Abstract
Mechanical cell stretching may be an attractive strategy for the tissue engineering of mechanically functional tissues. It has been demonstrated that cell growth and differentiation can be guided by cell stretch with minimal help from soluble factors and engineered tissues that are mechanically stretched in bioreactors may have superior organization, functionality, and strength compared with unstretched counterparts. This review explores recent studies on cell stretching in both two-dimensional (2D) and three-dimensional (3D) setups focusing on the applications of stretch stimulation as a tool for controlling cell orientation, growth, gene expression, lineage commitment, and differentiation and for achieving successful tissue engineering of mechanically functional tissues, including cardiac, muscle, vasculature, ligament, tendon, bone, and so on. Custom stretching devices and lab-specific mechanical bioreactors are described with a discussion on capabilities and limitations. While stretch mechanotransduction pathways have been examined using 2D stretch, studying such pathways in physiologically relevant 3D environments may be required to understand how cells direct tissue development under stretch. Cell stretch study using 3D milieus may also help to develop tissue-specific stretch regimens optimized with biochemical feedback, which once developed will provide optimal tissue engineering protocols.
Introduction
Mechanical stretching has been utilized to enhance the organization, functionality, and strength of engineered tissues.1–3 At the cellular level, mechanical stretch has demonstrated vital control over cell morphology, proliferation, lineage commitment, and differentiation.4–8 Cellular responses to stretch may vary by cell type and loading mode. Also, stretch stimulation of cells may depend on the properties of extracellular matrix (ECM) and the presence of soluble factors. Mechanotransduction, the conversion of mechanical signal into intracellular biochemical activity, occurs due to external tensile forces (outside in) and forces generated in cytoskeletons (inside out), which act as regulatory and exploratory cues, respectively.9 Signaling pathways of stretch-induced mechanotransduction have been examined using two-dimensional (2D) cultures, but few studies in three-dimensional (3D) constructs have explored mechanisms relevant for optimizing stretch-conditioned tissues. This review seeks to highlight and compare data of cell stretching for tissue engineering in both 2D and 3D environments, discuss the stretching devices employed, and briefly overview proposed mechanotransduction pathways.
Mechanical Cue and Homeostasis
Mechanical signals play a crucial role in homeostasis and tissue development. A disruption in the ability to properly respond to mechanical cues results in diseases, including arthritis, osteoporosis, developmental disorders, and cancer.10–13 Functional tissue engineering seeks to take advantage of the cell response to mechanical cues. Mechanical strain and stress in vivo are the key regulatory mechanical cues that guide cell morphogenesis and affect the healthy maintenance of tissues.14 The range of beneficial strain and stress varies with cell type, stage of cell development, and loading mode. For example, bone cells in vivo are exposed to compressive, tensile, and torsional stresses due to bone loading and to shear stress from interstitial flow.15 The magnitude of strain that developing woven bone tolerates from each stress mode varies from the strain magnitude necessary to increase lamellar bone mass. The maintenance of bone mass and microstructure in response to physiologically “healthy” strain and stress is achieved by proper osteocytic guidance of osteoblast and osteoclast activity.16 Outside the healthy strain and stress, bone resorption by osteoclasts overwhelms bone formation by osteoblasts, reducing bone mass and restructuring microarchitecture.17 Mechanically driven tissue remodeling is not unique to bone but is common throughout the tissues as an ongoing optimization process.
An example of this optimization process is the response of cardiac cells to mechanical cues in development processes, for both normal and pathological conditions. When a healthy equilibrium cannot be maintained, cardiac development is often perpetuated by a positive feedback loop produced by mechanical stimulation.18 The strain applied to and generated by cardiac cells regulates the structure of the heart at both cellular and organ levels through mechanisms involved in mechanotransduction. Similarly, vascular endothelial cells share characteristics of hemodynamic loading. Under cyclic stretch, endothelial cells increase stress filament area in response to shear stress and regulate autocrine and paracrine signaling for angiogenesis and vascular remodeling.19,20 These results on vascular homeostasis are significant for revealing the mechanism of mechanical control of vascular growth, regeneration, and remodeling in vivo.21 More in-depth reviews on mechanically driven homeostasis are available for cardiac,22,23 vasculature,20,24 bone,25 muscle,26 and tendon cells.27
Cell- and Tissue-Stretching Devices
Tensile stretching may elicit different cell responses than other stimulus modalities induce.6,28 Maul et al.6 recently compared the effects of stretch, shear stress, and pressure on mesenchymal stem cells (MSCs) and observed significant differences in cell morphology and lineage specification. MSCs subjected to cyclic stretch expressed smooth muscle cell phenotype in a dose-dependent manner coinciding with elongated cell shape, while MSCs exposed to fluid-flow-induced shear stress exhibited similar elongated morphology but did not express smooth muscle proteins. Compressed MSCs failed to express smooth muscle phenotype in cell shape and protein expression. Thus, well-defined experimental protocols with fully characterized devices for each stimulus mode are in need to compare cell-response data.
Stretching devices vary widely depending on the purpose and the mode of operation, ranging from basic devices used in research labs for brief stretching studies to bioreactors that aim to mechanically condition functional tissues over long time periods based on biophysical and biochemical feedback. Many lab-specific devices have been tailored to experimental needs. However, since the strain profile is affected by numerous factors, including the dimension of the stretchable membrane, tissue/scaffold properties, clamping method, mode of loading, and so on, each device requires strict characterization of the strain profile. However, this step has been often overlooked making data comparison among studies difficult. Table 1 lists the capabilities of some commercial and lab-specific stretching devices.
Table 1.
Selected Cell and Tissue Stretching Devices and Bioreactors
| Company or author | Mode of operation | Test parameters | Culture conditions | Sample size | Comments |
|---|---|---|---|---|---|
| Bose ElectroForce | Motor driven clamps | Force: up to 200 N; displacement: 6.35 mm; frequency: 0–2 Hz | Cell culture incubator compatible | Variable, bioreactor chamber dependent | Supports perfusion, torsional, and compressive loading; independent media loops; optional sensors for media pH, oxygen, etc. |
| Tissue Growth Technologies | Motor driven clamps | Force: up to 40 N; displacement: 20 mm | Cell culture incubator compatible | Multiple chamber sizes. 0.5 cm diameter, 15 cm long. 7×2×0.2 cm | Supports perfusion and compressive loading |
| Altman et al.36 | Motor driven clamps | Displacement: 2 mm; frequency: 0.0167 Hz; | Temperature control, gas exchanger | Greater than 6 mm diameter, 3 cm long | Supports perfusion, sheath flow, and torsional loading |
| Flexcell | Pneumatically deformed membrane | Strain: 1%–33%; frequency: 0.01–5 Hz; equibiaxial/uniaxial | Cell culture incubator compatible, no perfusion | 3 cm diameter membrane, ∼200 mm3 tissue | Supports attachment of 3D constructs to membranes |
| Wu et al.32 | Pneumatically deformed membrane | Strain: 0%–12%; frequency: 1 Hz; equibiaxial | Integrated perfusion system | 7 mm diameter membrane | High-throughput microbioreactor |
| Iwadate et al.33 | Membrane stretched by shape memory alloy | Strain: 0%–30%; frequency: 0.2 Hz | Cell culture incubator compatible | 4×2.2 cm membrane | Small and inexpensive; reduces unwanted vibrations |
| Pang et al.34 | Electromagnetical stretch | Strain: 20%; frequency: 1 Hz; uniaxial | Cell culture incubator compatible | 4×2×0.7 cm | Noncontact operation; may be used in standard labware; scalable |
3D, three-dimensional.
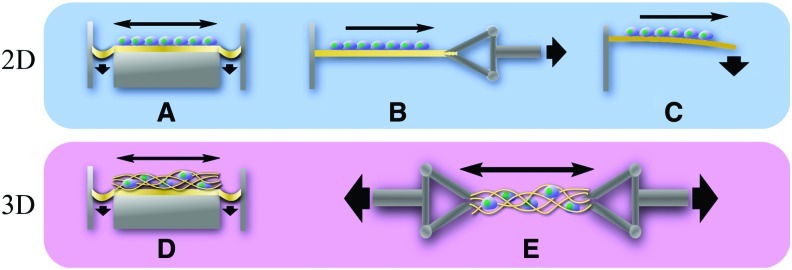
The most basic stretching devices involve pneumatically or electromechanically deformed membranes on which cells are seeded as a 2D layer (Fig. 1A, B) or to which 3D tissues and cell-cultured scaffolds are anchored (Fig. 1D). Most membrane-based devices are configured to deliver a uniform strain over the center of the membrane but the strain varies greatly outside the uniform strain region. These devices have typically low throughput and are suitable for small-scale studies since the tissue size is limited to a few cm2 and nutrient diffusion is hampered by the bounding membrane. Another 2D cell layer stretching employs a cantilevered beam subjected to bending (Fig. 1C),29,30 although this has not been commonly used for tissue engineering. For the regeneration of 3D functional tissues, a clamping method has been widely used (Fig. 1E).
