Abstract
Lung endothelium is believed to be a quiescent tissue with the potential to exhibit rapid and effective repair after injury. Endothelial progenitor cells derived from the bone marrow have been proposed as one source of new endothelial cells that may directly contribute to pulmonary endothelial cell homeostasis and repair. Here we use bone marrow transplantation models, using purified hematopoietic stem cells (HSCs) or unfractionated whole marrow, to assess engraftment of cells in the endothelium of a variety of tissues. We find scant evidence for any contribution of bone marrow–derived cells to the pulmonary endothelium in the steady state or after recovery from hyperoxia-induced endothelial injury. Although a rare population of CD45−/CD31+/VECadherin+ bone marrow–derived cells, originating from HSCs, can be found in lung tissue after transplantation, these cells are not readily found in anatomic locations that define the pulmonary endothelium. Moreover, by tracking transplanted bone marrow cells obtained from donor transgenic mice containing endothelial lineage–selective reporters (Tie2-GFP), no contribution of bone marrow–derived cells to the adult lung, liver, pancreas, heart, and kidney endothelium can be detected, even after prolonged follow-up periods of 11 months or after recovery from hyperoxic pulmonary endothelial injury. Our findings argue against any significant engraftment of bone marrow–derived cells in the pulmonary vascular endothelium.
Keywords: endothelium, vasculature, hyperoxia, EPC, bone marrow
Clinical Relevance
So-called “endothelial progenitor cells” (EPCs) derived from the bone marrow have been proposed as one source of new endothelial cells that may directly contribute to pulmonary endothelial cell homeostasis and repair. We find scant evidence for any contribution of bone marrow–derived cells to the pulmonary endothelium in the steady state or after recovery from hyperoxia-induced endothelial injury. Our findings argue against the existence of bone marrow–derived EPCs.
The pulmonary vasculature continuously receives the entire cardiac output, and the thin layer of endothelial cells lining this vasculature requires precise homeostatic mechanisms to effectively maintain life-long circulation and gas exchange. Although it is well known that subtle alterations in pulmonary vascular homeostasis can lead to disease states such as pulmonary hypertension or alveolar hemorrhage, the origins of new pulmonary endothelial cells during homeostatic maintenance or after pulmonary endothelial injury remains unclear. Until recently, in adult animals the repair of any injured endothelium or formation of new vessels had been widely accepted as involving the paradigm of angiogenesis, a mechanism whereby division of existing endothelial cells within a vessel network produces new cells. Accumulating evidence from several groups has challenged this paradigm with findings that claim adult mammals have circulating endothelial progenitor cells (EPCs) that may be derived from the bone marrow and may become incorporated as endothelial cells into the vasculature of organs or tumors (1–9).
Regarding the pulmonary endothelium, several investigators have claimed a role for so-called “bone marrow–derived EPCs” in endothelial maintenance and repair (10–20). These studies define EPCs as precursors of cells meeting molecular and morphologic criteria of mature endothelial cells, such as expression of a diversity of endothelial lineage marker genes and a flattened shape of cells lining pulmonary vascular lumens. For example, bone marrow cells were suggested to contribute to the mouse lung's alveolar capillary endothelium after intratracheal elastase injury (10). Furthermore, in human lung samples obtained at various time points after bone marrow transplantation, an average of 40% of recipients’ pulmonary endothelium has been claimed to be donor-derived (12). Compelling investigations also suggest a potential role for circulating EPCs in lung disease pathogenesis, including contributions to endothelial remodeling and participation in lung cancer angiogenesis (21, 22). Circulating EPCs have also been proposed as biomarkers for lung disease. For example, increased numbers of circulating cells expressing putative EPC markers have been found in patients with bacterial pneumonia (11), and increased numbers of similar circulating cells are associated with improved survival in acute lung injuries (13).
The potential contribution of bone marrow–derived EPCs to the pulmonary endothelium would be especially intriguing if confirmed because this putative novel paradigm raises the prospect of using these easily accessible cells for cell-based therapies to reconstitute injured or diseased lung endothelium. Indeed, rescue of monocrotaline-induced pulmonary hypertension has been demonstrated by using infusions of similar bone marrow–derived endothelial-like progenitor cells (14), and these findings have served as the basis for a “first-in-humans” clinical trial of cell-based therapy for pulmonary vascular disease (23).
Most recently, the contribution of any bone marrow–derived cell type, including EPCs, to adult endothelium has been called into question by several studies that have failed to find any significant contribution of bone marrow–derived cells to endothelial cells in several organs (24–28). Here we attempt to address the potential role of bone marrow–derived cells in lung endothelial maintenance and repair. Using mice expressing reporter genes ubiquitously or under regulatory control of endothelial-selective promoters, we find that a rare population of bone marrow–derived cells that do not express the pan-hematopoietic marker CD45 but express endothelial markers that lack complete specificity (i.e., CD45−/CD31+/VECadherin+ cells) can be found in the lung for at least 1 year after bone marrow transplantation. We find scant evidence, however, to support the contention that these cells or any other bone marrow–derived cells give rise to true pulmonary endothelial cells because no bone marrow–derived cells can be found in anatomic compartments that define the pulmonary endothelium. Moreover, when bone marrow–derived cells are tracked using lineage-selective reporters rather than antibody-based methods, no contribution of bone marrow–derived cells to the endothelium of the adult lung, heart, liver, pancreas, or kidney is observed, even after hyperoxic injury and recovery. Our findings argue against a direct structural contribution of bone marrow–derived cells, previously termed “EPCs,” to lung endothelial maintenance or repair in adults.
