by tail vein, after which recipient mice were exposed to inhaled LPS and examined at 24 hours as described above.
Calcium Flux Assays
Calcium flux in marrow neutrophils was measured in response to 25 ng/ml KC using Indo-1/AM (Molecular Probes, Carlsbad, CA) as described (30).
Determination of Neutrophil Surface CXCR2 Expression
CXCR2 surface expression on marrow neutrophils was measured using an LSR II flow cytometer (BD, San Jose, CA) after dual-staining the cells with anti–Gr-1-Pacific Blue monoclonal antibody, and Alexa-Fluor 647 anti-mouse CD182 (CXCR2; Biolegend, San Diego, CA) or isotype-control monoclonal antibodies, and the resulting data were analyzed by FloJo software (TreeStar, Ashland, OR) as described (30).
Statistical Analysis
Correlations between weight and BAL neutrophil levels, as well as covariates that might affect BAL neutrophil levels (e.g., age and weight), were analyzed by linear regression using STATA 10.0 (College Station, TX). All other data were analyzed with the Student’s or Welch’s t test using Prism 5 software (GraphPad, La Jolla, CA). Results are reported with SEM in the case of t test analysis and with 95% confidence intervals for linear regressions.
Results
Obesity Attenuates Pulmonary Neutrophilia and Capillary Leak yet Increases Blood Neutrophilia in Established ALI
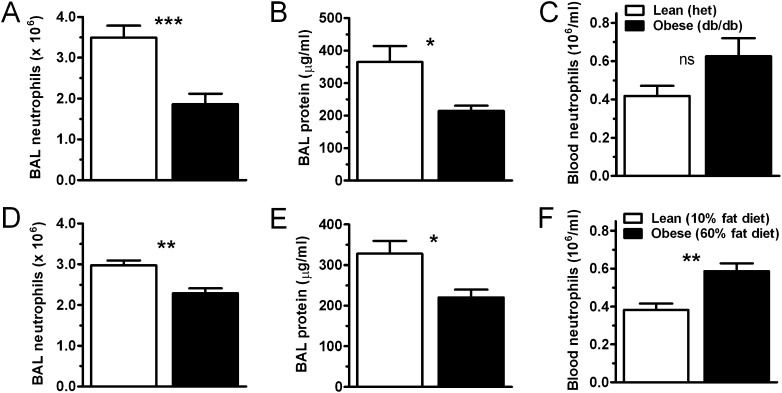
To investigate possible mechanisms for obesity-induced attenuation of ALI, we examined LPS injury in db/db (leptin-resistant mice spontaneously obese due to hyperphagia) and diet-induced (fed high-fat chow for 20 wk) obese mice. Twenty-four hours after LPS exposure, this injury model reproduces many of the features of established human ALI, including the often extreme blood and pulmonary neutrophilia implicated in the pathogenesis of the disease as well as the formation of proteinaceous alveolar edema, the sine qua non of ALI (2). Mice from both obesity models examined in these studies were found to weigh significantly more than their lean controls at the time of LPS exposure: lean heterozygous db mice weighed 22.2 ± 0.9 g, db/db weighed 41.8 ± 1.7 g (P < 0.0001); mice fed a 10% fat diet weighed 32.1 ± 0.9 g, and mice fed a 60% fat diet weighed 47.5 ± 0.8 g (P < 0.0001). Twenty-four hours after LPS exposure, airspace neutrophilia was diminished in db/db and diet-induced obese (DIO) mice compared with the lean control mice (Figures 1A and 1D). In addition, BAL fluid total protein content (a marker of alveolar injury and capillary leak) was decreased in db/db and DIO mice relative to lean mice after injury (Figures 1B and 1E). Histological examination of lungs from injured animals (see Figure E1 in the online supplement) demonstrated less injury in obese mice, with decreased airspace neutrophilia and serum protein leak seen in the obese db/db and, to a slightly lesser degree, in the obese DIO compared with lean mice. Total lung tissue neutrophil content as gauged by whole-lung myeloperoxidase activity was decreased in the injured obese mice compared with lean mice in the db/db model of obesity but was similar between lean and obese mice in the DIO model (Figure E2). Similar to our findings in human ALI (19), higher levels of blood neutrophilia were present in db/db and DIO mice (Figures 1C and 1F) relative to lean mice after injury. Obese and lean mice exposed to nebulized saline solution showed no evidence of inflammation or injury (data not shown).
Figure 1.
Obesity attenuates airspace neutrophilia and lung injury in db/db and diet-induced obese mice. Acute lung injury was induced by the inhalation of LPS in genetically obese (db/db) versus lean (heterozygous littermate control) mice (A–C), and diet-induced obese (60% fat diet) versus lean (10% fat diet) mice (D–F). Mice were exposed to nebulized Escherichia coli LPS (3 mg/ml; 15 min) 24 hours before determining bronchoalveolar lavage (BAL) (A, D) and blood (C, F) neutrophil levels by cell counter. BAL protein content (B, E) was determined by Bradford assay. n = 8 (diet-induced) or 12 (db/db) mice per condition. *P < 0.05; **P < 0.01; ***P < 0.001. ns = not significant.
