Abstract
BACKGROUND AND PURPOSE
Endocannabinoid systems are strongly implicated in the physiological control of appetite and eating behaviour, with cannabinoid CB1 receptor agonists and antagonists, respectively, increasing or decreasing food intake. This study examined the acute actions of the putative endocannabinoid noladin ether on food intake and eating motivation, assessing how it affects the amount of work expended by animals to obtain food.
EXPERIMENTAL APPROACH
Non-deprived male rats were injected systemically with noladin ether to assess its acute effects on ad libitum feeding of a standard laboratory diet. Additionally, the effects of noladin on lever pressing for palatable food were determined using a progressive ratio (PR) operant paradigm.
KEY RESULTS
Noladin dose dependently increased 2 h food intake, with a significant effect over 1 h after a dose of 0.5 mg·kg−1. In the PR test, this hyperphagic dose of noladin ether promoted sustained high rates of responding and significantly increased the total number of lever presses and break-point. These latter effects were prevented by pretreatment with 1.0 mg·kg−1 of the selective CB1 antagonist surinabant (SR147778), that alone had no effect on responding.
CONCLUSIONS AND IMPLICATIONS
This is the first report of hyperphagia induced by acute noladin administration, and the first description of behavioural actions in rats. Consistent with prevailing notions about the role of endocannabinoids in appetite, a hyperphagic dose of noladin markedly increased efforts expended by animals to obtain food. Thus, noladin exerts a specific action on eating motivation; possibly promoting eating by increasing the incentive value of food.
Keywords: progressive ratio, break-point, appetite, wanting, cannabinoid, feeding, hyperphagia, orexigen, surinabant, obesity
Introduction
Since the discovery of the endocannabinoid-cannabinoid receptor system, there has been much interest in its role in behavioural and motivational processes. In particular, there is substantial evidence for involvement of the endocannabinoids in the control of appetite and eating (see Kirkham and Williams, 2004; Di Marzo and Matias, 2005; Fride et al., 2005; Kirkham, 2005; 2009; Mechoulam et al., 2006; Kirkham and Rogers, 2010). Thus, in animal models, both exogenous and endogenous cannabinoid CB1 receptor agonists have been shown to induce hyperphagia (Williams et al., 1998; Williams and Kirkham, 1999; 2002a; Jamshidi and Taylor, 2001; Kirkham et al., 2002; Verty et al., 2005; Soria-Gómez et al., 2007; receptor nomenclature follows Alexander et al., 2011). By contrast, CB1 antagonists can exert an anorectic action, suppressing food intake and reducing body weight (Arnone et al., 1997; Colombo et al., 1998; Simiand et al., 1998; Rinaldi-Carmona et al., 2004). In addition, it is well established that intoxication with Cannabis sativa or its extracts promotes food craving and hyperphagia in humans (Kirkham and Williams, 2001a, b).
A variety of data indicate that stimulation of CB1 receptors specifically promotes food seeking or appetitive components of feeding motivation. For example, the latency to eat of spontaneously feeding animals is reduced by administration of both exogenous and endogenous cannabinoid receptor agonists; even well-satiated animals will quickly resume eating after agonist treatment (Williams and Kirkham, 2002b; Farrimond et al., 2010). Additionally, rats have been shown to work harder to obtain food or fluids after administration of CB1 receptor agonists. In an early demonstration of this action, Gallate and colleagues employed a progressive ratio (PR) paradigm to explore cannabinoid effects on responding for beer or sucrose solutions (Gallate et al., 1999). In the PR procedure, a progressively increasing number of responses must be made for each successive allocation of ingesta (Hodos, 1961). Gallate et al. (1999) found that break-point, the response requirement at which responding ceases (as defined by a fixed criterion), was dose-dependently increased by a synthetic cannabinoid, CP 55,940. This increased motivation to respond for ingesta was reversed by the selective cannabinoid CB1 receptor antagonist rimonabant (SR141716); while in a separate study, the antagonist alone reliably suppressed responding and reduced break-point (Gallate and McGregor, 1999). These effects on PR have also been replicated in animals lever pressing for food, with Δ9-tetrahydrocannabinol (THC) and rimonabant reported to respectively increase or reduce break-points (Higgs et al., 2005; Solinas and Goldberg, 2005). Further, CB1 receptor −/− mice have reduced sensitivity to the motivating properties of food, exhibiting reduced rates of responding for food and lower break points than wild-type mice (Sanchis-Segura et al., 2004).