FIG. 1.
Schematic of 2D and 3D stretching devices. Stretching of a 2D cell layer is achieved via pneumatic deformation of the membrane (A), clamp arm mechanism (B), and bending (C). Stretching of 3D cell–scaffold constructs is accomplished via pneumatic deformation (D) and clamping mechanism (E). 2D, two-dimensional; 3D, three-dimensional. Color images available online at www.liebertonline.com/teb
Novel stretch devices have been developed to increase the throughput and control over 2D stretching environments. Kamotani et al.31 developed a high-throughput stretching device, in which piezoelectric pins of a refreshable Braille display were programmed to actuate at specified frequencies to stretch microwell arrays of deformable membranes. Others have attempted to increase throughput and to make 2D stretch more relevant to tissue engineering by constructing bioreactors with small, pneumatically deformed membranes inside a perfusion system.32 Significant departures from pneumatic and motor-driven systems have used shape memory alloys or electromagnets to stretch the substrate.33,34 These new devices may offer benefits, such as reduction of external vibration for the case of shape memory and contact-free stretch capable within standard labware for the case of electromagnet. However, a potential disadvantage is that they could introduce more forms of unwanted interference, for example, transient temperature change from shape memory deformation or unwanted electromagnetic interference.
Devices that utilize clamping mechanism are more suited to condition a wider range of 3D functional tissues, since the tissue is not constricted by a membrane and the membrane does not interfere with the uniform diffusion of nutrients. Eliminating the membrane also obviates potential complications, for example, unwanted elastic recovery of the membrane and nonuniform strain that affects the edges of pneumatically deformed membrane. Motor-driven clamp mechanisms allow precise control over strain and strain rate, and the force is readily measured with common transducers. Possibly the largest advantage of the clamping system is the ability to stretch scaffolds with multiple loading modes. Altman et al.35 developed a stretch device housed in a reactor vessel with independent control over the applied stretch and torsion by stacking the linear actuator on the torsion platform. Stretching in 3D bioreactor system also posed a partial solution to the vascularization issue by creating a perfusion flow through the middle of the tissue as well as a sheath flow around the tissue.36
Many studies have used commercially available stretching devices, such as Flexcell (Flexcell International), ElectroForce (Bose), Strex (B-Bridge International), TGT Bioreactors (Tissue Growth Technologies), and so on. One of the advantages of these devices is these systems provide relatively well-characterized strain profiles.37 Adaptability to various stretching modes is also usually provided; for example, Flexcell device achieves uniaxial or equibiaxial stretch via using different loading posts and can perform 3D stretching by forming 3D gel in a pressure-driven temporary trough. However, these devices are generally low throughput, limiting the number of conditions to be tested simultaneously. Moreover, the tissue size to be stretched is often small and some systems offer nonlinear strain profiles causing uneven cell stimulation.
The strain that cells actually receive may vary with 2D and 3D stretches—cell location in the substrates, material properties and microstructure of the membrane and matrix, and the state of cell attachment. Matrix strain has been measured directly, or determined by using microscopy and finite element (FE) techniques.38–41 Cells receive varying degrees of compressive strain in a direction perpendicular to the stretch axis dependent on the Poisson ratio of the membrane or scaffold.42,43 Work in 3D FE has shown that the strain that cells actually receive can be much larger than that applied to the matrix due to variations in matrix microarchitecture.44 On the other hand, cell strain may be less than the given substrate elongation since cells are randomly distributed at arbitrary orientations with respect to the stretch direction. If cells are patterned to form predetermined 3D network structure or along the contact-printed 2D protein line pattern, one may precisely assess the effect of exact strain magnitude on cell response.45,46 If not, cell strain is usually smaller relative to membrane strain. A study analyzing strain homogeneity for the Flexcell device reported that although the cells in the middle portion of the membrane received relatively uniform strains, strain transferred to the cells was about half of that applied to the membrane.47 Stretching in 3D increases the difficulty in strain profile calculation, which requires rigorous characterizations using both imaging and FE approaches. A more focused review of 3D bioreactors is available in the literature.48–50
Stretching for Tissue Engineering
Cell stretching aims to engineer more functional and stronger tissues in regenerative medicine. A certain level of control over cell growth and differentiation and tissue integrity and strength may be accomplished through stretch stimulation alone.51 Stretch parameters, such as stretching mode (uniaxial, biaxial, and equiaxial), magnitude of strain, strain rate, frequency, stretch waveform (sinusoidal, ramp, and static), and insertion of rest periods, have been found to induce significant cell regulatory effects.52–58 These parameters have been studied vigorously in 2D, but relatively less systematic studies on these parameters as applied for the tissue engineering of 3D tissues have been done. This section reviews findings on 2D stretching that may be leveraged to engineer better tissues, and then highlights current 3D stretch studies.
2D stretching
Stretching of 2D cell layer has demonstrated that the organization of cells is affected by the nature of the loading applied, for example, frequency and strain rate. Cellular organization, an essential parameter for load bearing in tissue, affects the functionality of engineered tissues.59 A model developed by De et al.60 and verified and expanded upon by Hsu et al.61 attempted to explain the reorientation response of cells with bipolar morphologies to varying frequency of loading. The model predicted that cells reorganize focal adhesions and stress fibers to orient parallel to a static or quasi-static load with frequency < 1 Hz, but orient perpendicular to dynamic loads at frequencies of 1 Hz or more. It was proposed that cells seek to maintain stress/strain homeostasis and the orientation response is governed by the time scale required to remodel focal adhesions and stress fibers compared with the stretch frequency. These provided valuable insight into frequency-dependent orientation responses observed experimentally.42,62–64 Although strain homeostasis varies with cell type and is also influenced by cellular adaptive response to substrate stiffness, the saturation in frequency response seemed to occur around 1 Hz for various cells.62,65
Stretch frequency has also been shown to affect other cell behaviors than orientation, such as proliferation, apoptosis, and membrane permeability.66,67 Closely related to frequency, the strain rate of specific waveforms may be optimized to achieve desired results. Endothelial cells in vivo are subjected to unique mechanical stimuli consisting of flow-induced shear stress and tensile strain due to distension and stretching of the tissues. The 2D stretch study using human umbilical vein endothelial cells showed an intriguing observation that the asymmetric load waveform, which mimics the blood pressure wave by applying the velocity of extension to be greater than that of the release, induced most optimized endothelial remodeling.55 This was assessed by spindle-shaped cell morphology and increased cell orientation relative to symmetric load waveform data. Thus, establishing biomimicking stretch conditions with respect to strain rate and frequency would be beneficial for mechanical tissue regeneration.
Stretching may be used to control cell lineage commitment without the use of soluble factors. Differentiation to contractile cell lineages, such as skeletal, cardiac, and smooth muscle cells, may be aided or hindered by cyclic stretch depending on cell type and stretch regimen.68,69 Varying stretch modes induced differential effects on MSC lineage commitment; for example, uniaxial stretch transiently upregulated while equiaxial stretch downregulated smooth muscle contractile markers in MSCs, including smooth muscle α-actin and SM-22α.52 The differentiation markers along with changes in morphology and actin striation pattern indicated that stretch regimen (uniaxial vs. equiaxial) was sufficient to control MSC fate.