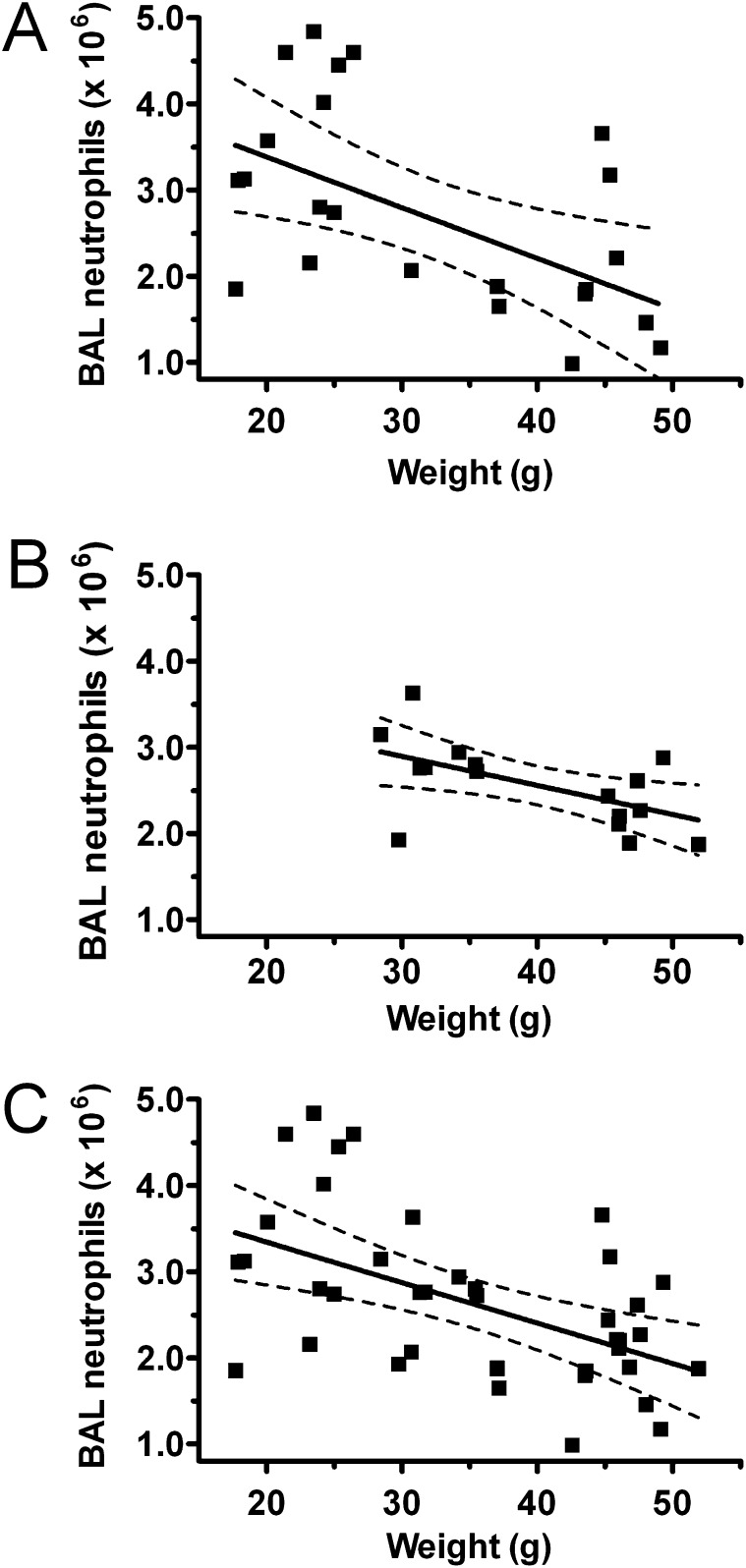
These findings suggest that the obese state in mice attenuates two cardinal features of ALI that have been shown to predict poor outcomes from this disease in humans (2). In further confirmation of the effect of weight on lung injury, we noted an inverse correlation between preinjury mouse weights and subsequent levels of airspace neutrophilia after injury in both obesity models (Figures 2A and 2B). Univariate regression analysis of the combined obese and lean mice from both mouse models (db/db and diet-induced) showed a highly significant relationship between BAL neutrophil levels and weight (P = 0.001; adjusted r2 = 0.25) (Figure 2C). There was no relationship between age or obesity model (db/db versus diet-induced obesity) and BAL neutrophil levels (P = 0.75 and 0.77, respectively).
Figure 2.
Airspace neutrophilia is inversely related to mouse weight in LPS-injured lean and obese mice. BAL neutrophil levels 24 hours after nebulized LPS exposure in genetically obese (db/db) and lean (heterozygous littermate control) mice (A) and diet-induced obese (60% fat diet) and lean (10% fat diet) mice (B) were graphed versus mouse weights measured immediately before injury. Db/db mice: r2 = 0.27, P = 0.0096 by linear regression; diet-induced mice: r2 = 0.32, P = 0.02. Combining lean and obese mice from db/db and diet-induced models (C), an inverse relationship between weight and airspace neutrophilia remained and was not affected by mouse age or obesity model (P = 0.001, adjusted r2 = 0.25). Dashed lines indicate 95% confidence intervals.
