Abstract
BACKGROUND AND PURPOSE
Muscarinic acetylcholine receptors (mAChRs) and β-adrenoceptors in the airways and lungs are clinically important in chronic obstructive pulmonary disease (COPD) and asthma. However, the quantitative and qualitative estimation of these receptors by radioligand binding approaches in human airways has not yet been reported because of tissue limitations.
EXPERIMENTAL APPROACH
The regional distribution and relative proportion of mAChR and β-adrenoceptor subtypes were evaluated in human bronchus and lung parenchyma by a tissue segment binding method with [3H]-N-methylscopolamine ([3H]-NMS) for mAChRs and [3H]-CGP-12,177 for β-adrenoceptors. Functional responses to carbachol and isoprenaline were also analysed in the bronchus.
KEY RESULTS
The M3 subtype predominantly occurred in the bronchus, but the density decreased from the segmental to subsegmental bronchus, and was absent in lung parenchyma. On the other hand, the M1 subtype occurred in the lung only, and the M2 subtype was distributed ubiquitously in the bronchus and lungs. β2-adrenoceptors were increased along the airways, and their densities in the subsegmental bronchus and lung parenchyma were approximately twofold higher than those of mAChRs in the same region. β1-adrenoceptors were also detected in lung parenchyma but not in the bronchus. The muscarinic contractions and adrenoceptor relaxations in both bronchial regions were mediated through M3-mAChRs and β2-adrenoceptors, respectively.
CONCLUSIONS AND IMPLICATIONS
From the present radioligand binding approach with intact tissue segments, we constructed a distribution map of mAChRs and β-adrenoceptors in human bronchus and lung parenchyma for the first time, providing important evidence for future pharmacotherapy and new drug development for respiratory disorders.
Keywords: human airway, bronchus, muscarinic acetylcholine receptor (mAChR), β-adrenoceptor, intact segment binding
Introduction
Airways, including the lung, are innervated by autonomic nerves and respiration is reciprocally regulated by parasympathetic and sympathetic nerves. The autonomic receptors in the airways are mainly muscarinic acetylcholine receptors (mAChRs) and β-adrenoceptors, and both receptors are activated by neuronal acetylcholine and noradrenaline (Barnes, 1986; Roux et al., 1998; Gosens et al., 2006). Acetylcholine is also synthesized in non-neuronal cells (bronchial epithelium and inflammatory cells) (Kawashima and Fujii, 2000; Wessler and Kirkpatrick, 2001; Gosens et al., 2004). As either an increase in the cholinergic activity or a decrease in the adrenergic activity is directly associated with bronchoconstriction and airway inflammation, both receptors are considered to be important in pathophysiological mechanisms of chronic obstructive pulmonary disease (COPD) and asthma (Gross and Skorodin, 1984; Broadley, 2006; Gosens et al., 2006). Thus, mAChR antagonists and β-adrenoceptor agonists are currently used for the treatment of the symptoms of these conditions (Goldie, 1990; Gosens et al., 2006; Barnes, 2007).
mAChRs and β-adrenoceptors are composed of distinct homogeneous families of five (M1–M5) or three (β1–β3) subtypes, respectively. The distribution of mAChRs and β-adrenoceptors in the airways has been mapped by receptor autoradiography and in situ hybridization (Spina et al., 1989; Mak and Barnes, 1990; Johnson, 1992; Mak et al., 1992; Hislop et al., 1998). The functional receptors in the airway smooth muscle are mainly M3 mAChR and β2-adrenoceptors, which are involved in bronchial constriction or dilatation, whereas the β2-adrenoceptor in lungs plays an important role in fluid clearance out of the alveolar airspace (Eglen et al., 1996; Shore and Moore, 2003; Barnes, 2004; Mutlu and Factor, 2008). Radioligand binding studies have also been applied to membrane preparations of trachea and bronchus isolated from large animals (dog, cow, horse and pig) (Barnes et al., 1983; Roffel et al., 1988; Abraham et al., 2003; D'Agostino et al., 2008) and to lung preparations from small and large animals, including humans (Roffel et al., 1990; Johnson, 1998). In bovine and horse tracheae, the majority of mAChRs are the M2 subtype and the presence of the M3 subtype is minimal (Roffel et al., 1988; Eglen et al., 1996; D'Agostino et al., 2008), whereas β1- and β2-adrenoceptors occur at a roughly 1:4 ratio in canine and equine trachea/bronchus (Barnes et al., 1983; Abraham et al., 2003). However, no quantitative or qualitative estimation of either autonomic receptor from a radioligand binding approach is yet available for human bronchus, due to the limitations of tissue isolation from lobectomy specimens.
As the receptor density, as well as the relative proportions of subtypes, may vary according to species and airway size (Johnson, 1998; Roux et al., 1998), regional quantification of mAChRs and β-adrenoceptors in human airways has been for some time desired for pharmacotherapy and drug development in respiratory disorders. Recently, a radioligand binding method with tissue segments was developed in lieu of the conventional binding method with membrane preparations (Tanaka et al., 2004; Muramatsu et al., 2005). In this method, with no homogenization or fractionation, it is not necessary to consider yield loss of the receptors, and therefore, there is no need to pool tissue samples from several individuals.
Here, for the first time, we applied this radioligand binding assay to human bronchus and lung segments prepared from lobectomy specimens and evaluated the mAChRs and β-adrenoceptors. The results clearly show that subtype-specific regional distribution of both mAChRs and β-adrenoceptors is unique in the human bronchus, in contrast to airways from large animals.
Methods
The present study was performed after getting full informed consent according to the guidelines of the Ethics Committee of the University of Fukui. Human lung was obtained from 19 male and 8 female patients (age range 50–78 years) with lung cancer who had undergone lobectomy or pneumonectomy. The patients were non-smokers and it was confirmed that they had not taken muscarinic or adrenergic drugs for at least two months before their operation. We used normal regions of the lung, confirmed by high-resolution computed tomography before the operations and by macroscopic analysis after isolation. The isolated lung and bronchi with cartilage (segmental bronchus: about 10 mm in outer diameter; subsegmental bronchus: 1 to 4 mm in outer diameter) were cleaned of connective tissue, as shown in Figure 1.
Figure 1.
