Abstract
BACKGROUND AND PURPOSE
α1-Adrenoceptor-induced contraction of prostate smooth muscle is mediated by calcium- and Rho kinase-dependent mechanisms. In addition, other mechanisms, such as activation of c-jun N-terminal kinase (JNK) may be involved. Here, we investigated whether JNK participates in α1-adrenoceptor-induced contraction of human prostate smooth muscle.
EXPERIMENTAL APPROACH
Prostate tissue was obtained from patients undergoing radical prostatectomy. Effects of the JNK inhibitors SP600125 (50 µM) and BI-78D3 (30 µM) on contractions induced by phenylephrine, noradrenaline and electric field stimulation (EFS) were studied in myographic measurements. JNK activation by noradrenaline (30 µM) and phenylephrine (10 µM), and the effects of JNK inhibitors of c-Jun phosphorylation were assessed by Western blot analyses with phospho-specific antibodies. Expression of JNK was studied by immunohistochemistry and fluorescence double staining.
KEY RESULTS
The JNK inhibitors SP600125 and BI-78D3 reduced phenylephrine- and noradrenaline-induced contractions of human prostate strips. In addition, SP600125 reduced EFS-induced contraction of prostate strips. Stimulation of prostate tissue with noradrenaline or phenylephrine in vitro resulted in activation of JNK. Incubation of prostate tissue with SP600125 or BI-78D3 reduced the phosphorylation state of c-Jun. Immunohistochemical staining demonstrated the expression of JNK in smooth muscle cells of human prostate tissue. Fluorescence staining showed that α1A-adrenoceptors and JNK are expressed in the same cells.
CONCLUSIONS AND IMPLICATIONS
Activation of JNK is involved in α1-adrenoceptor-induced prostate smooth muscle contraction. Models of α1-adrenoceptor-mediated prostate smooth muscle contraction should include this JNK-dependent mechanism.
Keywords: α1-adrenoceptor, benign prostate hyperplasia, JNK, mitogen-activated protein kinase, smooth muscle contraction, lower urinary tract symptoms
Introduction
α1-Adrenoceptors are important regulators of prostate smooth muscle tone (Andersson et al., 1997; Schilit and Benzeroual, 2009). In bladder outflow obstruction (BOO) caused by benign prostate hyperplasia (BPH), α1-adrenoceptor-mediated contraction of prostate smooth muscle (dynamic factor) may contribute to lower urinary tract symptoms (LUTS) in addition to prostate growth (static factor) and extra prostatic factors (Andersson et al., 1997; Schilit and Benzeroual, 2009). Consequently, α1-adrenoceptors in the lower urinary tract (LUT) represent an important target for the pharmacological treatment of LUTS (Andersson et al., 1997; Roehrborn and Schwinn, 2004; Schwinn and Roehrborn, 2008). Therefore, the mechanisms of α1-adrenoceptor-mediated contraction are of great theoretical as well as clinical interest.
α1-Adrenoceptor-induced contraction of prostate smooth muscle is known to involve activation of calcium- and Rho kinase-dependent mechanisms (Christ and Andersson, 2007). These mechanisms are also important contraction-mediating effectors of α1-adrenoceptors in other types of smooth muscle, for example, rat vascular smooth muscle, where c-jun N-terminal kinase (JNK) was suggested to be involved as well (Lee et al., 2006; Liu et al., 2007; Zhou et al., 2010). JNK represents a member of the family of MAPK (Johnson and Nakamura, 2007; Bogoyevitch et al., 2010). Different functions of JNK have been described, which may be cell or organ specific. JNK-dependent functions are of relevance for cell cycle, cellular survival, cell death, inflammation and differentiation (Johnson and Nakamura, 2007; Bogoyevitch et al., 2010). While the involvement of JNK in the contraction of vascular smooth muscle has been demonstrated (Lee et al., 2006; Liu et al., 2007; Zhou et al., 2010), the role of JNK in contraction of prostate smooth muscle has, to the best of our knowledge, not been investigated previously. In the present study, we have therefore examined the possible role of JNK in α1-adrenoceptor-mediated contraction of human prostate smooth muscle.
Methods
Human prostate tissue
Human prostate tissue was obtained from patients undergoing radical prostatectomy for prostate cancer (n = 47, mean age 67.4 years). Tissues for experiments were taken from the periurethral zone. Representative tissue sections did not exhibit histological signs of neoplasia, cancer or inflammation. In fact, most prostate tumours are located to the peripheral zone. In patients with prostate cancer, normal and hyperplastic tissues occur in very close proximity to each other, so that exact discrimination of these areas usually requires microscopic examination. Therefore, normal and hyperplastic areas were not separated. All procedures were approved by the Ethics Committee of the Ludwig-Maximilians-University, Munich, Germany. The research was carried out according to the World Medical Association Declaration of Helsinki.
Measurement of prostate contraction
For isometric tension measurements, human prostate strips (3 × 3 × 6 mm) were mounted in 5 mL aerated (95% O2 and 5% CO2) tissue baths (37°C, pH 7.4), containing Krebs–Henseleit solution. Mechanical activity was registered with a Grass Polygraph model 7E (Grass Technologies, West Warwick, RI, USA). Preparations were stretched to 0.5 g and left to equilibrate for 45 min to attain a stable resting tone. The inhibitors of JNK, SP600125 (50 µM) and BI-78D3 (30 µM), or vehicle [dimethyl sulfoxide (DMSO)] were applied 30 min before application of phenylephrine or noradrenaline, or the second cycle of electric field stimulation (EFS). The concentration of SP600125 used in our study is in the same range of that applied previously in studies with rat aortic rings (Lee et al., 2006; Zhou et al., 2010). After construction of concentration-response curves, the tissue chambers were washed three times with Krebs–Henseleit solution, and viability of the preparations was assessed by exposure to 80 mM KCl.