Early Pulmonary Cytokine Response to Injury Is Normal to Elevated in Obese Mice
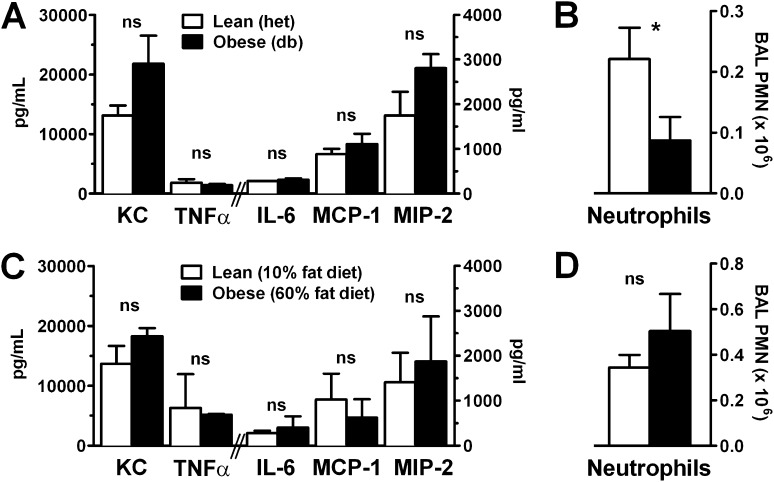
To determine whether the impaired pulmonary neutrophilia seen in established LPS-induced injury was caused by attenuated pulmonary cytokine release or by a primary defect in neutrophil chemotaxis, we examined BAL cytokine and neutrophil levels during the early phase of neutrophil recruitment (2 h after LPS injury). At this early time point, BAL levels of inflammatory cytokines, including the CXC chemokines KC and MIP-2, critical in pulmonary neutrophil recruitment, were normal to elevated in db/db and DIO obese mice compared with lean control mice (Figures 3A and 3C). However, despite this normal cytokine response, neutrophil recruitment into the airspace of obese mice was still significantly blunted at this early time point in the db/db model (Figure 3B), whereas it was not significantly different in the DIO obese mice (Figure 3D). Examination of the 6-hour time points showed similar findings (Figure E3). Because neutrophil recruitment to the lung is governed primarily by blood neutrophil mobilization, which we found to be elevated in obese mice (Figures 1C, 1F), pulmonary chemokine production (Figure 3A, also normal), and neutrophil chemotaxis, we questioned whether the obese state confers an intrinsic migratory defect upon the neutrophil.
Figure 3.
Initial lung cytokine response to injury is normal in obese mice, but early neutrophilia is decreased. BAL cytokine (A, C) and neutrophil (B, D) levels 2 hours after nebulized LPS exposure in lean compared with genetically obese (db/db) and diet-induced obese mice were determined by Bio-Plex and cell counter, respectively. n = 6 mice/condition. *P < 0.05. KC = keratinocyte cytokine; MCP-1 = monocyte chemotactic protein 1; MIP-2 = macrophage inflammatory protein; ns = not significant; PMN = polymorphonuclear leukocyte.
Obesity Attenuates Neutrophil Chemotaxis Response and Airspace Entry
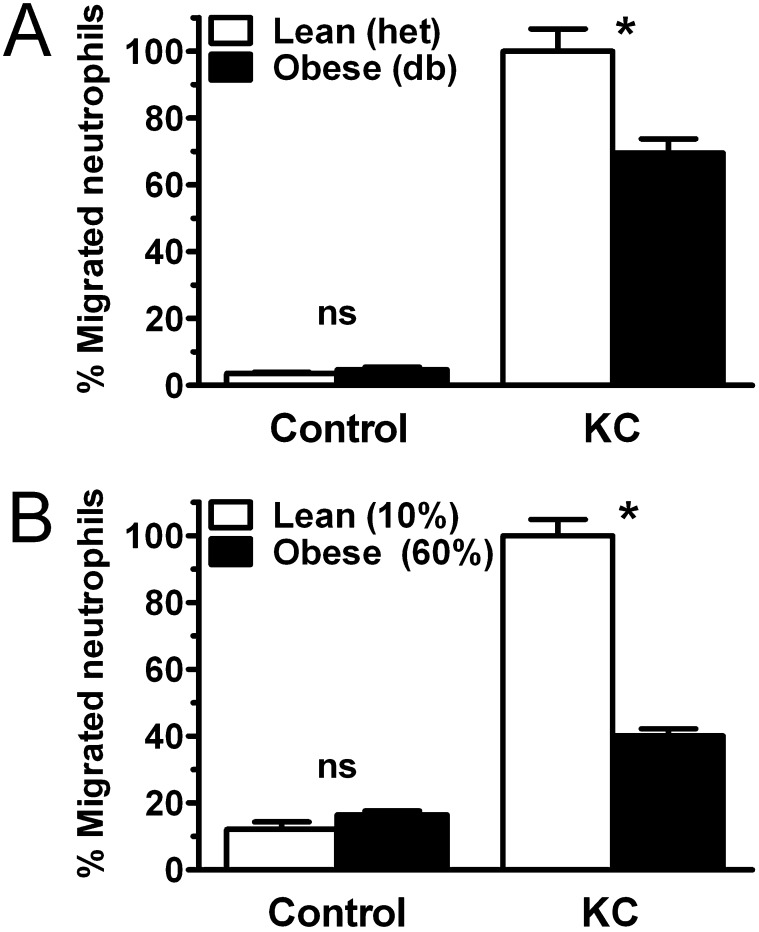
To investigate whether intrinsic defects in neutrophil function contribute to the attenuation of airspace neutrophil recruitment in obese mice, we examined chemoattractant response in neutrophils from lean and obese mice using modified Boyden chambers. Chemotaxis to the neutrophilic chemokine KC was found to be markedly attenuated in neutrophils from db/db and DIO mice compared with lean control mice (Figure 4). Examination of neutrophil chemotaxis response to a range of KC concentrations demonstrated that obese neutrophil response is subtly impaired at low KC concentrations and that this defect increases with rising concentrations of this chemokine (Figure E4), suggesting a rightward shift of the KC response curve in obese mouse neutrophils. To determine whether the obesity-associated defects in neutrophil airspace migration we found in vivo were conferrable using neutrophil adoptive transfer, we injected isolated marrow neutrophils from obese or lean mice into recipient lean mice, which were then exposed to inhaled LPS. At 24 hours, the resulting airspace neutrophilia was significantly lower in mice receiving obese compared with lean mouse neutrophils (Figure E5). Thus, obesity appears to be associated with previously undescribed intrinsic abnormalities in neutrophil function.