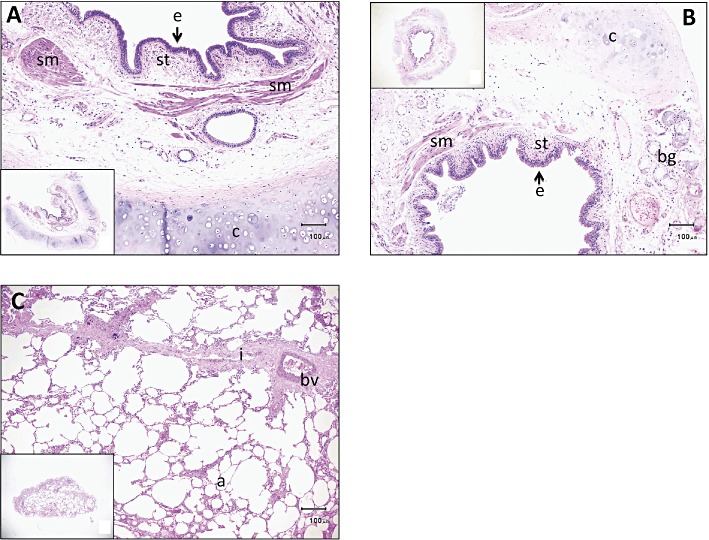
Histological sections of specimens prepared from human segmental (A) and subsegmental (B) bronchi and lung parenchyma (C). Haematoxylin and eosin staining. A part of each specimen was magnified. Insets show the whole specimens. Abbreviations: a, alveolar wall; bg, bronchial gland; bv, blood vessel; c, cartilage; e, epithelium; i, interstitial tissue; sm, smooth muscle; st, subepithelial tissue. Scale bar: 100 µm.
Histological experiments in human bronchus and lung
Specimens prepared from the isolated human bronchus and lung parenchyma were fixed in 10% neutral buffered formalin for 24 h and embedded in paraffin. The sections were stained with haematoxylin and eosin.
Tissue segment binding experiments using [3H]-N-methylscopolamine ([3H]-NMS) and [3H]-CGP-12,177
Radioligand binding experiments with intact tissue segments were conducted to identify mAChRs and β-adrenoceptors. Briefly, the isolated lung and bronchus were cut into small pieces in modified Krebs-Henseleit solution composed of (in mM): 120.7 NaCl, 5.9 KCl, 1.2 MgCl2, 2 CaCl2, 1.2 NaHPO4, 25.5 NaHCO3 and 11.5 glucose (pH 7.4) gassed with 95% O2 and 5% CO2, under a stereoscopic microscope. Usually, 150 or more pieces from lung (piece size: approximately 3 × 5 × 5 mm) and about 30 pieces from transversely cut bronchus (piece size of segmental bronchus: approximately 3–5 × 5 mm, piece size of subsegmental bronchus: approximately 1–4 × 2–4 mm) were prepared from a lobectomy specimen. Each piece was incubated at 4°C in 1 mL of Krebs incubation buffer with [3H]-NMS or [3H]-CGP-12,177 for 24 to 30 h to identify mAChRs or β-adrenoceptors. The composition of the incubation buffer was essentially the same as a modified Krebs-Henseleit solution, except the NaHCO3 concentration was reduced to 10.5 mM to adjust the pH to 7.4 in air. In saturation experiments, 50 to 2000 pM [3H]-NMS or [3H]-CGP-12,177 were used. In competition experiments, 500 pM of each radioligand was used. Binding in the presence of 1 µM atropine or 100 µM bupranolol was defined as non-specific binding of [3H]-NMS or [3H]-CGP-12,177, respectively. After the incubation, the tissue pieces were gently blotted and rinsed by vortexing for 45 s with 1.5 mL incubation buffer at 4°C. Each piece or segment (with cartilage in bronchus) was then blotted and dissolved in 1 mL of 0.3 M NaOH solution. Then, 0.5 mL of the solution was used to estimate the radioactivity, and 10 to 40 µL of the solution was used to measure protein content. As the size of each segment varied between preparations, the radioactivity in each solution was corrected to counts mg-1 protein before PRIZM analysis (Muramatsu et al., 2005). The specific binding was determined by subtracting the non-specific radioactivity bound mg-1 protein from total radioactivity bound mg-1 protein. Experiments were done in duplicate at each concentration of radioligand for saturation experiments, or at each concentration of competing ligand for competition experiments. Radioactivity was counted in a liquid scintillation counter using a scintillation fluid (ULTIMA GOLD™, Packard Bioscience, Groningen, the Netherlands). The protein concentration of each tissue segment in each tube was measured with a Bio-Rad commercial protein assay (Bio-Rad, Hercules, CA, USA).
Homogenate binding experiments using [3H]-NMS and [3H]-CGP-12,177
In order to compare the radioligand-binding capacity between intact tissue segments and homogenates, a part of the lung and bronchial pieces that had been prepared for the tissue segment binding experiments was homogenized in 10 volumes (v w-1) of the modified Krebs-Henseleit solution using a polytron homogenizer at 4°C. The resulting homogenates were directly (without fractionation) incubated with 2000 pM [3H]-NMS or [3H]-CGP-12,177 in 1 mL modified Krebs-Henseleit solution for 5 h at 4°C. Non-specific binding of [3H]-NMS or [3H]-CGP-12,177 was determined in the presence of 1 µM atropine or 100 µM bupranolol, respectively. The assay was terminated by rapid filtration over Whatman GF/C filters presoaked with 0.3% polyethyleneimine using a Brandel cell harvester, and filters were rapidly washed with 5 mL aliquots of ice-cold modified Krebs-Henseleit solution. The resulting filters were dried and the radioactivity retained on the filter paper was measured by liquid scintillation counting. The protein content of the homogenates was determined with a Bio-Rad protein assay.
Functional experiments with human bronchus
The isolated segmental bronchus (approximately 10 mm in outer diameter) was cut transversely, and small strips with cartilage (3–4 mm in width and 7–10 mm in length) were prepared. From the subsegmental bronchus (1–4 mm in outer diameter), ring preparations (2 mm in length) were made. Each preparation was placed at 37°C in an organ bath containing a modified Krebs-Henseleit solution gassed with 95% O2 and 5% CO2, and the tension changes were recorded isometrically. After a 2 h equilibration, the preparations were first exposed to 10 µM carbachol to confirm their reactivity. Thereafter, in the experiments with muscarinic antagonists, concentration–response curves for carbachol were obtained twice at 2 h intervals in each preparation, and the amplitude of the second concentration–response curve was normalized against the maximum amplitude of the contraction in the first concentration–response curve (obtained in the same preparation). Carbachol was applied cumulatively. A muscarinic antagonist was applied 40 min before and during the second concentration–response curve. In parallel with this experiment, a second concentration–response in the absence of antagonist was conducted in other preparations and was used as a time control. In the experiments with β-adrenoceptor antagonists, the preparation was again contracted with 10 µM carbachol 2 h after the first confirmation of tissue reactivity, and at the plateau phase of the carbachol-induced contracture, isoprenaline was applied cumulatively. In these experiments, the antagonist was also applied 40 min before and during the relaxation response. After the response to 100 µM isoprenaline had been recorded, 100 µM papaverine was added in order to detect the maximal relaxation, which was defined as 100% relaxation in each preparation. A time control was obtained from a parallel experiment without antagonist.