The differentiation response may also be controlled by strain magnitude, but as being dependent on the stage of development. Specifically, osteocytes in vivo experience amplification of the strain placed on the whole bone due to interstitial fluid drag force; for example, mathematical models have estimated that strain given to the bone can be amplified 20–100 times at osteocyte membrane.70 Given this and considering that bone homeostasis is determined by osteocyte guidance of the other bone cells,16 2D stretching studies have focused on the effect of strain magnitude on bone cells. Osteoblastic cells showed an increase in alkaline phosphatase (AP) activity at lower strain (∼0.8%) but the activity was unchanged at higher strain (∼3.2%), while an opposite trend was observed for osteocalcin, type I collagen, and core binding factor α1 (Cbfa1), which were greater at higher strain and corresponded to an increase in extracellular-signal-regulated kinase (ERK) phosphorylation.71 Reported strain levels for inducing MSC differentiation toward osteogenesis are not consistent, in which the stage of development and other environmental factors may play key roles. Applying equibiaxial stretch at or below 5% strain, MSCs displayed osteogenic differentiation markers such as increased AP activity and Cbfa1/Runx2 expression, while these markers were downregulated at higher strains of 10% and 15%.72 In another study, uniaxial strain of 0.2% has promoted the expression of osteogenic transcription markers (Cbfa1 and Ets-1) in MSCs.73
Cells may adapt to mechanical conditioning, resulting in unwanted long-term decline in stretch effects unless rest periods are strategically incorporated in the stretch regimen. To avoid this decline, stretch regimens for the tissue engineering of bone and ligament were optimized by incorporating rest periods.74–76 In a study using 2D and 3D stretching of adipose-derived MSCs and MG-63 osteoblasts,77 both cells displayed responses to short-term stretch (15 min to 1 h of stretch) by upregulating AP activity and osteogenic markers, but these effects returned to control level after 8 h of continuous stretch. When rest periods were inserted, cell mechanosensitivity could be recovered; for example, 8 h of rest period completely restored bone cell response to stretch and brief rest periods between each loading increased osteogenic response in stretched cells.58
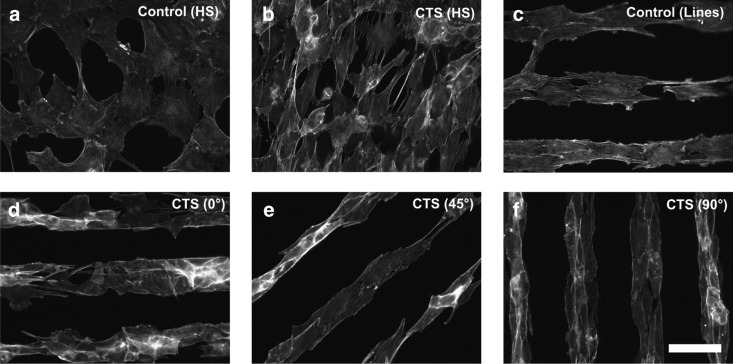
Directional dependence in 2D cell stretching is recently highlighted with micropatterned cells. Ahmed et al.46 patterned myoblasts on microcontact-printed fibronectin lines and applied cyclic stretch at 0°, 45°, and 90° to the patterned cell direction (Fig. 2). Interestingly, the greatest myoblast development was observed when stretch was applied at 45° of cell patterning direction, as was indicated by well-organized actin stress fibers and sarcomeric actin striation. Myoblasts that were stretched parallel to the cell patterning direction showed random cell orientation and actin stress fiber alignment oblique to the strain direction. Cells that were stretched perpendicular to the patterning direction did not display cell realignment to the stretch direction. Moreover, neither of parallel and perpendicular stretching induced striation patterns in actin. These data strongly suggest that the orientation of cells relative to stretching direction may greatly affect the “quality” of stretch in regulating cell functions.
FIG. 2.
Cell stretching in 2D affects cell orientation and cytoskeletal development, showing directional dependency. C2C12 myoblasts were cyclically stretched at 7% and 0.5 Hz in horizontal direction on membranes with micropatterned fibronectin lines. Staining with actin is shown. (a) Unpatterned (homogeneous [HS]), unstretched control had less well-developed actin fibers. (b) Stretching of unpatterned cells developed actin stress fibers oriented at an average angle of 72° (cyclic tensile strain [CTS]). (c) Patterned but not stretched cells showed actin fibers oriented along the fibronectin line pattern. (d) Stretch applied parallel to the patterned cell direction induced irregular cell orientation and formed actin stress fibers oblique to the strain direction (average actin fiber orientation of 48°). (e) Stretch applied to 45° of patterns caused an average actin fiber orientation of 52°. (f) Perpendicular stretch induced actin fiber orientation of 91°. Scale bar=50 μm. Reprinted with permission from Elsevier (Ahmed et al.).46
On which surfaces that cells are attached may also affect the “quality” of stretch stimulation. ECM environments affect cell spreading, growth, and differentiation through specific integrin binding and relevant signaling,78–81 and cell–matrix interaction is another vital parameter in stretch regulation of cells. Huang et al.51 examined the role of ECM protein on MSC osteogenesis under cyclic stretching and observed that ECM membrane coating on which cells are cultured significantly affected stretch-induced AP activity and mineralization, early and late osteogenesis markers, respectively. They observed that fibronectin and laminin exhibited the greatest benefit for stretch-induced MSC osteogenesis. The type of ECM decides which integrin is mainly involved in cell adhesion, for example, fibronectin-integrin α5β1, collagen-α2β1, vitronectin-αvβ3, and so on. This may potentially regulate cell reactivity to stretch stimulation. The concept of cell–substrate interaction control of cell mechanosensitivity is novel. We published data supporting this, though not for stretch.82 We demonstrated that mechanical responsiveness of MSCs to fluid flows, as measured by mechanically induced cytosolic calcium evolution, can be modulated by cell adhesion to substrate surfaces. Combined, enhancing cell mechanosensitivity via modulating cell–substrate interaction may be a novel way to achieve successful tissue engineering.
3D stretching
As evident in 2D studies, the nature of the stretch applied (strain magnitude, frequency and strain rate, uniaxial vs. equiaxial, rest period, etc.), correlation between cell orientation and stretch direction, and cell–substrate interaction affect cellular outcomes under stretch. In contrast with 2D studies, relatively less systematic studies on these parameters have been performed for 3D constructs. Instead, 3D stretching has been focusing on the engineering of functional tissues, for example, cardiac tissue,83,84 smooth muscle,85,86 vasculature,2,87 ligament and tendon,3,88 and bone,77,89 via stretching cells embedded in 3D scaffolds.
A great variety exists in the sophistication of 3D stretching design. Also, complications arise because ECM may be disintegrated due to stretch, potentially altering the strain the cells will receive. ECM constantly undergoes cell-mediated remodeling, with or without mechanical stimulation. These will alter the mechanical property of the matrix and consequently change the cell strain under stretch stimulation. Further, diffusion rates of soluble factors and nutrients through the matrix are affected following the changes in matrix integrity. Such dynamic cell–matrix interactions, which are characteristics of 3D culture relative to 2D layer situation, make the characterization of 3D cell stretch complicated. Specifically, signaling mechanisms for stretch-induced restructuring or remodeling of ECM remain almost not elucidated. For a brief review of stretch signaling identified so far, see the Stretch Signaling Pathways section.
Perhaps, the most obvious tissues to benefit from 3D mechanical stretch are contractile tissues. Stretch of contractile tissue constructs promotes a higher degree of differentiation, greater functionality, and more physiologically relevant structures. Contractile cells often align parallel to the stretch direction but the effects of various stretch regimens have not been fully established. Recent studies have shown that 3D geometries and mechanical cues could guide undifferentiated cells to form organized differentiated tissues. Guan et al.90 observed that MSCs significantly increased early cardiac markers in 3D cardiac-like environments compared with 2D cultures. They observed that static stretch aligned the cells and produced differentiation markers in a strain-dependent manner, for example, the most differentiation at 75% strain. Shimko and Claycomb84 observed similar advantages of 3D stretching of embryonic stem cells; for example, sarcomeric gene expression, such as α-skeletal actin, α-MHC, β-MHC, and α-cardiac actin, showed frequency-dependent up- and downregulation under stretch. Tobita et al.83 demonstrated that stretching 3D-engineered cardiac tissues promoted the tissues to retain native proliferative and contractile properties in vitro. They cultured white Leghorn chicken ventricular cells in collagen scaffolds and stretched to engineer fetal cardiac tissues. Cardiomyocytes began spontaneous contractions after 4 days in culture and synchronous contractions began after 6 days. Cyclic 3D stretching significantly elevated the active force of cardiomyocytes and increased the cardiomyocyte number per cross-sectional area.
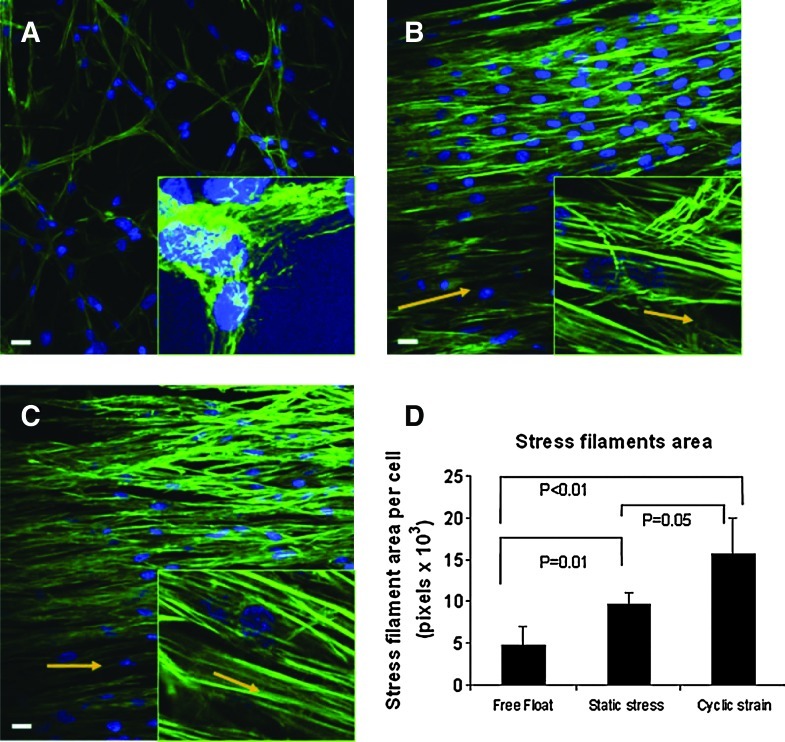
Dynamic versus static 3D stretch effects have been revealed by Nieponice et al.,86 in which bone-marrow-derived progenitors in a 3D fibrin scaffold were stretched (Fig. 3). The dynamic stretch group was subjected for 6 days to 10% strain at 1 Hz, a static stress group constrained between anchors but not dynamically stretched, and a free-floating group was used as a nonstretched control. The statically constrained group and, to a greater degree, the dynamic stretch group exhibited morphological changes characteristic of a smooth muscle lineage and stained positive for smooth muscle differentiation markers, α-actin and h1-calponin. The dynamic stretch group exhibited the greatest stress filament area per cell (Fig. 3D) and had the most realignment parallel to the stretch axis, indicating a greater force transmission. In the previous study by the same group,91 progenitor cells stretched in 2D showed an orientation perpendicular to the stretching axis, suggesting possible difference in 2D versus 3D stretch.