Figure 4.
Obesity impairs neutrophil chemotaxis. Chemotaxis of density centrifugation-isolated mature bone marrow neutrophils from genetically obese (db/db) (A) and diet-induced obese (B) mice was compared with lean control mice using a modified Boyden chamber with KC (25 ng/ml). Membrane counts were expressed as percentage of lean control neutrophil migration to KC for each experiment. Four separate experiments were performed on db/db and diet-induced obese mouse isolated neutrophils and respective control mice. *P < 0.01.
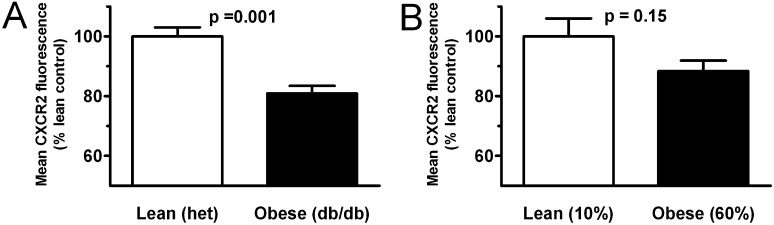
Obesity Is Associated with Blunted Neutrophil Signaling Response to KC and Decreased Neutrophil Surface Display of the Chemoattractant Receptor CXCR2
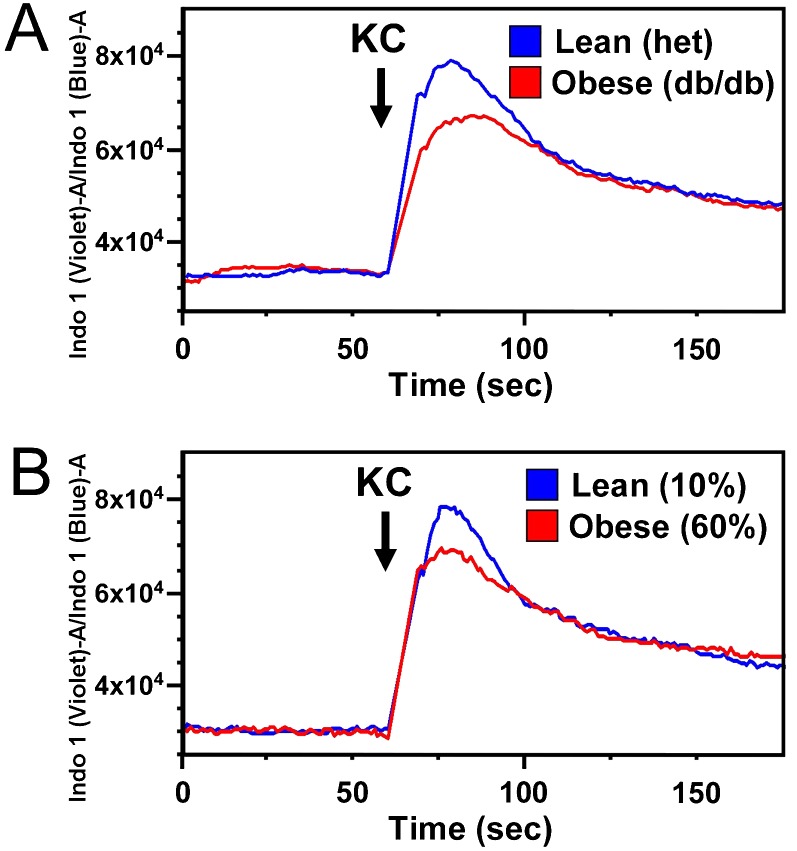
To explore the obesity-related defect in neutrophil chemotaxis, we examined cellular calcium flux, an early response to ligation of receptors driving neutrophil chemotaxis. Diminished calcium flux to KC was seen in neutrophils from obese db/db and DIO mice (Figure 5). To further dissect this defect, we examined neutrophil surface levels of CXCR2, the receptor for KC and MIP-2, in db/db and DIO mice compared with lean mice. Surface expression of CXCR2 was found to be significantly decreased in neutrophils from obese db/db animals compared with lean littermates (Figure 6A). This effect was less pronounced and did not reach significance in DIO mice (Figures 6B). Histographic representation of this data (Figure E6) demonstrates a broader range of surface CXCR2 staining intensity in neutrophils from obese mice as opposed to those from lean mice, which appear to express CXCR2 highly in a more uniform distribution. These histograms also illustrate differences between db/db and DIO neutrophil CXCR2 expression patterns in that DIO neutrophils, although appearing to stain less intensely than lean control mice, show a more narrow distribution in expression than do obese db/db mice.