Sampling and in vitro stimulation
Tissues were frozen or used for experiments directly after pathological examination of excised prostates, without any additional delay. For analysis by immunohistochemistry, samples of prostate tissue were shock frozen in liquid nitrogen after prostatectomy. For in vitro stimulation with adrenoceptor agonists or JNK inhibitors, samples of prostate tissue were prepared as small strips (2–3 mm × 1 mm) and allocated to three or four polyethylene tubes containing Krebs–Henseleit solution. During the experiments, tubes were kept at 37°C and continuously oxygenated with carbogen (95% O2, 5% CO2). Tissues were allowed to equilibrate for 20 min. For stimulation with phenylephrine or noradrenaline, 10 mM stock solutions were added at the required intervals and volumes to obtain a final concentration of 10 µM phenylephrine, or 30 µM noradrenaline. To avoid any effects due to different incubation periods, all samples were exposed to identical periods and experimental conditions. Therefore, stimulation was performed after the addition of phenylephrine or noradrenaline 20, 10 and 5 min before the end of the experiment. For incubation with SP600125 (50 µM) or BI-78D3 (30 µM), 10 mM stock solutions of inhibitors, or the equivalent volume of DMSO were added simultaneously, and incubation was performed for 2 h. At the end of each experiment, stimulated and unstimulated samples were simultaneously shock frozen in liquid nitrogen. Samples were stored at −80°C until Western blot analysis was performed.
Assessment of JNK activity
JNK is activated by phosphorylation at threonine183/tyrosine185 through MAPK kinase 4/7. For semi-quantitative assessment of JNK activity, the phosphorylation state of JNK was compared by Western blot analysis with a phospho-specific antibody. The total JNK content was compared by Western blot analysis with a non-phospho-specific antibody. After densitometric quantification, phospho-JNK, total JNK or phospho-c-Jun at 0 min or after DMSO, respectively, were set to 100%, and the contents in stimulated samples are expressed as % of the unstimulated or DMSO sample.
Western blot analysis
Frozen prostate tissues were homogenized in a buffer containing 25 mM Tris/HCl, 10 µM phenylmethanesulfonyl fluoride, 1 mM benzamidine and 10 µg mL-1 leupeptine hemisulfate, using a FastPrep®-24 system with matrix A (MP Biomedicals, Illkirch, France). After brief centrifugation, supernatants were assayed for protein concentration using the Dc-Assay kit (Biorad, Munich, Germany) and boiled for 10 min with sample buffer (Roth, Karlsruhe, Germany). Samples were subjected to SDS-PAGE (20 µg per lane), and proteins were blotted on nitrocellulose membranes. The membranes were blocked overnight, and subsequently incubated with primary antibodies. For detection, rabbit anti-phospho-stress-activated protein kinase (SAPK)/JNK (T183/Y185) antibody (98F2), rabbit anti-SAPK/JNK antibody (56G8) (Cell Signaling Technology, Danvers, MA, USA) or rabbit anti phospho-c-Jun (serine 63) antibody (sc-7980-R) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. Subsequently, membranes were washed with PBS containing 0.1% Tween 20, and incubated with secondary peroxidase-coupled antibody (Calbiochem, San Diego, CA, USA). Blots were developed with enhanced chemiluminescence (ECL) using ECL Hyperfilm (GE Healthcare, Freiburg, Germany). Intensities of the resulting bands were quantified using Image J (NIH, Bethesda, MD, USA).
Immunohistochemistry
Sections (6–8 µm) from frozen tissues were stained by an indirect immunoperoxidase technique. Sections were fixed with acetone, and endogenous peroxidase activity was subsequently blocked by 0.03% H2O2. Thereafter, sections were blocked with goat serum diluted 1:10 in PBS and incubated with primary rabbit anti-JNK (FL) antibody (sc-572) (Santa Cruz Biotechnology), or rabbit anti-phospho-SAPK/JNK (T183/Y185) antibody (98F2) (Cell Signaling Technology). Primary antibody was diluted 1:50 in PBS at room temperature and incubated with the sections overnight. After the sections had been washed three times in PBS, biotinylated secondary goat anti-rabbit antibody and avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA, USA) were sequentially applied for 30 min each. Staining was performed using the AEC peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA). Finally, all sections were counterstained with hemalaun. Control stainings without primary antibodies did not yield any signals.
Immunofluorescence
Human prostate specimens, embedded in optimal cutting temperature compound, were snap frozen in liquid nitrogen and kept at −80°C. Three sections (8 µm) were cut in a cryostat and collected on microscope slides (Superfrost®). Sections were postfixed in methanol at −20°C and blocked in 1% BSA before incubation with primary antibody overnight at room temperature. For immunofluorescence analysis, three sections per specimen were co-labelled for α1A-adrenoceptors (monoclonal mouse antibody, 1:50, sc-100291; Santa Cruz Biotechnology) and JNK (polyclonal rabbit antibody, D-2; Santa Cruz Biotechnology). Binding sites were visualized using Cy3- and Cy5-conjugated secondary antibodies (goat anti-mouse, AP124C, Millipore, Billerica, MA, USA, 1:1000; goat anti-rabbit, 1:1000, ab6564, Abcam, Cambridge, UK). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (1:50 000, D1306; Invitrogen, Camarillo, CA, USA) during incubation with the secondary antibody. Slides were coverslipped with Citiflour® mounting medium. Immunolabelled sections were analysed using a laser scanning microscope (Leica SP2, Wetzlar, Germany) equipped with an argon laser, a helium-neon laser and a diode laser. Laser scanning microscopy was performed with a 40× oil-immersion objective. Fluorescence was excited at 405 nm (DAPI), 488 nm (Cy5), and 483 nm (Cy3), and recorded with separate detectors. Control stainings without primary antibodies did not yield any signals.
Drugs and nomenclature
SP600125 and BI-78D3 are inhibitors of JNK. SP600125 and BI-78D3 (both from Tocris, Bristol, UK) were dissolved in DMSO and kept as 10 mM stock solution at −20°C until use. Aqueous stock solutions of the α1-adrenoceptor agonist phenylephrine (Sigma, St. Louis, MO, USA) (10 mM) were freshly prepared for each experiment. Nomenclature of receptors and enzymes conforms to BJP's ‘Guide to Receptors and Channels’ (Alexander et al., 2011).
