Abstract
β1 integrin regulates the response of both normal and cancer cells to their local environment. Although mis-localised in prostate cancer, the role β1 integrin plays in prostate development and carcinogenesis remains unknown. To assess the role of β1 integrin in vivo, we conditionally deleted β1 integrin from prostate epithelium and subsequently crossed these mice to the TRAMP prostate carcinogenesis model. Deletion of β1 integrin following castration and subsequent androgen supplementation resulted in an expansion of the p63-positive basal cell population and decreased differentiation. Consistent with these findings, deletion of β1 integrin in TRAMP mice decreased animal survival, decreased retention of normal prostate morphology, increased the percentage of tissue with poorly differentiated carcinoma, and increased cell proliferation. This study demonstrates that β1 integrin regulates several aspects of normal prostate development and in contrast to its role in several other tissues, its loss is associated with increased rates of prostate tumour progression.
Prostate cancer is responsible for the second highest number of cancer-related deaths, after lung cancer and statistically 1 in 33 men will die from the disease1. Prostate cancer is thought to arise in the secretory glandular cells of the prostate, predominately producing adenocarcinomas. Associated initially with the precursor lesion prostatic intra-epithelial neoplasia (PIN) the disease can progress through to castration-resistant metastatic disease, for which few therapeutic options exist. It is imperative therefore, to understand the gene and protein changes that contribute to the progression from normal prostate tissue, PIN, adenocarcinoma, and eventually, androgen-independence and metastases.
A cell's survival and proliferative ability is usually governed by its environment, mediated by extracellular matrix receptors, which provide positional context and homeostatic signals. Integrins connect a cell to its local environment, and although lacking intrinsic kinase activity, mediate signals through the recruitment of a multitude of additional proteins2. Aberrant integrin-growth factor signalling has been identified in most cancers, and is thought to modulate tumour initiation and progression, and ultimately, metastasis3. Integrins regulate cell polarity, adhesion, survival, and proliferation, all processes which when dys-regulated, influence tumour phenotype. Deletion of β1 integrin in mouse models of breast and pancreatic cancer impair tumourigenesis and metastasis4,5,6. While use of function-blocking β1 integrin antibodies in 3D cultures of breast cancer cell lines has been shown to revert cancer phenotype, resulting in cancer cells with a more normal morphology and decreased proliferation7,8,9, making integrins valid targets for therapeutics.
At a cellular level the prostate is comprised of a hormone-responsive polarised secretory luminal epithelium, surrounded by a myo-epithelial layer, in contact with a laminin-rich basement membrane. β1 integrin is widely expressed and known to control many developmental processes2, and in the adult prostate it is expressed predominantly on the baso-lateral surfaces of the epithelial cells10. Studies utilising either primary, or immortalised, prostate epithelial cells have demonstrated a role for β1 integrin in regulating differentiation in prostate cells. Function-blocking antibodies to β1 integrin in 3D cultures of RWPE-1 cells significantly reduces acinar formation11. Similarly, β1 integrin was demonstrated to negatively regulate prostate epithelial cell differentiation in primary human cell cultures12, indicating a potential, but untested, role for β1 integrin in prostate development and function in vivo.
β1 integrin expression is both up-regulated and mis-localised during progression of prostate cancer10,13, demonstrating the potential clinical importance of the undefined role of β1 integrin in prostate cancer. Although a number of studies have investigated the roles of β1 integrin using prostate cancer cell lines14,15,16,17,18,19, the role of β1 integrin in signalling between the melee of extracellular matrix proteins and the prostate cell remains undefined, making it imperative to examine this protein in the context of the normal (or in cancer, aberrant) cellular environment and extracellular matrix.
To redress this absence of in vivo data, we have used transgenic mouse models. The TRansgenic Adenocarcinoma of Mouse Prostate (TRAMP) model14 is one of the best characterised animal models of prostate tumourigenesis, effecting a reproducible and hormone-responsive model of prostate cancer which closely mimics the human disease. In this model the activity of the p53 and Rb tumour suppressor genes is abrogated in prostate epithelial cells by driving expression of the simian virus 40 early T/t antigen genes, from the prostate-specific probasin promoter. TRAMP mice develop PIN and proliferative lesions that progress from well-differentiated carcinoma (WDC) to poorly-differentiated carcinoma (PDC) and eventually undergo metastasis to the lungs and lymph nodes14,15,16. Using ARR2PBi-Cre17 transgenic mice, which drive high level Cre expression under the control of a composite androgen-responsive probasin promoter to all lobes of the prostate and LoxP-flanked β1 integrin (Itgb1fl/fl) mice18, we have specifically ablated β1 integrin in the prostate epithelium, enabling us to examine the effect of loss of this receptor during both normal tissue maintenance, and in the context of TRAMP-mediated prostate tumour formation and progression.
Given the evidence presented above, we hypothesised that β1 integrin signalling is required for normal prostate development, and furthermore, that by ablating its expression, prostate tumour progression can be delayed or moderated.