Figure 5.
Obesity attenuates neutrophil calcium flux in response to chemoattractants. Cellular calcium flux response to the CXC cytokine KC (25 ng/ml) was determined in mature bone marrow neutrophils isolated from genetically obese (db/db) (A) and diet-induced obese (B) mice and compared with lean control mice using Indo-1AM cytosolic dye-loading and flow cytometry. Three separate experiments were performed on db/db and diet-induced obese mouse isolated neutrophils and respective control mice; representative runs are shown for each source.
Figure 6.
Obesity is associated with decreased neutrophil surface expression of CXCR2. Cell surface levels of CXCR2 were determined on mature bone marrow neutrophils isolated from genetically obese (db/db) (A) and diet-induced obese (B) mice and compared with lean control mice using flow cytometry. Three separate experiments were performed on db/db and diet-induced obese mice and respective control mice, and reported results are normalized to control for each experiment.
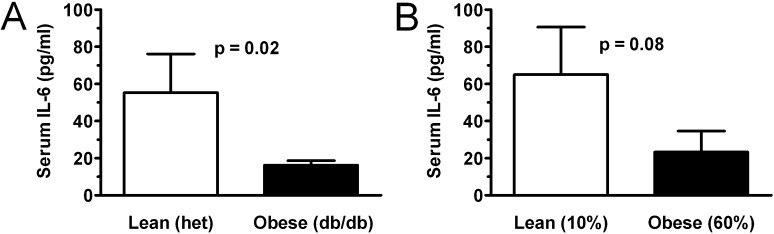
Obesity Is Associated with Decreased BAL and Plasma IL-6 Levels in LPS-Induced ALI
Although obesity is generally believed to confer an inflammatory cytokine environment, we have previously shown that plasma levels of the key inflammatory cytokine, IL-6, are decreased in obese patients with ALI (19). We therefore sought to determine whether obese mice might manifest a similar attenuation in the setting of established ALI. Similar to patients with ALI, plasma levels of IL-6 were reduced in db/db obese mice compared with lean control mice 24 hours after LPS-induced lung injury (Figure 7A). The reduction in IL-6 levels was less pronounced in DIO mice and did not reach significance (Figure 7B). Similar to findings at the 2- and 6-hour time points, 24-hour BAL levels of KC, TNF-α, and MIP-2 did not differ between lean and obese lung-injured mice (Figure E7). However, in contrast to early time points, BAL levels of IL-6 and MCP-1 appeared to be blunted by 24 hours in obese mice.
Figure 7.
Obesity attenuates plasma IL-6 response in db/db and diet-induced obese mice. Plasma IL-6 levels 24 hours after inhaled LPS lung injury were measured by Bio-Plex in genetically obese (db/db) (A) and diet-induced obese (B) mice and compared with lean control mice. n = 6 (diet-induced) or 12 (db/db) mice per condition.
Discussion
In the present study, we demonstrate an attenuating effect of obesity on LPS-induced lung injury and neutrophil trafficking in genetically hyperphagic (db/db) and diet-induced mouse models of obesity and implicate obesity-related defects in neutrophil chemotaxis in this diminished response. This is consistent with our previously published findings on obesity’s dampening effects on the inflammatory response in human ALI.
Previous work has suggested that obesity may have an attenuating effect on hyperoxic and ozone-induced lung injury models, although in the case of ozone exposure findings are mixed and appear to vary with the acuity of exposure and possibly the timing of examination (22, 32, 33). These findings, in light of the early evidence that obesity may have a protective effect in human ALI/ARDS (7, 34, 35), suggest that a clinically relevant alteration in the acute pulmonary inflammatory response may be associated with weight gain. Although limited work has explored this effect in animal models, leptin resistance (20) and alterations in IL-6 signaling (22) have been implicated in the attenuation of acute lung inflammation, but the possible role of obesity-associated neutrophil function defects has not previously been investigated.