Materials and Methods
Mice
Transplantation studies involving lineage-specific reporters used 6- to 8-week-old donor mice that express enhanced green fluorescent protein (GFP) under regulatory control of a Tie2 promoter (Tie2-GFP FVB/N mice, cat#003658; Jackson Labs, Bar Harbor, ME) or lacZ targeted to the thrombomodulin locus (TM-lacZ mice, generous gift of Dr. Robert D. Rosenberg, Massachusetts Institute of Technology, Boston, MA). Transplants involving ubiquitous reporters used 8–week-old donor mice that express GFP under control of the chicken β-actin promoter with cytomegalovirus enhancer C57BL/6j-Tg(ACTB-EGFP)1Osb/J (Jackson Labs). Recipient mice were congenic 8- to 10-week-old C57BL/6j mice (Jackson Labs) for β-actin or TM-lacZ transplants or FVB/N (Jackson Labs) for Tie2-GFP transplants. All animal studies were approved by the Institutional Animal Care and Use Committee of Boston University School of Medicine
Hematopoietic Stem Cell Purification
Hematopoietic stem cells (HSCs) for transplantation were purified from the bone marrow of mice by the Hoechst dye efflux method (29) with modifications published previously (30). Detailed methods are available in the online supplement. Single stem cell transplants were published in part previously (31), and archived fluorescence-activated cell sorting (FACS) raw data files and frozen tissue sections from those studies were stained and analyzed as indicated in the text.
Competitive Long-Term Blood Repopulation and Analysis of Peripheral Blood Chimerism
Recipient mice were lethally irradiated with 11 Gy of radiation in a single dose or 14 Gy delivered as two doses of 7 Gy given 3 hours apart on the day before transplantation. Where indicated in the text, 200 donor HSCs obtained from the indicated transgenic donor mice were mixed with 2 × 105 unfractionated unlabeled competitor bone marrow cells and intravenously injected retro-orbitally into unlabeled recipient mice. Robust long-term hematopoietic reconstitution (>50% blood chimerism for >3 mo after transplantation) was documented in all recipients before further analysis. More detailed methods are available in the online supplement.
Hyperoxic Lung Injury and Bromodeoxyuridine Labeling
For hyperoxic lung injury, mice were exposed to 95% oxygen versus room air for 52 hours. For in vivo bromodeoxyuridine (BrdU) labeling, injured or uninjured mice were recovered for 24 hours in room air before receiving drinking water containing 0.8 mg/ml BrdU (BrdU APC flow kit; BD Pharmingen, San Diego, CA) for 5 days. Mice that received BrdU water were killed with isofluorane, and cells from the lung and bone marrow were collected for analysis. Bleomycin injury was performed as previously published (40).
Immunostaining of Lung and Bone Marrow Samples for FACS
Lung tissue was harvested for FACS and tissue sectioning as previously published (31) and as detailed in the online supplement.
Lectin Staining and Confocal Microscopy
Frozen lung tissue sections were stained with biotinylated GSL 1-isolectin B4 (Vector Laboratories, Burlingame, CA) followed by streptavidin (Sav)-conjugated Cy3 (Invitrogen, Carlsbad, CA). Each analysis included negative control sections treated with CAS Block alone in place of lectin, followed by Sav-Cy3. Detailed methods for lectin staining and confocal microscopy analysis are available in the online supplement.
Results
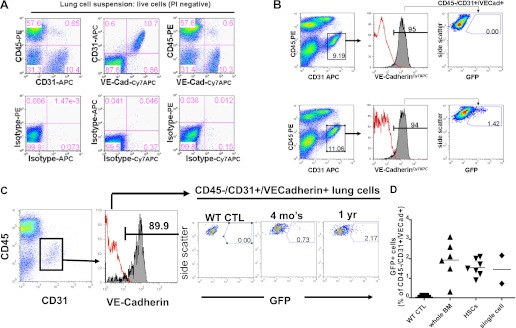
We previously reported that, in adult mouse recipients of bone marrow transplants, we found no detectable engraftment in the lung alveolar epithelium of bone marrow–derived cells (31, 32). In those experiments, more than 99% of cells detected in the lung after bone marrow transplantation were hematopoietic in lineage, as defined by cell surface expression of the hematopoietic marker, CD45 (31). Based on the presence of a rare bone marrow–derived CD45− population in the lung (representing <1% of cells; Figure 1), we sought to further phenotype this population in recipient lung tissue.
Figure 1.