In comparison to the large database on the effects of CB1 receptor antagonists on food intake and body weight, there is little detailed information on the consequences of endocannabinoid treatments on eating motivation. Current knowledge is primarily derived from a few reports on the actions of the prototypical endogenous CB1 receptor ligands, anandamide and 2-arachidonoylglycerol (2-AG) (Williams and Kirkham, 1999; Hao et al., 2000; Kirkham et al., 2002; Mahler et al., 2007; Soria-Gómez et al., 2007; Di Patrizio and Simansky, 2008). Unfortunately, studies of endocannabinoid effects on appetite have been largely confined to simple food intake measures. However, confirming the motivational actions of THC and CP 55,940, one study has shown that systemically administered 2-AG can increase the rate of responding and break-point in gestationally undernourished rats responding for sucrose pellets (Wakley and Rasmussen, 2009). Additionally, Touriño et al. (2010) have reported that mice deficient in fatty acid amide hydrolase (FAAH−/−), the principal degrading enzyme for anandamide, display elevated levels of responding for food under PR schedules compared with wild-type littermates. That these knockout mice have elevated levels of anandamide further supports the involvement of the endocannabinoid system in the motivation to eat.
Of the putative endocannabinoids, noladin ether (2-arachidonylglyceryl ether) is of particular interest (Hanus et al., 2001). Although debate continues as to whether noladin ether is a naturally occurring endogenous neuronal mediator in mammalian nervous systems (Fezza et al., 2002; Oka et al., 2003; Richardson et al., 2007; Páldyováet al., 2008), it is a metabolically stable agonist of CB1 receptors (Steffens et al., 2005) and so might be expected to affect appetite. Indeed, the compound has previously been reported to promote food intake with sub-chronic, low-dose, systemic administration in food-restricted mice (Avraham et al., 2005). However, so far, no more direct observations of the actions of noladin ether on feeding, or on appetite-related motivational indices, have been reported. Here, we present the first direct measures of the acute hyperphagic actions of noladin ether in ad libitum fed rats; report for the first time its stimulation of appetitive processes as measured using a PR analysis; and provide evidence to support the mediation of these actions by cannabinoid CB1 receptors.
Methods
Animals
All animal care and experimental procedures adhered to the guidelines of the United Kingdom Animals (Scientific Procedures) Act, 1986. Adult, male Lister Hooded rats (Harlan, Kent, UK), weighing approximately 250–300 g at the beginning of the experiments, were used throughout. Animals were individually housed and maintained at a temperature of 21 ± 2°C and 60 ± 5% humidity, under a 12:12 h light-dark cycle (lights on at 08:00), with all behavioural testing commencing from 1 h after lights on. All animals had ad libitum access to pelleted food (C.R.M., Special Diet Services, Witham, Essex, UK) and water at all times, unless otherwise stated.
Apparatus
PR testing was conducted in sound-proofed operant chambers controlled by MedPC software (Med Associates Inc., St Albans, VT), each of which had a grid floor, house light, two response levers (both active), a trough into which food pellets were delivered and a delivery light which was illuminated to indicate when food was available.
Experimental procedure
Experiment 1: Acute effects on short-term food intake of systemic noladin ether
Treatment with noladin ether began after habituation to handling and test procedures over 4 successive days. Animals (n= 10) were tested following injection of vehicle or noladin ether (0.5, 1.0 and 2.0 mg·kg−1), administered according to a counterbalanced schedule, with at least 48 h separating successive treatments. In the absence of any prior reports of the behavioural actions of noladin ether in rats, these doses were chosen on a molar basis to match the active, hyperphagic dose range previously reported for systemic administration of the related agents anandamide and 2-AG in this species (Williams and Kirkham, 1999; Higgs et al., 2003; Wakley and Rasmussen, 2009). On test days animals were injected i.p. at 09:00 and returned to their home cage for 20 min, during which period, food was withheld. Animals were then placed in individual test cages, identical to the home cages, containing a pre-weighed quantity of food (chow). Food intake was measured after 1 and 2 h, with appropriate correction for spillage. Intake data for each interval were analysed by one-way anova followed by Dunnett's multiple comparisons tests. Additionally, the behaviour of a single animal was video-recorded under each treatment for later observational analysis, using a 1 min time sampling technique, to obtain a preliminary indication of the principal adjustments to feeding and particularly to detect the occurrence of any non-specific effects of the drug.