Statistical analysis
Data are presented as means ± SEM with the indicated number (n) of patients. Student's two-tailed t-test was used for paired or unpaired observations. P values < 0.05 were considered statistically significant.
Results
Tension measurements
Noradrenaline induced concentration-dependent contractions of human prostate strips, with a maximum at 30 µM. The JNK inhibitor SP600125 (50 µM) significantly reduced noradrenaline-induced contractions (n = 7 patients) (Figure 1A). The inhibition was observed at 1, 3, 10 and 30 µM noradrenaline (Figure 1A).
Figure 1.
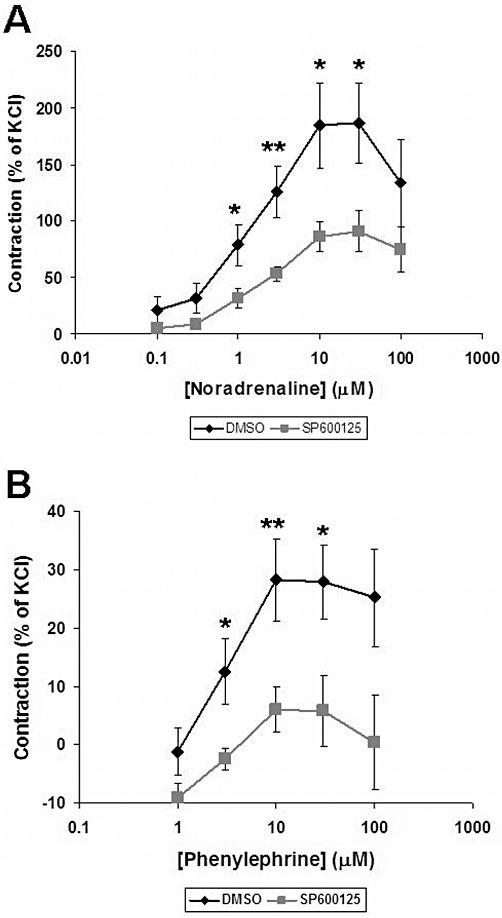
Effect of the JNK inhibitor SP600125 on adrenergic contraction of human prostate strips. Contraction of prostate tissue in response to noradrenaline (A) or phenylephrine (B) was determined by myographic measurements, and referred to maximal KCl-induced contraction. SP600125 (50 µM) or the vehicle DMSO was applied 30 min before the first dose of phenylephrine or noradrenaline. Shown are cumulative concentration- response curves for noradrenaline and phenylephrine. Data are means ± SEM from experiments with tissues from n = 6 (A) or n = 7 (B) patients. *P < 0.04, **P < 0.02 for DMSO versus SP600125.
Phenylephrine also induced concentration-dependent contractions of human prostate strips, with a maximum at 10 µM. SP600125 (50 µM) significantly reduced the contractions (n = 6 patients) (Figure 1B). The inhibition was observed at 3, 10 and 30 µM phenylephrine (Figure 1B).
In a separate set of experiments, the effects of another JNK inhibitor, BI-78D3 on noradrenaline- and phenylephrine-induced contractions was tested. Similar to SP600125, BI-78D3 (30 µM) significantly reduced the contractions induced by noradrenaline (n = 6 patients) and phenylephrine (n = 12 patients) (Figure 2). Inhibition of noradrenaline-induced contraction was observed at 0.3, 1, 3 and 10 µM noradrenaline (Figure 2A). Inhibition of phenylephrine-induced contraction was observed at 1, 3, 10 and 30 µM phenylephrine (Figure 2B).
Figure 2.
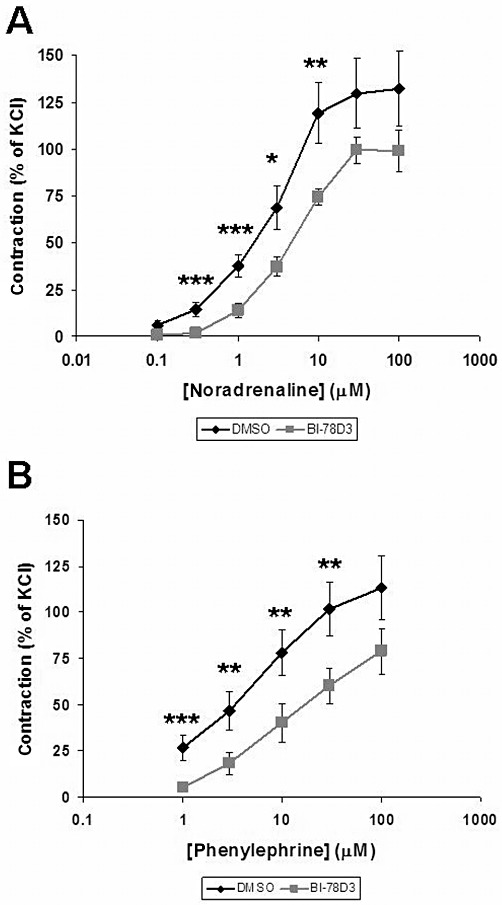
Effect of the JNK inhibitor BI-78D3 on adrenergic contraction of human prostate strips. Contraction of prostate tissue in response to noradrenaline (A) or phenylephrine (B) was determined by myographic measurements, and referred to maximal KCl-induced contraction. BI-78D3 (30 µM) or the vehicle DMSO was applied 30 min before the first dose of phenylephrine or noradrenaline. Shown are cumulative concentration response curves for noradrenaline and phenylephrine. Data are means ± SEM from experiments with tissues from n = 6 (A) or n = 12 (B) patients. *P < 0.04, **P < 0.03, ***P < 0.01 for DMSO versus BI-78D3.
EFS induced frequency-dependent contractions of the strips, with a maximum at 32 Hz (1.9 ± 0.5 g, n = 7 patients). SP600125 (50 µM) significantly reduced the contractions (n = 7 patients) (Figure 3). This inhibition of EFS-induced contraction was observed at 8, 16 and 32 Hz (Figure 3). In contrast, contractions of the first and second cycles were not different when DMSO was applied instead of SP600125 (Figure 3).
Figure 3.