Data analysis
Binding data were analysed using PRISM (Version 5.01, Graph Pad Software, La Jolla, CA, USA), as previously described (Anisuzzaman et al., 2011). Briefly, the data from saturation binding studies were fitted by a one-site saturation binding isotherm (Binding-Saturation Equation in PRISM), and the KD values and the binding capacity were then calculated. The abundance of mAChRs and β-adrenoceptors is presented as the maximum binding capacity mg-1 total tissue protein (Bmax). For the competition studies, data were analysed using the binding-competitive equation of PRISM. A two-site model was adopted only when the residual sums of squares were significantly less (P < 0.05) for a two-site fit to the data than for a one-site fit by F-test comparison. To validate the one-site and two-site model, Hill plot analyses were also performed.
In functional studies, antagonist affinity (pKB value) was estimated by a single concentration-ratio method (Furchgott, 1972). In the case of muscarinic antagonism, the concentration-ratio was compared at the 50% level of the maximum contraction in the first concentration–response curve for carbachol. However, in the case of β-adrenoceptor antagonism, the concentration-ratio was compared at the 40% level of the maximum relaxation induced by papaverine, because the maximal relaxation induced by isoprenaline was significantly less than that induced by papaverine in the same preparation.
Data are shown as the means ± SEM of a number of experiments (n). Data were statistically analysed using Student's t-test, when needed. A probability of less than 0.05 was considered significant.
Drug and molecular target nomenclature
The drug and molecular target nomenclature used conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Reagents
The following drugs were used in the present study: [3H]-NMS chloride (specific activity 3.00 TBq mmol-1) and [3H]-(-)-CGP-12,177 (specific activity: 46Ci mmol-1) (GE Health Care, Buckinghamshire, UK); atropine sulfate, carbachol, isoprenaline hydrochloride (Nacalai Tesque, Kyoto, Japan) and pirenzepine (Sigma-Aldrich, St. Louis, MO, USA); AF-DX 116, ICI-118,551 and SR59230A (Tocris Cookson Ltd, Bristol, UK); muscarinic toxin 3 (MT3, Peptide Institute, Osaka, Japan); darifenacin (Ono Pharmaceutical Co. Ltd, Osaka, Japan); andICI-89,406 (Imperial Chemical, Macclesfield, UK). Bupranolol was kindly provided by Schwarz Pharma (Mannheim, Germany).
Results
Histological observations of intact specimens prepared from human bronchus and lung
Histological pictures of intact specimens prepared from human isolated bronchus and lung are shown in Figure 1. In the specimens of segmental bronchus, epithelium, subepithelial tissue including inflammatory cells, smooth muscle and bronchial glands presented with cartilage. Essentially, there was no difference between segmental and subsegmental regions, except that the muscle bundle was larger in segmental bronchus than in subsegmental bronchus (Figure 1A,B). Contamination of lung parenchyma was minor or negligible in the bronchial specimens used in the present study. In the specimens of lung parenchyma, alveolar walls were predominantly present with interstitial tissue and blood vessel (Figure 1C).
[3H]-NMS and [3H]-CGP-12,177 binding to intact segments of human bronchus and lung
Before commencing the binding analysis with tissue segments, we preliminarily compared the binding capacities between homogenates and intact segments. Figure 2 shows specific binding at 2000 pM of [3H]-NMS and [3H]-CGP-12,177 in both preparations from three airway regions (segmental and subsegmental bronchus and lung) of humans. In all three regions, the specific bindings in the homogenates were significantly lower than those in the intact segments. In particular, the decrease in binding was greater for [3H]-NMS-binding (more than 10% reduction) than for [3H]-CGP-12,177-binding sites (20–50% reduction) in the three regions. This preliminary result implies that many airway samples should be pooled in the case of homogenate binding, and that homogenization causes a large loss in yield of airway tissue receptors, similar to other tissues and radioligands (Tanaka et al., 2004; Wang et al., 2011).
Figure 2.
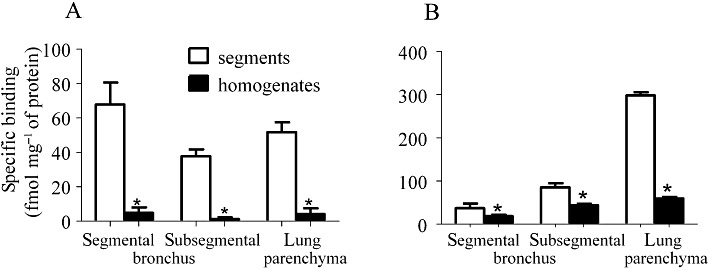
Specific binding of [3H]-NMS (A) and [3H]-CGP-12,177 (B) in the segments and homogenates prepared from human segmental and subsegmental bronchi and lung parenchyma. The binding of both radioligands was examined at 2000 pM. The specific binding was determined by subtracting the amount of [3H]-NMS or [3H]-CGP-12,177 bound in the presence of 1 µM atropine or 100 µM bupranolol, respectively. Mean ± SEM binding data from four individual samples are presented. *Significantly different from the binding in the segments (P < 0.05).
Next, binding experiments with intact segments of two bronchus regions (segmental and subsegmental bronchi) and lung were conducted. Figure 3A–C shows representative saturation curves for [3H]-NMS in the three regions, which were obtained from the same patient (male, 67 years old). [3H]-NMS (50–2000 pM) bound to the tissue segments in a concentration-dependent manner at 4°C for 24 to 30 h incubation. Non-specific binding of 1000 pM [3H]-NMS in the presence of 1 µM atropine was less than 20% of the total binding, indicating a clear estimation of specific binding of [3H]-NMS. The saturation curves fitted to a one-site model, and the calculated binding parameters are listed in Table 1. The KD values for [3H]-NMS were not much different among the three regions, but the mAChR density in the subsegmental bronchus was significantly lower than those in the segmental bronchus and lung parenchyma.