Experiment 2: Effects of noladin ether and surinabant on PR responding for food
A separate group of 15 rats were habituated to handling and injection procedures, as well as to the interior of the operant chamber, for 4 days. When operant training began, animals were restricted to 15 g each of chow overnight for 3 successive days to promote responding. Over this period, rats learned to respond for food under a fixed-ratio 1 schedule (FR1), where each lever press produced immediate delivery of a single, palatable 45 mg banana-flavoured ‘Formula P’ sucrose pellet (Research Diets Inc., New Brunswick, NJ). Subsequent to each training session, ad libitum food access was restored to animals in their home cages. Training continued for a further 4 days under a FR5 schedule.
Once responding consistently over each session on the FR5 training schedule, animals were given daily 1 h sessions under the PR test schedule. The schedule required an increasing number of responses for the delivery of each successive food pellet. A rapid exponential progression in the response requirement was determined using the equation 25n + 0.5e(n0.56). Rounded to the nearest integer, this produced the following response ratios for successive pellet deliveries: 1, 26, 52, 78, 105, 133, 164, 200, 244, 302, 385, 512, 714. Training on the PR schedule continued until stable baselines were obtained (i.e. when total responses for each individual varied by less than ±5% over three successive sessions).
Drug testing began using a repeated measures design, with all animals receiving each treatment according to a counterbalanced schedule. Animals were injected i.p and returned to their home cage (with the food removed) for 20 min before being placed in the operant chamber. Each test session lasted 1 h, with each animal being tested at the same time on each test day. The treatments were: vehicle-vehicle, vehicle-noladin, vehicle-surinabant and noladin-surinabant. At least 48 h separated consecutive treatments. On the intervening days, animals were run again under the PR schedule to ensure a return to baseline levels of responding.
A single dose of 0.5 mg·kg−1 noladin ether was selected for testing on the basis of the results of Experiment 1, indicating that this was an effective hyperphagic dose. Surinabant is a high affinity, specific CB1 receptor antagonist, with a pharmacological and behavioural profile that is similar to that of its structural analogue, rimonabant (Rinaldi-Carmona et al., 2004; Doggrell, 2005). Surinabant was administered at 1.0 mg·kg−1, chosen on the basis that this dose was previously reported to be sub-threshold for anorectic effects in rats, having no significant effect on spontaneous feeding in non-deprived or fasted animals, nor on consumption of a palatable sucrose solution (Rinaldi-Carmona et al., 2004). Higher doses of the drug have been reported to reliably suppress food intake after acute administration (Gessa et al., 2005). Additionally, this dose of surinabant was shown in our own pilot experiments to be devoid of significant effects on food intake under the test conditions described for Experiment 1 (doses of 0.05, 0.1, 0.5 and 1.0 mg·kg−1 failed to exert any reliable effect on 1 h chow intake; intake ranged between a maximum of 6.2 ± 0.6 g after vehicle and a minimum of 5.0 ± 0.5 g after surinabant; F4,36= 0.714, NS).
Data for the total number of responses, break-point (last ratio completed within the 1 h test), etc. were analysed by one-way anova followed by Dunnett's multiple comparisons tests. Treatment and time effects on responding over the course of the test were assessed by two-way anova.
Materials
Noladin ether (2-[(5Z,8Z,11Z,14Z)-eicosatetraenyloxy]-1,3-propanediol; Tocris Bioscience, Bristol, UK) and surinabant (SR147778; [5-(4-bromophenyl)-1-(2,4-dichloro-phenyl)-4-ethyl-N-(1-piperidinyl)-1H-pyrazole-3-carboxamide]; Sanofi-Aventis, Montpelier, France) were suspended by sonication in 0.9% saline and injected i.p. in a volume of 1 mL·kg−1.