Presence of green fluorescent protein (GFP)+ bone marrow hematopoietic stem cell–derived cells in lung tissue after bone marrow transplantation. Frozen lung tissue sections examined by fluorescence microscopy without the use of antibody staining reveal GFP+ cells easily detected in the lung tissue of recipient animals after transplantation of GFP+ bone marrow cells (representative section and flow cytometry shown in B and D, compared with recipient controls in A and C that did not receive GFP+ cells). The majority of GFP+ cells are CD45+, indicating their hematopoietic lineage. A small fraction (0.48% of all live lung cells analyzed in B do not express CD45. Br = bronchus; CTL = control; PI = propidium iodide. *Vascular lumen
Using transgenic donor mice that ubiquitously express a GFP reporter gene (β-actin GFP), we transplanted 2 × 106 unfractionated marrow cells, 200 purified HSCs (purified as Hoechst effluxing bone marrow cells that are exclusively CD45+ and express HSC markers Sca1+ckit+ and Lin−, also referred to as SP cells; see Figure E4 in the online supplement) (29, 30, 33), or single HSCs into lethally irradiated wild-type recipients. The 200 HSC donor number was chosen based on the expected 200 HSCs that would be found in a population of 2 × 106 unfractionated marrow cells, given the frequency of SP cells of this phenotype in the bone marrow is 1/10,000 cells (30). At 1 to 12 months after transplantation, we analyzed the recipient lungs by FACS and fluorescence microscopy of frozen tissue sections. Consistent with our previous report (31), after transplantation of unfractionated marrow (n = 45), 200 purified HSCs (n = 16), or single stem cells (n = 2), GFP+ labeled cells were easily detected in recipient lung tissue with the predominance of engraftment found as blood cells expressing the hematopoietic marker CD45 (Figure 1). To screen for putative bone marrow–derived endothelial cells in recipient lungs, we used FACS analysis to isolate cells expressing a surface phenotype that selectively identifies lung endothelial cells: CD45−/CD31+/VECadherin+ (Figure 2). An average of 1.74 ± 0.75% (±SD) of all CD45−/CD31+/VECadherin+ lung cell suspensions were GFP+, suggesting their origin from bone marrow cells. Because 200 purified HSCs, single HSCs, or unfractionated bone marrow transplants were all able to give rise to this CD45−/CD31+/VECadherin+/GFP+ population, this suggested their origin from bone marrow HSCs (Figure 2). Four additional secondary recipients transplanted with whole marrow taken from one of the primary recipients were similarly followed for 4 months after this serial transplant (Figure E1), and these secondary recipients of the original single GFP+ bone marrow stem cell also exhibited similar evidence of CD45−/CD31+/VECadherin+/GFP+ progeny in the lung tissues of these recipients analyzed by FACS (0.4–2.0% of all lung CD45−/CD31+/VECadherin+ cells). Based on a prior report of liver endothelial engraftment arising from a single transplanted HSC (34), we also evaluated single cell suspensions from the livers of these recipients and found a similar frequency of engrafted GFP+/CD45−/CD31+/VECadherin+ cells deriving from the single transplanted GFP+ HSCs (data not shown).
Figure 2.
Fluorescence-activated cell sorting (FACS) screening for potential lung engraftment of GFP-labeled endothelial cells after transplantation of bulk bone marrow or purified hematopoietic stem cells (HSCs). (A) FACS analysis of live single-cell suspensions from wild-type control lung digests demonstrating antibody specificity used to identify cells expressing candidate endothelial cell surface markers CD31 and VECadherin. (B) FACS data from one representative experiment illustrating gating algorithm to determine the percentage of GFP+ cells present within live single-cell suspensions gated on CD31+/CD45−/VECadherin+ putative lung endothelial cells. Note the absence of GFP+ cells in the endothelial compartment of the wild-type control lung compared with 1.42% GFP+ cells in the transplant recipient of GFP+ bone marrow. (C) Putative engraftment over time of GFP+ cells within the lung population defined as live/CD31+/CD45−/VECadherin+ cells in recipients of single GFP+ HSC transplants examined 4 months and 1 year after transplantation. (D) Summary graph of mouse recipients (as shown in B and C) transplanted with GFP+ unfractionated whole marrow, 200 purified HSCs, or a single purified HSC. Each dot indicates results from an individual mouse recipient, quantifying the percentage of GFP+ cells within the lung's CD45−/CD31+/VECadherin+ population by FACS. Results are representative of four repeated experiments analyzing a total of 61 recipients harvested 1 to 12 months after transplantation. WT = wild type.
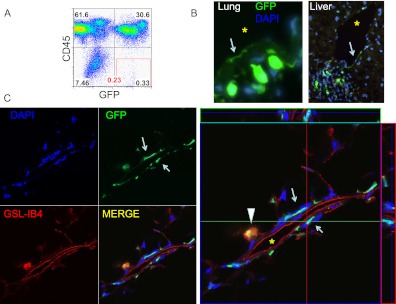
Because a defining characteristic of vascular endothelial cells is their anatomic location lining vascular lumens, we assessed frozen lung tissue sections for the presence of GFP+ bone marrow–derived cells by fluorescence microscopy. As in previous reports using bone marrow transplants from donor transgenic mice carrying ubiquitously expressed GFP reporter genes (β-actin GFP) (31), due to the presence of hematopoietic (CD45+) lineages within the lung, we found an abundance of GFP+ cells within recipient lung tissue (Figure 1), making it difficult to clearly distinguish any true rare endothelial progeny on tissue sections. Indeed, only very rare cells (<1 per tissue section on fluorescence microscopy) initially appeared to exhibit an appropriate anatomic location suggestive of lung or liver endothelial cells in each respective organ (Figures 1 and 3). On further review of lung sections, however, using confocal microscopy analysis of GFP expression in combination with red fluorescent lectin staining (GSL-IB4) to identify the luminal surface of endothelial cells, all bone marrow–derived cells that initially appeared to mimic the morphology or location of lung endothelia were separated from vessel lumens by at least one cell layer typically consisting of a native (GFP negative) endothelial cell (Figures 3 and E3). No cells of true pulmonary endothelial morphology and anatomic location could be found in any recipient. These observations called into question the existence of any bona fide lung endothelial cells deriving from donor GFP+ bone marrow cells.
Figure 3.
