Abstract
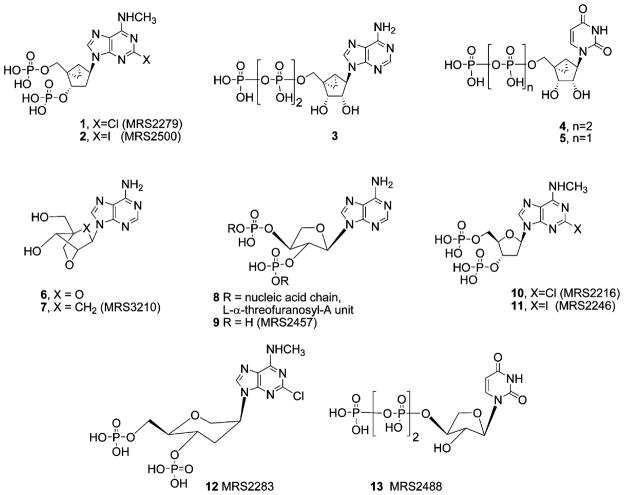
The ribose moiety of adenine nucleotide 3′,5′-bisphosphate antagonists of the P2Y1 receptor has been successfully substituted with a rigid methanocarba ring system, leading to the conclusion that the North (N) ring conformation is preferred in receptor binding. Similarly, at P2Y2 and P2Y4 receptors, nucleotides constrained in the (N) conformation interact equipotently with the corresponding ribosides. We now have synthesized and examined as P2Y receptor ligands nucleotide analogues substituted with two novel ring systems: (1) a (N) locked-carbocyclic (cLNA) derivative containing the oxabicyclo[2.2.1]heptane ring system and (2) L-α-threofuranosyl derivatives. We have also compared potencies and preferred conformations of these nucleotides with the known anhydrohexitol-containing P2Y1 receptor antagonist MRS2283. A cLNA bisphosphate derivative MRS2584 21 displayed a Ki value of 22.5nM in binding to the human P2Y1 receptor, and antagonized the stimulation of PLC by the potent P2Y1 receptor agonist 2-methylthio-ADP (30nM) with an IC50 of 650nM. The parent cLNA nucleoside bound only weakly to an adenosine receptor (A3). Thus, this ring system afforded some P2Y receptor selectivity. A L-α-threofuranosyl bisphosphate derivative 9 displayed an IC50 of 15.3μM for inhibition of 2-methylthio-ADP-stimulated PLC activity. L-α-Threofuranosyl-UTP 13 was a P2Y receptor agonist with a preference for P2Y2 (EC50 = 9.9μM) versus P2Y4 receptors. The P2Y1 receptor binding modes, including rotational angles, were estimated using molecular modeling and receptor docking.
Keywords: Nucleoside, Purine, Pyrimidine, G protein-coupled receptor, Carbocyclic, Phospholipase C, Radioligand binding, Molecular model
1. Introduction
The ribose rings of nucleosides and nucleotides exist in an equilibrium covering a range of conformations, which has been described as a pseudorotational cycle.1 The North ((N), 2′-exo, 3′-endo) and South ((S), 2′-endo, 3′-exo) conformations are the most relevant to the biological activities observed for nucleosides and nucleotides in association with DNA, RNA, and various enzymes.1–6
Various ribose ring substitutions have been incorporated into biologically active nucleosides and nucleic acids. A methanocarba modification introduced by Marquez and co-workers2,3 has been used to constrain the pseudosugar (cyclopentane) ring of carbocyclic nucleosides in various systems. The methanocarba ring system consists of a fused cyclopropane and cyclopentane rings, to attain either a (N) or (S) conformation depending on the position of fusion. Methanocarba analogues helped to define the role of sugar puckering in stabilizing the active bound conformation at both purine and pyrimidine receptors and thereby allowed identification of a favored conformation.4–6 Using such conformationally constrained analogues, a preference for the (N) conformation of ribose at the P2Y1 receptor5,6 was deduced. The bisphosphate derivatives 1 (MRS2279) and 2 (MRS2500), which are locked in a (N) conformation by the bicyclo[3.1.0]hexane ring system, are the most potent known antagonists of the P2Y1 receptor (Chart 1).6–9 Subsequently we discovered that P2Y2, P2Y4, and P2Y11 receptors can recognize (N)-methanocarba nucleoside 5′-triphosphates 3 and 4 roughly as potently as the corresponding ribosides.5 However, in the case of P2Y6 receptors, the (N)-methanocarba equivalent 5 of the natural nucleotide activator, UDP, was inactive.5
Chart 1.