Results
Acute effects of noladin ether on short-term food intake
As Figure 1 illustrates, testing during the light phase resulted in low baseline food intake, evenly distributed across the 2 h test period. Noladin ether administration produced a dose-related increase in total 2 h chow intake (F3,27= 3.679, P= 0.024). This overall increase was predominantly attributable to an elevation of intake during the first hour of testing (F3,27= 4.011, P= 0.0175). Both 0.5 and 1.0 mg·kg−1 produced a greater than twofold increase in chow consumption during this interval, with the most robust hyperphagia apparent after administration of 0.5 mg·kg−1 (q = 2.919, P < 0.05). Although the effect of 1.0 mg·kg−1 noladin during hour 1 was not statistically significant in this analysis (q = 2.451, NS), increased food intake was apparent in 7 out of 10 rats (pairwise Bonferroni comparison against control: t = 2.45, P < 0.05). No reliable effects on intake were observed during hour 2 (F3,27= 1.015, NS). The highest 2.0 mg·kg−1 dose had no reliable effect on intake at either measurement point. The specimen behavioural analysis indicated that during hour 1 the frequency of observations of exploratory locomotor activity – the most prevalent category under control conditions, representing 65% of all observations in this period – was halved by both 0.5 and 1.0 mg·kg−1 noladin (data not shown). There was a corresponding small increase in the frequency of observations of eating at these doses, with the onset of feeding occurring during the first hour of testing, rather than in hour 2 as after vehicle. By contrast, after 2.0 mg·kg−1 the most marked change was an increase in the frequency of resting/inactivity during hour 1, with this category accounting for approximately 23% of all observations, compared with only 3% in the control condition. On the basis of the food intake data, the 0.5 mg·kg−1 dose of noladin was selected for testing in the second experiment.
Figure 1.
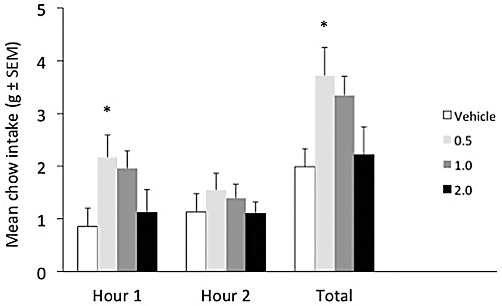
Dose-related effects of intraperitoneal injection of noladin ether (0.5, 1.0 and 2.0 mg·kg−1) on food intake in non-deprived rats (n= 10) tested during the light phase. Data represent chow intake during hours 1 and 2, and total 2 h consumption (mean ± SEM). *P < 0.05, different from vehicle-vehicle control.
Noladin ether effects on PR responding
As may be seen from the representation of cumulative response curves in Figure 2, under control conditions animals initially responded at a high rate for the palatable food pellets. Over the course of the 1 h test, and with the accelerating increments in response requirement of the PR schedule, response rate was gradually curtailed. Noladin ether counteracted this typical decline in responding, so that the initial higher response rates were sustained longer throughout the test when compared with the control condition (treatment × time interaction, F11,308= 2.70, P= 0.0025). Significant separation between cumulative response curves for control and noladin ether was evident from approximately 35 min into the test session (time bin 8; P < 0.05). Consequently, significant treatment effects were obtained for total lever presses (F3,42= 3.235, P= 0.032), with noladin producing a marked 36% increase over baseline (P < 0.5): total presses (mean ± SEM), vehicle-vehicle = 1112.1 ± 145.5; vehicle-noladin = 1514.1 ± 221.8; surinabant-vehicle = 1235.0 ± 156.0; surinabant-noladin = 1215.4 ± 199.5. More particularly, noladin ether produced a significant increase in the total number of responses (q = 2.973, P < 0.05). This effect of noladin ether was abolished by surinabant pretreatment, so that in the surinabant-noladin condition the cumulative response curve was very similar to that in the vehicle-vehicle control (q = 0.764, NS). Consistent with the sub-anorectic nature of this dose of surinabant, the drug had no effect on PR responding when administered alone (q = 0.909, NS).
Figure 2.
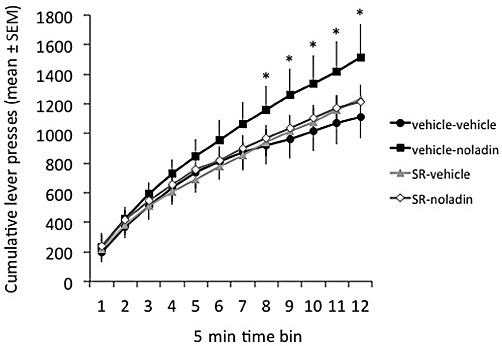
Effects of a hyperphagic dose of noladin ether (0.5 mg·kg−1, i.p.), alone or in combination with a sub-anorectic dose of the CB1 receptor antagonist surinabant (1.0 mg·kg−1), on cumulative lever presses in rats responding for palatable 45 mg food pellets under a progressive ratio schedule. All data are means (±SEM) for 15 rats. *P < 0.05, different from vehicle-vehicle control.