FIG. 3.
Cell stretching in 3D affects actin stress fiber formation depending on static or dynamic stretching condition. Rat bone-marrow-derived progenitor cells were cultured in fibrin scaffolds. (A) Free-float, unstretched control showed random cell orientation. (B) Static stress group (constrained between anchors but not stretched) oriented actin fibers parallel to the direction of strain (arrows). (C) Cyclic stretch (10%, 1 Hz, for 6 days) oriented actin fibers parallel to the strain direction (arrows). (D) Quantified stress filament area per cell shows an increase in the order of free float<static constrained<dynamic stretch. Blue is DAPI staining and green is F-actin staining. Scale bar=10 μm (insets are at 100× magnification). Reprinted with permission from Wiley (Nieponice et al.).86 Color images available online at www.liebertonline.com/teb
One potential issue that must be addressed in tissue engineering is the formation of vasculature within the engineered tissue. In vivo tissue engineering will be limited if there is no active nutrient and oxygen supply via vasculature. The maintenance of healthy vasculature and the development of new vasculature through angiogenesis and vasculogenesis are controlled by a complex interplay between mechanical and biochemical factors.92 Just as cardiac and myoblastic cells, the connected vasculature may also be regulated by mechanical signals. Ideally, a stretch regimen would have a positive effect on the growth of vasculature in tandem with other tissues. Three vessel cells (endothelial cells, smooth muscle cells, and fibroblasts) have been tested in 3D stretch assays.85,93,94 Cell stretch resulted in regulatory signaling cascades of vasodilators and realigned endothelial cells perpendicular to stretch direction.95 Biochemical factors, such as vascular endothelial growth factor (VEGF) and transforming growth factor-β family, and integrin-mediated adhesion regulated 3D stretch control of vascular structure and property.96–99 Stretching human endothelial cells seeded in a fibrin gel provided alignment cues necessary to form linearly organized lumens parallel to the direction of stretch.100
Each vasculature layer cells respond to stretch cue uniquely but the mechanical and biochemical signals may be shared among adjacent cell types. However, there have been very few co-culture stretch studies. The importance of co-culture stretching assay may be suggested from the study by Lee et al.,2 in which axial stretch elevated intimal hyperplasia in arterial grafts subjected to pulsatile flow when endothelial cell layer was denudated but this effect was not observed for intact grafts. Van der Schaft et al.101 observed that muscle cells co-cultured in a 3D hydrogel with endothelial cells directed the formation of vasculature through VEGF signaling when uniaxial stretch was applied.
Engineered ligaments and tendons are in demand due to the lack of regeneration of the native tissues and complications associated with current tissue graft practices. Although there are differences in ligament and tendon, tissue engineering of these tissues has used similar strategies, that is, stretch-mediated functional tissue regeneration. One difficulty in ligament and tendon tissue engineering is that the high force that will be applied to newly implanted tissues creates unique challenge in choosing a scaffold material. Also, the lack of consensus on tenocyte and ligament phenotypic markers makes the choice of cell source difficult. However, there have been some markers identified, for example, scleraxis, Col1A2, and tenascin-C expression for tendon.102
Stretching in 3D could successfully commit undifferentiated cells to express genes characteristic of ligament.36 Stretch also improved mechanical properties of engineered ligament by producing organized collagen bundles compared with unstretched control.3 Similar results have been found for rabbit patella tendons stretched in a bioreactor. The gene expression of collagen types I and III was greatly increased and the stiffness of the tissues was improved.103 Garvin et al.88 cultured tenocytes in a 3D collagen matrix and subjected to cyclic stretch. They observed that 3D stretch of engineered tendon increased the expression of collagen XII and prolyl hydroxylase over time resulting in a stronger tendon. The increase in hydroxylase may be responsible for time-dependent strengthening of the engineered tendon, as hydroxylase stabilizes collagen. A recent study attempted to optimize 3D strain magnitude, duty cycle, and duration of conditioning for tendon tissue engineering,104 in which the duration of conditioning was observed to affect the mechanical properties of the tendon but load magnitude did not.
Regenerating bone via inducing MSCs toward bone phenotype using 3D construct stretching has long been pursued given that bone is a dynamic mechanoresponsive tissue.77,89,105 Mauney et al.89 proposed an interesting concept on the co-regulatory role of stretch and soluble cues. MSCs cultured on 3D partially demineralized bone scaffolds were stretched using a four-point bending apparatus and observed that stretching after 10 nM dexamethasone treatment promoted MSC osteogenesis by significantly elevating AP activity, osteogenic transcription, and matrix mineralization over static controls. They proposed that the presence and concentration of osteogenic hormonal inducers may regulate the ability of MSCs to sense and respond to stretch stimulation during osteogenesis. If cell mechanosensitivity can be increased with help from soluble factor, this will enhance the mechanical regeneration of functional tissue.
Stretching 2D versus 3D constructs
The results of 2D stretching may be utilized for guiding 3D stretch and elucidating mechanotransduction pathways involved. However, it is important to note that there may be striking differences in cell behavior in 2D and 3D. Simply moving from 2D to 3D milieus, even under static culture, may have vital effects on integrin-mediated focal adhesion, cytoskeletal structure and tension, gene expression, and differentiation.106–109 This results from complex 3D cell–cell interaction and communication; altered transport phenomena for media, nutrient, and growth factors; and difference in cell–matrix binding in 3D versus 2D.110 These differences may be even magnified under external mechanical stimulations. Though very little is known as regards the difference in cell behavior for 2D versus 3D stretch, it is speculated that 3D stretch may induce potentially more biomimetic effects than does 2D stretch. As evidence, 3D stretch induced the reorganization of tissues with mature sarcomeres and produced a more physiologically relevant myofilament composition relative to 2D stretch.111
Stretching of 2D versus 3D constructs also involves the issue on how easy one can assess cell behavior.112 Many measurement methods developed for 2D culture can be applied to 3D, but at times the 2D measurements are more appropriate. For example, in the vasculature formation assay, endothelial cells migrate through vascular guidance tunnels that resemble 2D culture conditions, while the other cell types are embedded in the ECM where 3D measurements are beneficial.113 Traditional imaging techniques used in 2D can also be used in 3D for sufficiently thin or translucent scaffolds. The development of fluorescent dyes for 2D applications typically requires the fixation of the cells, but ideal measurements of engineered tissues should be nondestructive. Optical contrast tomography (OCT) has been employed to image cell morphology and migration in tissues noninvasively. However, the resolution of OCT is relatively poor compared with destructive methods.114 Attempts using microtomography have successfully quantified scaffold pore size and interconnectivity and mineralization resulting from osteoblast activity.115 Cell migration trajectories may be determined from imaged tissues and by using methods exist for estimating cell traction forces from imaged displacement fields.116,117 Other work has quantified cell viability in 3D culture in a noninvasive way using optical coherence phase microscopy.118
Signaling pathways responsible for stretch mechanotransduction have been studied using 2D cultures due to easiness in molecular biology assays. However, one potential dilemma is that mechanotransduction pathways revealed through 2D stretch assays may not necessarily be applied the same to more complex 3D milieus. Cell stretch within 3D constructs is recognized as more physiologically relevant than 2D stretch, as supported by the data showing enhanced tissue regeneration for 3D situations.86,111 However, a number of 3D stretch-based tissue engineering studies have often failed to complete thorough mechanistic studies. To engineer more robust and biomimetic tissues via optimizing 3D stretch regimens and to reveal responsible mechanisms, studies examining signaling cascades triggered in physiologically relevant 3D stretching are highly required.
Stretch Signaling Pathways
The study of stretch signaling pathways in 3D is relevant to understand how cells direct tissue organization and development under stretch and is also necessary to develop tissue-specific stretch regimens optimized with biochemical feedback. Optimizing stretching conditions may be a relatively simple yet effective tool for controlling the structural integrity and functional strength of tissue-engineered constructs. Recent studies have proposed the optimization of stretch conditions using stretch-stimulated molecular marker expression. It was shown that 2D stretch regimens can be optimized for bone growth and 3D stretch conditions for ligament tissue engineering by monitoring ERK1/2 phosphorylation.71,76 ERK is a relatively well-established signaling cascade through which mechanical signals may exert stimulatory effects on several cellular processes, including osteoblastic differentiation.119 Similar approaches using relevant protein and kinase marker expression may be attempted to optimize 3D stretch regimens. This may provide a novel way to provide feedback information for assessing the status of engineered 3D tissue “health” at the cellular level.
