Abstract
Chronic obstructive pulmonary disease (COPD) is the third leading cause of mortality in the United States. The major cause of COPD is cigarette smoking. Extensive leukocyte influx into the lungs, mediated by chemokines, is a critical event leading to COPD. Although both resident and myeloid cells secrete chemokines in response to inflammatory stimuli, little is known about the role of epithelial-derived chemokines, such as CXC chemokine ligand (CXCL)5, in the pathogenesis of cigarette smoke–induced inflammation. To explore the role of CXCL5, we generated CXCL5 gene–deficient mice and exposed them to secondhand smoke (SHS) for 5 hours/day for 5 days/week up to 3 weeks (subacute exposure). We observed a reduced recruitment of leukocytes to the lungs of CXCL5−/− mice compared with their wild-type (WT) counterparts, and noted that macrophages comprised the predominant leukocytes recruited to the lungs. Irradiation experiments performed on CXCL5−/− or WT mice transplanted with WT or CXCL5−/− bone marrow revealed that resident but not hematopoietic cell–driven CXCL5 is important for mediating SHS-induced lung inflammation. Interestingly, we observed a significant reduction of monocyte chemotactic protein–1 (MCP-1/CC chemokine ligand 2) concentrations in the lungs of CXCL5−/− mice. The instillation of recombinant MCP-1 in CXCL5−/− mice reversed macrophage recruitment. Our results also show the reduced activation of NF-κB/p65 in the lungs, as well as the attenuated activation of C-Jun N-terminal kinase, p42/44, and p38 mitogen-activated protein kinases and the expression of intercellular adhesion molecule-1 in the lungs of SHS-exposed CXCL5−/− mice. Our findings suggest an important role for CXCL5 in augmenting leukocyte recruitment in SHS-induced lung inflammation, and provide novel insights into CXCL5-driven pathogenesis.
Keywords: smoke, CXCL5/LIX, macrophages, chemokines, cytokines
Clinical Relevance
This basic investigation demonstrates the important role of CXC chemokine ligand 5 in second-hand smoke–induced lung inflammation.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States (1–3). According to recent reports, the prevalence of COPD in the United States involves more than 12 million, with an annual mortality of 120,000 (1–3). COPD is an irreversible disease characterized by airway inflammation (chronic bronchitis) and airspace enlargement, along with the destruction of lung parenchyma (emphysema) (4, 5). Although the molecular and cellular mechanisms that contribute to the development of COPD are not well understood, substantial recruitment and activation of inflammatory cells into the airspaces and lung parenchyma has been documented in humans with COPD and implicated in its pathogenesis (4, 5).
Cigarette smoking accounts for most of the debilitating effects of COPD, but other environmental risk factors include air pollution and chronic occupational exposure to various dusts (6). Why only a small proportion (10–20%) of smokers develops COPD remains unclear (7). Cigarette smoke (CS) contains more than 4,700 chemicals (8), and is a powerful inducer of inflammatory mediators, including oxidants and proteases, that are believed to play a major role in causing lung damage (4, 5, 9–11). In patients with COPD, neutrophils and macrophages accumulate around small and large airways (12, 13), whereas in murine models, CS induces neutrophil and macrophage infiltration into the airways and lung parenchyma (14, 15). The macrophages are derived from circulating blood monocytes that traffic into the lung, where they undergo differentiation and maturation. The macrophage phagocytosis of tar in tobacco smoke triggers the production of numerous proinflammatory mediators that regulate a broad range of inflammatory processes implicated in the pathogenesis of COPD (9–11).
Chemokine production is a critical step associated with leukocyte accumulation in the lungs. Chemokines exert their biological functions via binding to their receptors (16). The binding of monocyte chemotactic protein–1 (MCP-1/CCL2) to its receptor CCR2 results in the recruitment of monocytes and dendritic cells (DCs) to the lungs. CXC chemokine ligand (CXCL)1/keratinocyte-derived chemoattractant (KC), CXCL2/macrophage inflammatory protein (MIP)-2, and CXCL5/lipopolysaccharide-induced CXC chemokines (LIX), which act via binding to CXCR2, are important for neutrophil accumulation in the lungs (16, 17). In humans with COPD, elevated concentrations of IL-8, CXCL5/epithelium-derived neutrophil-activating peptide–78 (ENA-78), and CXCL1/growth-related oncogene (GRO)–α were detected in the lungs (18, 19). In murine models, CS enhances the production of several proinflammatory cytokines/chemokines, including keratinocyte-derived chemokine (CXCL1), CXCL2, and IL-6 (17, 20).
The absence of a murine IL-8 homologue makes mice an excellent model to study the contribution of chemokines to leukocyte influx in murine lungs (16, 17, 20). In this context, keratinocyte cell–derived chemokine (CXCL1), CXCL2, CXCL5, and lungkine have been identified as essential neutrophil chemoattractants in mice during lung inflammation/infection (16, 17, 20). Whereas CXCL1 and CXCL2 are mainly produced by myeloid cells, including macrophages and neutrophils, lungkine is produced by bronchial epithelial cells (16, 17, 20). In previous studies, we demonstrated that CXCL5 is produced by alveolar epithelial Type II (AEII) cells, but not by myeloid cells, in response to LPS (21). In additional studies with specific blocking antibodies, we demonstrated that CXCL5 is important for inducing neutrophil accumulation in the lungs after LPS challenge (22). In studies with CXCL5−/− mice, we found that CXCL5 contributed to LPS-induced, neutrophil-dependent lung inflammation (23). Despite the potential role of CXCL5 in pulmonary inflammation, the role of CXCL5 in tobacco smoke–induced lung inflammation has not been explored, to the best of our knowledge. Although epidemiological studies indicated an association between SHS exposures and health outcomes for patients with COPD (24, 25), only a limited number of studies point to a mechanistic role for SHS in lung inflammation.