In line with the above changes, there was a significant effect of treatment on break-point (F3,42= 5.409, P= 0.003; Figure 3). Noladin alone produced a significant elevation of break-point, increasing the size of the final completed ratio by some 37%, from a control level of 150 ± 13 (mean ± SEM) to 205 ± 23 (q = 3.848, P < 0.01). Again, this effect was prevented by surinabant pretreatment, so that break-point after the combination was not different from control (q = 1.866, NS); while surinabant alone was without effect (q = 0.933, NS).
Figure 3.
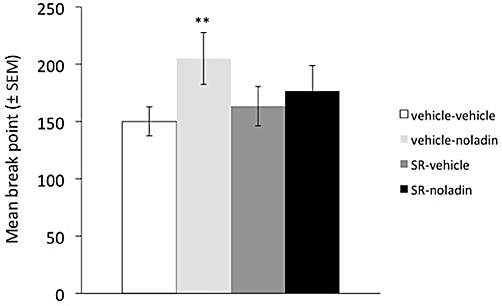
Systemic noladin ether increases break-point (last ratio completed under a progressive ratio schedule over 1 h), an effect which is prevented by surinabant pretreatment. **P < 0.01, different from vehicle-vehicle control.
Discussion and conclusions
These data represent the first demonstration of dose-related hyperphagic actions of noladin ether in the rat, and suggest that this substance shares with other CB1 receptor agonists a propensity to accentuate the appetitive aspects of eating motivation. Our findings therefore extend previous motivational analyses that support a significant role of endocannabinoid systems in the processes that give rise to appetite for food, promote food seeking and stimulate consumption.
Although relatively modest compared with the systemic effects of an exogenous cannabinoid such as THC (Williams et al., 1998), the overall hyperphagic effect of a low dose of noladin ether was more marked than the effects previously reported for systemic (Williams and Kirkham, 1999; Hao et al., 2000), or even intrahypothalamic administration of anandamide in rats (Jamshidi and Taylor, 2001). Arguably, the relatively greater effectiveness of noladin ether in this study reflects not only its nature as a full agonist at CB1 receptors, but also the greater in vivo stability of ethers compared with esters such as anandamide (Mechoulam et al., 1998).
It is notable that our effects on feeding were obtained at higher noladin doses than those reported by Avraham et al. (2005) to be effective in female mice (0.5 mg·kg−1 here, compared with 0.001 mg·kg−1 in the mouse experiment). However, it is difficult to make further comparisons between the effects of noladin ether in the two studies, because (in addition to the obvious species and gender differences) in that original report mice were chronically food restricted (with only 2.5 h food access per day), received the drug over several days, and acute effects on feeding in individual animals were not reported (daily food intake was calculated for pair-housed mice, and only total of 9-day food intake data was reported). The same group has also reported that a very low dose of anandamide (0.001 mg·kg−1·day−1) is hyperphagic in the same repeated-dosing, food-restriction mouse model (Hao et al., 2000), suggesting species differences or a particular sensitivity of that paradigm to cannabinoid actions. However, the hyperphagic actions of systemic noladin ether in the present study were obtained at doses comparable with those of the related compounds anandamide and 2-AG previously reported to be effective at promoting ingestive behaviour in non-deprived rats after acute, systemic administration (Williams and Kirkham, 1999; Higgs et al., 2003; Wakley and Rasmussen, 2009).
Although some non-significant elevation of intake was evident during the second hour of testing, the most marked effects of noladin ether were apparent during the first hour. This early hyperphagic effect is consistent with previous data indicating that CB1 receptor agonists tend to promote an earlier than normal onset of eating (Williams and Kirkham, 2002b; Farrimond et al., 2010). Importantly, the data from the second experiment also consolidate previous findings indicating that CB1 receptor agonists can directly increase the motivation to work to obtain food (Gallate and McGregor, 1999; Gallate et al., 1999; Higgs et al., 2005; Solinas and Goldberg, 2005; Wakley and Rasmussen, 2009). In line with those reports, in our animals, lever pressing for sweet food pellets under a PR schedule, a hyperphagic dose of noladin ether reliably increased break-point, total number of lever presses, and maintained longer the higher initial rates of responding observed under control conditions. Mediation of these effects by cannabinoid CB1 receptors was supported by their abolition by pretreatment with a sub-anorectic dose of the selective CB1 receptor antagonist surinabant which had no effect on responding when administered alone (Rinaldi-Carmona et al., 2004).