Screening for candidate GFP+ lung endothelial engraftment after bone marrow transplantation. (A) FACS analysis of live lung single-cell suspension suggesting 0.23% of live cells are GFP+ and not hematopoietic (CD45−). (B) Conventional fluorescence microscopy suggesting the rare presence of rare flat GFP+ cells (arrow) in a location apparently suggestive of an endothelial cell lining a vascular lumen in a lung tissue section and in a liver tissue section. (C) Confocal microscopy analysis of two candidate endothelial cells (arrows) similar to those shown in B demonstrate that the GFP+ flat cells near a vascular lumen are lectin negative and are separated from this lumen by an intervening flat endogenous (GFP−) endothelial cell that binds the GSL-IB4 lectin, indicating the GFP+ cell is not an endothelial cell. No bona fide GFP+ endothelial cells were detected. Arrowhead indicates a round bone marrow−derived alveolar macrophage that is GFP+ and also stains for the GSL-IB4 lectin. DAPI = 4',6-diamidino-2-phenylindole nuclear stain; GFP = green fluorescence protein; GSL-IB4 = endothelial cell surface marker, Griffonia simplicifolia, isolectin B4. *Vascular lumen.
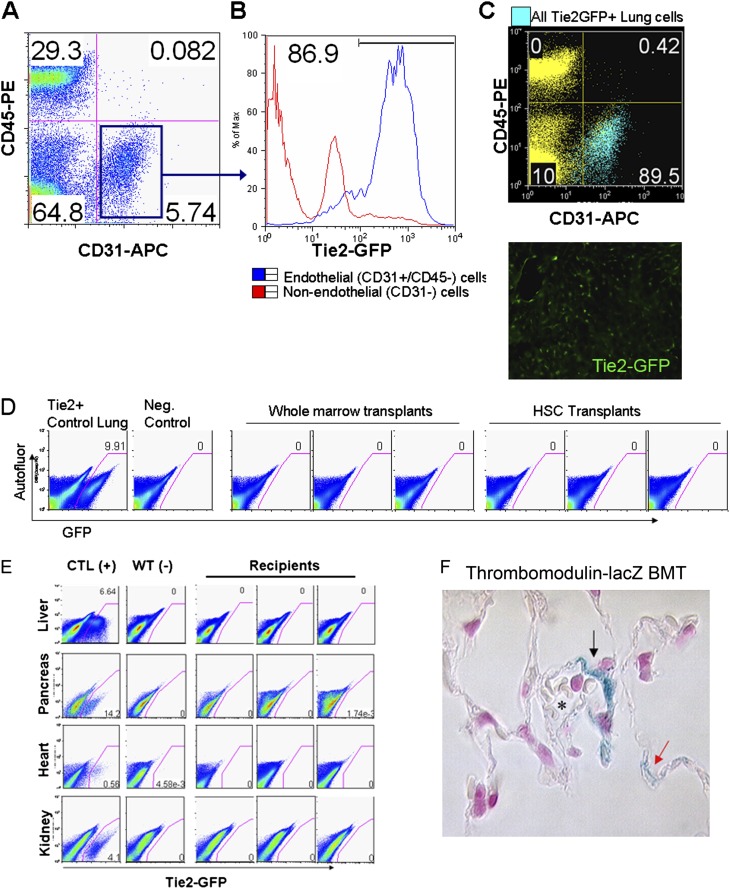
We have previously reported the use of bone marrow transplantation from transgenic lineage-specific reporter mice as a powerful technique that enables antibody-independent methods for the identification of particular lung lineages (31, 32). The use of lineage-specific reporters to track the progeny of bone marrow–derived cells minimizes the considerable hematopoietic background signal that accompanies engraftment in lung of cells derived from mice ubiquitously expressing reporter genes. We screened two published endothelial reporter mice (Tie2-GFP [35] and thrombomodulin [TM]-lacZ [36]) whose GFP or lacZ reporter genes have been described as selectively distinguishing endothelial cells from cells of other lineages, such as hematopoietic cells (Figure 4). Four mice received unfractionated marrow transplants from a donor TM-lacZ mouse that contained a lacZ reporter gene targeted to the thrombomodulin locus. This locus is selectively active in all endothelial cells (36). Four months after hematopoietic reconstitution, two recipients were exposed to intratracheal bleomycin, an agent known to injure lung epithelial and endothelial cells (37–39). Five months after transplantation (1 mo after bleomycin injury), we performed X-gal staining of all recipient lungs and used a whole mount screening technique (40) to identify a small blue patch in one lobe of one uninjured recipient for further study by paraffin sectioning (Figure 4). No blue patches were observed in the other three mice, including the injured recipients. Histologic assessment revealed that the single blue patch consisted of approximately four cells lining a small vessel and alveolar capillary lumen, consistent with the morphology and location of pulmonary vascular endothelial cells expressing TM-lacZ. The remainder of all lobes from this recipient and all lobes from the other three recipients showed no detectable engraftment of lacZ+ cells.
Figure 4.
Little to no engraftment detected in endothelium of multiple tissues of mouse recipients of bone marrow transplants from donor mice carrying endothelial lineage-selective reporters. (A) FACS characterization of lung cells from the control donor Tie2-GFP transgenic mouse. Note 87% percent of live lung cells (defined as PI−/CD45−/CD31+ cells) express the Tie2 promoter–driven GFP reporter gene. Alternatively, of all GFP+ cells from the lungs of these mice, 89.5% fall within the endothelial gate (C) defined as CD45−/CD31+ cells. Frozen lung tissue sections indicate expression of the Tie2-GFP reporter throughout the macro- and microvasculature of the donor control mouse's lungs. (D) FACS analysis of live lung cells from recipients 11 months after receiving bone marrow transplants from Tie2-GFP donors. Three recipients of whole marrow transplants and three recipients of purified HSC transplants are shown, indicating no evidence of engraftment of Tie2-GFP+ lung cells. Note 10% of all lung cells are Tie2-GFP+ in the lungs of the donor control mouse. (E) No evidence of endothelial Tie2-GFP+ engraftment was detected by FACS in the livers, hearts, kidneys, or pancreases of the three recipients of whole bone marrow transplants shown in D. (F) Very rare engraftment of lacZ+ cells was found in lung tissue of one out of four recipients of whole bone marrow transplants from donor thrombomodulin-lacZ knock-in mice. Shown are three of the four lacZ+ (blue; arrows) cells found after screening of all lungs from these mice. *Vascular lumen. No lacZ+ staining was observed in lungs from mice transplanted with wild-type cells (see Figure E2 for additional negative controls and gating algorithms.)