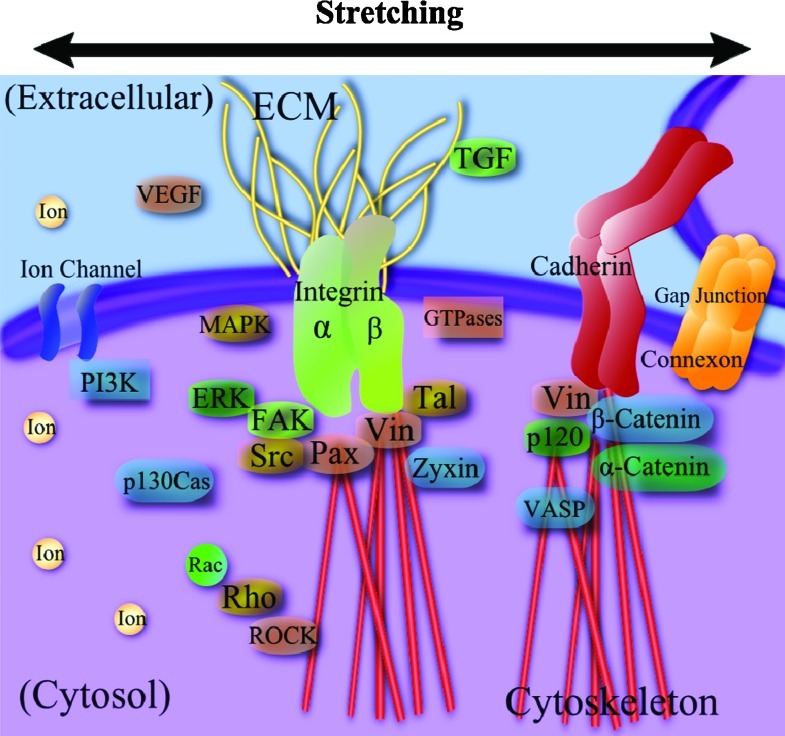
Cells employ numerous mechanisms to sense external forces and translate them into biochemical signals. Several key mechanotransduction elements identified so far are illustrated in Figure 4. Cytoskeletons are the primary mechanical component responsible for load bearing and maintaining mechanical homeostasis.120,121 Cytoskeletal elements of actin, microtubules, and intermediate filaments anchor intracellular organelles and provide mechanical links to nucleus. Cytoskeletal reorganization and adaptation are the main processes in cell reorientation, migration, and morphological change during mechanically induced differentiation and tissue development. Cytoskeletal tension signaling, such as RhoA and RhoA kinase (ROCK), is involved in stress fiber regulation, including the organization of actin filaments and phosphorylation of myosin light chain, playing a key role as a dynamic mechanosensor.9 Development of actin stress filaments in contractile cells under stretch typically indicates higher possible force generation, which may be used as a first indicator of mechanical tissue development.
FIG. 4.
Major stretch-induced mechanotransduction signaling elements, including integrins, force-sensitive kinases and proteins, cytoskeletal elements, stretch-activated ion channels, membranes, ions, and so on. ERK, extracellular-signal-regulated kinase; FAK, focal adhesion kinase; MAPK, mitogen-activated protein kinase; Pax, paxillin; ROCK, RhoA kinase; Tal, talin; TGF, transforming growth factor; VASP, vasodilator-stimulated phosphoprotein; VEGF, vascular endothelial growth factor; Vin, vinculin. Color images available online at www.liebertonline.com/teb
ECM may function as a memory storage device, storing mechanical information as cells “write” to it by secreting ECM proteins and other matrix factors and “reading” the information through mechanotransduction.10 Cells bind to ECM proteins through transmembrane integrins that have ECM protein binding sites (e.g., arginine-glycine-aspartic acid, RGD) and are linked to cytoskeletal elements via focal adhesion proteins (vinculin, paxillin, talin, α-actinin, etc.).122 Talin is force sensitive and reveals binding sites for signaling factors, and vinculin provides a force-dependent link to actomyosin, actin, and other focal adhesion proteins.9,10 The number and density of focal adhesions and the relative strength of focal adhesion signaling, for example, focal adhesion kinase (FAK), may depend on static substrate characteristic and also on dynamic mechanical stimulation.123,124 Stretch-based tissue engineering strategies that target specific integrin binding dynamics may guide tissue development. Integrins have been shown to regulate morphogenesis, angiogenesis, heart remodeling, and neuritogenesis.125–127 For example, integrin α5 and αV were not required in endothelial cells for initial vasculogenesis and angiogenesis but they were significantly involved in the remodeling of the heart and great vessels.126 It was also shown that β1 integrin played a critical role in skeletal myoblast response to stretch by activating the downstream effector FAK.128
FAK activates GTPases and other signaling cascades involving mitogen-activated protein kinase and ERK,10 which has been utilized to optimize stretch regimen as described previously.71,76 FAK activation at specific phosphorylation sites (e.g., pY397) may act as a sensitive marker revealing cell–substrate interaction in line with specific integrins (αv relative to α5), as we reported previously for static culture.123 Under mechanical stimulation, FAK is extensively involved in stretch signaling pathways and mediates various cell functions, such as proliferation, migration, and cell cycle regulation.129–131 FAK is an important target for contractile cell and tissue studies due to its role in muscle tissue hypertrophy, organization of sarcomeres via p130Cas, and the regulation of attachment forces through integrins.132,133 FAK is also of significant interest to ligament and tendon engineering as it has been shown to regulate the realignment and differentiation of human MSCs toward a tenocyte lineage with application of stretch.134
Stretch-activated ion channels also play a role in stretch mechanotransduction by selectively regulating the permeability of the cell membrane to charged molecules in response to strain. An alteration in ion gradients results in numerous physiological responses affecting cell performance. Endothelial cell reorientation in response to stretch has recently been found to be mediated by stress-activated ion channel, TRPV4, which triggers PI3K.135 PI3K activates β1 integrin and related signaling molecules, which in turn triggers RhoA/ROCK. This causes stress fiber and focal adhesion remodeling, finally resulting in cell reorientation. The knockdown of other ion channels, TRPV2 and TRPC1, did not affect the cytoskeletal reorganization or change cytosolic calcium influx. Additional studies are required to elucidate the relationship of stretch-activated channels and the other ion channels with downstream mechanotransduction effectors under stretch conditions, for both 2D and 3D. The other mechanosensors, for example, cytosolic calcium concentration, β-catenin, G protein-coupled receptors, prostaglandin E2, nitric oxide, cyclooxygenase-2, cadherin-mediated cell–cell adhesion, connexon-based gap junction intercellular communication, and so on,136–140 are also of interest as stretch signaling pathways.
Conclusions
Mechanical stretch is an important regulator in functional tissue engineering due to its ability to elicit beneficial cell responses and increase the organization and strength of engineered tissues. Stretching has been shown to have a unique role in regulating cell behavior and elicits differing responses compared with other mechanical stimulation modalities. The dimensions of the cellular environment (2D and 3D) and the direction of stretch with respect to cellular alignment have been found to affect cell responses, such as orientation, migration, differentiation, and stress fiber development. Noncontractile cells have often shown a frequency-dependent alignment response to cyclic stretch with saturation occurring around 1 Hz. Differentiation of contractile and bone cells has displayed strain-magnitude-dependent behavior and the stretch regimens have been optimized by incorporating strategic rest periods. Bioreactors and custom stretching devices have been developed to apply multimodal stretch stimulations to cells and engineered tissues. However, most systems have low throughput, are limited in the size of tissue that can be stretched, and have diffusion issues due to the interference of a bounding membrane or lack of a perfusion system. The strain profile of each device must be characterized but this step has been often overlooked. While 3D stretching has provided fruitful data on the regeneration of 3D tissues for the tissue engineering of cardiac, muscle, vasculature, ligament, tendon, bone, and so on, signaling pathways involved in regulating cell responses to stretch have been studied mostly using 2D stretches. Mechanotransduction pathway studies using 3D stretch will allow enhanced physiological control over tissue development if proper 3D stretch optimization protocols with gene and molecular marker feedbacks could be established. This will provide a new insight on functional tissue engineering and also on developmental mechanobiology.
Acknowledgments
The authors would like to thank the support by AO Foundation Research Grant (S-10-7L, Lim) and Nebraska DHHS Stem Cell Research Grant (Stem Cell 2011–05, Lim).
Disclosure Statement
No competing financial interests exist.