It is possible that increased rates and persistence of PR responding in noladin-treated rats might have reflected some non-specific stimulatory action of the drug. However, our limited behavioural observations in Experiment 1 indicate this to be unlikely, because the most obvious non-feeding effect of the drug was a tendency for reduced locomotor activity and, particularly at the highest dose, an increase in resting/inactivity. Indeed, locomotor depression has been reported to occur in mice at higher systemic doses of noladin ether (Hayase et al., 2005) and may be a possible factor in our failure to detect increased food intake at the highest dose in Experiment 1. It is also noteworthy that all of the food pellets that rats obtained in each condition were consumed, suggesting that increased motivation to eat, rather than non-specific, stereotypic lever pressing, underlies the observed hyperphagic effects of noladin ether.
Overall, the effects described here are consistent with noladin ether, like other CB1 receptor agonists, acting to amplify appetitive aspects of eating motivation – or wanting. That the drug caused lever pressing to be more enduring may indicate an action to enhance the incentive value of food, possibly reflecting the normal role of the endocannabinoids in energizing and guiding behaviour in response to physiological factors, such as hunger arising from changing energetic status. Indeed, it is noteworthy that fasting – which is associated with elevated brain anandamide and 2-AG levels in rats (Kirkham et al., 2002) – will produce similar changes in PR responding to those induced by noladin ether (Solinas and Goldberg, 2005). There are also data to indicate that cannabinoids can accentuate food palatability, or liking (Higgs et al., 2003; Jarrett et al., 2005; Mahler et al., 2007). An additional action of noladin ether to enhance the hedonic evaluation of food could therefore also contribute to the increased incentive salience of food and the persistence of high rates of responding seen here. Further experiments are required to explore the involvement of cannabinoid-mediated processes in these separate motivational components, and to characterize more precisely the behavioural adjustments induced by noladin ether.
To conclude, this is the first report of the ability of noladin ether to promote hyperphagia in rats, and the first demonstration of its capacity to specifically enhance the motivational effects of food as measured using a PR paradigm. These data thus provide important supporting evidence for a physiological role of the endocannabinoids in the control of appetite for food, and suggest that noladin ether may be a useful tool for the further investigation of the underlying neural mechanisms.
Acknowledgments
This work was supported by a grant from the UK BBSRC (to TCK).
Glossary
- 2-AG
2-arachidonoylglycerol
- FAAH
fatty acid amide hydrolase
- PR
progressive ratio
- THC
Δ9-tetrahydrocannabinol
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiébot M, Poncelet M, Soubrié P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Avraham Y, Ben Menachem A, Okun A, Zlotarav O, Abel N, Mechoulam R, et al. Effects of the endocannabinoid noladin ether on body weight, food consumption, locomotor activity, and cognitive index in mice. Brain Res Bull. 2005;65:117–123. doi: 10.1016/j.brainresbull.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Di Patrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulate feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggrell SA. Will the new CB1 cannabinoid receptor antagonist SR-147778 have advantages over rimonabant? Expert Opin Investig Drugs. 2005;14:339–342. doi: 10.1517/13543784.14.3.339. [DOI] [PubMed] [Google Scholar]
- Farrimond JA, Whalley BJ, Williams CM. A low-Δ9tetrahydrocannabinol cannabis extract induces hyperphagia in rats. Behav Pharmacol. 2010;21:769–772. doi: 10.1097/FBP.0b013e328340a062. [DOI] [PubMed] [Google Scholar]
- Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, Di Marzo V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513:294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- Fride E, Bregman T, Kirkham TC. Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood. Exp Biol Med (Maywood) 2005;230:225–234. doi: 10.1177/153537020523000401. [DOI] [PubMed] [Google Scholar]
- Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl) 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Avraham Y, Mechoulam R, Berry EM. Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur J Pharmacol. 2000;392:147–156. doi: 10.1016/s0014-2999(00)00059-5. [DOI] [PubMed] [Google Scholar]