Examining an LPS-induced lung injury model, we find that db/db and DIO animals show decreased airspace neutrophilia and attenuated capillary leak. Elevated levels of circulating neutrophils are seen in the obese mice in our studies, similar to findings we have previously reported in obese patients with ARDS (19). This suggests that neutrophil mobilization in response to injury is not impeded in obesity, implicating a defect in the recruitment of blood-borne neutrophils to the airspace as the cause of attenuated injury and neutrophilia. Although such a finding could result from an abnormal pulmonary cytokine response, in our models the proinflammatory cytokine response appears to be normal. Furthermore, in the case of the db/db model of obesity, airspace neutrophilia is blunted even in this early phase of recruitment, suggesting that defects in neutrophil response may exist in obese animals, leading to impaired neutrophil migration into the lung. This is further suggested by our adoptive transfer studies in which neutrophils from obese animals show significantly impaired airspace migration when infused into lung-injured, lean recipients. Thus, obesity appears to confer an intrinsically impaired neutrophil migratory response that is independent of additional host defects that may accompany obesity.
Neutrophil diapedesis into the lung is a complex process, requiring endovascular rolling, adhesion, and subsequent chemokine-directed tissue migration to the alveolar space (36). Although defects in neutrophil chemotaxis may arise from alteration in multiple cellular processes, we find that impaired response to the CXC chemokine KC is evident in obese neutrophils during calcium flux, which is the earliest signaling event that initiates chemotaxis. Associated with this impairment, we find evidence of significantly decreased surface levels of the CXCR2 receptor on neutrophils from db/db obese mice and to a lesser degree in DIO mice. The cause of this reduction in CXCR2 is unclear. Although obesity is known to be accompanied by chronic, low-level systemic inflammation, which could lead to CXC cytokine-mediated reduction in neutrophil CXCR2 display, plasma inflammatory cytokine levels including CXCR2 ligands KC and MIP-2 are not significantly different between naive obese and lean mice in either model of obesity (Figures E8). Even in the case of db/db-derived neutrophils, unknown mechanisms other than reduced CXCR2 display must contribute to obesity-associated neutrophil chemotaxis defects, given the disproportionate magnitude of this defect in relation to the observed reduction in surface CXCR2.
The finding of an obesity-linked primary defect in neutrophil function adds to the growing list of obesity-associated defects suggested to contribute to the attenuated pulmonary inflammatory response, including leptin resistance and abnormalities in IL-6 signaling, and is notably similar to our previously published findings examining lean mouse models of dyslipidemia (37), a condition known to accompany obesity. In our previous studies, lean mice with diet-induced hypercholesterolemia demonstrated a small but significant reduction in pulmonary neutrophilia 24 hours after exposure to nebulized LPS, which was associated with defects in neutrophil chemotaxis as well as decreased neutrophil surface levels of CXCR2 (37). Although the effects of hypercholesterolemia on neutrophil trafficking were less substantial than those we report here in obese mice, such findings suggest that hypercholesterolemia, present in both models of obesity in this report (Figure E9), may contribute to the obesity-associated defects in neutrophil chemotaxis.
Although the current literature is inconclusive, there are suggestions that obesity may affect the recruitment of neutrophils to the lung differently from recruitment to other sites. For instance, peritoneal recruitment of neutrophils may be augmented in obesity in sterile peritonitis (38). How this may be reconciled with our current finding of obesity-associated impairment of neutrophil chemotaxis is unclear. We have previously described a similar paradox in hypercholesterolemic mice (37) in which we found isolated neutrophils to have similar defects in chemotaxis associated with increased recruitment to the inflamed peritoneum despite impaired recruitment to the lung using the same inflammatory agents (LPS, Klebsiella infection). The etiology of this difference is unclear but may involve augmented cytokine response in the peritoneum compared with the lung.
Examination of inflammatory cytokine levels in our models demonstrates that, although the initial pulmonary cytokine response in obese animals appears normal, a reduction in plasma IL-6 levels is seen in more established injury (24 h). This finding is similar to our previous findings in patients with ALI in which plasma IL-6 was found to be decreased in obese patients. MCP-1 and to a lesser degree IL-6 are reduced in the airspace of obese animals with lung injury at 24 hours (Figure E9). It is unclear whether this occurrence reflects a downstream effect of attenuated neutrophil recruitment (because neutrophils are an important source of IL-6 and MCP-1 release after lung injury [39]) or an evolving defect in the monocyte/macrophage or pulmonary epithelial response during the course of lung injury. In the case of IL-6, this decrease in alveolar cytokine release appears to mirror the defect seen in systemic cytokine response.
Differences are evident between our two mouse models of obesity. The db/db and diet-induced obesity models showed similar defects in neutrophil chemotaxis and comparable attenuations in airspace neutrophilia and capillary leak in the setting of established lung injury (24 h). However, these models manifest subtle differences in other aspects of the inflammatory response, possibly attributable to their disparate mechanisms and durations of obesity. Although db/db mice are primarily hyperphagic and rapidly develop obesity on normal chow within 4 to 6 weeks of birth, DIO mice develop obesity as a product of high fat chow over the course of 20 weeks. Thus, full manifestations of the metabolic syndrome, such as vascular activation and injury, are likely to be greater at baseline in the DIO model compared with the db/db model. Such endothelial activation may account for the normal to increased early neutrophil recruitment seen in injured obese DIO compared with lean mice (Figure 3D) (as well as the relatively normal lung myeloperoxidase content) that occurs in this model despite the demonstrated obesity-associated defects in neutrophil chemotaxis.