References
- 1.Powell H.M. McFarland K.L. Butler D.L. Supp D.M. Boyce S.T. Uniaxial strain regulates morphogenesis, gene expression, and tissue strength in engineered skin. Tissue Eng Part A. 2010;16:1083. doi: 10.1089/ten.TEA.2009.0542. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y.U. Hayman D. Sprague E.A. Han H.C. Effects of axial stretch on cell proliferation and intimal thickness in arteries in organ culture. Cell Mol Bioeng. 2010;3:286. doi: 10.1007/s12195-010-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhardt H.A. Cosgriff-Hernandez E.M. The role of mechanical loading in ligament tissue engineering. Tissue Eng Part B Rev. 2009;15:467. doi: 10.1089/ten.TEB.2008.0687. [DOI] [PubMed] [Google Scholar]
- 4.Lee W.C. Maul T.M. Vorp D.A. Rubin J.P. Marra K.G. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6:265. doi: 10.1007/s10237-006-0053-y. [DOI] [PubMed] [Google Scholar]
- 5.Kelly D.J. Jacobs C.R. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90:75. doi: 10.1002/bdrc.20173. [DOI] [PubMed] [Google Scholar]
- 6.Maul T.M. Chew D.W. Nieponice A. Vorp D.A. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2011;10:939. doi: 10.1007/s10237-010-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang K.S. Lee S.J. Lee H.S. Moon W.K. Cho D.W. Effects of combined mechanical stimulation on the proliferation and differentiation of pre-osteoblasts. Exp Mol Med. 2011;43:367. doi: 10.3858/emm.2011.43.6.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L. Chen M. Deng L. Mao Y. Wu W. Chang M. Chen H. The effect of mechanical stimulation on the expression of α2, β1, β3 integrins and the proliferation, synthetic function in rat osteoblasts. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2003;20:187. [PubMed] [Google Scholar]
- 9.Holle A.W. Engler A.J. More than a feeling: discovering, understanding, and influencing mechanosensing pathways. Curr Opin Biotechnol. 2011;22:648. doi: 10.1016/j.copbio.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufort C.C. Paszek M.J. Weaver V.M. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaalouk D.E. Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingber D.E. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 13.Butcher D.T. Alliston T. Weaver V.M. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard J. Grill S.W. Bois J.S. Turing's next steps: the mechanochemical basis of morphogenesis. Nat Rev Mol Cell Biol. 2011;12:400. doi: 10.1038/nrm3120. [DOI] [PubMed] [Google Scholar]
- 15.Burr D.B. Robling A.G. Turner C.H. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- 18.Omens J.H. Stress and strain as regulators of myocardial growth. Prog Biophys Mol Biol. 1998;69:559. doi: 10.1016/s0079-6107(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 19.Yung Y.C. Chae J. Buehler M.J. Hunter C.P. Mooney D.J. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci U S A. 2009;106:15279. doi: 10.1073/pnas.0905891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 21.Boerckel J.D. Uhrig B.A. Willett N.J. Huebsch N. Guldberg R.E. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci U S A. 2011;108:E674. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCain M.L. Parker K.K. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 2011;462:89. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- 23.Jacot J.G. Martin J.C. Hunt D.L. Mechanobiology of cardiomyocyte development. J Biomech. 2010;43:93. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egginton S. Invited review: activity-induced angiogenesis. Pflugers Arch. 2009;457:963. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 25.Ozcivici E. Luu Y.K. Adler B. Qin Y.X. Rubin J. Judex S. Rubin C.T. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam C. Endogenous musculoskeletal tissue engineering—a focused perspective. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1234-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Sharir A. Zelzer E. Tendon homeostasis: the right pull. Curr Biol. 2011;21:R472. doi: 10.1016/j.cub.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Zhong Z. Zeng X.L. Ni J.H. Huang X.F. Comparison of the biological response of osteoblasts after tension and compression. Eur J Orthod. 2011 doi: 10.1093/ejo/cjr016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Rumsey J.W. Das M. Bhalkikar A. Stancescu M. Hickman J.J. Tissue engineering the mechanosensory circuit of the stretch reflex arc: sensory neuron innervation of intrafusal muscle fibers. Biomaterials. 2010;31:8218. doi: 10.1016/j.biomaterials.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das M. Wilson K. Molnar P. Hickman J.J. Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat Protoc. 2007;2:1795. doi: 10.1038/nprot.2007.229. [DOI] [PubMed] [Google Scholar]
- 31.Kamotani Y. Bersano-Begey T. Kato N. Tung Y.C. Huh D. Song J.W. Takayama S. Individually programmable cell stretching microwell arrays actuated by a Braille display. Biomaterials. 2008;29:2646. doi: 10.1016/j.biomaterials.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu M.H. Wang H.Y. Liu H.L. Wang S.S. Liu Y.T. Chen Y.M. Tsai S.W. Lin C.L. Development of high-throughput perfusion-based microbioreactor platform capable of providing tunable dynamic tensile loading to cells and its application for the study of bovine articular chondrocytes. Biomed Microdevices. 2011;13:789. doi: 10.1007/s10544-011-9549-z. [DOI] [PubMed] [Google Scholar]
- 33.Iwadate Y. Yumura S. Cyclic stretch of the substratum using a shape-memory alloy induces directional migration in Dictyostelium cells. Biotechniques. 2009;47:757. doi: 10.2144/000113217. [DOI] [PubMed] [Google Scholar]
- 34.Pang Q. Zu J.W. Siu G.M. Li R.K. Design and development of a novel biostretch apparatus for tissue engineering. J Biomech Eng. 2010;132:014503. doi: 10.1115/1.3005154. [DOI] [PubMed] [Google Scholar]
- 35.Altman G.H. Lu H.H. Horan R.L. Calabro T. Ryder D. Kaplan D.L. Stark P. Martin I. Richmond J.C. Vunjak-Novakovic G. Advanced bioreactor with controlled application of multi-dimensional strain for tissue engineering. J Biomech Eng. 2002;124:742. doi: 10.1115/1.1519280. [DOI] [PubMed] [Google Scholar]
- 36.Altman G.H. Horan R.L. Martin I. Farhadi J. Stark P.R. Volloch V. Richmond J.C. Vunjak-Novakovic G. Kaplan D.L. Cell differentiation by mechanical stress. FASEB J. 2002;16:270. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 37.Vande Geest J.P. Di Martino E.S. Vorp D.A. An analysis of the complete strain field within Flexercell membranes. J Biomech. 2004;37:1923. doi: 10.1016/j.jbiomech.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Freyman T.M. Yannas I.V. Yokoo R. Gibson L.J. Fibroblast contraction of a collagen-GAG matrix. Biomaterials. 2001;22:2883. doi: 10.1016/s0142-9612(01)00034-5. [DOI] [PubMed] [Google Scholar]
- 39.Marquez J.P. Genin G.M. Zahalak G.I. Elson E.L. The relationship between cell and tissue strain in three-dimensional bio-artificial tissues. Biophys J. 2005;88:778. doi: 10.1529/biophysj.104.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gladilin E. Micoulet A. Hosseini B. Rohr K. Spatz J. Eils R. 3D finite element analysis of uniaxial cell stretching: from image to insight. Phys Biol. 2007;4:104. doi: 10.1088/1478-3975/4/2/004. [DOI] [PubMed] [Google Scholar]
- 41.Dado D. Levenberg S. Cell-scaffold mechanical interplay within engineered tissue. Semin Cell Dev Biol. 2009;20:656. doi: 10.1016/j.semcdb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Hayakawa K. Hosokawa A. Yabusaki K. Obinata T. Orientation of smooth muscle-derived A10 cells in culture by cyclic stretching: relationship between stress fiber rearrangement and cell reorientation. Zool Sci. 2000;17:617. doi: 10.2108/zsj.17.617. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L. Kahn C.J. Chen H.Q. Tran N. Wang X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol Int. 2008;32:344. doi: 10.1016/j.cellbi.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Breuls R.G. Sengers B.G. Oomens C.W. Bouten C.V. Baaijens F.P. Predicting local cell deformations in engineered tissue constructs: a multilevel finite element approach. J Biomech Eng. 2002;124:198. doi: 10.1115/1.1449492. [DOI] [PubMed] [Google Scholar]
- 45.Khetan S. Burdick J.A. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010;31:8228. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed W.W. Wolfram T. Goldyn A.M. Bruellhoff K. Rioja B.A. Moller M. Spatz J.P. Saif T.A. Groll J. Kemkemer R. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials. 2010;31:250. doi: 10.1016/j.biomaterials.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 47.Bieler F.H. Ott C.E. Thompson M.S. Seidel R. Ahrens S. Epari D.R. Wilkening U. Schaser K.D. Mundlos S. Duda G.N. Biaxial cell stimulation: a mechanical validation. J Biomech. 2009;42:1692. doi: 10.1016/j.jbiomech.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Brown T.D. Techniques for mechanical stimulation of cells in vitro: a review. J Biomech. 2000;33:3. doi: 10.1016/s0021-9290(99)00177-3. [DOI] [PubMed] [Google Scholar]