In the present study, aimed at further investigating the conformational preferences of P2Y receptors, we have explored additional substitutions of the ribose moiety in P2Y receptor agonists and antagonists.
One such substitution is based on the ‘locked nucleic acids’ (LNAs), for example, containing monomer 6, which were introduced several years ago10–15 and which hybridize effectively to natural RNA. Recently, we reported the synthesis of a novel ring system corresponding to the ring methylene equivalent of the LNAs, that is, carbocyclic LNAs (cLNAs), for example, 7.16 cLNAs, which contain the oxabicyclo[2.2.1]heptane ring system, are expected to be more stable than oxygen LNAs because of their nonglycosidic nature, as has been discussed for methanocarba ribose in comparison with natural ribose.4,5,17 The cLNA would prefer an approximate (N)-conformation,16 but is expected to adopt a different pseudorotational angle from that of the (N)-methanocarba ring system.
Another novel ribose substitution adapted to P2Y receptor ligands in the present study is the L-α-threofuranosyl ring. Eschenmoser and colleagues have demonstrated that Watson–Crick pairing in RNA is not exclusively dependent on the ribofuranosyl ring system. 18,19 The unnatural threofuranosyl ring system (leading to threofuranosyl nucleic acids, TNAs, for example, 8), which is lacking one carbon in comparison to the ribosyl moiety, is capable of base pairing to RNA strands with a high strength.
Here we report the first synthesis of bisphosphate antagonists of the P2Y1 receptor 21 and 9, derived from carbocyclic locked adenine 7 and L-α-threofuranosyl adenine 22, respectively. These novel ring structures are compared both in biological assays and in P2Y1 receptor model20 docking with 9-riboside analogues, for example, 10 and 11, and with a previously reported anhydrohexitol bisphosphate antagonist 12 of the P2Y1 receptor.7 L-α-Threofuranosyl-UTP 13 was also synthesized and assayed at P2Y2, P2Y4, and P2Y6 receptors.
Since the A3 adenosine receptor (AR)4 also showed a preference for the (N) conformation of the ribose, we tested the nucleoside precursors of 2, 9, and 21 in binding studies at the ARs.
2. Results
2.1. Chemical synthesis
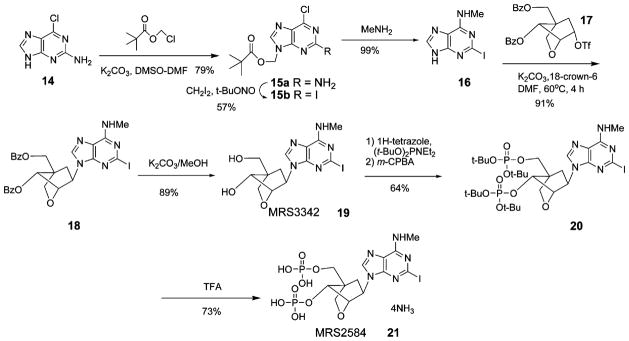
We prepared cLNA analogues of bisphosphate derivatives (Scheme 1), in which the bicyclo[2.2.1]heptane ring system fixed the pseudoribose moiety in a rigid (N)-envelope conformation. Identification of compounds was confirmed by NMR (1H and 31P) and by high-resolution mass spectrometry (HRMS), and purity was demonstrated with high-performance liquid chromatography (HPLC) in two different solvent systems.
Scheme 1.
In a previous publication,16 we reported an efficient synthetic procedure for formation of the bicyclo[2.2.1]heptane system. Generally, the formation of carbocyclic nucleosides requires more synthetic steps than does the preparation of the corresponding natural nucleosides. In the reported LNA synthesis10,11 the ribose ring was cyclized after introduction of the base. This required more synthetic steps and also made it difficult to synthesize numerous analogues. The coupling of sugar and base moieties at a later stage (Scheme 1) provided greater versatility in the efficient preparation of nucleoside and nucleotide derivatives.