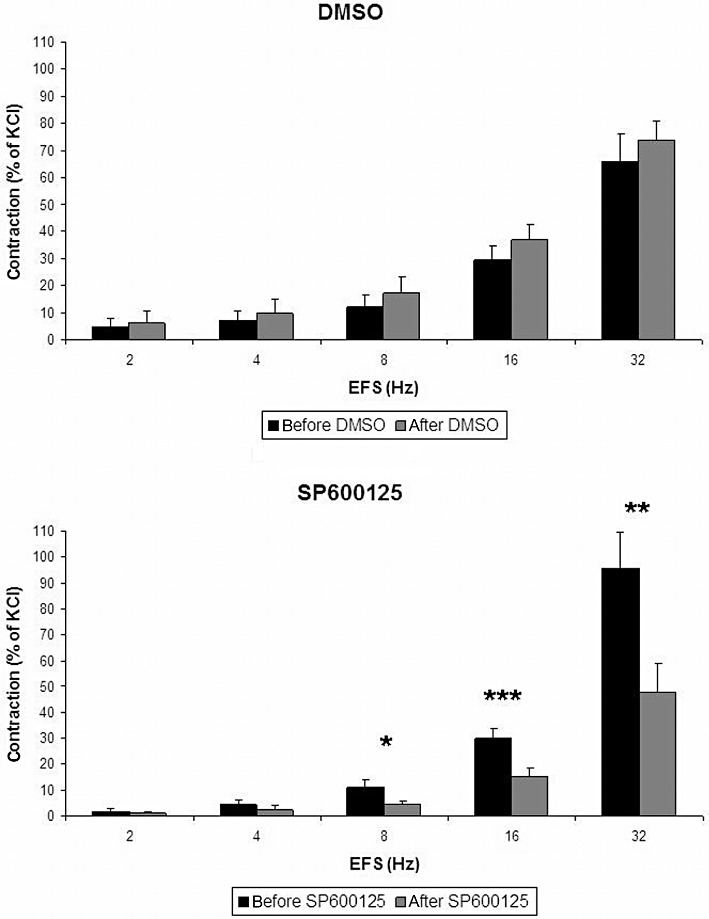
Effect of the JNK inhibitor SP600125 on EFS-induced contraction of human prostate strips. Contraction of prostate tissue in response to EFS was determined by myographic measurements, and referred to maximal KCl-induced contraction. SP200125 (50 µM) or the vehicle DMSO was added between two cycles of EFS (30 min before the second cycle). Shown are frequency-dependent concentrations. Data are means ± SEM from experiments with tissues from n = 7 patients. *P < 0.05, **P < 0.03, ***P < 0.01 before and after SP600125.
JNK activity
Stimulation of human prostate tissue with noradrenaline (30 µM) increased the phosphorylation of JNK, reflecting activation of JNK (Figure 4). This phosphorylation was observed 5, 10 and 20 min after stimulation (Figure 4). In contrast, the total JNK content in prostate tissue did not change during the stimulation experiments (Figure 4).
Figure 4.
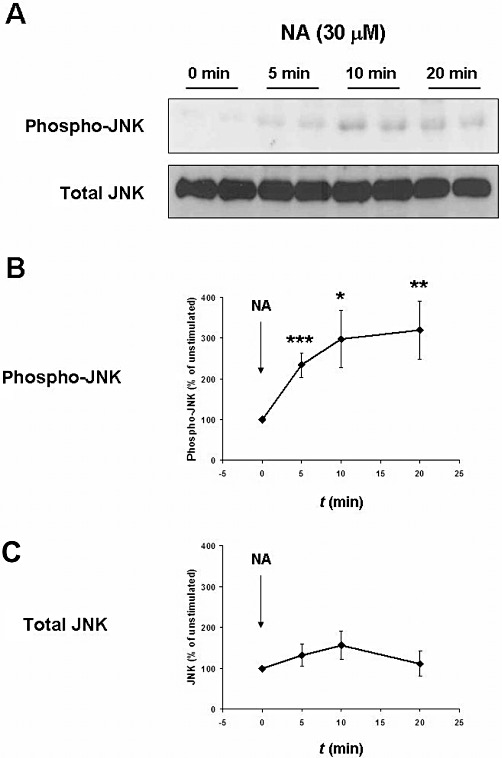
JNK activation by noradrenaline in human prostate tissue. Samples of human prostate tissue were stimulated in vitro with noradrenaline (30 µM) for 0, 5, 10 or 20 min. All samples including the unstimulated (‘0 min’) were exposed for identical periods to experimental conditions and simultaneously shock frozen. The phosphorylation state of JNK was assessed by Western blot analysis with a phospho-specific antibody, and reflects JNK activity. (A) Representative Western blots for phosphorylated JNK and total JNK. (B and C) Results from densitometric quantification of all experiments (data are means ± SEM from independent experiments with tissues from n = 8 patients). *P < 0.02, **P < 0.01, ***P < 0.001 versus ‘0 min’.
Stimulation of human prostate tissue with phenylephrine (10 µM) increased the phosphorylation of JNK, reflecting activation of JNK (Figure 5). The phosphorylation was observed 10 min after stimulation (Figure 5). In contrast, the total JNK content in prostate tissue did not change during the stimulation experiments (Figure 5).
Figure 5.