Several other notable differences between obesity models exist. Mice with diet-induced obesity appear to have less pronounced alterations in neutrophil recruitment, calcium flux, and CXCR2 expression compared with the db/db model of obesity, whereas lean mice in the diet-induced model have a blunted response to LPS injury compared with lean heterozygous db mice. Several factors may account for these findings. Lean mice in the diet-induced model are significantly heavier than lean mice in the db/db model (22.2 ± 0.9 g versus 32.1 ± 0.9 g; P < 0.0001), suggesting that differences in weight might affect BAL neutrophilia even in the “lean” groups. Diet composition, which differs substantially between models, also has been shown to alter the inflammatory response (40) and may contribute to the differences seen between models. Although we do not find a significant correlation between airspace neutrophilia and mouse age in our lung injury model, age has been shown to impair neutrophil chemotaxis response in mice and humans, independent of weight, through unclear mechanisms (41–45), and this may augment the defect in DIO neutrophils independent of CXCR2 expression levels.
The development of spontaneous diabetes in the db/db mouse model is well known, and although our experiments were designed to limit the development of frank diabetes in the animals by using mice on a nondiabetogenic background (B6) and examining them at an age before the typical onset of diabetes (46, 47), we cannot exclude the possibility that early diabetes may have influenced inflammatory response in the obese db/db mice. Thus, we might expect that the multiple differences between the db/db and DIO obesity models would alter how obesity-associated defects in neutrophil function and recruitment are expressed. Despite this, the shared phenotype of impaired pulmonary inflammatory response and neutrophil dysfunction in both models suggests that the obese state itself has an overarching effect on the pathogenesis of lung injury.
In summary, we show that obesity has an attenuating effect on LPS-induced lung injury and neutrophil trafficking in two mouse models of obesity. This occurs despite an apparently normal early pulmonary cytokine response and with elevated levels of circulating neutrophils. Further examination revealed that the witnessed attenuation on pulmonary neutrophilia in both models may in part be due to obesity-related abnormalities in neutrophil CXCR2 signaling with associated defects in neutrophil chemotaxis. Taken together, these results suggest that neutrophil dysfunction may play a prominent role in what appears to be a complex, multifactorial process underlying the attenuation of lung injury in obesity. Further studies are warranted to better characterize and dissect these obesity-related alterations in neutrophil function.
Supplementary Material
Acknowledgments
The authors thank Lawrence Rudel, Ph.D., and Martha Wilson, Ph.D., at the Lipoprotein Analysis Laboratory, Department of Pathology, Wake Forest University School of Medicine for assistance in determining murine plasma LDL levels.
Footnotes
This work was supported by National Institutes of Health grants R01 HL084200, R01 HL089177, and NCRR P20RR15557 and by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0334OC on March 15, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 2.Suratt BT, Parsons PE. Mechanisms of acute lung injury/acute respiratory distress syndrome. Clin Chest Med 2006;27:579–589 [DOI] [PubMed] [Google Scholar]
- 3.Tate RM, Repine JE. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis 1983;128:552–559 [DOI] [PubMed] [Google Scholar]
- 4.Baughman RP, Gunther KL, Rashkin MC, Keeton DA, Pattishall EN. Changes in the inflammatory response of the lung during acute respiratory distress syndrome: prognostic indicators. Am J Respir Crit Care Med 1996;154:76–81 [DOI] [PubMed] [Google Scholar]
- 5.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS: pPlasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995;107:1062–1073 [DOI] [PubMed] [Google Scholar]
- 6.Ware LB. Prognostic determinants of acute respiratory distress syndrome in adults: impact on clinical trial design. Crit Care Med 2005;33(Suppl)S217–S222 [DOI] [PubMed] [Google Scholar]
- 7.O'Brien JM, Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med 2006;34:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris AE, Stapleton RD, Rubenfeld GD, Hudson LD, Caldwell E, Steinberg KP. The association between body mass index and clinical outcomes in acute lung injury. Chest 2007;131:342–348 [DOI] [PubMed] [Google Scholar]
- 9.O'Brien JM, Jr, Welsh CH, Fish RH, Ancukiewicz M, Kramer AM. Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med 2004;140:338–345 [DOI] [PubMed] [Google Scholar]
- 10.Garrouste-Orgeas M, Troche G, Azoulay E, Caubel A, de Lassence A, Cheval C, Montesino L, Thuong M, Vincent F, Cohen Y, et al. Body mass index: an additional prognostic factor in ICU patients. Intensive Care Med 2004;30:437–443 [DOI] [PubMed] [Google Scholar]
- 11.Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest 2003;123:1202–1207 [DOI] [PubMed] [Google Scholar]
- 12.Nasraway SA, Jr, Albert M, Donnelly AM, Ruthazer R, Shikora SA, Saltzman E. Morbid obesity is an independent determinant of death among surgical critically ill patients. Crit Care Med 2006;34:964–970 [DOI] [PubMed] [Google Scholar]
- 13.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:515–521 [DOI] [PubMed] [Google Scholar]