Figure 3.
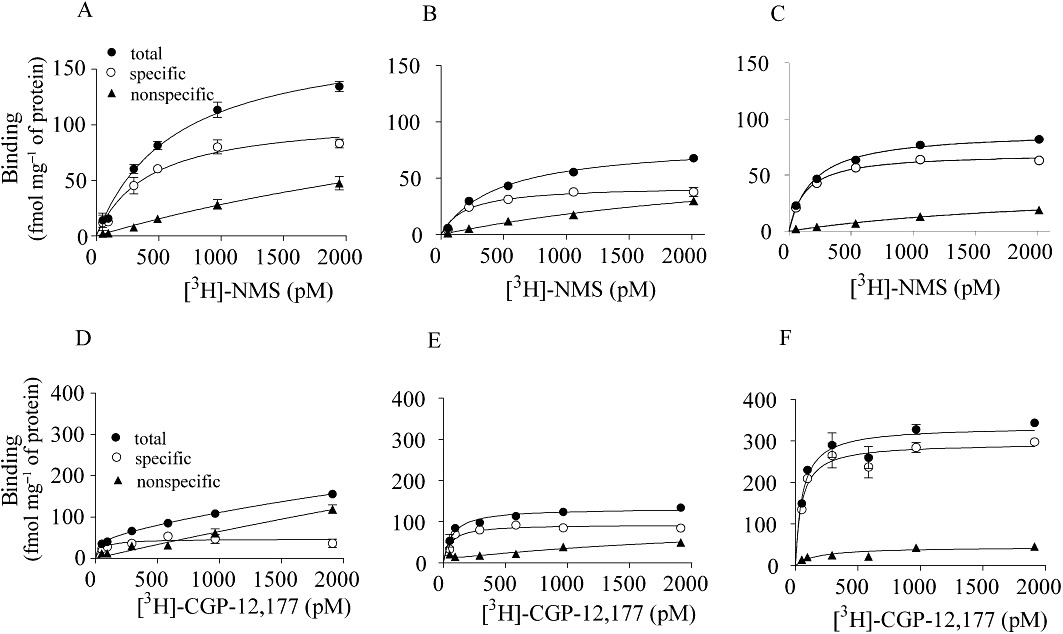
Representative saturation curves of [3H]-NMS and [3H]-CGP-12,177 binding to intact segments of segmental (A,D) and subsegmental (B,E) bronchi and lung parenchyma (C,F) prepared from human lobectomy specimens. The ordinate represents the level of [3H]-NMS or [3H]-CGP-12,177 binding mg-1 tissue protein. Specific binding was determined by subtracting the non-specific binding from the total binding. Each point represents the mean of duplicate determinations in a representative experiment.
Table 1.
Binding parameters of [3H]-NMS and [3H]-CGP-12,177 in human bronchus and lung
| Region of the airway | Bmax (fmol·mg−1 of protein) | KD (pM) | |
|---|---|---|---|
| [3H]-NMS binding sites | Segmental bronchus | 97 ± 11* | 220 ± 28 |
| Subsegmental bronchus | 43 ± 3* | 105 ± 26 | |
| Lung parenchyma | 71 ± 7* | 130 ± 14 | |
| [3H]-CGP-12,177 binding sites | Segmental bronchus | 47 ± 9* | 100 ± 37 |
| Subsegmental bronchus | 90 ± 10* | 81 ± 30 | |
| Lung parenchyma | 320 ± 26* | 98 ± 10 |
Saturation binding experiments with intact tissue segments were carried out at 4°C.
Bmax: binding density mg−1 total protein of the segments.
Data represent means ± SEM of four to five experiments.
Significantly different from the other regions (P < 0.05).
[3H]-CGP-12,177 also bound to the intact segments of the three regions. Although the non-specific binding was slightly higher than that in the [3H]-NMS binding experiment, specific binding was clearly detected (Figure 3D–F). Computer analysis indicated a single set of [3H]-CGP-12,177 binding sites in the three regions (Table 1). The β-adrenoceptor density increased in peripheral tissues without changing the binding affinity for [3H]-CGP-12,177.
Effects of various ligands on [3H]-NMS and [3H]-CGP-12,177 binding sites
The pharmacological properties of mAChRs identified by [3H]-NMS were first examined by three subtype-selective ligands (pirenzepine for M1-subtype, AF-DX 116 for M2 and M4-subtypes, and darifenacin for M3-subtype). Pirenzepine competed with 500 pM [3H]-NMS binding biphasically in lung parenchyma, better fitting a two-site model in computer analysis (Figure 4C). Thus, high affinity sites for pirenzepine (pKi= 8.5) were detected in lung parenchyma (Table 2). In contrast, competition curves for pirenzepine in two regions of the bronchus were monophasic, resulting in low affinity estimates (pKi∼7) (Figure 4A,B). AF-DX 116 showed shallow curves in all three regions, with high and low affinity sites being detected (Figure 4A–C, Table 2). On the other hand, darifenacin competed for [3H]-NMS binding biphasically in the bronchus but not in lung parenchyma. MT 3 (a specific antagonist for the M4-subtype) failed to inhibit [3H]-NMS binding (data not shown).
Figure 4.