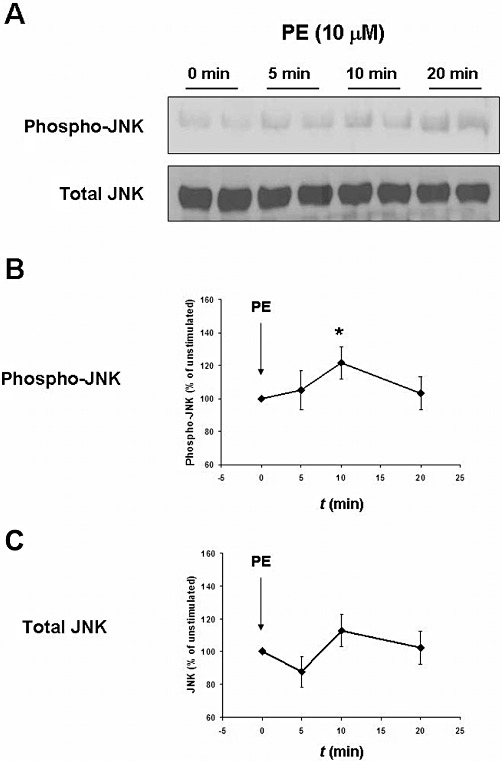
JNK activation by phenylephrine (PE) in human prostate tissue. Samples of human prostate tissue were stimulated in vitro with phenylephrine (10 µM) for 0, 5, 10 or 20 min, and assessed for JNK activation as described in Figure 4. (A) Representative Western blots for phosphorylated JNK and total JNK. (B and C) Results from densitometric quantification of all experiments (data are means ± SEM from independent experiments with tissues from n = 9 patients). *P < 0.05 versus ‘0 min’.
Incubation of human prostate tissue with SP600125 (50 µM) or BI-78D3 (30 µM) for 2 h reduced the phosphorylation state of the JNK substrate, c-Jun at serine 63 (Figure 6). This reflects inhibition of JNK activity by SP600125 and BI-78D3.
Figure 6.
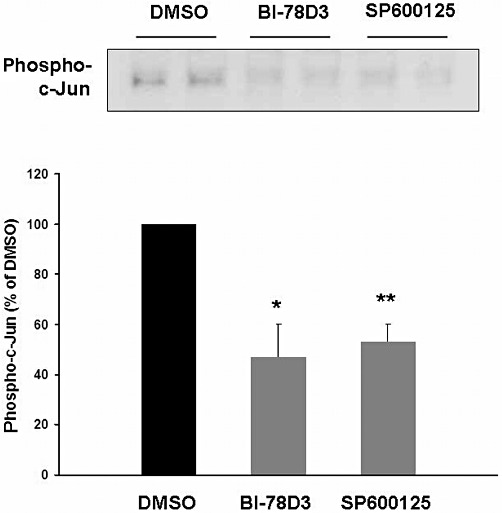
Effect of the JNK inhibitors BI-78D3 and SP600125 on phosphorylation of the JNK substrate, c-Jun at serine 63. Samples of human prostate tissue were stimulated in vitro with SP600125 (50 µM) and BI-78D3 (30 µM) for 2 h, assessed for phospho-JNK by Western blot analysis. Shown is a representative Western blot for phospho-JNK, and densitometric quantification of all experiments (data are means ± SEM from independent experiments with tissues from n = 5 patients). *P < 0.01, **P < 0.001 versus DMSO.
Immunohistochemistry
JNK staining was found in perinuclear and nuclear regions of prostate smooth muscle cells, and in the perinuclear regions of glandular cells (Figure 7). Faint immunoreactivity after staining with a phospho-specific JNK antibody was observed in smooth muscle cells (Figure 7). Control stainings, where the primary antibody was replaced by PBS, did not show any immunoreactivity (Figure 7).
Figure 7.
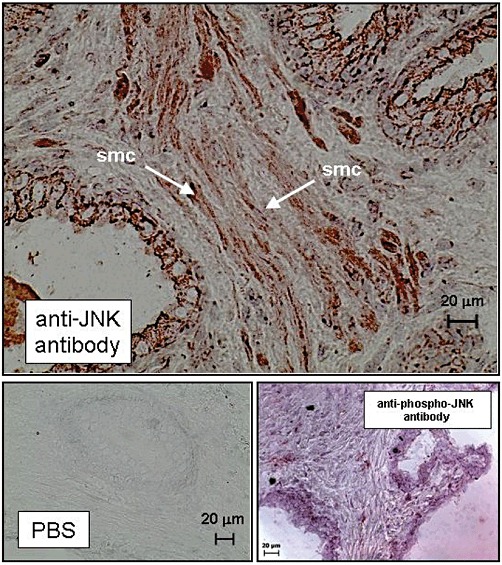
Immunohistochemical detection of JNK expression and phospho-JNK in human prostate tissue. Sections of human prostate tissue were stained by the peroxidase technique using antibodies for JNK or phospho-JNK. In control stainings, the primary antibody was replaced by ‘PBS’ (lower panel). Shown are representative stainings from experiments with tissues from n = 5 patients with similar results. Examples of smooth muscle cells (‘smc’) are indicated by arrows.
Immunofluorescence
Fluorescence staining revealed immunoreactivity for JNK and α1A-adrenoceptors in prostate smooth muscle cells (Figure 8). Overlaid images showed regions with co-localization of JNK and α1A-adrenoceptors, as indicated by yellow colour in merged pictures (Figure 8). Control stainings, where the primary antibodies were replaced by PBS, did not show immunoreactivity (Figure 8).
Figure 8.
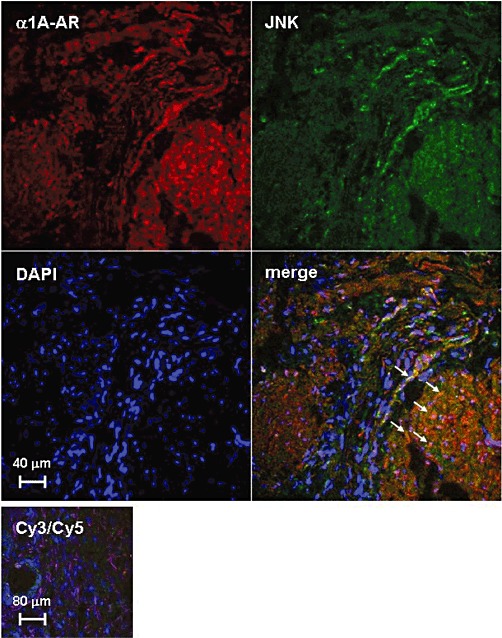
Co-localization of α1A–adrenoceptors (α1A-ARs) and JNK in human prostate smooth muscle cells. Using an immunofluorescence technique, prostate sections were double stained for α1A-adrenoceptors and JNK. In control stainings (‘Cy3/Cy5’, lower panel), the primary antibodies were replaced by PBS. Arrows indicate examples of areas with strong signals for co-localization. Shown are representative stainings from experiments with tissues from n = 5 patients with similar results.