- 49.Martin Y. Vermette P. Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials. 2005;26:7481. doi: 10.1016/j.biomaterials.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 50.Martin I. Wendt D. Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Huang C.H. Chen M.H. Young T.H. Jeng J.H. Chen Y.J. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem. 2009;108:1263. doi: 10.1002/jcb.22356. [DOI] [PubMed] [Google Scholar]
- 52.Park J.S. Chu J.S. Cheng C. Chen F. Chen D. Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 53.Matheson L.A. Maksym G.N. Santerre J.P. Labow R.S. Differential effects of uniaxial and biaxial strain on U937 macrophage-like cell morphology: influence of extracellular matrix type proteins. J Biomed Mater Res A. 2007;81:971. doi: 10.1002/jbm.a.31117. [DOI] [PubMed] [Google Scholar]
- 54.Qu M.J. Liu B. Wang H.Q. Yan Z.Q. Shen B.R. Jiang Z.L. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J Vasc Res. 2007;44:345. doi: 10.1159/000102278. [DOI] [PubMed] [Google Scholar]
- 55.Haghighipour N. Tafazzoli-Shadpour M. Shokrgozar M.A. Amini S. Effects of cyclic stretch waveform on endothelial cell morphology using fractal analysis. Artif Organs. 2010;34:481. doi: 10.1111/j.1525-1594.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 56.Boonen K.J. Langelaan M.L. Polak R.B. van der Schaft D.W. Baaijens F.P. Post M.J. Effects of a combined mechanical stimulation protocol: value for skeletal muscle tissue engineering. J Biomech. 2010;43:1514. doi: 10.1016/j.jbiomech.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 57.Hanson A.D. Marvel S.W. Bernacki S.H. Banes A.J. van Aalst J. Loboa E.G. Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng. 2009;37:955. doi: 10.1007/s10439-009-9648-7. [DOI] [PubMed] [Google Scholar]
- 58.Robling A.G. Burr D.B. Turner C.H. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204:3389. doi: 10.1242/jeb.204.19.3389. [DOI] [PubMed] [Google Scholar]
- 59.Engler A.J. Humbert P.O. Wehrle-Haller B. Weaver V.M. Multiscale modeling of form and function. Science. 2009;324:208. doi: 10.1126/science.1170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De R. Zemel A. Safran S.A. Dynamics of cell orientation. Nat Phys. 2007;3:655. [Google Scholar]
- 61.Hsu H.J. Lee C.F. Kaunas R. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One. 2009;4:e4853. doi: 10.1371/journal.pone.0004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jungbauer S. Gao H. Spatz J.P. Kemkemer R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J. 2008;95:3470. doi: 10.1529/biophysj.107.128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu H.J. Lee C.F. Locke A. Vanderzyl S.Q. Kaunas R. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One. 2010;5:e12470. doi: 10.1371/journal.pone.0012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J.H. Goldschmidt-Clermont P. Wille J. Yin F.C. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech. 2001;34:1563. doi: 10.1016/s0021-9290(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 65.De R. Zemel A. Safran S.A. Theoretical concepts and models of cellular mechanosensing. Methods Cell Biol. 2010;98:143. doi: 10.1016/S0091-679X(10)98007-2. [DOI] [PubMed] [Google Scholar]
- 66.Nishimura K. Blume P. Ohgi S. Sumpio B.E. The effect of different frequencies of stretch on human dermal keratinocyte proliferation and survival. J Surg Res. 2009;155:125. doi: 10.1016/j.jss.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 67.Cohen T.S. Cavanaugh K.J. Margulies S.S. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J. 2008;32:854. doi: 10.1183/09031936.00141007. [DOI] [PubMed] [Google Scholar]
- 68.Ghazanfari S. Tafazzoli-Shadpour M. Shokrgozar M.A. Effects of cyclic stretch on proliferation of mesenchymal stem cells and their differentiation to smooth muscle cells. Biochem Biophys Res Commun. 2009;388:601. doi: 10.1016/j.bbrc.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 69.Iijima Y. Nagai T. Mizukami M. Matsuura K. Ogura T. Wada H. Toko H. Akazawa H. Takano H. Nakaya H. Komuro I. Beating is necessary for transdifferentiation of skeletal muscle-derived cells into cardiomyocytes. FASEB J. 2003;17:1361. doi: 10.1096/fj.02-1048fje. [DOI] [PubMed] [Google Scholar]
- 70.You L. Cowin S.C. Schaffler M.B. Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34:1375. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J. Zhang X. Wang C. Peng X. Zhang X. Different magnitudes of tensile strain induce human osteoblasts differentiation associated with the activation of ERK1/2 phosphorylation. Int J Mol Sci. 2008;9:2322. doi: 10.3390/ijms9122322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koike M. Shimokawa H. Kanno Z. Ohya K. Soma K. Effects of mechanical strain on proliferation and differentiation of bone marrow stromal cell line ST2. J Bone Miner Metab. 2005;23:219. doi: 10.1007/s00774-004-0587-y. [DOI] [PubMed] [Google Scholar]
- 73.Qi M.C. Hu J. Zou S.J. Chen H.Q. Zhou H.X. Han L.C. Mechanical strain induces osteogenic differentiation: Cbfa1 and Ets-1 expression in stretched rat mesenchymal stem cells. Int J Oral Maxillofac Surg. 2008;37:453. doi: 10.1016/j.ijom.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Partap S. Plunkett N.A. Kelly D.J. O'Brien F.J. Stimulation of osteoblasts using rest periods during bioreactor culture on collagen-glycosaminoglycan scaffolds. J Mater Sci Mater Med. 2010;21:2325. doi: 10.1007/s10856-009-3966-z. [DOI] [PubMed] [Google Scholar]
- 75.Plunkett N.A. Partap S. O'Brien F.J. Osteoblast response to rest periods during bioreactor culture of collagen-glycosaminoglycan scaffolds. Tissue Eng Part A. 2010;16:943. doi: 10.1089/ten.TEA.2009.0345. [DOI] [PubMed] [Google Scholar]
- 76.Paxton J.Z. Hagerty P. Andrick J.J. Baar K. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 2012;18:277. doi: 10.1089/ten.tea.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diederichs S. Bohm S. Peterbauer A. Kasper C. Scheper T. van Griensven M. Application of different strain regimes in two-dimensional and three-dimensional adipose tissue-derived stem cell cultures induces osteogenesis: implications for bone tissue engineering. J Biomed Mater Res A. 2010;94:927. doi: 10.1002/jbm.a.32772. [DOI] [PubMed] [Google Scholar]
- 78.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 79.Reilly G.C. Engler A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Petrie T.A. Capadona J.R. Reyes C.D. Garcia A.J. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 81.Martino M.M. Mochizuki M. Rothenfluh D.A. Rempel S.A. Hubbell J.A. Barker T.H. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salvi J.D. Lim J.Y. Donahue H.J. Increased mechanosensitivity of cells cultured on nanotopographies. J Biomech. 2010;43:3058. doi: 10.1016/j.jbiomech.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobita K. Liu L.J. Janczewski A.M. Tinney J.P. Nonemaker J.M. Augustine S. Stolz D.B. Shroff S.G. Keller B.B. Engineered early embryonic cardiac tissue retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H1829. doi: 10.1152/ajpheart.00205.2006. [DOI] [PubMed] [Google Scholar]
- 84.Shimko V.F. Claycomb W.C. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008;14:49. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao S.W. Hida K. Park J.S. Li S. Mechanical regulation of matrix reorganization and phenotype of smooth muscle cells and mesenchymal stem cells in 3D matrix. Conf Proc IEEE Eng Med Biol Soc. 2004;7:5024. doi: 10.1109/IEMBS.2004.1404388. [DOI] [PubMed] [Google Scholar]
- 86.Nieponice A. Maul T.M. Cumer J.M. Soletti L. Vorp D.A. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J Biomed Mater Res A. 2007;81:523. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- 87.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 88.Garvin J. Qi J. Maloney M. Banes A.J. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 89.Mauney J.R. Sjostorm S. Blumberg J. Horan R. O'Leary J.P. Vunjak-Novakovic G. Volloch V. Kaplan D.L. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74:458. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 90.Guan J. Wang F. Li Z. Chen J. Guo X. Liao J. Moldovan N.I. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials. 2011;32:5568. doi: 10.1016/j.biomaterials.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamilton D.W. Maul T.M. Vorp D.A. Characterization of the response of bone marrow-derived progenitor cells to cyclic strain: implications for vascular tissue-engineering applications. Tissue Eng. 2004;10:361. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- 92.Patel-Hett S. D'Amore P.A. Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol. 2011;55:353. doi: 10.1387/ijdb.103213sp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raeber G.P. Lutolf M.P. Hubbell J.A. Part II: fibroblasts preferentially migrate in the direction of principal strain. Biomech Model Mechanobiol. 2008;7:215. doi: 10.1007/s10237-007-0090-1. [DOI] [PubMed] [Google Scholar]