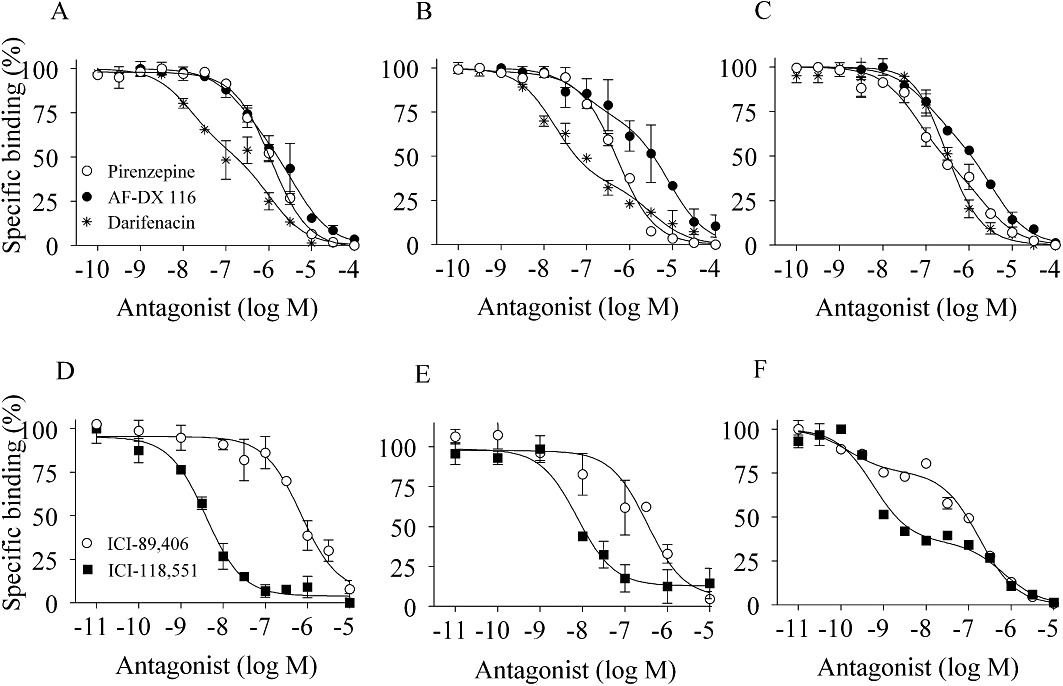
Competition curves for muscarinic antagonists and β-adrenoceptor antagonists at [3H]-NMS (upper panels) or [3H]-CGP-12,177 (lower panels) binding sites in segmental (A,D) and subsegmental bronchi (B,E) and lung parenchyma (C,F) prepared from human lobectomy specimens. The concentration of [3H]-NMS and [3H]-CGP-12,177 used was 500 pM. Muscarinic antagonists: pirenzepine, AF-DX 116 and darifenacin. β-Adrenoceptor antagonists: ICI-89,406 and ICI-118,551. Each point represents the mean of duplicate determinations from four to five representative experiments.
Table 2.
Pharmacological profiles of [3H]-NMS and [3H]-CGP12,177 binding sites in human bronchus and lung
| [3H]-NMS binding sites | [3H]-CGP-12,177 binding sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pirenzepine (M1- subtype) | AF-DX 116 (M2- subtype) | Darifenacin (M3- subtype) | ICI-89,406 (β1- subtype) | ICI-118,551 (β2- subtype) | ||||||
| Region of the airway | pKihigh (%) | pKilow | pKihigh (%) | pKilow | pKihigh (%) | pKilow | pKihigh (%) | pKilow | pKihigh (%) | pKilow |
| Segmental bronchus | 7.4 ± 0.2 | 7.3 ± 0.1 (44 ± 6%) | 5.8 ± 0.2 | 8.2 ± 0.1 (55 ± 5%) | 6.6 ± 0.1 | 6.8 ± 0.2 | 8.3 ± 0.5 | |||
| Subsegmental bronchus | 6.9 ± 0.2 | 7.1 ± 0.2 (38 ± 3%) | 5.8 ± 0.2 | 8.6 ± 0.3 (62 ± 5%) | 6.8 ± 0.1 | 6.2 ± 0.8 | 8.8 ± 0.2 | |||
| Lung parenchyma | 8.5 ± 0.3 (53 ± 4%) | 5.9 ± 0.1 | 7.1 ± 0.2 (37 ± 8%) | 5.2 ± 0.2 | 7.2 ± 0.1 | 10.2 ± 0.2 (35 ± 2%) | 7.1 ± 0.1 | 9.2 ± 0.2 (66 ± 1%) | 6.8 ± 0.2 | |
Competition binding experiments with intact tissue segments were carried out at 4°C. The concentrations of [3H]-NMS and [3H]-CGP-12,177 used were 500 pM.
pKihigh and pKilow: negative logarithm of the equilibrium constants (pKi) at high and low-affinity sites for tested drugs. (%): percentage of high-affinity sites.
Data represent means ± SEM of 4–5 experiments.
Next, the pharmacological profiles of β-adrenoceptors were also examined. In lung parenchyma, ICI-89,406 (β1-selective antagonist) and ICI-118,551 (β2-selective antagonist) competed for 500 pM [3H]-CGP-12,177 binding biphasically, whereas both antagonists showed a simple competition with low affinity for ICI-89,406 and with high affinity for ICI-118,551 in bronchus preparations (Figure 4, Table 2). SR59230A (β3-selective antagonist) showed a single pKi (approximately 6) in the three regions (data not shown).
From the total density of [3H]-NMS and [3H]-CGP-12,177 binding sites (Table 1), and the proportion showing different affinities for competitors (Table 2), regional populations of each subtype of mAChRs and β-adrenoceptors were calculated and are shown in Figure 5A and B, respectively.
Figure 5.
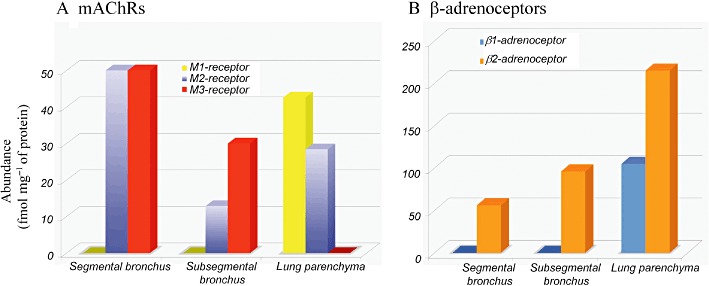
Distribution map of mAChR subtypes (A) and β-adrenoceptor subtypes (B) in human segmental and subsegmental bronchi and lung parenchyma. The population of each subtype was extrapolated from Bmax values (Table 1) and the subtype ratio (Table 2) in each airway region. Note the different ordinate scales between (A) and (B).
Functional responses to carbachol and isoprenaline in human bronchus
Carbachol produced concentration-dependent contractions in the segmental and subsegmental bronchus (Figure 6A,B). The EC50 values for carbachol were 0.42 ± 0.05 µM and 0.50 ± 0.11 µM in the segmental and sebsegmental bronchus, respectively, with no significant difference between the two regions. Darifenacin at 1 µM shifted the concentration–response curves to the right, resulting in high pKB estimates (Table 3). The same concentration (1 µM) of pirenzepine slightly competed with the carbachol response, whereas inhibition by 1 µM AF-DX 116 was negligible or minor in both bronchial regions. The antagonist affinities estimated from the functional assay were close to the binding affinities (Tables 2 and 3).
Figure 6.