Discussion
As mentioned in the Introduction, it is widely accepted that α1-adrenoceptor-induced contraction of prostate smooth muscle is caused by activation of calcium- and Rho kinase-dependent pathways (Christ and Andersson, 2007). In the present study, we identified an additional mechanism contributing to α1-adrenoceptor-mediated prostate smooth muscle contraction. This is based on two main findings. Firstly, the JNK inhibitors SP600125 and BI-78D3 inhibited contractions of human prostate strips induced by α1-adrenoceptors and EFS. Secondly, stimulation with noradrenaline or the α1-adrenoceptor agonist, phenylephrine, resulted in activation of JNK in the tissue. Together, this suggests that JNK activation is involved in α1-adrenoceptor-induced contraction, in addition to the established mechanisms (Christ and Andersson, 2007). α1-Adrenoceptor-mediated tone represents an important target for the pharmacological therapy of LUTS in patients with BOO secondary to BPH (Andersson et al., 1997; Roehrborn and Schwinn, 2004; Schwinn and Roehrborn, 2008). Treatment with α1-adrenoceptor blockers causes smooth muscle relaxation in the LUT, including the prostate (Andersson et al., 1997; Schilit and Benzeroual, 2009). Reduced prostate smooth muscle tone may improve urinary flow and symptoms due to reduced urethral resistance (Andersson et al., 1997; Schilit and Benzeroual, 2009). As SP600125 and BI-78D3 prevented α1-adrenoceptor-mediated contraction of prostate tissue, future in vivo studies in animals are required to find out whether JNK represents a reasonable target for therapy of LUTS.
A similar role of JNK for α1-adrenoceptor-mediated contraction has been previously assumed for vascular smooth muscle. Thus, the JNK inhibitor SP600125 blocked the noradrenaline-induced contraction of rat aortic rings (Lee et al., 2006; Zhou et al., 2010). This was confirmed by in vivo studies, where JNK inhibitors caused hypotension and decreased peripheral vascular resistance in anaesthetized rats (Liu et al., 2007; Zhou et al., 2010). We speculate that JNK is of similar relevance for the contraction of prostate smooth muscle as in vascular smooth muscle. In fact, SP600125 and BI-78D3 blocked the contraction of human prostate strips, regardless of whether the contraction was elicited by phenylephrine, noradrenaline or EFS. Reduction of prostate smooth muscle tone is an important strategy for the treatment of LUTS in patients with BOO and LUTS.
Although SP600125 has been described as an inhibitor of JNK, its specificity may be limited (Bennett et al., 2001; Bain et al., 2003). To confirm the involvement of JNK in α1-adrenoceptor-mediated prostate contraction, we tested the effect of BI-78D3 on noradrenaline- and phenylephrine-induced contraction of prostate strips. BI-78D3 is a JNK inhibitor, which is structurally unrelated to SP600125 (Stebbins et al., 2008). Similar to SP600125, BI-78D3 inhibited both noradrenaline- and phenylephrine-induced contractions. This supports the conclusion that JNK is involved in α1-adrenoceptor-mediated contraction of prostate smooth muscle. Finally, inhibition of JNK by both inhibitors was confirmed by Western blot analyses using a phospho-specific antibody for the JNK substrate, c-Jun. Application of SP600125 or BI-78D3 to prostate tissues resulted in reduced phosphorylation of c-Jun.
In vitro kinase assays using recombinant enzymes showed that SP600125 inhibits the three isoforms JNK1, -2 and -3 with similar potency (Bennett et al., 2001). IC50 values during its application in intact tissues or cultured cells may differ considerably from those in biochemical assays. In a study investigating the effects of SP600125 on noradrenaline-induced contraction of rat aortic rings, 1 µM of SP600125 was without effect, while 10 and 100 µM dose-dependently inhibited contraction (Lee et al., 2006). Therefore, we used an intermediate concentration from that range (i.e. 50 µM).
The present study shows that the current models of intracellular α1-adrenoceptor signalling in the human prostate have to be extended. To the best of our knowledge, our findings show for the first time that a JNK-dependent mechanism may be involved in α1-adrenoceptor-mediated prostate smooth muscle contraction, in parallel with the calcium- and Rho kinase-dependent mechanisms. Substrates, which are used by JNK to mediate prostate contraction, remain to be identified. In vascular smooth muscle, JNK may lead to contraction by phosphorylation of caldesmon (Zhou et al., 2010). In fact, caldesmon is expressed and regulated by α1-adrenoceptors in the human prostate (Walther et al., 2012). Together, these findings demonstrate that intracellular signalling induced by prostate α1-adrenoceptors is still not completely understood to date, despite the fact that expression and subtype distribution of α1-adrenoceptors in the prostate have been widely studied (Michel and Vrydag, 2006).
JNK is a member of the MAPK family (Maroni et al., 2004). Non-motoric JNK functions may differ between cell types and organs. The function of JNK in prostate cells has been investigated using different cell lines with diverse results (Maroni et al., 2004). For non-malignant, epithelial human prostate cells, not only a pro-apoptotic, antiproliferative role of JNK activation but also JNK-dependent survival has been described (Uzgare and Isaacs, 2004; Wadsworth et al., 2004). Proliferation after JNK activation has been reported from non-malignant, human stromal prostate cells (Koochekpour et al., 2005). Several studies using different lines of prostate tumour cells have suggested a pro-apoptotic role in these cells (Sanchez et al., 2007; Ho et al., 2009; Liou et al., 2009; Zhang et al., 2009). What these studies have in common is that they all support the role of JNK in survival and growth of prostate cells.