An important aspect in the preparation was the nucleobase synthesis. In this synthesis, we applied a method featuring N9-protection, as described previously for the synthesis of 2-iodo-6-chloroadenine.8 N9-Protection of the adenine precursor 14 by the pivaloyloxymethyl group, which improved the solubility, enabled various transformation reactions. The 2-iodination of 15a was then performed by the method of Nair and co-workers.21 Treatment of 15b with methylamine gave 2-iodo-N6-methyladenine 16 in good yield.
The key step of this synthesis was the coupling of the nucleobase 16 with a triflate intermediate16 17 to provide 18. In a previous study of cLNAs,16 we tried several coupling procedures, but most were unsuccessful because the leaving group was at the concave face of the cLNA scaffold, which decreased the reactivity. Only the triflate ester 17 could be applied to this coupling reaction. Also, the coupling step required the use of well-dried potassium carbonate. In the course of this synthesis, the triflate 17 was crystallized from ethyl acetate/petroleum ether. This enabled a large-scale preparation and storage of the triflate substrate as a common intermediate, which is an important advantage in synthetic nucleoside chemistry and in parallel synthesis.
The benzoyl groups of the resulting 18 were hydrolyzed to give 19. Compound 19 was phosphorylated by the phosphoramidite method to give 20. The tert-butyl protecting group of 20 was removed using trifluoroacetic exchange resin chromatography, to obtain the target compound 21.
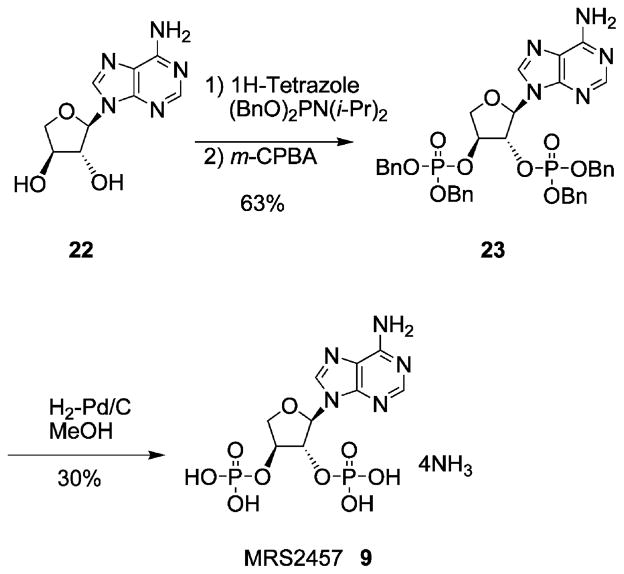
The TNA bisphosphate 9 was prepared as shown in Scheme 2. The synthesis of the UTP analogue 13 was by standard methods of triphosphate formation.34
Scheme 2.
2.2. Pharmacological activity
We recently developed [3H]MRS2279 1 as a high-affinity and selective radioligand for quantification of the P2Y1 receptor.9 Therefore, the human P2Y1 receptor can be expressed from a baculovirus to high levels in Sf9 insect cells, and membranes prepared from these cells can be used with [3H]1 to directly assess the affinity of newly synthesized molecules at the P2Y1 receptor. Human P2Y1 receptor-expressing membranes were incubated with approximately 20nM [3H]1 and a wide range of concentrations of 2-substituted (N)-methanocarba bisphosphate analogues. The novel cLNA adenine nucleotide MRS2584 21 interacted with the P2Y1 receptor, as shown by its capacity to inhibit [3H]1 binding, and this inhibition occurred with kinetics consistent with interaction at a single site by the law of mass action kinetics. IC50 values were determined from each competition curve, and a Ki value was calculated as 22.5nM according to the relationship Ki = IC50/1 + [[3H]1]/Kd of [3H]1. The Ki value for compounds 9, 10, and 12 were also determined, indicating that the riboside 10, bound more potently than the anhydrohexitol derivative 12 and the TNA bisphosphate 9, by factors of 5 and 77, respectively.