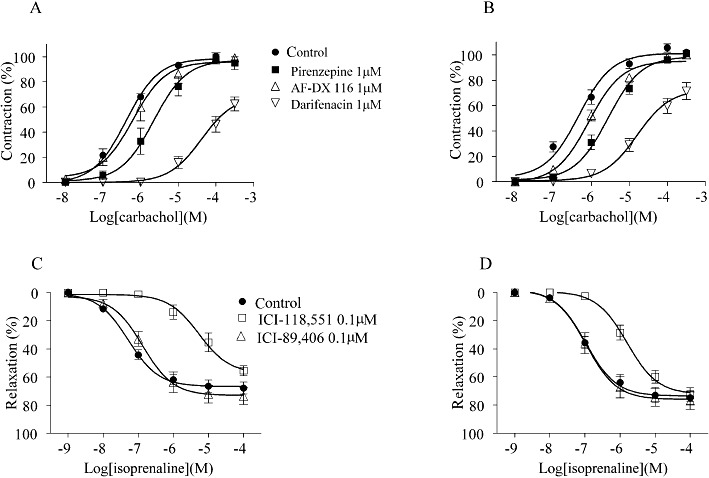
The concentration–response curves for carbachol (A,B) and for isoprenaline (C,D) in the absence or presence of antagonists in human segmental (left) and subsegmental bronchi (right). Upper panels: as described in the Methods, the maximum contraction in the first concentration–response curve for carbachol was taken as 100% in each preparation, and the antagonist was applied for 40 min before and during the second concentration–response curve in the same preparation. Lower panels: papaverine (100 µM)-induced relaxation was taken as 100%. The β-adrenoceptor antagonist was applied for 40 min before and during the concentration–response curve for isoprenaline in each preparation. Control means time control without antagonist treatment, which was carried out in parallel with antagonist experiments. Means ± SEM of four to six preparations isolated from four individuals are presented.
Table 3.
Functional affinities of various muscarinic receptor antagonists and β-adrenoceptor antagonists in human bronchus
| pKB | ||
|---|---|---|
| Segmental bronchus | Subsegmental bronchus | |
| Carbachol-induced contraction | ||
| Pirenzepine (1 µM) | 6.5 ± 0.4 | 6.6 ± 0.2 |
| AF-DX 116 (1 µM) | No inhibition | 6.1 ± 0.2 |
| Darifenacin (1 µM) | 8.4 ± 0.2 | 8.3 ± 0.1 |
| Isoprenaline-induced relaxation | ||
| ICI-89, 406 (0.1 µM) | No inhibition | No inhibition |
| ICI-118, 551 (0.1 µM) | 9.1 ± 0.3 | 8.3 ± 0.3 |
pKB values were calculated from the competition curves in the absence or presence of a single concentration of antagonist described in this table, according to concentration-ratio method (Furchgott, 1972).
Results represent mean ± SEM of 4–6 experiments.
Isoprenaline relaxed the bronchial strips or ring preparations that had been contracted by carbachol. The relaxation was concentration-dependent (EC50= 0.26 ± 0.10 µM in segmental bronchus and 0.48 ± 0.13 µM in subsegmental bronchus), but the maximum response was less than the relaxation induced by papaverine for the same preparation (Figure 6C,D). The relaxation responses to isoprenaline were inhibited by 0.1 µM ICI-118,551, but not by 0.1 µM ICI-89,406 (Table 3).
Discussion
In the present study, mAChRs and β-adrenoceptors in human airways were quantified by radioligand binding assays with airway segments for the first time. Regardless of the presence or absence of cartilage, the airway segments were directly incubated with radioligands in physiological solution. As epithelial and muscle layers were not scraped from the cartilage, and in contrast to a marked reduction of [3H]-NMS and [3H]-CGP-12,177 binding in homogenates (Figure 2), the present segment binding assay is less prone to loss of receptors and to modification of the natural environment/conformation of the receptors (Wang et al., 2011). However, the airway segments used in the present study were heterogeneous tissues that included various types of cells, as shown in Figure 1. Thus, it is likely that the quantification and qualification obtained here reflect the inherent distribution/property of both mAChRs and β-adrenoceptors in different regions of human airways, although the cell types expressing each receptor cannot be identified, unlike with autoradiography (Muramatsu et al., 2005; Anisuzzaman et al., 2011).
Regional quantification of mAChR subtypes and β-adrenoceptor subtypes is shown in Figure 5. As a whole, the distribution maps clearly show that the total density of each receptor and their subtype ratio varied according to airway size and region. The mAChRs were more dense in the larger than in the small bronchi, whereas the β-adrenoceptors were increased in peripheral tissues: segmental bronchus < subsegmental bronchus < lung parenchyma. Thus, the total densities of β-adrenoceptors were approximately two- and fourfold higher than those of mAChRs in subsegmental bronchus and lung parenchyma, respectively, indicating that distal airways and lungs may be predominantly regulated by β-adrenoceptor activity (Table 1, Figure 5).
Among the subtypes of mAChRs, the M3-subtype is dominant in human bronchus. This result is in contrast to the major distribution of the M2 subtype (70 to 80% of the total mAChRs) reported in bovine, horse and porcine bronchi (Roffel et al., 1988; Eglen et al., 1996; Abraham et al., 2003; 2007; D'Agostino et al., 2008). Autoradiography studies have demonstrated abundant distribution of mAChRs in the smooth muscle of human bronchus, although mAChRs are also localized to airway epithelium and submucosal glands (Mak and Barnes, 1990; Roux et al., 1998; Barnes, 2004). The high density of the M3 subtype in human bronchus supports the hypothesis that human airway smooth muscle is mainly contracted through the M3 subtype (Roffel et al., 1990; Eglen et al., 1996; Barnes, 2004). In the present study, carbachol-induced contraction in isolated human bronchus was also more effectively inhibited by darifenacin (M3-antagonist) than by pirenzepine (M1-antagonist) or AF-DX 116 (M2-antagonist). A comparable amount of the M2 subtype in the segmental bronchus further suggests that this subtype may be particularly important in maintaining sustained contraction in large airways (Haddad el and Rousell, 1998; Ehlert et al., 1999).
In contrast to the bronchi, both M1 and M2 subtypes are predominantly expressed in human lungs. A lack of the M3 subtype indicates that this subtype is not expressed in human lungs and also suggests that possible contamination of the bronchioles was negligible in the lung segments prepared in the present study. Indeed, no contractile response to carbachol was observed in human lung strips (I. Muramatsu, unpubl. obs.). The histological study showed that alveolar walls were present in the lung parenchyma specimens (Figure 1C). The presence of the M1 subtype was previously reported in conventional binding assays with membrane preparations of human lungs (Gies et al., 1989; Roux et al., 1998; Gosens et al., 2006). Although the physiological roles of mAChRs in lungs are not well known, it is interesting to note that an anticholinergic drug, tiotropium bromide, markedly reduces the functional decline of COPD patients (Anzueto et al., 2005). As tiotropium bromide is a ‘kinetically selective’ antagonist for M1 and M3 subtypes, it is possible that, in addition to its inhibitory actions on bronchoconstriction and bronchial mucus secretion, its antagonist activity at the M1 subtype in lung parenchyma may be involved in its clinical effect in COPD patients, suggesting that the M1 subtype might be an additional target for COPD or pulmonary oedema therapy.