We speculate that JNK may be of relevance for further functions besides contraction in prostate smooth muscle cells. In non-prostate smooth muscle cells, JNK participates in the regulation of growth and differentiation. In vascular and airway smooth muscle cells, JNK activation is involved in proliferation (Kim and Iwao, 2003; Zhai et al., 2004). Interestingly, a role for JNK activation in vascular neointimal hyperplasia and in hyperplasia of airway smooth muscle has been proposed (Kim and Iwao, 2003; Xie et al., 2007). In the bladder, mechanical stress leads to JNK-mediated hypertrophy (Yamaguchi, 2004). Together with our finding that α1-adrenoceptors in the human prostate activate JNK, this suggests that α1-adrenoceptor-mediated JNK activation in prostate smooth muscle cells may be of relevance for prostate hyperplasia. In fact, previous studies in rodents or using cultured prostate cells proposed that α1-adrenoceptors represent one of many regulators of prostate growth (Golomb et al., 1998; Kanagawa et al., 2003; Marinese et al., 2003). However, any clinical effect of α1-blockers on prostate volume may be prevented by other important regulators such as androgens, growth factors or cytokines, which may cover the α1-adrenoceptor-dependent component of growth (Royuela et al., 1998; Lucia and Lambert, 2008).
In our experiments, we assessed agonist-induced changes in phospho-JNK by semi-quantitative comparisons between bands of the same blot in each experiment. We did not perform any comparisons between bands of different blots or films. Therefore, any variations in intensities of ‘0 min’ samples, due to different exposure times of films or different levels of constitutive phospho-JNK, did not influence our measurements. Similar procedures were applied to examine agonist-induced phosphorylation of other targets in recent studies (Hennenberg et al., 2011).
Our immunohistochemical stainings using a peroxidase technique demonstrated the expression of JNK in smooth muscle cells of human prostate tissue. This confirms results from previous studies (Royuela et al., 2002; Ricote et al., 2003), and supports the idea that α1-adrenoceptor-mediated JNK activation is at least partially located in smooth muscle cells. This was in fact confirmed by double stainings using an immunofluorescence technique, where immunoreactivity for α1A-adrenoceptors and JNK co-localized in smooth muscle cells. JNK was shown to be expressed in cultured prostate smooth muscle and stroma cells (Kanagawa et al., 2003). However, in contrast to our study, adrenergic regulation was not observed in cultured cells (Kanagawa et al., 2003). Thus, important cellular functions may get lost during cell culture. In our stainings, we focussed on α1A-adrenoceptors, as this subtype is responsible for smooth muscle contraction in the human prostate (Marshall et al., 1995; Roehrborn and Schwinn, 2004; Michel and Vrydag, 2006; Schwinn and Roehrborn, 2008).
Conclusion
Our findings provide evidence for a JNK-dependent mechanism contributing to α1-adrenoceptor-mediated human prostate smooth muscle contraction. Current models of intracellular signalling in prostate smooth muscle contraction should be extended to include this JNK-dependent pathway, which may represent a potential target for the treatment of LUTS in patients with BPH.
Acknowledgments
We thank B. Rutz and M. Perutka for excellent technical assistance. This study was supported by grants from the ‘Gester Foundation’, the Swedish Research Council, and the ‘Förderprogramm für Forschung und Lehre’ (FöFoLe) of the Ludwig-Maximilians University, Munich, Germany (Grant Reg.-Nr. 654).
Glossary
- BI-78D3
4-(2,3-dihydro-1,4-benzodioxin-6-yl)-2,4-dihydro-5-[(5-nitro-2-thiazolyl)thio]-3H-1,2,4-triazol-3-one
- BOO
bladder outflow obstruction
- BPH
benign prostate hyperplasia
- DMSO
dimethyl sulfoxide
- LUT
lower urinary tract
- LUTS
lower urinary tract symptom
- SP600125
anthra[1-9-cd]pyrazol-6(2H)-one
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE, Lepor H, Wyllie MG. Prostatic alpha 1-adrenoceptors and uroselectivity. Prostate. 1997;30:202–215. doi: 10.1002/(sici)1097-0045(19970215)30:3<202::aid-pros9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371((Pt 1)):199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Andersson KE. Rho-kinase and effects of Rho-kinase inhibition on the lower urinary tract. Neurourol Urodyn. 2007;26(6) Suppl.:948–954. doi: 10.1002/nau.20475. [DOI] [PubMed] [Google Scholar]
- Golomb E, Kruglikova A, Dvir D, Parnes N, Abramovici A. Induction of atypical prostatic hyperplasia in rats by sympathomimetic stimulation. Prostate. 1998;34:214–221. doi: 10.1002/(sici)1097-0045(19980215)34:3<214::aid-pros9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Hennenberg M, Strittmatter F, Walther S, Hedlund P, Andersson KE, Stief CG, et al. alpha1-adrenoceptor activation induces phosphorylation of beta2-adrenoceptors in human prostate tissue. BJU Int. 2011;108:922–928. doi: 10.1111/j.1464-410X.2010.10021.x. [DOI] [PubMed] [Google Scholar]
- Ho KK, Rosivatz E, Gunn RM, Smith ME, Stavropoulou AV, Numbere MG, et al. The novel molecule 2-[5-(2-chloroethyl)-2-acetoxy-benzyl]-4-(2-chloroethyl)-phenyl acetate inhibits phosphoinositide 3-kinase/Akt/mammalian target of rapamycin signalling through JNK activation in cancer cells. FEBS J. 2009;276:4037–4050. doi: 10.1111/j.1742-4658.2009.07112.x. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Nakamura K. The c-Jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa K, Sugimura K, Kuratsukuri K, Ikemoto S, Kishimoto T, Nakatani T. Norepinephrine activates P44 and P42 MAPK in human prostate stromal and smooth muscle cells but not in epithelial cells. Prostate. 2003;56:313–318. doi: 10.1002/pros.10267. [DOI] [PubMed] [Google Scholar]
- Kim S, Iwao H. Stress and vascular responses: mitogen-activated protein kinases and activator protein-1 as promising therapeutic targets of vascular remodeling. J Pharmacol Sci. 2003;91:177–181. doi: 10.1254/jphs.91.177. [DOI] [PubMed] [Google Scholar]