Next we sought to more precisely quantify and screen for any potential engraftment of bone marrow–derived endothelial cells by combining microscopy with FACS-based analysis of live lung endothelial cells in recipients of marrow transplants from Tie2-GFP mice. Irradiated recipient wild-type mice were transplanted with unfractionated marrow or purified HSCs prepared from donor mice that carry a GFP reporter gene under control of a Tie2 promoter, which is selectively active in the majority of endothelial cells (35). For analysis of engraftment, we applied FACS-based methods developed to analyze large numbers of living lung cells without the use of any antibodies or staining methods that might introduce background artifacts in rare cells (31). Propidium iodide (PI) staining to exclude dead cells has been shown to be useful in these methods because it minimizes the background autofluorescence of dead cells and debris (Figure E2). We first confirmed pan-endothelial GFP reporter gene expression in the lungs of the donor Tie2-GFP mice by fluorescence microscopy as well as FACS analysis of single-cell suspensions (Figure 4). More than 85% of live lung endothelial cells (defined as PI−/CD45−/CD31+ cells) expressed the Tie2-GFP reporter gene, whereas less than 0.5% of all nonendothelial lung cells (defined as all other PI− cells) expressed Tie2-GFP. In single-cell suspensions prepared from heart, kidney, liver, and pancreas tissue, the Tie2-GFP reporter was also expressed in the vast majority of endothelial cells analyzed by FACS (Figure 4). In contrast, the Tie2-GFP reporter was expressed in less than 1% of cells in the unfractionated marrow compartment, findings in keeping with prior reports (27).
We analyzed single-cell suspensions from the lungs, hearts, livers, pancreases, and kidneys of irradiated recipient mice 11 months after hematopoietic reconstitution with 10 × 106 unfractionated bone marrow cells from Tie2-GFP donor mice. Simultaneous positive controls were prepared consisting of single-cell suspensions of these organs from age-matched Tie2-GFP reporter (positive control) and wild-type (negative control) mice. More than 500,000 cells from each tissue of each recipient was analyzed with this method, which is able to specifically and sensitively detect GFP expression in as few as 1 in 640,000 cells (31). We found no evidence of engraftment of GFP-expressing cells in the lungs, hearts, livers, pancreases, and kidneys of any recipient (n = 7; Figure 4). Similarly, in additional irradiated recipients of purified HSC transplants from Tie2-GFP donors, we detected no evidence of engraftment of GFP+ cells in recipient lung tissue (n = 3; Figure 4). No Tie2−GFP+ cells were detected in lung frozen tissue sections from any of the 10 recipients (Table 1).
TABLE 1.
SUMMARY OF EXPERIMENTS SCREENING FOR BONE MARROW–DERIVED LUNG ENDOTHELIAL CELLS
| Donor Reporter | Donor Cells | Lung Injury | T after Transplant | T after Injury | Recipients (n) | Engrafted Endothelial Cells | Analysis Method |
| Tie2-GFP | 107 BM | — | 11 mo | N/A | 7 | 0, 0 | FACS, Histo |
| Tie2-GFP | 200 HSC | — | 11 mo | N/A | 3 | 0, 0 | FACS, Histo |
| Tie2-GFP | 107 BM | Hyperoxia | 6 mo | 5 d | 3 | 0, 0 | FACS, Histo |
| Tie2-GFP | 107 BM | — | 6 mo | N/A | 3 | 0, 0 | FACS, Histo |
| Tie2-GFP | 107 BM | Hyperoxia | 7 mo | 16 d | 3 | 0, 0 | FACS, Histo |
| Tie2-GFP | 107 BM | — | 7 mo | N/A | 6 | 0, 0 | FACS, Histo |
| TM-lacZ | 107 BM | Bleomycin | 5 mo | N/A | 2 | 0 | X-gal Histo |
| TM-lacZ | 107 BM | — | 5 mo | N/A | 2 | 4 | X-gal Histo |
| β-actin-GFP | 107 BM | Hyperoxia | 7 mo | 16 d | 2 | 0 | Confocal Histo |
| Total | 31 | 4 cells |
Definition of abbreviations: BM = bone marrow; FACS = fluorescence-activated cell sorting; Histo = histology; HSC = hematopoietic stem cells; N/A = not available; T = time.