The present study had two aims. First, we investigated the effects of cigarette smoke extract (CSE) on CXCL5 production by AEII cells, in vitro. Second, we studied the role of subacute SHS exposure on CXCL5-mediated lung responses in CXCL5−/− and wild-type (WT) mice. We found that CSE induces significant CXCL5 from AEII cells. In our in vivo experiments, we observed that subacute SHS exposure reduced macrophage numbers in the bronchoalveolar lavage fluid (BALF) of CXCL5−/− mice, compared with their littermate control mice. We also found that CXCL5 regulates the production of CCL2 and TNF-α, the activation of NF-κB and mitogen-activated protein kinases (MAPKs), and the expression of intercellular adhesion molecule ICAM-1 in the lungs after SHS exposure.
Materials and Methods
Animals
Eight- to 10-week-old CXCL5−/− and CXCL5+/+ female mice on a mixed background (C57BL/6 X 129) were used (23). All animal studies were approved by the Louisiana State University Institutional Animal Care and Use Committee. The mice ranged from 19–25 g in weight.
CSE Challenge
CSE was prepared as a stock solution in DMSO containing 40 mg/ml particulate matter and 6% nicotine. A working solution of 80 μg/ml was prepared immediately before use (26–29). The isolation of AEII cells (21, 30) and of bone marrow–derived macrophages (BMMs) (31) was performed as described in previous reports. In total, 2.5 × 106 AEII cells were maintained on a collagen/matrigel system. Both the apical and basolateral surfaces were challenged with 80 μg/ml CSE for 18 hours at 37°C. Bone marrow (BM) cells were differentiated using Dulbecco's Modified Eagle's Medium (DMEM) containing 10% FBS and macrophage colony-stimulating factor (M-CSF) for 7 days. BM cells were differentiated in culture dishes by supplementing DMEM with M-CSF on Days 3 and 5. On Day 7, BMMs were treated with CSE.
SHS Exposure
For smoke inhalation, sidestream smoke comprised approximately 90% of SHS, and the remaining 10% consisted of exhaled mainstream smoke (32–34). Here, sidestream smoke served as a surrogate for SHS (32–34). A 30-port smoking machine (AMESA Technologies, Geneva, Switzerland) generated smoke from 3R4F filtered research cigarettes (University of Kentucky, Lexington, KY). We diluted SHS with HEPA-filtered air to establish a steady-state suspended particle load of 10 mg/m3. We exposed 8- to 10-week-old CXCL5−/− and CXCL5+/+ mice to subacute SHS (14 air changes/hour, 5 hours/day, 5 days/week, for 3 consecutive weeks) in 1.3-m3 stainless steel and Plexiglas dynamic exposure chambers (71° ± 1.5°F; relative humidity, 53% ± 3%). Control HEPA-filtered air exposures were performed simultaneously in adjacent exposure chambers.
Instillation of Recombinant CCL2/MCP-1
CCL2−/− mice were treated intratracheally with exogenous CCL2 (10 μg/mouse) for 1 hour before SHS exposure, and control mice were treated with an equal volume of PBS with 0.1% BSA every day for 5 days (1 week). After SHS exposure, BALF was collected and processed for cellular enumeration.
BALF Collection
BALF was collected, and total and differential cell counts and cytokine/chemokine concentrations were determined. Approximately 3 ml of lavage fluid were retrieved per mouse. Total leukocytes in BALF were determined using a hemocytometer. Cytospin samples were subsequently prepared from BALF cells and stained with Diff-Quik (Fisher Scientific, Chicago, IL). Differential cell counts were determined by the direct counting of stained slides. The remainder (2 ml) of the undiluted cell-free BALF was passed via a 0.22-μm filter and used immediately, or stored at −80°C (21, 31).
Bone Marrow Transplantation
Donor and recipient mice (6–8 wk old) were used to generate chimeras, as described earlier (30, 31). We found that more than 90% of blood leukocytes were derived from donor mice at the time the mice were used for experiments (data not shown). Irradiated mice that were not transplanted with donor cells died between Days 19 and 24 after transplantation (data not shown).
Cytokine and Chemokine Determination
BALF and lung homogenates were prepared in a similar manner as described in our previous studies (21, 35). Concentrations of CXCL1 and CXCL2 were quantified using ELISA kits from R&D Systems, Inc. (Minneapolis, MN). The minimum detection limit in each case was 8 pg/ml cytokine protein (21, 35).
NF-κB DNA Binding Assay
Nuclear proteins were extracted from 50–80 mg lung tissue collected from mice after 1, 2, and 3 weeks of SHS exposure. In total, 7.5 μg of nuclear extract were used as described in our earlier studies (31, 35, 36).