- 14.Hogue CW, Jr, Stearns JD, Colantuoni E, Robinson KA, Stierer T, Mitter N, Pronovost PJ, Needham DM. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med 2009;35:1152–1170 [DOI] [PubMed] [Google Scholar]
- 15.Aldawood A, Arabi Y, Dabbagh O. Association of obesity with increased mortality in the critically ill patient. Anaesth Intensive Care 2006;34:629–633 [DOI] [PubMed] [Google Scholar]
- 16.Martino JL, Stapleton RD, Wang M, Day AG, Cahill NE, Dixon AE, Suratt BT, Heyland DK. Extreme obesity and outcomes in critically ill patients. Chest 2011;140:1198–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos EJ, Xu Y, Romanova I, Middleton F, Chen C, Quinn R, Inui A, Das U, Meguid MM. Is obesity an inflammatory disease? Surgery 2003;134:329–335 [DOI] [PubMed] [Google Scholar]
- 18.Desai MY, Dalal D, Santos RD, Carvalho JA, Nasir K, Blumenthal RS. Association of body mass index, metabolic syndrome, and leukocyte count. Am J Cardiol 2006;97:835–838 [DOI] [PubMed] [Google Scholar]
- 19.Stapleton RD, Dixon AE, Parsons PE, Ware LB, Suratt BT. The association between bmi and plasma cytokine levels in patients with acute lung injury. Chest 2010;138:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 2007;175:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barazzone-Argiroffo C, Muzzin P, Donati YR, Kan CD, Aubert ML, Piguet PF. Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol Lung Cell Mol Physiol 2001;281:L1150–L1156 [DOI] [PubMed] [Google Scholar]
- 22.Shore SA, Lang JE, Kasahara DI, Lu FL, Verbout NG, Si H, Williams ES, Terry RD, Lee A, Johnston RA. Pulmonary responses to subacute ozone exposure in obese vs. lean mice. J Appl Physiol 2009;107:1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang JE, Williams ES, Mizgerd JP, Shore SA. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol 2008;294:L1013–L1020 [DOI] [PubMed] [Google Scholar]
- 24.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol 2007;150:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J Immunol 2002;168:4018–4024 [DOI] [PubMed] [Google Scholar]
- 26.Mancuso P. Obesity and lung inflammation. J Appl Physiol 2010;108:722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudmann DG, Moore MW, Tepper JS, Aldrich MC, Pfeiffer JW, Hogenesch H, Tumas DB. Modulation of allergic inflammation in mice deficient in tnf receptors. Am J Physiol Lung Cell Mol Physiol 2000;279:L1047–L1057 [DOI] [PubMed] [Google Scholar]
- 28.Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol 2007;178:8148–8157 [DOI] [PubMed] [Google Scholar]
- 29.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol 2001;281:L913–L921 [DOI] [PubMed] [Google Scholar]
- 30.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 2004;104:565–571 [DOI] [PubMed] [Google Scholar]
- 31.Petty JM, Paulbinskas M, Lenox CC, Engelken D, Suratt BT. VLA-4 and VCAM are critical for neutrophil homeostasis. Am J Respir Crit Care Med 2007;175:A493 [Google Scholar]
- 32.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L856–L865 [DOI] [PubMed] [Google Scholar]
- 33.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol 2008;104:1727–1735 [DOI] [PubMed] [Google Scholar]
- 34.McCallister JW, Adkins EJ, O'Brien JM., Jr Obesity and acute lung injury. Clin Chest Med 2009;30:495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marik PE. The paradoxical effect of obesity on outcome in critically ill patients. Crit Care Med 2006;34:1251–1253 [DOI] [PubMed] [Google Scholar]
- 36.Pilewski JM, Albelda SM. Adhesion molecules in the lung: an overview. Am Rev Respir Dis 1993;148:S31–S37 [DOI] [PubMed] [Google Scholar]
- 37.Madenspacher JH, Draper DW, Smoak KA, Li H, Griffiths GL, Suratt BT, Wilson MD, Rudel LL, Fessler MB. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent tlr response phenotypes. J Immunol 2010;185:1660–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pini M, Gove ME, Sennello JA, van Baal JW, Chan L, Fantuzzi G. Role and regulation of adipokines during zymosan-induced peritoneal inflammation in mice. Endocrinology 2008;149:4080–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, Frank JA. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol 2009;182:8056–8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dandona P, Ghanim H, Chaudhuri A, Dhindsa S, Kim SS. Macronutrient intake induces oxidative and inflammatory stress: potential relevance to atherosclerosis and insulin resistance. Exp Mol Med 2010;42:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biasi D, Carletto A, Dell'Agnola C, Caramaschi P, Montesanti F, Zavateri G, Zeminian S, Bellavite P, Bambara LM. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation 1996;20:673–681 [DOI] [PubMed] [Google Scholar]
- 42.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. J Leukoc Biol 2000;67:40–45 [DOI] [PubMed] [Google Scholar]
- 43.Niwa Y, Kasama T, Miyachi Y, Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci 1989;44:1655–1664 [DOI] [PubMed] [Google Scholar]
- 44.Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev 2001;122:1521–1535 [DOI] [PubMed] [Google Scholar]
- 45.Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol 2009;30:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svenson KL, Von Smith R, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol 2007;102:2369–2378 [DOI] [PubMed] [Google Scholar]
- 47.Nishina PM, Lowe S, Wang J, Paigen B. Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 1994;43:549–553 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.