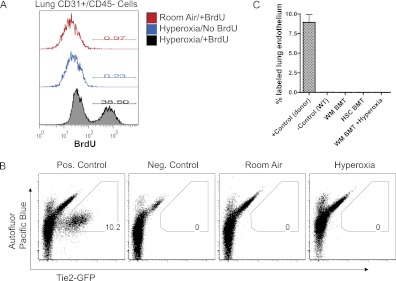
We speculated that lung endothelial injury may be necessary for the engraftment of bone marrow–derived cells in the lung, although screening studies of bleomycin-injured recipients had not revealed engraftment of TM-lacZ–labeled marrow–derived cells. We selected the mouse model of hyperoxic lung injury because it is a well characterized model resulting in diffuse injury to lung alveolar epithelium and endothelium (41–51), with rapid necrosis and apoptosis of lung endothelial cells followed by rapid proliferation and recovery within 2 weeks after injury. We confirmed exposure to hyperoxia as a valid model of lung endothelial injury in our hands by exposing mice to 60 hours of 95% oxygen (versus room air control) followed by a 5- or a 14-day recovery period in room air. On Days 1 through 5 of recovery, the mice received BrdU-supplemented water to label cells undergoing DNA synthesis, reflective of proliferation or repair of DNA damage during the recovery period. On Day 5 or 14 of recovery, mice were harvested, and lung cell suspensions were analyzed by flow cytometry. We found that 38% of lung endothelial cells (CD31+/CD45− lung cells) showed BrdU labeling during the first 5 days of recovery from hyperoxia. In contrast, control uninjured mice exhibited BrdU labeling in less than 1% of their lung endothelium (Figure 5), emphasizing the quiescence of the pulmonary endothelium in the normal uninjured steady state. As expected, the vast majority of bone marrow cells in all BrdU-exposed mice were labeled during this period, consistent with the known high proliferation rates of hematopoietic progenitors and indicating that injured and uninjured mice had similarly ingested sufficient quantities of the BrdU labeling agent. Additional control mice exposed to hyperoxia but not BrdU showed no labeling (Figure 5).
Figure 5.
Lack of evidence for endothelial engraftment of bone marrow–derived cells after hyperoxic lung endothelial injury. (A) BrdU labeling index of lung endothelial cells after 6 days of recovery from hyperoxic lung injury. BrdU was administered to each mouse for 5 days after recovery from exposure to hyperoxia versus room air control. (B) Representative FACS plots of live lung cells from recipient mice after bone marrow transplantation from Tie2-GFP donor mice. FACS gating based on GFP fluorescence versus Pacific Blue autofluorescence is designed to detect true GFP fluorescence versus autofluorescence. Note expression of the Tie2-GFP reporter in 10% of live lung cells from the control transgenic donor mouse and the absence of detectable reporter expression in lung tissue of uninjured or hyperoxia-injured recipient mice. (C) Bar graph summarizing lung endothelial engraftment rates (defined as Tie2-GFP+ lung cells identified by FACS of 1 million events) after transplantation of bone marrow from Tie2-GFP mice. Further subgroups based on follow up times are detailed in Table 1. Bars indicate average ± SEM. +control n = 4 individual mice; wild type (WT), n = 6; whole marrow (WM) bone marrow transplant (BMT), n = 16; HSC BMT, n = 3; BMT+hyperoxic injury, n = 6.
Having confirmed the utility of hyperoxic injury to damage a significant portion of lung endothelial cells, we exposed recipients of Tie2-GFP unfractionated bone marrow transplants to hyperoxia versus room air (n = 8 per group), followed by 5 or 14 days of recovery in room air before analysis (n = 4 per group). For these studies, hyperoxic injury was initiated 6 months after hematopoietic reconstitution of irradiated recipients. Surprisingly, no GFP+ cells were detected in single-cell suspensions prepared from the lung tissue of any recipient. To verify hyperoxic injury of the recipients’ endothelium, the experiment was repeated with BrdU labeling during the 5-day recovery period (n = 4 per group). Five days after recovery from hyperoxic injury, the BrdU labeling indices of lung endothelial cells were 13 ± 6% (average ± SD) in injured recipients versus 1.2 ± 0.3% in uninjured recipients (student's t test; P = 0.03). The increased pulmonary endothelial BrdU labeling index confirmed endothelial injury; however, no GFP+ cells were detected in the lungs of any recipient after recovery from this injury (Figure 5; Table 1).
Discussion
Although bone marrow–derived EPCs have been described as contributing to the endothelium of multiple tissues, including the lung, heart, and liver, we found little evidence to support this phenomenon using lineage-selective transgenic reporter mice. Similar to previous reports, we detected the presence of bone marrow–derived cells in these tissues that appear to express a compelling constellation of nonspecific markers considered to selectively identify endothelial cells (e.g., CD31+, VECadherin+, and CD45−). Many markers used to identify endothelial cells are known to be expressed on a variety of other cell lineages (52–54), and the lack of specificity in available endothelial markers may explain the presence of CD31+/VECadherin+ bone marrow–derived cells in the lung that in our hands do not appear to express the full molecular phenotype or correct anatomical location that defines lung endothelial cells. Using these markers to identify putative EPC colonies during culturing of blood or bone marrow is similarly problematic because cells of myeloid or other nonendothelial lineage appear to express a variety of these markers after culture expansion (52).
The bone marrow–derived cells found in lung tissue in our studies may have important indirect roles in maintenance and repair of lung tissue. Indeed, the potential of a variety of blood- or bone marrow–derived leukocytes to modulate repair of a variety of somatic tissue beds, whether through paracrine or other mechanisms, is widely supported by many investigations (27, 55–57). However, our results add to a growing number of investigations questioning whether any of these bone marrow–derived cells directly contribute to the maintenance of vascular integrity in adults by contributing engrafted endothelial cells to the luminal lining of vessels.