Results
Deletion of β1 integrin in prostate epithelium
Prostate epithelial specific β1 integrin null mice were generated by crossing Itgβ1fl/fl18 mice to ARR2PBi-Cre (Cre)17 transgenic mice. The specificity of Cre-mediated recombination was determined using PCR analysis, and showed that DNA from wild-type mice Itgβ1fl/fl;Cre+/+ (Itgβ1fl/fl;WT) yields only the 280 bp product from the floxed allele, whereas Itgβ1fl/fl;Cretg/+ (Itgβ1fl/fl;Cre) animals also yield a recombination product of approximately 300 bp, in addition to the Cre-transgene product (100 bp) (Figure 1a). Immuno-histochemical analyses of wild-type prostatic tissue shows the expected baso-lateral staining of epithelial cells (arrows), as well as expression in the stroma (Figure 1b). Examination of β1 integrin protein by immuno-histochemistry in Itgβ1fl/fl;WT and Itgβ1fl/fl;Cre animals reveal specific ablation of β1 integrin expression in the luminal epithelial cells, but β1 integrin retention within the stromal tissue in Cre-expressing animals (Figures 1c and d). This is consistent with the presence of Cre staining within the epithelial cells, but not the stroma (Figures 1e and f).
Figure 1. Specific deletion of β1 integrin in prostate epithelium.
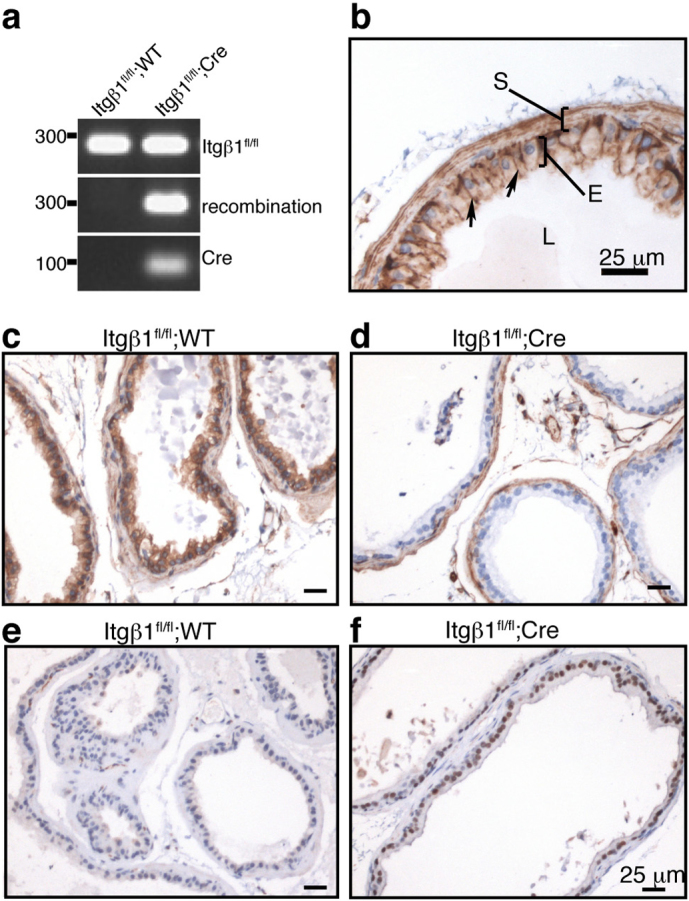
a) PCR analysis of Cre-mediated recombination in prostate glands of Itgβ1fl/fl;WT and Itgβ1fl/fl;Cre animals at 26 weeks of age. The 280 bp product labelled Itgβ1fl/fl indicates the presence of the floxed allele. Also shown are the 300 bp amplicon resulting from recombination of the floxed allele, and the 100 bp product demonstrating the presence of the Cre transgene. b) β1 integrin immuno-staining in the dorsal prostate of a 20 wk WT mouse. β1 integrin is present predominantly on the baso-lateral surfaces of the luminal epithelial (E) cells (arrows), but is also present at the cell membrane of stromal cells (S). c & d) β1 integrin staining of the ventral prostates of 26 wk old Itgβ1fl/fl;WT (c) and Itgβ1fl/fl;Cre (d) animals, demonstrating almost complete ablation of β1 integrin within the epithelium (d), but intact stromal staining. e) Cre staining of prostates of 26 wk old Itgβ1fl/fl;WT (e) and Itgβ1fl/fl;Cre (f) animals. Scale bars in b, c and d, 25 μm.