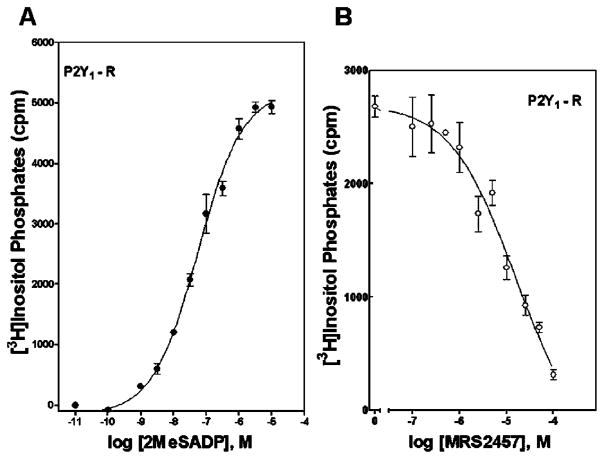
We also tested the activities of bisphosphate analogues as agonists and antagonists at the human P2Y1 receptor stably expressed in 1321N1 human astrocytoma cells. Agonist activity was tested by measuring the capacity of the molecules to increase inositol phosphate accumulation by activating the phospholipase C (PLC)-coupled P2Y1 receptor, and antagonist activity at the P2Y1 receptor was assessed by measuring the capacity of these molecules to inhibit 2-MeSADP (30nM)-stimulated inositol phosphate accumulation. None of the bisphosphate analogues exhibited agonist activity. The cLNA bisphosphate derivative 21 antagonized the P2Y1 receptor with an IC50 value of 650nM. The TNA bisphosphate 9 only weakly antagonized the human P2Y1 receptor with an IC50 value of 15.3μM (Fig. 1). Antagonist potencies at the P2Y1 receptor for the previously reported7 antagonists 10–12 are presented in Table 1 for comparison. Included among the archival compounds was the known anhydrohexitol-containing P2Y1 receptor antagonist MRS2283 12.7
Figure 1.
Activation of the human P2Y1 receptor (A) and competitive antagonism (B) of 2-MeSADP-promoted activation. PLC activity was measured as described in Section 4 in 1321N1 human astrocytoma cells stably expressing the human P2Y1 receptor. Assays were in the presence of the indicated concentrations of the agonist 2-MeSADP alone or in the presence of 100nM–100μM MRS2457 9. The data are the means of triplicate determinations and are representative of results obtained in three separate experiments.
Table 1.
In vitro pharmacological data at P2Y1 receptors in binding (human receptor expressed in Sf9 cells)9and for inhibition of PLC activity stimulated by 30nM 2-MeSADP (human receptor expressed in 1321N1 astrocytoma cells or in turkey erythrocytes)
| Compound | R = (2-position) | R′ = (N6-position) | Binding, Ki, nMa | Antagonism, IC50, nMb |
|---|---|---|---|---|
| 1 MRS2279c | Cl | CH3 | 2.5 ± 0.4 | 51.6 ± 0.8 (t) |
| 2 MRS2500c | I | CH3 | 0.78 ± 0.08 | 8.4 ± 0.8 (h) |
| 9 MRS 2457 | H | H | 666 ± 333 | 15,300 ± 300 (h) |
| 10 MRS 2216 | Cl | CH3 | 8.6 ± 2.6 | 206 ± 53 (t) |
| 11 MRS 2246 | I | CH3 | ND | 891 ± 233 (t) |
| 12 MRS 2283 | Cl | CH3 | 43 ± 10 | 566 ± 224 (t)c |
| 21 MRS 2584 | I | CH3 | 22.5 ± 10.4 | 650 ± 100 (h) |
Mean ± s.e.m. is given for three separate determinations. None of the compounds displayed agonist effects.
The affinities were determined by using [3H]1 in a radioligand binding assay, as recently described.9 The human P2Y1 receptor was expressed to high levels in Sf9 insect cells with a recombinant baculovirus. Membranes prepared from these cells were incubated for 30min at 4°C in the presence of ~20nM [3H]1.
Antagonist IC50 values represent the concentration needed to inhibit by 50% the effect elicited by 30nM 2-MeSADP. n = 3, unless otherwise indicated in parentheses. t = turkey, h = human.