- 94.Joung I.S. Iwamoto M.N. Shiu Y.T. Quam C.T. Cyclic strain modulates tubulogenesis of endothelial cells in a 3D tissue culture model. Microvasc Res. 2006;71:1. doi: 10.1016/j.mvr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 95.Kaunas R. Nguyen P. Usami S. Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A. 2005;102:15895. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanjaya-Putra D. Bose V. Shen Y.I. Yee J. Khetan S. Fox-Talbot K. Steenbergen C. Burdick J.A. Gerecht S. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Erba P. Miele L.F. Adini A. Ackermann M. Lamarche J.M. Orgill B.D. D'Amato R.J. Konerding M.A. Mentzer S.J. Orgill D.P. A morphometric study of mechanotransductively induced dermal neovascularization. Plast Reconstr Surg. 2011;128:288e. doi: 10.1097/PRS.0b013e3182268b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng W. Christensen L.P. Tomanek R.J. Stretch induces upregulation of key tyrosine kinase receptors in microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H2739. doi: 10.1152/ajpheart.00410.2004. [DOI] [PubMed] [Google Scholar]
- 99.Wilson E. Sudhir K. Ives H.E. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest. 1995;96:2364. doi: 10.1172/JCI118293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsumoto T. Sasaki J. Alsberg E. Egusa H. Yatani H. Sohmura T. Three-dimensional cell and tissue patterning in a strained fibrin gel system. PLoS One. 2007;2:e1211. doi: 10.1371/journal.pone.0001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van der Schaft D.W. van Spreeuwel A.C. van Assen H.C. Baaijens F.P. Mechanoregulation of vascularization in aligned tissue-engineered muscle: a role for vascular endothelial growth factor. Tissue Eng Part A. 2011;17:2857. doi: 10.1089/ten.TEA.2011.0214. [DOI] [PubMed] [Google Scholar]
- 102.Taylor S.E. Vaughan-Thomas A. Clements D.N. Pinchbeck G. Macrory L.C. Smith R.K. Clegg P.D. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord. 2009;10:27. doi: 10.1186/1471-2474-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Juncosa-Melvin N. Matlin K.S. Holdcraft R.W. Nirmalanandhan V.S. Butler D.L. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13:1219. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 104.Woon C.Y. Kraus A. Raghavan S. Pridgen B.C. Megerle K. Pham H. Chang J. Three-dimensional-construct bioreactor conditioning in human tendon tissue engineering. Tissue Eng Part A. 2011;17:2561. doi: 10.1089/ten.TEA.2010.0701. [DOI] [PubMed] [Google Scholar]
- 105.Vunjak-Novakovic G. Meinel L. Altman G. Kaplan D. Bioreactor cultivation of osteochondral grafts. Orthod Craniofac Res. 2005;8:209. doi: 10.1111/j.1601-6343.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 106.Barron M.J. Tsai C.J. Donahue S.W. Mechanical stimulation mediates gene expression in MC3T3 osteoblastic cells differently in 2D and 3D environments. J Biomech Eng. 2010;132:041005. doi: 10.1115/1.4001162. [DOI] [PubMed] [Google Scholar]
- 107.Tseng P.C. Young T.H. Wang T.M. Peng H.W. Hou S.M. Yen M.L. Spontaneous osteogenesis of MSCs cultured on 3D microcarriers through alteration of cytoskeletal tension. Biomaterials. 2012;33:556. doi: 10.1016/j.biomaterials.2011.09.090. [DOI] [PubMed] [Google Scholar]
- 108.Green J.A. Yamada K.M. Three-dimensional microenvironments modulate fibroblast signaling responses. Adv Drug Deliv Rev. 2007;59:1293. doi: 10.1016/j.addr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong H. Stegemann J.P. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J Biomater Sci Polym Ed. 2008;19:1279. doi: 10.1163/156856208786052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cukierman E. Pankov R. Stevens D.R. Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 111.Zimmermann W.H. Schneiderbanger K. Schubert P. Didie M. Munzel F. Heubach J.F. Kostin S. Neuhuber W.L. Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 112.Decaestecker C. Debeir O. Van Ham P. Kiss R. Can anti-migratory drugs be screened in vitro? A review of 2D and 3D assays for the quantitative analysis of cell migration. Med Res Rev. 2007;27:149. doi: 10.1002/med.20078. [DOI] [PubMed] [Google Scholar]
- 113.Stratman A.N. Saunders W.B. Sacharidou A. Koh W. Fisher K.E. Zawieja D.C. Davis M.J. Davis G.E. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith L.E. Smallwood R. Macneil S. A comparison of imaging methodologies for 3D tissue engineering. Microsc Res Tech. 2010;73:1123. doi: 10.1002/jemt.20859. [DOI] [PubMed] [Google Scholar]
- 115.Jones J.R. Atwood R.C. Poologasundarampillai G. Yue S. Lee P.D. Quantifying the 3D macrostructure of tissue scaffolds. J Mater Sci Mater Med. 2009;20:463. doi: 10.1007/s10856-008-3597-9. [DOI] [PubMed] [Google Scholar]
- 116.Wang J.H. Lin J.S. Cell traction force and measurement methods. Biomech Model Mechanobiol. 2007;6:361. doi: 10.1007/s10237-006-0068-4. [DOI] [PubMed] [Google Scholar]
- 117.Wilkes R. Zhao Y. Cunningham K. Kieswetter K. Haridas B. 3D strain measurement in soft tissue: demonstration of a novel inverse finite element model algorithm on MicroCT images of a tissue phantom exposed to negative pressure wound therapy. J Mech Behav Biomed Mater. 2009;2:272. doi: 10.1016/j.jmbbm.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 118.Bagnaninchi P.O. Holmes C. Drummond N. Daoud J. Tabrizian M. Two-dimensional and three-dimensional viability measurements of adult stem cells with optical coherence phase microscopy. J Biomed Opt. 2011;16:086003. doi: 10.1117/1.3606561. [DOI] [PubMed] [Google Scholar]
- 119.Boutahar N. Guignandon A. Vico L. Lafage-Proust M.H. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 120.Tamada M. Sheetz M.P. Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 121.Asparuhova M.B. Gelman L. Chiquet M. Role of the actin cytoskeleton in tuning cellular responses to external mechanical stress. Scand J Med Sci Sports. 2009;19:490. doi: 10.1111/j.1600-0838.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 122.DeMali K.A. Wennerberg K. Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 123.Lim J.Y. Dreiss A.D. Zhou Z. Hansen J.C. Siedlecki C.A. Hengstebeck R.W. Cheng J. Winograd N. Donahue H.J. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials. 2007;28:1787. doi: 10.1016/j.biomaterials.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 124.Katsumi A. Naoe T. Matsushita T. Kaibuchi K. Schwartz M.A. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 125.Jung Y. Kissil J.L. McCarty J.H. Beta8 integrin and band 4.1B cooperatively regulate morphogenesis of the embryonic heart. Dev Dyn. 2011;240:271. doi: 10.1002/dvdy.22513. [DOI] [PubMed] [Google Scholar]
- 126.van der Flier A. Badu-Nkansah K. Whittaker C.A. Crowley D. Bronson R.T. Lacy-Hulbert A. Hynes R.O. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupton S.L. Gertler F.B. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18:725. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang S.J. Truskey G.A. Kraus W.E. Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA. Am J Physiol Cell Physiol. 2007;292:C2057. doi: 10.1152/ajpcell.00493.2006. [DOI] [PubMed] [Google Scholar]
- 129.Cary L.A. Han D.C. Polte T.R. Hanks S.K. Guan J.L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitra S.K. Hanson D.A. Schlaepfer D.D. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 131.Franchini K.G. Focal adhesion kinase—the basis of local hypertrophic signaling domain. J Mol Cell Cardiol. 2012;52:485. doi: 10.1016/j.yjmcc.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 132.Kovacic-Milivojevic B. Roediger F. Almeida E.A. Damsky C.H. Gardner D.G. Ilic D. Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy. Mol Biol Cell. 2001;12:2290. doi: 10.1091/mbc.12.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Michael K.E. Dumbauld D.W. Burns K.L. Hanks S.K. Garcia A.J. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol Biol Cell. 2009;20:2508. doi: 10.1091/mbc.E08-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu B. Song G. Ju Y. Effect of focal adhesion kinase on the regulation of realignment and tenogenic differentiation of human mesenchymal stem cells by mechanical stretch. Connect Tissue Res. 2011;52:373. doi: 10.3109/03008207.2010.541961. [DOI] [PubMed] [Google Scholar]
- 135.Thodeti C.K. Matthews B. Ravi A. Mammoto A. Ghosh K. Bracha A.L. Ingber D.E. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iqbal J. Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun. 2005;328:751. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 137.Liedert A. Kaspar D. Blakytny R. Claes L. Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 138.Hoffman B.D. Grashoff C. Schwartz M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eyckmans J. Boudou T. Yu X. Chen C.S. A hitchhiker's guide to mechanobiology. Dev Cell. 2011;21:35. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Donahue H.J. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26:417. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]