β1 integrin regulates the basal epithelial cell population during castration/ testosterone-driven development
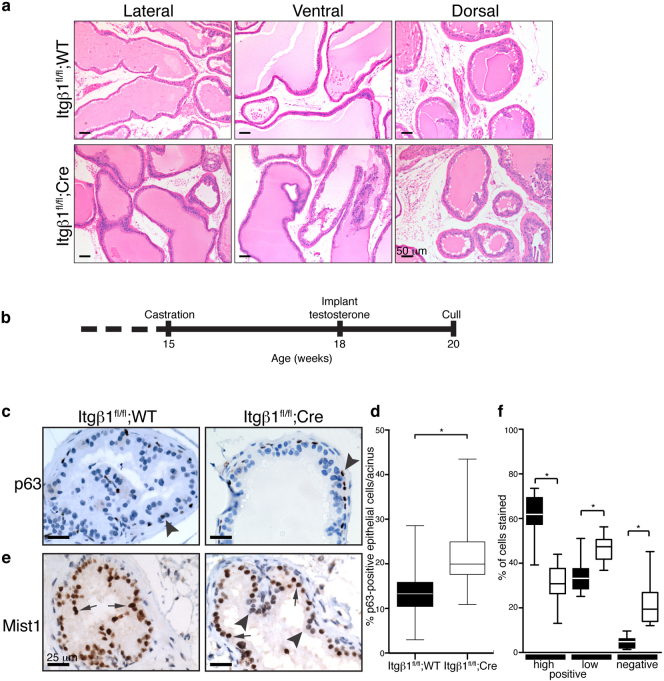
Expression of ARR2PBi-Cre is strongest post-puberty, as a result of the androgen-β1 integrin plays in normal prostate tissue maintenance, we examined ventral, lateral, and dorsal prostate lobes at 9, 26 (Figure 2a), and 60 weeks of age, using H&E staining. These analyses reveal no gross morphological differences between the two genotypes, indicating no essential requirement for β1 integrin in post-pubertal prostate development, nor in prostate tissue maintenance.
Figure 2. Ablation of β1 integrin does not affect prostate tissue maintenance, but perturbs epithelial cell population numbers and differentiation in androgen-rescued castrated mice.
a) H&E staining of 26 wk old Itgβ1fl/fl;WT and Itgβ1fl/fl;Cre prostates reveal no gross morphological differences between the genotypes. Scale bar, 50 μm. b) Schematic representation of the timeline used for castration, and subsequent testosterone supplementation. c) p63 immuno-staining of dorsal prostates reveal an up-regulation of the number of basal epithelial (p63-positive, arrowheads) cells in Itgβ1fl/fl;Cre animals. Scale bar, 25 μm. d) Quantitation of the number of p63- stained cells per acinus was performed, and is represented as a percentage of total epithelial cells. * p<0.0001. e) Immuno-staining using anti-Mist1 antibodies reveals almost all luminal epithelial cells to be strongly stained in Itgβ1fl/fl;WT tissue (arrows), whereas in Itgβ1fl/fl;Cre samples, a number of luminal cells have low/absent staining (arrowheads), possibly indicating a delay or defect in differentiation caused by loss of β1 integrin. f) Quantitation of Mist1 staining in Itgb1fl/fl;WT (dark boxes) and Itgβ1fl/fl;Cre animals (white boxes). Cells were judged to be positive (high and low) or negative for Mist1 staining. *p<0.0001 Scale bar, 25 μm.
We next used castration and subsequent testosterone supplementation (Figure 2b) as a means to recapitulate the programme of growth and differentiation that usually occurs during development, in the absence of β1 integrin. No differences in the masses of the prostate lobes between Itgβ1fl/fl;WT and Itgβ1fl/fl;Cre animals (data not shown), nor gross morphological alterations between the two genotypes (see Supplementary Fig. 1 online) were observed. However, histological analysis revealed an apparent increase in the number of cells with an epithelial basal morphology in Itgβ1fl/fl;Cre mice. p63 is a marker of the basal cell population19,20,21,22, and IHC staining for p63 (Figure 2c, arrowhead) and subsequent quantification (Figure 2d) revealed an increase in the percentage of p63-stained epithelial cells/acinus (22.14±0.91 vs 13.6±0.65, p<0.0001, Figure 2d) in mice lacking β1 integrin compared to control mice.
The basic helix-loop-helix transcription factor, Mist1, has been proposed to regulate serous exocrine cell differentiation23. IHC using an antibody against Mist1 showed that the majority of luminal epithelial cells in Itgβ1fl/fl;WT animals were positive for Mist1 expression (Figure 2e, arrows), whereas Itgβ1fl/fl;Cre tissue reveals a significant number of cells with low or absent expression (34.01±2.03% and 4.51±0.70% versus 46.55±1.63% and 22.11±2.86%, Itgβ1fl/fl;Cre and Itgβ1fl/fl;WT, respectively; Figure 2e, arrowheads; and quantified in Figure 2f), indicating that loss of β1 integrin alters prostate epithelial cell populations and results in a reduction in the number of differentiated luminal epithelial cells.