Western Blotting
Lungs were collected at designated time points, and the lung homogenates were used for immunoblotting, as described earlier (36, 37). All primary antibodies (Abs) were used at a 1:1,000 dilution except for total p38 and glyceraldehyde 3–phosphate dehydrogenase (GAPDH), were used at a 1:5,000 dilution, whereas secondary Abs were used at a dilution of 1:2,000. Blots were stripped and reprobed with an Ab specific for GAPDH or total p38. The intensity of immunoreactive bands was determined using gel digitizing software (UN-SCAN-IT gel; Silk Scientific, Inc., Orem, UT).
Statistical Analysis
Data are expressed as mean ± SE. Data were analyzed by ANOVA, followed by Bonferroni post hoc analysis. The experiments were repeated at least three times. All statistical calculations were performed using Stat software and GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA). Differences were considered statistically significant at *P < 0.05 when compared with controls.
Results
CSE Induces CXCL5 Expression in AEII Cells but Not Macrophages
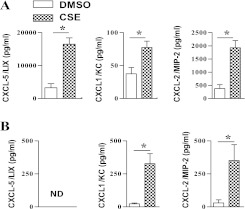
In previous studies, we reported that AEII cells are the predominant source of CXCL5 in the lungs during LPS-induced inflammation (21). Because tobacco smoke exposure also induces lung inflammation, we first asked whether CSE can induce significant CXCL5 in AEII cells maintained on a collagen/matrigel substrate. Using ELISA, we found that CSE-stimulated AEII cells produce approximately 4-fold more CXCL5 than DMSO-exposed control cells (Figure 1A). The CSE-exposed AEII cells also produced approximately twofold more CXCL1 and CXCL2 than did control cells (Figure 1A). Although CSE-treated bone marrow–derived macrophages produce increased amounts of CXCL1 and CXCL2 in response to CSE (80 μg/ml), these cells did not produce detectable CXCL5 (Figure 1B).
Figure 1.
Expression of the chemokines CXC chemokine ligand (CXCL)5/lipopolysaccharide-induced CXC chemokine (LIX), CXCL1/keratinocyte-derived chemokine (KC), and CXCL2/macrophage inflammatory protein (MIP)-2 in the culture media of wild-type (WT) alveolar Type II epithelial cells (A) and bone marrow–derived macrophages (B) after cigarette smoke extract (CSE) stimulation (80 μg/ml) in vitro. Experiments were performed in triplicate wells (n = 3–4 mice/group). *P < 0.05, compared with DMSO control. ND, not detected.
CXCL5 Deficiency Causes Reduced Macrophage Recruitment to the Lungs in Response to SHS Exposure
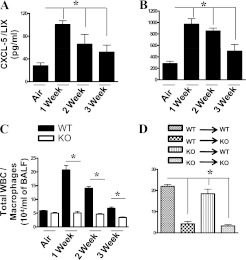
Next, we examined whether exposure to SHS can increase concentrations of CXCL5 in the lung. We found that SHS exposure significantly increased CXCL5 concentrations in the BALF (Figure 2A) and lung homogenates (Figure 2B) of WT mice. To determine the requirements of CXCL5 in cellular recruitment, we exposed CXCL5−/− and WT control mice to subacute SHS (10 mg/m3). Groups of mice were killed at the end of 1 week, 2 weeks, or 3 weeks after exposure. We found reduced leukocyte recruitment to the lungs of CXCL5−/− mice compared with their WT counterparts (Figure 2C). All of the recruited cells were macrophages (Figure 2C).
Figure 2.
CXCL5 concentrations in bronchoalveolar lavage fluid (BALF) (A) and lungs (B) after secondhand smoke (SHS) exposure. WT (C57Bl/6) mice were exposed to SHS or HEPA-filtered air, and BALF and lung homogenates were used to detect CXCL5 concentrations by sandwich ELISA (n = 4–5 mice/group; P < 0.05). (C) Total leukocyte/macrophage numbers in the lungs of CXCL5+/+ and CXCL5−/− mice upon cigarette smoke exposure. Mice were exposed to HEPA-filtered air–diluted SHS (10 mg/m3 for 5 hours/day/week) for up to 3 weeks. Control animals were exposed to HEPA-filtered air. At the end of each week, CXCL5−/− and WT mice were killed to determine the number of cells recruited to the lungs, and to perform further analyses as described in Materials and Methods. For cellular enumeration, lungs were lavaged, and the BALF was obtained at 1, 2, and 3 weeks after exposure (n = 8 mice/group; P < 0.05). (D) Total leukocyte/macrophage numbers in the airspaces of bone marrow chimeras of CXCL5+/+ (WT) and CXCL5−/− (knockout; KO) mice after smoke exposure (n = 6–8 mice/group.) WBC, white blood cells. *P < 0.05, compared with CXCL5−/− mice.
Resident Cell–Derived CXCL5 Is Important for Macrophage Recruitment to the Lungs after SHS Exposure
Because we observed reduced macrophage numbers in CXCL5−/− mice, we next determined whether this effect was attributable to myeloid cell–driven or resident cell–driven CXCL5. To identify the cell types responsible for the production of CXCL5 in the lungs, bone marrow chimeras were generated by radioablation, reconstituted with bone marrow cells obtained from WT and CXCL5−/− mice, and transplanted mice were exposed to SHS. We observed reduced macrophage numbers in CXCL5−/− mice, irrespective of whether donor bone marrow transplantations were derived from CXCL5−/− or WT mice (Figure 2D). These findings demonstrate that the CXCL5 produced by resident lung (structural) cells is important for macrophage recruitment to the lungs after SHS exposure.