Topographical differences were also observed in the distribution of β-adrenoceptor subtypes (Figure 5B). The β2-adrenoceptor obviously increases in the distal regions in human respiratory tissues. The exclusive existence of β2-adrenoceptors in human bronchus is unique, because the β1-adrenoceptor was shown to co-exist with the β2-adrenoceptor in both the canine and equine bronchi (Barnes et al., 1983; Abraham et al., 2003). Bronchodilatation is mediated through the β2-adrenoceptor (present study, and Shore and Moore, 2003; Barnes, 2004). Thus, the present results strongly suggest that in human bronchus, β2-adrenoceptor activation would be a powerful treatment for asthma and COPD, where small airways are involved (Barnes, 2004; Broadley, 2006). Further, the high density of β2-adrenoceptors in distal bronchi may either serve as a receptor reserve or cause a lack of functional desensitization upon repeated application of sympathomimetic bronchodilators (Giembycz, 2009). Autoradiographic studies of human airways have demonstrated that β2-adrenoceptors are widely distributed, occurring not only in airway smooth muscle, but also on other cells, such as epithelial cells and mast cells (Johnson, 1998; Barnes, 2004).
In human lungs, β2-adrenoceptors are known to occur predominantly in alveolar cells and to regulate fluid clearance (Mutlu and Factor, 2008). Like previous membrane binding assays with lungs from many species, including humans (Roffel et al., 1990; Abraham et al., 2003; Broadley, 2006), the present segment binding study also demonstrated that the human lung parenchyma expresses abundant β1- and β2-adrenoceptors, roughly in a 1:2 ratio. Predominant and wide distributions of β2-adrenoceptors in human lungs have been reported in autoradiographic mapping and in situ hybridization studies (Carstairs et al., 1985; Hamid et al., 1991). The minor but significant occurrence of the β1-subtype in lungs suggests that this subtype might have a compensatory function under pathophysiological conditions such as COPD, asthma or pulmonary oedema. Given this, it is interesting to note the change of function β-adrenoceptors in remodelling of the heart, where the β2-subtype can work alternatively as a main receptor when the function of the β1-subtype declines in congestive heart failure (Nikolaev et al., 2010). In human lungs and in contrast to the heart, β2-adrenoceptors are usually dominant, but it is possible that co-existing β1-adrenoceptors are also involved in the remodelling of the lung in COPD patients. This possibility should be explored in future studies.
Another interesting finding in the present study was the presence of a greater number of β-adrenoceptors in the lung as compared with the bronchus (Figure 5B). As excess receptors are known not only to act as physiological receptors, but also to serve to buffer large amounts of transmitters or drugs (Fukuroda et al., 1994; Carpenter et al., 2003), the abundance of β-adrenoceptors in the lung may also function to bind excess amounts of inhaled sympathomimetic bronchodilators and then protect against systemic side effects such as tachycardia.
In summary, we quantified mAChRs and β-adrenoceptors in human bronchus and lung by a tissue segment binding approach and constructed a distribution map of their receptor subtypes. The unique distribution of both autonomic receptors in human airways is obviously important for future pharmacotherapy and new drug development for respiratory disorders.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS), by a grant from the University of Fukui, and by a grant from the Smoking Research Foundation of Japan.
Glossary
- AF-DX 116
11-({2-[(diethylamino) methyl]-1-piperdinyl} acetyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-one
- COPD
chronic obstructive pulmonary disease
- ICI-118,551
3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol
- ICI-89,406
N-{2-[3-(2-cyanophenoxy)-2-hydroxypropylamino]ethyl}-N′-phenylurea
- mAChR
muscarinic acetylcholine receptor
- NMS
N-methylscopolamine
Conflict of interest
The authors have no conflict of interest.
References
- Abraham G, Kottke C, Dhein S, Ungemach FR. Pharmacological and biochemical characterization of the β-adrenergic signal transduction pathway in different segments of the respiratory tract. Biochem Pharmacol. 2003;66:1067–1081. doi: 10.1016/s0006-2952(03)00460-x. [DOI] [PubMed] [Google Scholar]
- Abraham G, Kottke C, Ammer H, Dhein S, Ungemach FR. Segment-dependent expression of muscarinic acetylcholine receptors and G-protein coupling in the equine respiratory tract. Vet Res Commun. 2007;31:207–226. doi: 10.1007/s11259-006-3396-z. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC). 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisuzzaman AS, Nishimune A, Yoshiki H, Uwada J, Muramatsu I. Influence of tissue integrity on pharmacological phenotypes of muscarinic acetylcholine receptors in the rat cerebral cortex. J Pharmacol Exp Ther. 2011;339:186–193. doi: 10.1124/jpet.111.182857. [DOI] [PubMed] [Google Scholar]
- Anzueto A, Tashkin D, Menjoge S, Kesten S. One-year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulm Pharmacol Ther. 2005;18:75–81. doi: 10.1016/j.pupt.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Neural control of human airways in health and disease. Am Rev Respir Dis. 1986;134:1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Distribution of receptor targets in the lung. Proc Am Thorac Soc. 2004;1:345–351. doi: 10.1513/pats.200409-045MS. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J. 2007;29:587–595. doi: 10.1183/09031936.00080306. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Nadel JA, Skoogh BE, Roberts JM. Characterization of beta adrenoceptor subtypes in canine airway smooth muscle by radioligand binding and physiological responses. J Pharmacol Exp Ther. 1983;225:456–461. [PubMed] [Google Scholar]