Expression of β1 integrin during prostate carcinogenesis in TRAMP mice parallels β1 integrin expression in human prostate cancer
β1 integrin has been identified in a number of studies as being both mis-localised and up-regulated in expression during prostate cancer progression10,13. To investigate the role that this protein plays in prostate cancer and the validity of utilising the TRAMP model with respect to β1 integrin function during prostate carcinogenesis, we examined β1 integrin protein levels during prostate cancer progression in TRAMP mice. Prostates were dissected out, and one half paraffin-embedded, the other half lysed for protein. Using H&E analyses to grade the prostate tumour morphology, protein was extracted from the corresponding half and subjected to western analysis. Consistent with the previous observations of Goel and colleagues24, β1 integrin was expressed in normal mouse prostate (Figure 1b), with protein levels increasing during the progression from prostatic intraepithelial neoplasia into well-differentiated carcinoma. However, as the tumour progresses, and becomes poorly differentiated (poorly-differentiated carcinoma), β1 integrin expression is lost (24 and see Supplementary Fig. 2 online)
Loss of β1 integrin in the TRAMP prostate cancer model decreases animal survival
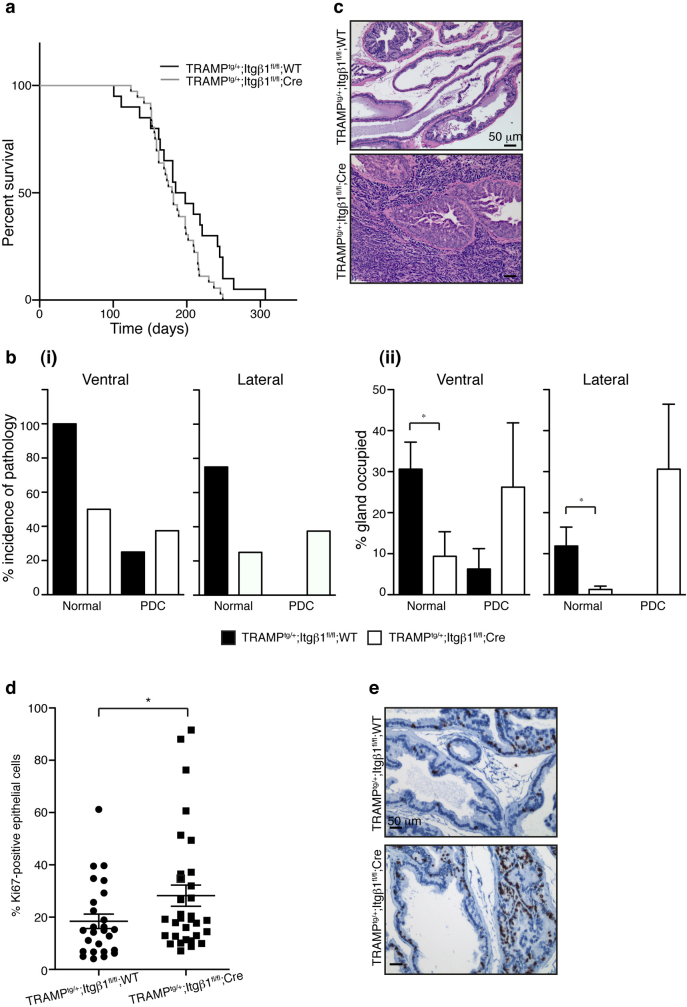
To investigate the role of β1 integrin in prostate cancer progression, Itgβ1fl/fl;WT and Itgβ1fl/fl;Cre mice were crossed to TRAMPtg/+ mice14. Longitudinal survival analysis studies were performed. Kaplan-Meier analysis revealed that loss of β1 integrin in this mouse model of prostate cancer resulted in a small decrease in survival (Figure 3a; p = 0.05), with a median survival time of 191.5 vs. 181.0 days (TRAMPtg/+;Itgβ1fl/fl;WT (n = 20) versus TRAMPtg/+;Itgβ1fl/fl;Cre (n = 36)). No difference in the occurrence of lung, liver, or peri-aortic lymph node, metastases, upon visual inspection was observed between the genotypes (data not shown).
Figure 3. Loss of β1 integrin enhances progression of prostate cancer in TRAMPtg/+ mice.
a) Kaplan-Meier analysis of survival of TRAMPtg/+;Itgβ1fl/fl;WT versus TRAMPtg/+;Itgβ1fl/fl;Cre mice. Ablation of β1 integrin results in a decreased median survival time, from 191.5 days (TRAMPtg/+;Itgβ1fl/fl;WT) to 181 days (TRAMPtg/+;Itgβ1fl/fl;Cre), p = 0.05. b) Analysis of 15 wk old prostates reveals less normal-appearing epithelium in the lateral and ventral prostates of TRAMPtg/+;Itgβ1fl/fl;Cre animals, and more poorly differentiated carcinoma (PDC), than their TRAMPtg/+;Itgβ1fl/fl;WT littermates, as measured by both incidence of the pathology (i), and percentage of the gland occupied (ii). c) H&E staining of 15 wk old TRAMPtg/+;Itgβ1fl/fl;WT and TRAMPtg/+;Itgβ1fl/fl;Cre ventral prostates, showing presence of normal and low grade PIN in the former, and PDC in the latter. d) Quantitation of Ki67-stained epithelial cells from 18 wk old prostates, represented as a percentage of total epithelial cells. *p = 0.0613 e) Ki67 staining of 18 wk old TRAMPtg/+;Itgβ1fl/fl;WT and TRAMPtg/+;Itgβ1fl/fl;Cre ventral prostates. Scale bars 50 μm.
Loss of β1 integrin results in earlier, more aggressive tumours
To further characterise the effect of deleting β1 integrin in TRAMP-mediated prostate carcinogenesis, we performed a cross-sectional analysis. Prostate lobes were collected, paraffin-embedded, and histological examination performed using H&E staining. Tissue was graded according to Gingrich et al.,15. We first scored the percentage of mice within the cohort presenting with a particular pathology (ie normal, PIN, WDC, PDC) regardless of the percentage of the gland occupied by the pathology/lesion. At 15 weeks, the ventral prostates of all of the TRAMPtg/+;Itgβ1fl/fl;WT mice retained at least some normal-appearing prostate epithelium, whereas only 50% of TRAMPtg/+;Itgβ1fl/fl;Cre mice retained regions of normal prostate tissue (Figure 3b(i)). Within the same cohort of animals, 37.5% of the TRAMPtg/+;Itgβ1fl/fl;Cre mice had progressed to PDC, (Figure 3b(i)) in the lateral prostate, a pathology not observed in the lateral prostates of TRAMPtg/+;Itgβ1fl/fl;WT mice (Figure 3c).