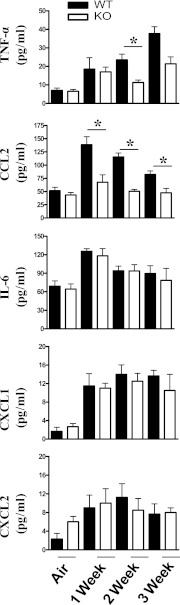
To determine whether CXCL5 regulates the expression of other cytokines/chemokines after SHS exposure, we determined the concentrations of TNF-α, CCL2 IL-6, CXCL1, and CXCL2 in BALF after subacute SHS exposure. We observed decreased TNF-α expression in the BALF of CXCL5−/− mice at the end of the second and third weeks of SHS exposure, compared with WT controls (Figure 3). Furthermore, CCL2 concentrations were reduced in the CXCL5−/− mice after the first, second, and third weeks of SHS exposure when compared with SHS-exposed WT control mice. However, IL-6, CXCL1, and CXCL2 concentrations were not significantly altered in CXCL5−/− mice after SHS exposure (Figure 3).
Figure 3.
Cytokine and chemokine concentrations in the lungs after cigarette smoke exposure for up to 3 weeks. Mice were exposed to cigarette smoke, and cell-free BALF was used to determine concentrations (pg/ml) of TNF-α, CCL2, IL-6, CXCL1, and CXCL2 by sandwich ELISA. Asterisks indicate a significant difference between CXCL5+/+ and CXCL5−/− mice (n = 8 mice in each group at each time-point). MCP-1, monocyte chemotactic protein–1. P < 0.05.
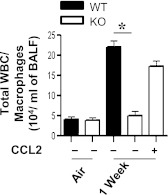
CCL2 Regulates Macrophage Recruitment in a CXCL5-Dependent Manner
Because we observed reduced macrophage numbers in BALF associated with reduced CCL2 concentrations in CCL2−/− mice, we wanted to determine whether CCL2 treatment can rescue macrophage influx after SHS exposure. In this regard, we treated CXCL5−/−− mice intratracheally with 10 μg CCL2, 1 hour before SHS exposure. We observed that intrapulmonary CCL2 restored macrophage recruitment to the lungs in response to SHS exposure (Figure 4).
Figure 4.
Effects of exogenous CCL2 on macrophage influx in the lungs of CXCL5−/− mice after SHS exposure. Cellular infiltration was evident in airspaces at 1 week after SHS exposure with MCP-1 (10 μg/mouse) or vehicle (BSA) control. Macrophage influx in BALF from CXCL5−/− or WT mice infected with administration of CCL2 or vehicle (BSA), followed by SHS exposure. For all experiments, n = 5−8 mice/group. *P < 0.05, compared with BSA–administered mice.
CXCL5 Regulates Activation of NF-κB and MAPK and Expression of ICAM-1 after SHS Exposure
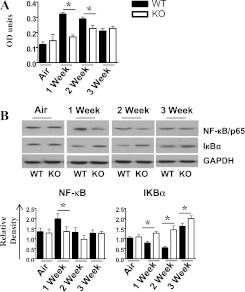
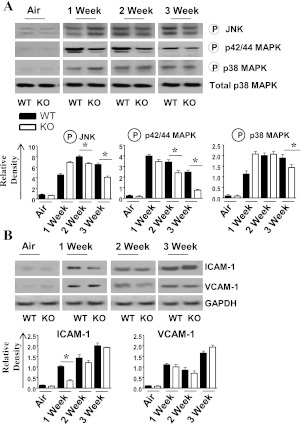
The expression of cytokines and chemokines involves the activation of transcription factors and MAPKs (38, 39). Because NF-κB and MAPKs play a critical role in the expression of proinflammatory mediators in response to multiple stimuli (17, 40), we examined the activation of NF-κB and MAPKs in whole-lung homogenates after SHS exposure. We observed a decreased activation of NF-κB after 1 and 2 weeks of SHS exposure in CXCL5−/− mice, whereas IKBα concentrations were increased every week in CXCL5−/− mice compared with control mice (Figures 5A and 5B). Furthermore, the activation of p38, C-Jun N-terminal kinase, and P42/44 (extracellular regulated kinase) MAPKs was reduced in CXCL5−/− mice after 2 and 3 weeks of SHS exposure (Figure 6A). Moreover, ICAM-1 up-regulation, unlike that of VCAM-1, was decreased after 1 week of SHS exposure in the lungs of CXCL5−/− mice, compared with their littermate control mice (Figure 6B).
Figure 5.