- Koochekpour S, Sartor O, Hiraiwa M, Lee TJ, Rayford W, Remmel N, et al. Saposin C stimulates growth and invasion, activates p42/44 and SAPK/JNK signaling pathways of MAPK and upregulates uPA/uPAR expression in prostate cancer and stromal cells. Asian J Androl. 2005;7:147–158. doi: 10.1111/j.1745-7262.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- Lee YR, Lee CK, Park HJ, Kim H, Kim J, Lee KS, et al. c-Jun N-terminal kinase contributes to norepinephrine-induced contraction through phosphorylation of caldesmon in rat aortic smooth muscle. J Pharmacol Sci. 2006;100:119–125. doi: 10.1254/jphs.fp0050777. [DOI] [PubMed] [Google Scholar]
- Liou SF, Lin HH, Liang JC, Chen IJ, Yeh JL. Inhibition of human prostate cancer cells proliferation by a selective alpha1-adrenoceptor antagonist labedipinedilol-A involves cell cycle arrest and apoptosis. Toxicology. 2009;256:13–24. doi: 10.1016/j.tox.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhao H, Liu B, Xin Z, Liu M, Serby MD, et al. Hemodynamic effects of potent and selective JNK inhibitors in anesthetized rats: implication for targeting protein kinases in metabolic diseases. Bioorg Med Chem Lett. 2007;17:495–500. doi: 10.1016/j.bmcl.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Lucia MS, Lambert JR. Growth factors in benign prostatic hyperplasia: basic science implications. Curr Urol Rep. 2008;9:272–278. doi: 10.1007/s11934-008-0048-6. [DOI] [PubMed] [Google Scholar]
- Marinese D, Patel R, Walden PD. Mechanistic investigation of the adrenergic induction of ventral prostate hyperplasia in mice. Prostate. 2003;54:230–237. doi: 10.1002/pros.10170. [DOI] [PubMed] [Google Scholar]
- Maroni PD, Koul S, Meacham RB, Koul HK. Mitogen activated protein kinase signal transduction pathways in the prostate. Cell Commun Signal. 2004;2:5. doi: 10.1186/1478-811X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I, Burt RP, Chapple CR. Noradrenaline contractions of human prostate mediated by alpha 1A-(alpha 1c-) adrenoceptor subtype. Br J Pharmacol. 1995;115:781–786. doi: 10.1111/j.1476-5381.1995.tb15001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl. 2):S88–119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Royuela M, Garcia-Tunon I, Bethencourt FR, Paniagua R, Fraile B. Pro-apoptotic tumor necrosis factor-alpha transduction pathway in normal prostate, benign prostatic hyperplasia and prostatic carcinoma. J Urol. 2003;170:787–790. doi: 10.1097/01.ju.0000082712.41945.17. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–1035. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- Royuela M, De Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Paniagua R. Transforming growth factor beta 1 and its receptor types I and II. Comparison in human normal prostate, benign prostatic hyperplasia, and prostatic carcinoma. Growth Factors. 1998;16:101–110. doi: 10.3109/08977199809002121. [DOI] [PubMed] [Google Scholar]
- Royuela M, Arenas MI, Bethencourt FR, Sanchez-Chapado M, Fraile B, Paniagua R. Regulation of proliferation/apoptosis equilibrium by mitogen-activated protein kinases in normal, hyperplastic, and carcinomatous human prostate. Hum Pathol. 2002;33:299–306. doi: 10.1053/hupa.2002.32227. [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Malagarie-Cazenave S, Olea N, Vara D, Chiloeches A, Diaz-Laviada I. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis. 2007;12:2013–2024. doi: 10.1007/s10495-007-0119-z. [DOI] [PubMed] [Google Scholar]
- Schilit S, Benzeroual KE. Silodosin: a selective alpha1A-adrenergic receptor antagonist for the treatment of benign prostatic hyperplasia. Clin Ther. 2009;31:2489–2502. doi: 10.1016/j.clinthera.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Schwinn DA, Roehrborn CG. Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int J Urol. 2008;15:193–199. doi: 10.1111/j.1442-2042.2007.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins JL, De SK, Machleidt T, Becattini B, Vazquez J, Kuntzen C, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci U S A. 2008;105:16809–16813. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgare AR, Isaacs JT. Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res. 2004;64:6190–6199. doi: 10.1158/0008-5472.CAN-04-0968. [DOI] [PubMed] [Google Scholar]
- Wadsworth TL, Carroll JM, Mallinson RA, Roberts CT, Roselli CE. Saw palmetto extract suppresses insulin-like growth factor-I signaling and induces stress-activated protein kinase/c-Jun N-terminal kinase phosphorylation in human prostate epithelial cells. Endocrinology. 2004;145:3205–3214. doi: 10.1210/en.2003-1716. [DOI] [PubMed] [Google Scholar]
- Walther S, Strittmatter F, Roosen A, Heinzer F, Rutz B, Stief CG, et al. Expression and alpha1-adrenoceptor regulation of caldesmon in human prostate smooth muscle. Urology. 2012;79:745.e5–745.e12. doi: 10.1016/j.urology.2011.10.053. [DOI] [PubMed] [Google Scholar]
- Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L245–L253. doi: 10.1152/ajplung.00068.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi O. Response of bladder smooth muscle cells to obstruction: signal transduction and the role of mechanosensors. Urology. 2004;63(3) Suppl. 1:11–16. doi: 10.1016/j.urology.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Zhai W, Eynott PR, Oltmanns U, Leung SY, Chung KF. Mitogen-activated protein kinase signalling pathways in IL-1 beta-dependent rat airway smooth muscle proliferation. Br J Pharmacol. 2004;143:1042–1049. doi: 10.1038/sj.bjp.0705971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YX, Kong CZ, Wang HQ, Wang LH, Xu CL, Sun YH. Phosphorylation of Bcl-2 and activation of caspase-3 via the c-Jun N-terminal kinase pathway in ursolic acid-induced DU145 cells apoptosis. Biochimie. 2009;91:1173–1179. doi: 10.1016/j.biochi.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Zhou MS, Schulman IH, Chadipiralla K, Raij L. Role of c-Jun N-terminal kinase in the regulation of vascular tone. J Cardiovasc Pharmacol Ther. 2010;15:78–83. doi: 10.1177/1074248409354603. [DOI] [PubMed] [Google Scholar]