We next scored the percentage of the prostate containing a particular pathology. The percentage of the glands occupied by normal-appearing prostate tissue was decreased in both the ventral (9.4±6% TRAMPtg/+;Itgβ1fl/fl;Cre vs 30.6±6.6% TRAMPtg/+;Itgβ1fl/fl;WT p = 0.0141 and lateral (1.3±1% TRAMPtg/+;Itgβ1fl/fl;Cre vs 11.9±4.6% TRAMPtg/+;Itgβ1fl/fl;WT p = 0.0206) prostate of TRAMPtg/+;Itgβ1fl/fl;Cre animals compared to controls (Figure 3b(ii)). In addition, 26.3±15.6% and 30.6±15.8% of the TRAMPtg/+;Itgβ1fl/fl;Cre ventral and lateral prostates respectively were scored as PDC compared to 6.3±5% in the ventral and not present in the lateral prostates of TRAMPtg/+;Itgβ1fl/fl;WT mice. These data indicate that the decreased average survival time observed in β1 integrin null mice is, presumably due to the increased proportion of tissue with higher grade lesion.
Deletion of β1 integrin increases prostate epithelial cell proliferation
β1 integrin deletion has been shown to suppress cell proliferation in two mouse mammary tumourigenesis models4,6 subsequently resulting in impaired tumourigenesis. Decreased survival times and an enhancement in progression of pathological grade following loss of β1 integrin in the prostate prompted an examination of the proliferative status of these tumours. Quantification of Ki67 expression levels by IHC (representative images of Ki67 staining are shown in Figure 3f), demonstrated a consistent increase in cell proliferation in TRAMPtg/+;Itgβ1fl/fl;Cre prostates relative to controls at 15 (data not shown) and 18 weeks (28.2±7.7% TRAMPtg/+;Itgβ1fl/fl;Cre vs 18.4±2.4% TRAMPtg/+;Itgβ1fl/fl;WT p = 0.0613) (Figure 3d), consistent with the increased tumour progression observed in our cross-sectional and survival analysis.
Discussion
We have detailed herein the first conditional deletion of β1 integrin from mouse prostate epithelium, allowing us to investigate the role of this extra-cellular matrix receptor in the context of the signalling and extra-cellular environment of the prostate during development, in tissue maintenance, and utilised the TRAMP model to gain insight into its role in prostate cancer.
β1 integrin has been shown to play an essential role in the regulation of cell phenotype and function in a number of different systems (reviewed in25) including mammary gland, where conditional deletion has demonstrated a critical role for β1 integrin in maintaining the structural integrity of alveoli and regulating epithelial cell proliferation and differentiation during glandular development26,27. In contrast to these tissues, histological analyses of animals lacking expression of β1 integrin in prostate epithelium reveal no requirement for the receptor in normal tissue maintenance, possibly indicating functional compensation from other β integrins expressed in prostate epithelium (reviewed by Goel and colleagues28), or that β1 integrin is functionally redundant once an appropriate basal lamina has formed in development.
To further test the requirement of β1 integrin in the prostate, we used castration and subsequent androgen supplementation, to force prostate regeneration and remodeling in the absence of β1 integrin. The defect in differentiation observed is consistent with studies implicating β1 integrin as required for terminal differentiation. The role of β1 integrin in keratinocyte differentiation has been extensively studied, revealing that reduction of β1 integrin is required to both reduce adhesion to the basement membrane, and in turn, initiate differentiation29. This has been further dissected to show that adhesion to extra-cellular matrix proteins suppresses terminal differentiation30,31. Studies performed by Bagutti and colleagues32 indicate that β1 integrin is required to enhance sensitivity of keratinocytes to factors secreted by dermal fibroblasts, such as Keratinocyte Growth Factor (KGF or FGF7), indirectly demonstrating an interaction between β1 integrin and the KGF receptor (FGFR2IIIb). KGF and β1 integrin have also been shown to act in concert to regulate differentiation of primary human prostate epithelial cells in culture12. KGF is a paracrine regulator of ductal growth and branching morphogenesis of the developing prostate33, and treatment of primary human prostate epithelial cells with KGF induces both a decrease in α2β1 integrin expression, as well as an increase in the proportion of cells expressing prostate epithelial differentiation markers. This differentiation effect is restricted to the CD133− population, thought to represent the transit amplifying cells, thus enabling the prostate to continue to produce both a differentiated epithelial compartment, as well as maintain the (CD133+) stem cell population. The increase in p63-positive basal epithelial cells observed in our Itgβ1fl/fl;Cre animals after castration and testosterone supplementation may be indicative of a temporary increase in proliferation (although no differences in Ki67 were observed (data not shown), similar to that observed with β1 integrin blockade by Heer and colleagues12) which, with sufficient exposure to paracrine factors such KGF, results in delayed, but not inhibited, differentiation, as evidenced by the decrease in Mist1 staining.