Activation of NF-κB in whole-lung homogenates after smoke exposure in CXCL5−/− mice. (A) Nuclear lysates from CXCL5−/− mice and control mice were prepared at 1, 2, and 3 weeks after smoke exposure. The NF-κB binding assay was performed in nuclear extracts from the lungs. OD, optical density. (B) Lung homogenates were prepared and total protein from lungs was resolved on an SDS-PAGE, and the membranes were blotted with antibodies against the activated/phosphorylated form of NF-κB and mitogen-activated protein kinases (MAPKs), as described in Materials and Methods. Upper panel in B: Blots are representative of three independent experiments with identical results. Lower panel in B: Relative densities, normalized against glyceraldehyde 3–phosphate dehydrogenase (GAPDH), are representative of three independent blots/experiments. For experiments in both A and B, n = 5–6 mice/group; *P < 0.05, compared with CXCL5−/− mice.
Figure 6.
(A) Activation of MAPKs in the lungs after SHS exposure in CXCL5−/− mice. Total proteins in the lungs of CXCL5−/− mice and control mice were isolated and resolved on an SDS-PAGE, and the membranes were blotted with the antibodies against activated/phosphorylated or inactive forms of MAPKs, as described in Materials and Methods. Above: Representative of three separate experiments with identical results. Below: Relative densities were normalized against total p38 MAPK, and are representation of three different blots. Circled “P” indicates phosphorylated form of the protein. (B) Expression of intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in the lung homogenates after subacute smoke exposure. The lungs were homogenized, and total proteins were resolved on SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membranes were blotted with antibodies against ICAM-1, VCAM-1, and GAPDH. This blot is representative of three separate blots with identical results. Below: Densitometric analysis was performed in three blots to demonstrate the expression of ICAM-1 and VCAM-1. For experiments in both panels, n = 4–6 mice/group. *P < 0.05, compared with CXCL5−/− mice.
Discussion
Acute and chronic inflammation in the lungs results from exposure to cigarette smoke, chemicals, and infections. Cigarette smoke–induced inflammation of the airways and lung parenchyma can lead to chronic lung diseases in humans, including COPD (4, 5). Currently, no specific therapies are available to treat underlying causes of COPD, but treatments are available to help reduce symptoms. It is therefore critical to understand the role of inflammatory mediators involved in the pathogenesis of COPD, to define better therapies.
CS exposure initiates the infiltration of inflammatory cells into the airways and lung parenchyma, which eventually destroys alveolar structure and function, resulting in COPD and emphysema. Of the mechanisms associated with inflammation in the lungs, the most important is the successful recruitment of leukocytes from the blood. Numerous innate and adaptive immune cells, such as neutrophils, macrophages, dendritic cells, and CD8 lymphocytes, were implicated in the pathogenesis of COPD (4, 13, 41). Both macrophage and neutrophil numbers were reported to be substantially increased in the BALF and sputum of patients with COPD, which correlates well with the severity of COPD (42–44). In a murine model of lung inflammation, the role of macrophages and neutrophils in the pathogenesis of emphysema is well documented (45–47).
Cellular recruitment to the lungs is tightly regulated by the production of chemokines. Chemokines have a complex network of signaling that can be redundant, synergistic, or antagonistic (38–40). In humans and mice, monocytes can be recruited from the circulation to the alveoli by monocyte chemoattractants, such as CCL2, whereas neutrophil recruitment can be regulated by CXC chemokines (4, 12, 48). In humans, chemokines involved in neutrophil chemotaxis include IL-8; neutrophil-activating peptide–2, GRO-α, GRO-β, GRO-γ, ENA-78, and granulocyte chemotactic protein–2 (4, 48, 49).
Regarding chemokines in mice, the murine homologue of human ENA-78 is CXCL5/LIX. CXCL5 is produced by alveolar Type II epithelial cells in response to LPS exposure (21). When alveolar Type II cells were exposed to CS, an enhanced expression of CXCL5/LIX was observed, as compared with the expression of other potent lung chemokines such as CXCL1 and CXCL2. In addition, SHS exposure enhanced the expression of CXCL5 in the airways and lung parenchyma. These findings suggest that CXCL5 can play an important role in the pathogenesis of CS-induced airway inflammation and emphysema. To understand the role of CXCL5/LIX further, we generated CXCL5−/− mice and exposed them to a subacute dose of SHS (for up to 3 weeks). Our data demonstrate that CCL2 concentrations and macrophage numbers were significantly reduced in the lungs of CXCL5−/− mice. The reduction of macrophage numbers can be attributed to either (decreased) direct chemotaxis or indirectly, via the reduced CXCL5-dependant induction of proinflammatory mediators such as CCL2. Circulating monocytes express CXCR2, the receptor for CXCL5/LIX (4). Our findings demonstrate that the inefficient recruitment of monocytes in the lungs of CXCL5−/− mice is caused by CXCL5 deficiency. These data support the direct and indirect roles of CXCL5 in monocyte recruitment to the lungs, via the regulation of CCL2 expression during SHS exposure.