- Hayase T, Yamamoto Y, Yamamoto K. Persistent anxiogenic effects of a single or repeated doses of cocaine and methamphetamine: interactions with endogenous cannabinoid receptor ligands. Behav Pharmacol. 2005;16:395–404. doi: 10.1097/00008877-200509000-00012. [DOI] [PubMed] [Google Scholar]
- Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after Δ9-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology (Berl) 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Higgs S, Barber DJ, Cooper AJ, Terry P. Differential effects of two cannabinoid receptor agonists on progressive ratio responding for food and free-feeding in rats. Behav Pharmacol. 2005;16:389–393. doi: 10.1097/00008877-200509000-00011. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett MM, Limebeer CL, Parker LA. Effect of Δ9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav. 2005;86:475–479. doi: 10.1016/j.physbeh.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol. 2005;16:297–313. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Endocannabinoids and the non-homeostatic control of appetite. Curr Top Behav Neurosci. 2009;1:231–253. doi: 10.1007/978-3-540-88955-7_9. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Rogers EK. Endocannabinoids in the aetiopathology of obesity – central mechanisms. Drug Discov Today Dis Mech. 2010;7:e163–e168. [Google Scholar]
- Kirkham TC, Williams CM. Endocannabinoids: neuromodulators of food craving? In: Hetherington M, editor. Food Cravings and Addiction. Leatherhead, Surrey: Leatherhead Publishing; 2001a. pp. 85–120. [Google Scholar]
- Kirkham TC, Williams CM. Endogenous cannabinoids and appetite. Nutr Res Rev. 2001b;14:65–86. doi: 10.1079/NRR200118. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Endocannabinoid receptor antagonists. Treat Endocrinol. 2004;3:1–16. doi: 10.2165/00024677-200403060-00003. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Hanus E, Fride E. Towards cannabinoid drugs: revisited. Prog Med Chem. 1998;35:199–243. doi: 10.1016/s0079-6468(08)70037-7. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Berry EM, Avraham Y, Di Marzo V, Fride E. Endocannabinoids, feeding and suckling – from our perspective. Int J Obes. 2006;30:S24–S28. doi: 10.1038/sj.ijo.0803274. [DOI] [PubMed] [Google Scholar]
- Oka S, Tsuchie A, Tokumura A, Muramatsu M, Suhara Y, Takayama H, et al. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J Neurochem. 2003;85:1374–1381. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- Páldyová E, Bereczki E, Sántha M, Wenger T, Borsodi A, Benyhe S. Noladin ether, a putative endocannabinoid, inhibits mu-opioid receptor activation via CB2 cannabinoid receptors. Neurochem Int. 2008;52:321–328. doi: 10.1016/j.neuint.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360:216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Congy C, Martinez S, Oustric D, Pério A, et al. SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)-1H-pyrazole-3-carboxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;310:905–914. doi: 10.1124/jpet.104.067884. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Cline BH, Marsicano G, Lutz B, Spanagel R. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology (Berl) 2004;176:223–232. doi: 10.1007/s00213-004-1877-8. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane P, Soubrié P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Soria-Gómez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens M, Zentner J, Honegger J, Feuerstein TJ. Binding affinity and agonist activity of putative endogenous cannabinoids at the human neocortical CB1 receptor. Biochem Pharmacol. 2005;69:169–178. doi: 10.1016/j.bcp.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Touriño C, Oveisi F, Lockney J, Piomelli D, Maldonado R. FAAH deficiency promotes energy storage and enhances the motivation for food. Int J Obes. 2010;34:557–568. doi: 10.1038/ijo.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty ANA, McGregor IS, Mallet PE. Paraventricular hypothalamic CB1 cannabinoid receptors are involved in the feeding stimulatory effects of Δ9-tetrahydrocannabinol. Neuropharmacology. 2005;49:1101–1109. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Rasmussen EB. Effects of cannabinoid drugs on the reinforcing properties of food in gestationally undernourished rats. Pharmacol Biochem Behav. 2009;94:30–36. doi: 10.1016/j.pbb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Reversal of Δ9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav. 2002a;7:333–340. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol Behav. 2002b;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral Δ9-THC. Physiol Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]