Utilising the TRAMP model of prostate carcinogenesis, increased percentages of higher tumour grades were observed in β1 integrin-deleted animals (Figure 3b), as were higher proliferative rates (Figure 3c). These alterations are of pathological relevance, resulting in a small decrease in median survival time, as indicated by Kaplan-Meier survival analysis (Figure 3a). Using a second mouse model of prostate cancer (in which PTEN is deleted from prostate epithelium34) to further investigate the role of β1 integrin, suggests that similar to the TRAMP model, deletion of β1 enhances progression of tumourigenesis (preliminary results; data not shown). This is in contrast to several studies performed using β1 integrin deletion in mouse tumourigenesis models, such as breast, in which loss of β1 results in a decrease in proliferation, a block of tumour induction6, or a decrease in metastatic potential4. However, it should be noted, that unlike in prostate cancer, where β1 integrin levels initially increase with loss of differentiation10,13, β1 integrin levels decrease in breast cancer35, and that β1 integrin levels do not correlate with patient survival in breast cancer mRNA expression arrays36. β1 integrin also regulates normal mammary epithelial cell proliferation and morphology during mammopoiesis26, whereas we have demonstrated here that ablation of β1 integrin in the prostate has no effect on proliferation, or gland morphology, and a mild effect on differentiation following castration/androgen supplementation.
A number of factors complicate the study of individual integrins both in vitro and in vivo. Firstly, integrins are obligate heterodimers, requiring an alpha and a beta sub-unit. The functional redundancy of specific α/β heterodimers has been well characterised. In the mouse mammary gland, deletion of either α3 or α6, both partners of β1 integrin, has no effect on mammary gland development37, whereas deletion of β1 integrin, which results in ablation of both α3β1 and α6β1, has a significant mammary phenotype27. In addition, while complete deletion of β1 integrin inhibits tumour growth and metastasis, targeting of the α2β1 heterodimer has the opposite effects in breast cancer cells36, indicating both context and heterodimer specific roles for individual integrins. In prostate cancer, the likely β1 integrin heterodimers present are α2β1 and α6β1, given the aberrant expression of both α2 and α6 sub-units (reviewed in28), deletion of the β1 sub-unit is likely to have knock-on effects. The increase in tumour progression and cell proliferation observed in this study following β1 integrin deletion in the prostate, is consistent with α2β1 integrin being the dominant integrin heterodimer in the prostate.
Finally, β1 integrin also has a number of different isoforms, of which β1a and β1c have been reported in human prostate38,39,40. The β1c isoforms however, are not present in the mouse genome41,42. Differing in the cytoplasmic tail, and therefore altering binding partners, experiments performed in prostate cell lines indicate opposing roles for these isoforms, inducing and inhibiting cellular proliferation, and decreasing and enhancing adhesion to the basement membrane, respectively24,43,44,45,46. While the roles played by individual isoforms of β1 integrin have yet to be elucidated, our results indicate that β1 integrin acts to modulate proliferation in prostate cancer, with its loss resulting in enhanced tumour progression in the TRAMP mouse model. From work performed on human prostate cancer cell lines, Goel and colleagues44 have hypothesised that re-expression of the β1c integrin isoforms may be sufficient to revert a neoplastic phenotype to a non-proliferative and highly adherent phenotype. Our results presented here are consistent with the idea that human prostate tumour progression is driven not by increasing expression of β1a integrin, but rather, by the loss of β1c integrin.
We conclude that although previous studies have indicated a requirement for β1 integrin in tumour cell proliferation and differentiation, our studies in mouse prostate epithelium indicate that β1 integrin regulates epithelial cell specification and differentiation in normal prostate, and that deletion of β1 integrin in prostate cancer can result in enhanced tumourigenesis and proliferation. Therefore, therapeutic strategies aimed at inhibiting this receptor in prostate cancer may prove not to be beneficial, and that downstream pathways, such as those mediated by Integrin-linked kinase or Focal adhesion kinase, should be investigated.
Methods
Mouse strains and breeding
The animal experimentation described within this study was approved by the Garvan Institute and St Vincent's Hospital Animal Ethics Committee.
The Itgβ1fl/fl, TRAMPtg/+, and ARR2PBi-Cre mice have been described previously14,17,18. Animals were crossed as described in the relevant sections, and where possible, the Cre and TRAMP transgenes were carried by the female parent to avoid any potential issues with fertility.
Genotyping was performed for Itgβ1fl/fl, TRAMPtg/+, and ARR2PBi-Cre, as outlined by the Jackson Laboratory. Recombination of the Itgβ1fl/fl locus was confirmed as previously described47 using primers: b1-5 Forward 5′-CGCAGAACAATAGGTGCTGAAATTAC-3′ and b1-3 Reverse 5′-CCACAACTTTCCCAGTTAGCTCTC-3′ which are located either side of the loxP insertion sites. A 300 bp amplicon is produced when recombination has occurred.