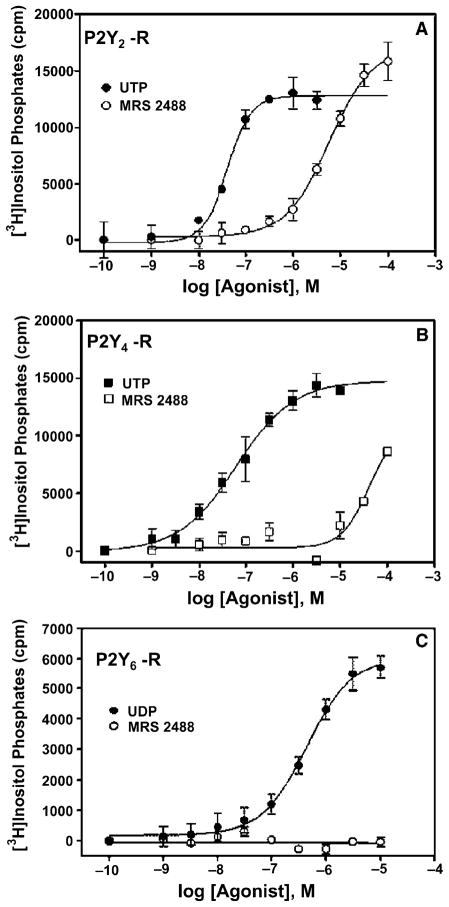
Activity of the UTP analogue in the TNA series, compound 13, was also examined at the PLC-coupled human P2Y2 and P2Y4 receptors stably expressed in 1321N1 human astrocytoma cells. The EC50 values for receptor activation by 13 were found to be 9.9 ± 2.1μM (n = 3) and 26μM (n = 2) at human P2Y2 and P2Y4 receptors, respectively (Fig. 2). The corresponding EC50 values for the riboside, UTP, were 8 and 49nM, respectively.5 Although 13 was a full agonist at the P2Y4 receptor, it was completely inactive at the human P2Y6 receptor.
Figure 2.
Activation of the human P2Y2 (A), P2Y4 (B), and P2Y6 (C) receptors by the uracil nucleotides UDP and UTP and by the L-α-threofuranosyl analogue of UTP (MRS2488 13). PLC activity was measured as described in Section 4 in 1321N1 human astrocytoma cells stably expressing the human P2Y receptors. The data are the means of triplicate determinations and are representative of results obtained in three separate experiments.
The adenine nucleosides precursors of 2, 9, and 21 were examined in binding to human ARs. Compound 7 (MRS3210), that is, the unsubstituted cLNA adenosine, at 10μM had no effect in binding at two ARs (hA1, hA2A) and displaced ~30% binding at the hA3AR. We also tested the corresponding 2-iodo-N6-methyl nucleoside analogue 19 (MRS3342) in binding assays, and obtained a Ki value of 4.90μM at the hA3AR. The threofuranosyl adenine nucleoside 22 (which was the precursor of 9) showed no measurable affinity at 10μM in binding to the hA1, A2A, A3ARs or in functional agonism of the hA2BAR. However, the (N)-methanocarba nucleosides typically bind more readily to adenosine receptors and have already been shown to favor selectivity of binding to the A3AR over other subtypes. (N)-Methanocarba-2-chloro-N6-methyladenosine, which may be considered the 2′-hydroxy equivalent of the nucleoside precursor of 1, and thus an (N)-methanocarba analogue of 19 (MRS3342), had a Ki value of 23nM at the hA3AR and bound more weakly to the rA1AR.22 Even the parent (N)-methanocarba-adenosine, which was comparable to 7 (MRS3210), had a Ki value of 404nM in binding to the hA3AR.4
2.3. Molecular modeling
With the goal of understanding the molecular features important for the binding of MRS2279 1, we studied with computational and molecular biology tools the interactions of 1 with the P2Y1 receptor.20,23 As already stated in the introduction, MRS2279 1 is a potent and selective P2Y1 receptor antagonist. We also studied by means of computational techniques the interactions between our rhodopsin-based P2Y1 model20 and the nonriboside antagonists 2, 9, 12, and 21. Like 1, all of these P2Y1 antagonists may be regarded as analogues of the 2′-deoxyadenosine-3′,5′-bisphosphate in which the 2′-deoxyribose is replaced by various pseudosugar moieties.