It has been suggested that the many conflicting reports on the contribution of bone marrow–derived cells to the endothelium of tissues or tumors is due to differences in histologic techniques or differences in the injury (or tumor) models used by various investigators (31, 57). The methods used in our studies may be limited if the transgenic reporters were not faithfully activated. Our inclusion of transgenic (Tie2-GFP) and knock-in (TM-lacZ) reporter mice ensures that partial promoter fragments or positional effects are not limiting the faithfulness of reporter gene expression because the lacZ reporter in the TM-lacZ mouse has been targeted to the endogenous thrombomodulin locus known to be active in endothelial cells. Our studies may also be limited by the possibility that radiation exposure affected the capacity of the lung endothelium to mobilize marrow cells after hyperoxic injury. Using parabiotic mice rather than irradiated recipients in future studies may be a more sensitive mouse model for studying marrow–derived endothelial reconstitution, although a recent report did not detect any bone marrow–derived contribution to a variety of endothelia in parabiotic mouse models (28). Our results cannot exclude the presence in the bone marrow compartment of endothelial progenitor cells that reside outside of the transplantable HSC compartment or that transiently incorporate in the endothelium at different or earlier time points than those we studied. Indeed, beyond HSCs, there are a variety of additional cell types in the bone marrow, such as marrow stromal cells and nonhematopoietic endothelial cells, some of which may be difficult to transplant with the myeloablative procedures used in this study. However, our myeloablative techniques and use of marrow and HSC transplantation reproduce the techniques of previous investigators who observed the progeny of transplantable marrow-derived EPCs in a variety of tissue endothelial beds. Our findings of no detectable transplantable marrow-derived endothelia engrafted in these tissues differ significantly from these prior works. The prior report observing no marrow-derived endothelial engraftment in studies using parabiotic mouse models (28) suggests that this is not simply due to failure to reconstitute a transplant-resistant nonhematopoietic or other marrow subcompartment.
Taken together, we believe our results support a model where adult lung endothelia are maintained primarily by cells that originate from other endothelial cells rather than from bone marrow cells. The low BrdU labeling index observed in the lung endothelium of uninjured mice confirms previous reports that the resting pulmonary endothelium is quiescent (54, 58), and the significant increase in the BrdU labeling index of lung endothelial cells during recovery from hyperoxic injury suggests that local proliferation of endothelial cells is an important component of endothelial reconstitution after injury, as suggested by others (reviewed in Reference 59). Our results do not exclude a contribution to the pulmonary endothelium from a local endothelial progenitor cell or from circulating cells that may be mobilized from tissue beds other than the hematopoietic bone marrow (52, 60). Nevertheless, our findings suggest that the term “bone marrow–derived EPC” should be discouraged in clinical trials without definitive evidence supporting the presence of cells in the bone marrow with endothelial reconstituting capacity.
The absence of evidence in support of a marrow-derived circulating EPC in adults is surprising given the common developmental origin of hematopoietic and endothelial cells from primordial hemangioblasts found early in the developing embryo (61). Because blood and endothelial lineages develop from the mesodermal germ layer and appear to share a common developmental origin in a variety of in vivo and in vitro studies, the presence of a multipotent hemangioblast in adults has been long postulated. However, in contrast to previous reports, our results do not support the residual presence in adult animals of a multipotent hemangioblast cell that is able to readily contribute to endothelial maintenance or repair.
Supplementary Material
Acknowledgments
The authors thank Anne Hinds and Letty Kwok of the Boston University Pulmonary Center for technical support and Dr. Ross Summer for insightful discussion and advice.
Footnotes
This work was supported by National Institutes of Health grant 1R21HL086610-01A1 and by an Evans Foundation Pilot Award from the Boston University Department of Medicine.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0180OC on February 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Isner JM. Endothelial progenitor cells for vascular regeneration. J Hematother Stem Cell Res 2002;11:171–178 [DOI] [PubMed] [Google Scholar]
- 3.Isner JM, Kalka C, Kawamoto A, Asahara T. Bone marrow as a source of endothelial cells for natural and iatrogenic vascular repair. Ann N Y Acad Sci 2001;953:75–84 [DOI] [PubMed] [Google Scholar]
- 4.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000;97:3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003;107:461–468 [DOI] [PubMed] [Google Scholar]
- 6.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002;105:3017–3024 [DOI] [PubMed] [Google Scholar]
- 7.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, et al. Impaired cxcr4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res 2005;97:1142–1151 [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998;92:362–367 [PubMed] [Google Scholar]
- 9.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. Vegf contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 1999;18:3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249–252 [DOI] [PubMed] [Google Scholar]
- 11.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax 2005;60:410–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:318–322 [DOI] [PubMed] [Google Scholar]
- 13.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 2005;172:854–860 [DOI] [PubMed] [Google Scholar]
- 14.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and enos gene therapy in established disease. Circ Res 2005;96:442–450 [DOI] [PubMed] [Google Scholar]
- 15.Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, Karibe A, Minegishi N, Suzuki N, Yamamoto M, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation 2006;113:1442–1450 [DOI] [PubMed] [Google Scholar]