Morphological, histological, and immuno-histochemical analyses
Lateral, ventral, dorsal, and anterior (coagulating gland) prostate lobes were removed from mice, and one of each lobe fixed in 10% buffered formalin for 4 hours, transferred to 70% ethanol overnight, before being embedded in paraffin (the remaining lobes were flash frozen with liquid nitrogen). Morphological analyses were performed by examination of 4 μm sections stained with haematoxylin and eosin (H&E). For immunohistochemical analysis, endogenous peroxidase activity was blocked using 3% hydrogen peroxide. Antibodies and the retrieval methods used were: β1 integrin, 1/20000 dilution (Millipore, Clone MB1.2) Dako s2367, pressure cooker retrieval; Mist1, (C. Pin, University of Western Ontario, Canada) 0.05% citraconic anhydride, pressure cooker retrieval; p63, 1/100 dilution (Dako Clone 4A4) 0.05% citraconic anhydride, pressure cooker retrieval; and Ki67, 1/100 dilution (Lab Vision Neomarkers, Clone SP6), Dako s1699, pressure cooker retrieval. Primary antibodies were detected using HRP-conjugated secondary antibody, and DAB detection. Sections were counter-stained with haemotoxylin.
Quantification of Ki67 and p63 was performed by taking either >6 random low magnification images, or images of >10 acini, respectively, and using ImageJ to count the stained and unstained epithelial cells.
Castration and testosterone supplementation
Five animals of each genotype (Itgβ1fl/fl;WT and Itgβ1fl/fl;Cre) were castrated at 15 weeks of age. Three weeks post-surgery, testosterone pellets (5 mg, 21 day release; Innovative Research of America) were implanted subcutaneously in the upper back of mice. Animals were sacrificed 2 weeks post-supplementation for analysis. A small number of castrated animals were sacrificed at 18 weeks to assess the extent of involution.
Survival and statistical analysis
For survival analyses, animals were sacrificed once tumour burden was either determined to reach 10% initial body weight, or cause distress to the animals (as dictated by the Garvan Animal Ethics guidelines, including hunched posture, poor coat condition, difficulty breathing, and failure to respond to stimuli), requiring euthanasia. For cross-sectional analyses, lateral, ventral, dorsal, and anterior prostate lobes were collected at 9, 12, 15, 18, and 24 weeks of age, with at least 8 animals of each genotype for each time point. Liver, lung, kidney, and peri-aortic lymph nodes were examined visually at necropsy for metastases.
Data, where indicated, are represented as mean±SEM, and statistical analysis performed using Student's t-test and GraphPad Prism software. Kaplan-Meier survival analysis and the Mantel-Cox log-rank test were performed using GraphPad Prism software.
Author Contributions
KMJ and MN conceived and designed the experiments. KMJ and AL executed the experiments. KMJ, AL and MN analysed the data. KMJ and MN wrote the manuscript. All authors reviewed the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We would like to thank Dr Christopher Pin (University of Western Ontario, Canada) for provision of the Mist1 antibody, Dr Fen Wang (Texas A&M Health Science Center, USA) for the provision of the ARR2PBi-Cre mice, Alice Boulghourjian (Garvan Institute, Australia) for histological support, and A/Prof Chris Ormandy (Garvan Institute, Australia) for his helpful discussion in relation to this study. Financial support for this study was provided by the Cancer Council NSW, Cancer Institute NSW, Prostate Cancer Foundation of Australia, Australian Cancer Research Foundation and the National Health & Medical Research Council.
References
- Jemal A. et al. Cancer statistics, 2009. CA Cancer J Clin 59, 225–249 (2009). [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002). [DOI] [PubMed] [Google Scholar]
- Guo W. & Giancotti F. G. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 5, 816–826 (2004). [DOI] [PubMed] [Google Scholar]
- Huck L., Pontier S. M., Zuo D. M. & Muller W. J. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci U S A 107, 15559–15564 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren A. et al. Increased tumor cell dissemination and cellular senescence in the absence of beta1-integrin function. EMBO J 26, 2832–2842 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. E. et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170 (2004). [DOI] [PubMed] [Google Scholar]
- Park C. C. et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res 66, 1526–1535 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Hansen R. K., Radisky D., Yoneda T., Barcellos-Hoff M. H., Petersen O. W., Turley E. A. & Bissell M. J. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst 94, 1494–1503 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver V. M. et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137, 231–245 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J. D. et al. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol 145, 167–174 (1994). [PMC free article] [PubMed] [Google Scholar]
- Bello-DeOcampo D., Kleinman H. K., Deocampo N. D. & Webber M. M. Laminin-1 and alpha6beta1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cells. Prostate 46, 142–153 (2001). [DOI] [PubMed] [Google Scholar]
- Heer R., Collins A. T., Robson C. N., Shenton B. K. & Leung H. Y. KGF suppresses alpha2beta1 integrin function and promotes differentiation of the transient amplifying population in human prostatic epithelium. J Cell Sci 119, 1416–1424 (2006). [DOI] [PubMed] [Google Scholar]
- Murant S. J. et al. Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur J Cancer 33, 263–271 (1997). [DOI] [PubMed] [Google Scholar]