Numerous studies demonstrated that the exposure of mice to environmental tobacco smoke (ETS) (TSP = 60–120 mg/m3) (Teague Smoke Machine, Woodland, CA) induces the infiltration of as much as 85–95% of the cell population as macrophages into the lungs of mice (46, 50, 51). However, mainstream cigarette smoke exposure (TSP = 250–300 mg/m3) was shown to induce the infiltration of inflammatory cells, predominantly neutrophils, into the lungs of mice (52, 53). These studies suggest that the method of CS exposure determines the type of inflammatory cell population in the lungs. In the present study, we exposed WT and CXCL5−/− mice to ETS (11% mainstream smoke and 89% air; TSP = 10 mg/m3), using a 30-port smoking machine (AMESHA Technologies, Geneva, Switzerland) for 5 hours/day, 5 days/week, up to 3 consecutive weeks. BAL cell count analysis revealed the presence of macrophages in the lungs upon SHS exposure. The present findings are consistent with our earlier reports (29–31) as well as with other studies using ETS exposure (46, 50, 51). Interestingly, the increased infiltration of macrophages into the lungs is clearly associated with the enhanced production of the macrophage-attracting chemokine, CCL2, in the lungs of CS-exposed WT mice in a CXCL5-dependent manner. Nevertheless, the exposure of mice to ETS did not significantly induce the expression of neutrophil chemoattractants (CXCL1/KC and CXCL2/MIP-2), which could explain the absence of neutrophils in the lungs of cigarette smoke-exposed mice.
TNF-α, in concert with other cytokines and chemokines, can promote the generation of reactive oxygen species, elastase, and matrix metalloproteases, which can impose serious lung damage (4, 54, 55). Concentrations of TNF-α are increased in the lungs when neutrophils or macrophages are recruited from the bloodstream during inflammation (41, 56–58). In agreement with these findings, a significantly inhibited SHS-induced production of TNF-α (although at a low concentration) was observed in CXCL5−/− mice, which exhibited reduced leukocyte recruitment. In this regard, a previous study showed that mice lacking TNF-α receptors and exposed to mainstream smoke displayed fewer neutrophils and macrophages (57).
During infection and inflammation in the lungs, both bone marrow and resident cells produce proinflammatory mediators (17, 40). Our present data suggest that hematopoietic cells produce neutrophil chemoattractants, such as CXCL1 and CXCL2, whereas resident cells produce the neutrophil chemoattractants LIX and lungkine. The present study reveals that CXCL5 derived from resident cells is essential for CS-induced macrophage recruitment to the lungs. MyD88, derived from hematopoietic cells, was previously shown to be more important for the LPS-induced production of TNF-α and IL-12p40 (59), although both hematopoietic cell–derived and resident cell–derived MyD88 are essential for LPS-induced neutrophil influx (60–62). Similarly, we demonstrated that both hematopoietic cell–derived and resident cell–derived myeloid differentiation protein-2 is essential to induce neutrophil influx after Escherichia coli–induced inflammation (30). In additional studies, we showed that both hematopoietic and resident cells are important for CXCL1-induced neutrophil accumulation in the lungs in response to Klebsiella pneumoniae–induced inflammation (31).
The expression of cytokines and chemokines require the activation of MAPKs and multiple transcription factors (17, 40). In this study, we explored whether CXCL5 regulates the expression and activation of NF-κB, a well-studied transcription factor that regulates the expression of inflammatory cytokines and chemokines (17, 40). Our data suggest that CXCL5 does play a role in regulating NF-κB expression in the lungs, and NF-κB activation was induced in lungs after 1 and 2 weeks of smoke exposure in WT mice, but such activation was defective in the knockout mice. The other important observation regarding lung inflammation in our study involves the activation of MAPKs, which was found to be regulated by CXCL5 during the second and third weeks of smoke exposure. These results bring up the speculation that NF-κB is important for early cellular recruitment, and that MAPKs are important for subsequent cellular influx. These findings are consistent with previous reports indicating that the activation of NF-κB and MAPKs in the lungs occurs at different concentrations of mainstream smoke exposure (63, 64). However, our findings are the first, to the best of our knowledge, to show the regulation of NF-κB and MAPKs by CXCL5 in the lungs during SHS exposure. Because CXCR2 is expressed on both myeloid and resident cells in the lungs, the activation of NF-κB and MAPKs by CXCL5 likely occurs via both autocrine and paracrine mechanisms.
Leukocyte sequestration within capillaries and migration into lung parenchyma and eventually to the alveolar spaces during inflammation constitute a multistep process that involves leukocyte stiffening, retention in the capillaries, firm adhesion, and migration into the alveolus (17, 40). The expression of cell adhesion molecules on endothelial venules is critical in leukocyte recruitment (17). Leukocytes bind to ICAM-1, E-selectin, and VCAM-1, expressed on endothelial venules (17, 40). In particular, VCAM-1 and ICAM-1 are inducible in endothelia by LPS and other inflammatory mediators, such as TNF-α (17, 38, 39). We also observed that CXCL5−/− deficiency in mice resulted in a reduced SHS-induced expression of cellular adhesion molecule ICAM-1.
We conclude that CXCL5-dependent signaling cascades are essential for the recruitment of macrophages to the lungs upon subacute SHS exposure. Our results suggest that the interaction of CXCL5 with its receptor CXCR2 leads to CS-induced pulmonary inflammation via the activation of NF-κB and MAPK and the expression of ICAM-1. The specific targeting of CXCL5 may be a feasible option to attenuate excessive macrophage recruitment to the lung during SHS-induced lung inflammation. However, future studies are warranted to determine the effects of CXCL5 in chronic SHS-induced emphysema.