- 16.Fadini GP, Schiavon M, Cantini M, Avogaro A, Agostini C. Circulating cd34+ cells, pulmonary hypertension, and myelofibrosis. Blood 2006;108:1776–1777; author reply 1777 [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, Avogaro A, Agostini C. Pathophysiology of circulating progenitor cells in pulmonary disease and parallels with cardiovascular disease. Am J Respir Cell Mol Biol 2006;35:403–404 [DOI] [PubMed] [Google Scholar]
- 18.Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 2006;24:1806–1813 [DOI] [PubMed] [Google Scholar]
- 19.Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in copd. Eur Respir J 2006;27:529–541 [DOI] [PubMed] [Google Scholar]
- 20.Fadini GP, Schiavon M, Rea F, Avogaro A, Agostini C. Depletion of endothelial progenitor cells may link pulmonary fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 2007;176:724–725, author reply 725 [DOI] [PubMed] [Google Scholar]
- 21.Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Woll E, Kahler CM. Cd133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol 2004;57:965–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res 2006;66:7341–7347 [DOI] [PubMed] [Google Scholar]
- 23.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther 2010;126:1–8 [DOI] [PubMed] [Google Scholar]
- 24.Stadtfeld M, Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development 2005;132:203–213 [DOI] [PubMed] [Google Scholar]
- 25.Voswinckel R, Ziegelhoeffer T, Heil M, Kostin S, Breier G, Mehling T, Haberberger R, Clauss M, Gaumann A, Schaper W, et al. Circulating vascular progenitor cells do not contribute to compensatory lung growth. Circ Res 2003;93:372–379 [DOI] [PubMed] [Google Scholar]
- 26.Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: Bone marrow-derived cells do not contribute to tumor endothelium. Blood 2004;104:1769–1777 [DOI] [PubMed] [Google Scholar]
- 27.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med 2003;9:789–795 [DOI] [PubMed] [Google Scholar]
- 28.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 2008;105:6620–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotton DN, Fabian AJ, Mulligan RC. A novel stem cell population in adult liver with potent hematopoietic reconstitution activity. Blood 2005;106:1574–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol 2005;33:335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of cd34 antigen exist in multiple species. Nat Med 1997;3:1337–1345 [DOI] [PubMed] [Google Scholar]
- 34.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 2004;103:13–19 [DOI] [PubMed] [Google Scholar]
- 35.Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, et al. Universal gfp reporter for the study of vascular development. Genesis 2000;28:75–81 [DOI] [PubMed] [Google Scholar]
- 36.Weiler-Guettler H, Aird WC, Husain M, Rayburn H, Rosenberg RD. Targeting of transgene expression to the vascular endothelium of mice by homologous recombination at the thrombomodulin locus. Circ Res 1996;78:180–187 [DOI] [PubMed] [Google Scholar]
- 37.Shen AS, Haslett C, Feldsien DC, Henson PM, Cherniack RM. The intensity of chronic lung inflammation and fibrosis after bleomycin is directly related to the severity of acute injury. Am Rev Respir Dis 1988;137:564–571 [DOI] [PubMed] [Google Scholar]
- 38.Adamson IY, Bowden DH. The pathogenesis of bloemycin-induced pulmonary fibrosis in mice. Am J Pathol 1974;77:185–197 [PMC free article] [PubMed] [Google Scholar]
- 39.Adamson IY, Bowden DH. Bleomycin-induced injury and metaplasia of alveolar type 2 cells: relationship of cellular responses to drug presence in the lung. Am J Pathol 1979;96:531–544 [PMC free article] [PubMed] [Google Scholar]
- 40.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188 [DOI] [PubMed] [Google Scholar]
- 41.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration: a cytodynamic study in mice after exposure to oxygen. Lab Invest 1974;30:35–42 [PubMed] [Google Scholar]
- 42.Adamson IY, Bowden DH, Wyatt JP. Oxygen poisoning in mice: ultrastructural and surfactant studies during exposure and recovery. Arch Pathol 1970;90:463–472 [PubMed] [Google Scholar]
- 43.Bowden DH, Adamson IY, Wyatt JP. Reaction of the lung cells to a high concentration of oxygen. Arch Pathol 1968;86:671–675 [PubMed] [Google Scholar]
- 44.Bowman CM, Butler EN, Repine JE. Hyperoxia damages cultured endothelial cells causing increased neutrophil adherence. Am Rev Respir Dis 1983;128:469–472 [DOI] [PubMed] [Google Scholar]
- 45.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 1980;122:123–143 [DOI] [PubMed] [Google Scholar]
- 46.Crapo JD, Peters-Golden M, Marsh-Salin J, Shelburne JS. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest 1978;39:640–653 [PubMed] [Google Scholar]
- 47.D'Amore PA, Sweet E. Effects of hyperoxia on microvascular cells in vitro. In Vitro Cell Dev Biol 1987;23:123–128 [DOI] [PubMed] [Google Scholar]
- 48.Hu LM, Jones R. Injury and remodeling of pulmonary veins by high oxygen: a morphometric study. Am J Pathol 1989;134:253–262 [PMC free article] [PubMed] [Google Scholar]
- 49.Jones R, Zapol WM, Reid L. Pulmonary arterial wall injury and remodelling by hyperoxia. Chest 1983;83(Suppl):40S–42S [DOI] [PubMed] [Google Scholar]
- 50.O'Reilly MA, Staversky RJ, Stripp BR, Finkelstein JN. Exposure to hyperoxia induces p53 expression in mouse lung epithelium. Am J Respir Cell Mol Biol 1998;18:43–50 [DOI] [PubMed] [Google Scholar]
- 51.Staversky RJ, Watkins RH, Wright TW, Hernady E, LoMonaco MB, D'Angio CT, Williams JP, Maniscalco WM, O'Reilly MA. Normal remodeling of the oxygen-injured lung requires the cyclin-dependent kinase inhibitor p21(cip1/waf1/sdi1). Am J Pathol 2002;161:1383–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood 2005;106:1525–1531 [DOI] [PubMed] [Google Scholar]
- 53.Schmeisser A, Graffy C, Daniel WG, Strasser RH. Phenotypic overlap between monocytes and vascular endothelial cells. Adv Exp Med Biol 2003;522:59–74 [DOI] [PubMed] [Google Scholar]
- 54.Ezaki T, Baluk P, Thurston G, La Barbara A, Woo C, McDonald DM. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol 2001;158:2043–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211–226 [DOI] [PubMed] [Google Scholar]
- 56.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation workshop. Proc Am Thorac Soc 2006;3:193–207 [DOI] [PubMed] [Google Scholar]
- 57.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2008;5:637–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer 1984;49:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Reilly MA. DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol 2001;281:L291–L305 [DOI] [PubMed] [Google Scholar]
- 60.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 2000;105:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 2004;432:625–630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.