- Broadley KJ. β-adrenoceptor responses of the airways: for better or worse? Eur J Pharmacol. 2006;533:15–27. doi: 10.1016/j.ejphar.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Carpenter T, Schomberg S, Steudel W, Ozimek J, Colvin K, Stenmark K, et al. Endothelin B receptor deficiency predisposes to pulmonary edema formation via increased lung vascular endothelial cell growth factor expression. Circ Res. 2003;93:456–463. doi: 10.1161/01.RES.0000090994.15442.42. [DOI] [PubMed] [Google Scholar]
- Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of β-adrenoceptor subtypes in human lung. Am Rev Respir Dis. 1985;132:541–547. doi: 10.1164/arrd.1985.132.3.541. [DOI] [PubMed] [Google Scholar]
- D'Agostino G, Condino AM, Gioglio L, Zonta F, Tonini M, Barbieri A. Isolated porcine bronchi provide a reliable model for development of bronchodilator anti-muscarinic agents for human use. Br J Pharmacol. 2008;154:1611–1618. doi: 10.1038/bjp.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- Ehlert FJ, Sawyer GW, Esqueda EE. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999;64:387–394. doi: 10.1016/s0024-3205(98)00584-0. [DOI] [PubMed] [Google Scholar]
- Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- Furchgott RF. The classification on adrenoceptors (adrenergic receptors): an evaluation from the standpoint of receptor theory. In: Blaschko H, Muscholl E, editors. Handbuch der Experimentellen Pharmacology. Vol. 3. New York: Springer; 1972. pp. 283–335. [Google Scholar]
- Giembycz MA. An estimation of β 2-adrenoceptor reserve on human bronchial smooth muscle for some sympathomimetic bronchodilators. Br J Pharmacol. 2009;158:287–299. doi: 10.1111/j.1476-5381.2009.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gies JP, Bertrand C, Vanderheyden P, Waeldele F, Dumont P, Pauli G, et al. Characterization of muscarinic receptors in human, guinea pig and rat lung. J Pharmacol Exp Ther. 1989;250:309–315. [PubMed] [Google Scholar]
- Goldie RG. Receptors in asthmatic airways. Am Rev Respir Dis. 1990;141:S151–S156. doi: 10.1164/ajrccm/141.3_Pt_2.S151. [DOI] [PubMed] [Google Scholar]
- Gosens R, Zaagsma J, Grootte Bromhaar M, Nelemans A, Meurs H. Acetylcholine: a novel regulator of airway smooth muscle remodelling? Eur J Pharmacol. 2004;500:193–201. doi: 10.1016/j.ejphar.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311:421–425. doi: 10.1056/NEJM198408163110701. [DOI] [PubMed] [Google Scholar]
- Haddad el B, Rousell J. Regulation of the expression and function of the M2 muscarinic receptor. Trends Pharmacol Sci. 1998;19:322–327. doi: 10.1016/s0165-6147(98)01231-0. [DOI] [PubMed] [Google Scholar]
- Hamid QA, Mak JC, Sheppard MN, Corrin B, Venter JC, Barnes PJ. Localization of β2-adrenoceptor messenger RNA in human and rat lung using in situ hybridization: correlation with receptor autoradiography. Eur J Pharmacol. 1991;206:133–138. doi: 10.1016/0922-4106(91)90021-9. [DOI] [PubMed] [Google Scholar]
- Hislop AA, Mak JC, Reader JA, Barnes PJ, Haworth SG. Muscarinic receptor subtypes in the porcine lung during postnatal development. Eur J Pharmacol. 1998;359:211–221. doi: 10.1016/s0014-2999(98)00585-8. [DOI] [PubMed] [Google Scholar]
- Johnson M. Beta-Agonists in the Treatment of Asthma. In: Costello JF, Mann RD, editors. Mechanisms of Action of β-Adrenoceptor Agonists. Carnforth: Parthenon; 1992. pp. 27–42. [Google Scholar]
- Johnson M. The β-adrenoceptor. Am J Respir Crit Care Med. 1998;158:S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Mak JC, Barnes PJ. Autoradiographic visualization of muscarinic receptor subtypes in human and guinea pig lung. Am Rev Respir Dis. 1990;141:1559–1568. doi: 10.1164/ajrccm/141.6.1559. [DOI] [PubMed] [Google Scholar]
- Mak JC, Baraniuk JN, Barnes PJ. Localization of muscarinic receptor subtype mRNAs in human lung. Am J Respir Cell Mol Biol. 1992;7:344–348. doi: 10.1165/ajrcmb/7.3.344. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Tanaka T, Suzuki F, Li Z, Hiraizumi-Hiraoka Y, Anisuzzaman AS, et al. Quantifying receptor properties: the tissue segment binding method – a powerful tool for the pharmacome analysis of native receptors. J Pharmacol Sci. 2005;98:331–339. doi: 10.1254/jphs.cpj05001x. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Factor P. Alveolar epithelial β2-adrenergic receptors. Am J Respir Cell Mol Biol. 2008;38:127–134. doi: 10.1165/rcmb.2007-0198TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, et al. β2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- Roffel AF, Elzinga CR, Van Amsterdam RG, De Zeeuw RA, Zaagsma J. Muscarinic M2 receptors in bovine tracheal smooth muscle: discrepancies between binding and function. Eur J Pharmacol. 1988;153:73–82. doi: 10.1016/0014-2999(88)90589-4. [DOI] [PubMed] [Google Scholar]
- Roffel AF, Elzinga CR, Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm Pharmacol. 1990;3:47–51. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- Roux E, Molimard M, Savineau JP, Marthan R. Muscarinic stimulation of airway smooth muscle cells. Gen Pharmacol. 1998;31:349–356. doi: 10.1016/s0306-3623(98)00007-x. [DOI] [PubMed] [Google Scholar]
- Shore SA, Moore PE. Regulation of β-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol. 2003;137:179–195. doi: 10.1016/s1569-9048(03)00146-0. [DOI] [PubMed] [Google Scholar]
- Spina D, Rigby PJ, Paterson JW, Goldie RG. Autoradiographic localization of β-adrenoceptors in asthmatic human lung. Am Rev Respir Dis. 1989;140:1410–1415. doi: 10.1164/ajrccm/140.5.1410. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Zhang L, Suzuki F, Muramatsu I. Alpha-1 adrenoceptors: evaluation of receptor subtype-binding kinetics in intact arterial tissues and comparison with membrane binding. Br J Pharmacol. 2004;141:468–476. doi: 10.1038/sj.bjp.0705627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Yoshiki H, Anisuzzaman ASM, Uwada J, Nishimune A, Lee KS, et al. Re-evaluation of nicotinic acetylcholine receptors in rat brain by a tissue-segment binding assay. Front Pharmacol. 2011;2:65. doi: 10.3389/fphar.2011.00065. doi: 10.3389/fphar.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler IK, Kirkpatrick CJ. The Non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther. 2001;14:423–434. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]