Supplementary Material
Acknowledgments
The authors thank Lindsey Clemons and Rui Xiao for their help in performing SHS exposure. The authors thank Pete Mottram at Louisiana State University for his critical reading of the manuscript. The authors also thank the Lung Biology Laboratory members Theivanthiran Balamayooran, Jin Liliang, K. Jeyagowri, and Keshalini Sabaratnam at Imperial College, London for helpful discussions and critical reading of the manuscript.
Footnotes
This work was supported by Scientist Award YCSA-062466 from the Flight Attendant Medical Research Institute, by grants R01 HL-091958 and R01 HL-091958S1 from the National Institutes of Health (S.J.), and by the Louisiana Governor's Biotechnology Initiative.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0260OC on February 23, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–773 [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Murray CCJL. The global burden of disease, 1990–2020. Nat Med 1998;4:1241–1243 [DOI] [PubMed] [Google Scholar]
- 3.Balkissoon R, Lommatzsch S, Carolan B, Make B. Chronic obstructive pulmonary disease: a concise review. Med Clin North Am 2011;95:1125–1141 [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672–688 [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano A, Caramori G, Ricciardolo FLM, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy 2004;34:1156–1167 [DOI] [PubMed] [Google Scholar]
- 6.Viegi G, Scognamiglio A, Baldacci S, Pistelli F, Carrozzi L. Epidemiology of chronic obstructive pulmonary disease (COPD). Respiration 2001;68:4–19 [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care 2003;48:1185–1191 [PubMed] [Google Scholar]
- 8.Stedman RL. The chemical composition of tobacco and tobacco smoke. Chem Rev 1968;68:153–207 [DOI] [PubMed] [Google Scholar]
- 9.Borgerding M, Klus H. Analysis of complex mixtures: cigarette smoke. Exp Toxicol Pathol 2005;57:43–73 [DOI] [PubMed] [Google Scholar]
- 10.Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 2011;8:613–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thielen A, Klus H, Müller L. Tobacco smoke: unraveling a controversial subject. Exp Toxicol Pathol 2008;60:141–156 [DOI] [PubMed] [Google Scholar]
- 12.MacNee W, Wiggs B, Belzberg AS, Hogg JC. The effect of cigarette smoking on neutrophil kinetics in human lungs. N Engl J Med 1989;321:924–928 [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax 2006;61:448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Profita M, Sala A, Bonanno A, Riccobono L, Ferraro M, La Grutta S, Albano GD, Montalbano AM, Gjomarkaj M. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. Am J Physiol Lung Cell Mol Physiol 2010;298:L261–L269 [DOI] [PubMed] [Google Scholar]
- 15.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke–induced emphysema in mice. Science 1997;277:2002–2004 [DOI] [PubMed] [Google Scholar]
- 16.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2005;288:L3–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balamayooran G, Batra S, Fessler MB, Happel KI, Jeyaseelan S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol 2010;43:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, Jeffery PK. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168:968–975 [DOI] [PubMed] [Google Scholar]
- 19.Morrison D, Strieter R, Donnelly S, Burdick M, Kunkel S, MacNee W. Neutrophil chemokines in bronchoalveolar lavage fluid and leukocyte-conditioned medium from nonsmokers and smokers. Eur Respir J 1998;12:1067–1072 [DOI] [PubMed] [Google Scholar]
- 20.Remick DG, Green LB, Newcomb DE, Garg SJ, Bolgos GL, Call DR. CXC chemokine redundancy ensures local neutrophil recruitment during acute inflammation. Am J Pathol 2001;159:1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 2005;32:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun 2004;72:7247–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, Worthen GS. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 2010;33:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisner M, Balmes J, Yelin E, Katz P, Hammond SK, Benowitz N, Blanc P. Directly measured secondhand smoke exposure and COPD health outcomes. BMC Pulm Med 2006;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisner MD, Wang Y, Haight TJ, Balmes J, Hammond SK, Tager IB. Secondhand smoke exposure, pulmonary function, and cardiovascular mortality. Ann Epidemiol 2007;17:364–373 [DOI] [PubMed] [Google Scholar]
- 26.Castro SM, Kolli D, Guerrero-Plata A, Garofalo RP, Casola A. Cigarette smoke condensate enhances respiratory syncytial virus–induced chemokine release by modulating NF-kappa B and interferon regulatory factor activation. Toxicol Sci 2008;106:509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke–induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol 2007;292:H130–H139 [DOI] [PubMed] [Google Scholar]
- 28.Huang Fu W-C, Liu J, Harty RN, Fuchs SY. Cigarette smoking products suppress anti-viral effects of Type I interferon via phosphorylation-dependent downregulation of its receptor. FEBS Lett 2008;582:3206–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni R, Rampersaud R, Aguilar JL, Randis TM, Kreindler JL, Ratner AJ. Cigarette smoke inhibits airway epithelial cell innate immune responses to bacteria. Infect Immun 2010;78:2146–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai S, Zemans RL, Young SK, Worthen GS, Jeyaseelan S. Myeloid differentiation protein–2–dependent and –independent neutrophil accumulation during Escherichia coli pneumonia. Am J Respir Cell Mol Biol 2009;40:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella infection via CXCL2, CXCL5, NF-κB, and MAPKs. J Immunol 2010;185:6214–6225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowles KS, Horohov DW, Paulsen DB, LeBlanc CJ, Littlefield-Chabaud MA, Ahlert T, Ahlert K, Pourciau SS, Penn A. Exposure of adult mice to environmental tobacco smoke fails to enhance the immune response to inhaled antigen. Inhal Toxicol 2005;17:43–51 [DOI] [PubMed] [Google Scholar]