- Greenberg N. M. et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A 92, 3439–3443 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J. R., Barrios R. J., Foster B. A. & Greenberg N. M. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis 2, 70–75 (1999). [DOI] [PubMed] [Google Scholar]
- Gingrich J. R. et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res 56, 4096-4102 (1996). [PubMed] [Google Scholar]
- Jin C., McKeehan K. & Wang F. Transgenic mouse with high Cre recombinase activity in all prostate lobes, seminal vesicle, and ductus deferens. Prostate 57, 160–164 (2003). [DOI] [PubMed] [Google Scholar]
- Raghavan S., Bauer C., Mundschau G., Li Q. & Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150, 1149–1160 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T., Medina R. T., Mills A. A. & Cunha G. R. Role of p63 and basal cells in the prostate. Development 131, 4955–4964 (2004). [DOI] [PubMed] [Google Scholar]
- Signoretti S. et al. p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci U S A 102, 11355–11360 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S. et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 157, 1769–1775 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A. et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2, 305–316 (1998). [DOI] [PubMed] [Google Scholar]
- Pin C. L., Bonvissuto A. C. & Konieczny S. F. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec 259, 157–167 (2000). [DOI] [PubMed] [Google Scholar]
- Goel H. et al. beta1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res 65, 6692–6700 (2005). [DOI] [PubMed] [Google Scholar]
- Brakebusch C. & Fassler R. beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev 24, 403–411 (2005). [DOI] [PubMed] [Google Scholar]
- Li N. et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J 24, 1942–1953 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M. J. et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 171, 717–728 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel H. L., Li J., Kogan S. & Languino L. R. Integrins in prostate cancer progression. Endocr Relat Cancer 15, 657–664 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A. J., Haase I. & Watt F. M. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci U S A 96, 6728–6733 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. C. & Watt F. M. Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature 340, 307–309 (1989). [DOI] [PubMed] [Google Scholar]
- Watt F. M., Kubler M. D., Hotchin N. A., Nicholson L. J. & Adams J. C. Regulation of keratinocyte terminal differentiation by integrin-extracellular matrix interactions. J Cell Sci 106 (Pt 1), 175–182 (1993). [DOI] [PubMed] [Google Scholar]
- Bagutti C., Hutter C., Chiquet-Ehrismann R., Fassler R. & Watt F. M. Dermal fibroblast-derived growth factors restore the ability of beta(1) integrin-deficient embryonal stem cells to differentiate into keratinocytes. Dev Biol 231, 321–333 (2001). [DOI] [PubMed] [Google Scholar]
- Sugimura Y. et al. Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. Int J Dev Biol 40, 941–951 (1996). [PubMed] [Google Scholar]
- Wang S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003). [DOI] [PubMed] [Google Scholar]
- Zutter M. M., Mazoujian G. & Santoro S. A. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol 137, 863–870 (1990). [PMC free article] [PubMed] [Google Scholar]
- Ramirez N. E. et al. The alphabeta integrin is a metastasis suppressor in mouse models and human cancer. J Clin Invest 121, 226–237 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinowska T. C. et al. Epithelial development and differentiation in the mammary gland is not dependent on alpha 3 or alpha 6 integrin subunits. Dev Biol 233, 449–467 (2001). [DOI] [PubMed] [Google Scholar]
- Fornaro M., Tallini G., Bofetiado C. J., Bosari S. & Languino L. R. Down-regulation of beta 1C integrin, an inhibitor of cell proliferation, in prostate carcinoma. Am J Pathol 149, 765–773 (1996). [PMC free article] [PubMed] [Google Scholar]
- Moro L., Perlino E., Marra E., Languino L. R. & Greco M. Regulation of beta1C and beta1A integrin expression in prostate carcinoma cells. J Biol Chem 279, 1692–1702 (2004). [DOI] [PubMed] [Google Scholar]
- Perlino E. et al. Regulation of mRNA and protein levels of beta1 integrin variants in human prostate carcinoma. Am J Pathol 157, 1727–1734 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino L. R. & Ruoslahti E. An alternative form of the integrin beta 1 subunit with a variant cytoplasmic domain. J Biol Chem 267, 7116–7120 (1992). [PubMed] [Google Scholar]
- Svineng G., Fassler R. & Johansson S. Identification of beta1C-2, a novel variant of the integrin beta1 subunit generated by utilization of an alternative splice acceptor site in exon C. Biochem J 330 (Pt 3), 1255–1263 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel H. L. et al. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by beta1 integrins. J Cell Biol 166, 407–418 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel H. L. et al. Beta1 integrins modulate cell adhesion by regulating insulin-like growth factor-II levels in the microenvironment. Cancer Res 66, 331–342 (2006). [DOI] [PubMed] [Google Scholar]
- Goel H. L., Underwood J. M., Nickerson J. A., Hsieh C. C. & Languino L. R. Beta1 integrins mediate cell proliferation in three-dimensional cultures by regulating expression of the sonic hedgehog effector protein, GLI1. J Cell Physiol 224, 210–217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed A., Alam N., Trerotola M. & Languino L. R. Insulin-like growth factor 1 stimulation of androgen receptor activity requires beta(1A) integrins. J Cell Physiol 227, 751–758 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S. et al. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci 26, 223–232 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information