- 33.Penn A, Snyder C. Inhalation of sidestream cigarette smoke accelerates development of arteriosclerotic plaques. Circulation 1993;88:1820–1825 [DOI] [PubMed] [Google Scholar]
- 34.Penn AL, Rouse RL, Horohov DW, Kearney MT, Paulsen DB, Lomax L. In utero exposure to environmental tobacco smoke potentiates adult responses to allergen in BALB/c mice. Environ Health Perspect 2007;115:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Toll–IL-1 receptor domain–containing adaptor protein is critical for early lung immune responses against Escherichia coli lipopolysaccharide and viable Escherichia coli. J Immunol 2005;175:7484–7495 [DOI] [PubMed] [Google Scholar]
- 36.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain–containing adaptor inducing IFN-beta (TRIF)–mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol 2007;178:3153–3160 [DOI] [PubMed] [Google Scholar]
- 37.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol 2009;183:6629–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balamayooran T, Balamayooran G, Jeyaseelan S. Review: Toll-like receptors and NOD-like receptors in pulmonary antibacterial immunity. Innate Immun 2010;16:201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003;31(Suppl 4)S195–S199 [DOI] [PubMed] [Google Scholar]
- 40.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 2009;77:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pessina GP, Paulesu L, Corradeschi F, Luzzi E, Stefano AD, Tanzini M, Matteucci G, Bocci V. Effects of acute cigarette smoke exposure on macrophage kinetics and release of tumour necrosis factor-alpha in rats. Mediators Inflamm 1993;2:119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanni S, Pelegrino N, Angeleli A, Correa C, Godoy I. Smoking status and tumor necrosis factor–alpha mediated systemic inflammation in COPD patients. J Inflamm 2010;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pauwels RA, Buist AS, Calverley PMA, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276 [DOI] [PubMed] [Google Scholar]
- 44.Dewar M, Curry RW., Jr Chronic obstructive pulmonary disease: diagnostic considerations. Am Fam Physician 2006;15:669–676 [PubMed] [Google Scholar]
- 45.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke–induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 2008;294:L612–L631 [DOI] [PubMed] [Google Scholar]
- 46.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke–induced emphysema in mice. Am J Pathol 2003;163:2329–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc 2008;5:475–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traves SL, Culpitt SV, Russell REK, Barnes PJ, Donnelly LE. Increased levels of the chemokines GRÕα and MCP-1 in sputum samples from patients with COPD. Thorax 2002;57:590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanino M, Betsuyaku T, Takeyabu K, Tanino Y, Yamaguchi E, Miyamoto K, Nishimura M. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax 2002;57:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of NRF2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J Clin Invest 2004;114:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rangasamy T, Misra V, Zhen L, Tankersley CG, Tuder RM, Biswal S. Cigarette smoke–induced emphysema in A/J mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am J Physiol Lung Cell Mol Physiol 2009;296:L888–L900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu F, Zhuang J, Wang R, Seagrave JC, March TH. Blunted ventilatory response to hypoxia/hypercapnia in mice with cigarette smoke–induced emphysema. Respir Physiol Neurobiol 2007;158:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang JW, Rajendrasozhan S, Yao H, Chung S, Sundar IK, Huyck HL, Pryhuber GS, Kinnula VL, Rahman I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke–induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol 2011;187:987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida T, Tuder RM. Pathobiology of cigarette smoke–induced chronic obstructive pulmonary disease. Physiol Rev 2007;87:1047–1082 [DOI] [PubMed] [Google Scholar]
- 55.Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke–induced inflammation via tumor necrosis factor–{alpha} release. Am J Respir Crit Care Med 2003;167:1083–1089 [DOI] [PubMed] [Google Scholar]
- 56.Keatings V, Collins P, Scott D, Barnes P. Differences in interleukin-8 and tumor necrosis factor–alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996;153:530–534 [DOI] [PubMed] [Google Scholar]
- 57.Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor–{alpha} is central to acute cigarette smoke–induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med 2002;166:849–854 [DOI] [PubMed] [Google Scholar]
- 58.Li Y-T, He B, Wang Y-Z. Exposure to cigarette smoke upregulates AP-1 activity and induces TNF-alpha overexpression in mouse lungs. Inhal Toxicol 2009;21:641–647 [DOI] [PubMed] [Google Scholar]
- 59.Noulin N, Quesniaux VF, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, Vargaftig BB, Ryffel B, Couillin I. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol 2005;175:6861–6869 [DOI] [PubMed] [Google Scholar]
- 60.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol 2007;178:1896–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol 2008;38:699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol 2004;287:L143–L152 [DOI] [PubMed] [Google Scholar]
- 63.Zhao J, Harper R, Barchowsky A, Di YPP. Identification of multiple MAPK-mediated transcription factors regulated by tobacco smoke in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L480–L490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birrell MA, Wong S, Catley MC, Belvisi MG. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J Cell Physiol 2008;214:27–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.