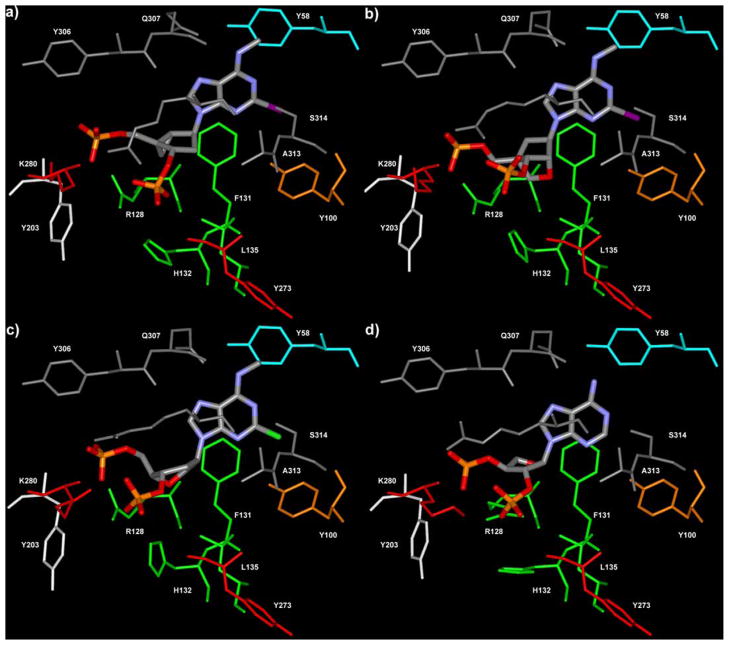
The ligands were first fully optimized by means of quenched molecular dynamics and energy minimization. Then they were flexibly superimposed, taking into account both steric and electronic features, to the bound conformation of 1, as derived from our docking experiments.20 Starting from these ligand/receptor complexes, we carried out automatic docking experiments at the P2Y1 receptor, to explore the interactions between amino acids in the receptor binding pocket and the various antagonists (Fig. 3). The docking was based on a Monte Carlo/simulated annealing approach, During the docking procedure the ligands and the binding site were treated as fully flexible. In this way we allowed both the receptor and the ligands to undergo the conformational changes necessary for mutual adaptation.
Figure 3.
P2Y1 receptor complexes with the ligands MRS2500 2 (a), MRS2584 21 (b), MRS2283 12 (c), and MRS2457 9 (d). The residues of the P2Y1 binding pocket are colored according to the following scheme: cyan (TM1), orange (TM2), green (TM3), red (TM6), gray (TM7), white (EL2).
To estimate the conformational changes that the ligands had to go through in order to bind to the receptor, we also studied the sugar puckering of the nucleotides before and after docking at the P2Y1 receptor by calculating the puckering coordinates of the pseudosugars. The puckering coordinates can be derived from the torsional angles of the rings and furnish a complete description of the puckering. All of the torsional angles could be calculated from the puckering coordinates by applying the inverse formulae. For a description of the puckering of the five-membered rings we used the coordinates P (phase angle of pseudorotation) and θm (puckering amplitude) as defined by Altona and Sundaralingham,1 while for the six-membered ring we used the coordinates Θ (phase angle of symmetrical interconversion), P2 (phase angle of pseudorotation), and Q (total puckering amplitude) as defined by Haasnoot.24
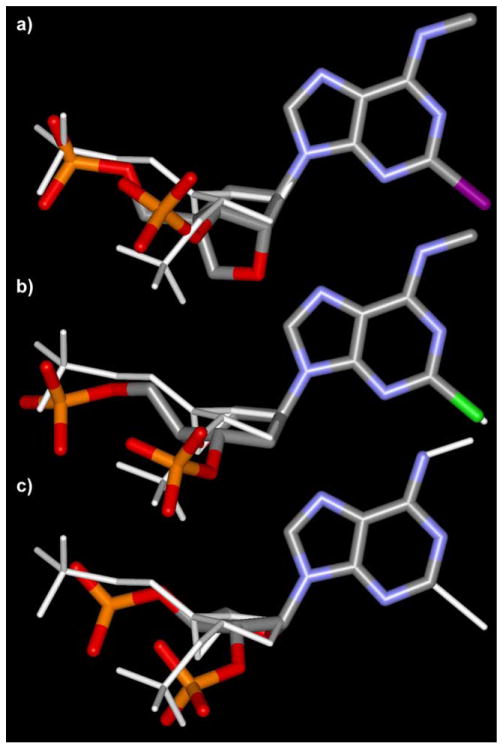
The special disposition and steric features of the pseudosugar and the phosphate moieties were studied after the superimposition of the adenine moieties of all the docked ligands. This involved analyzing the conformational similarities to the most potent compound 2 (Fig. 4).
Figure 4.
Superimpositions of the bound conformation of MRS2584 21 (a), MRS2283 12 (b), and MRS2457 9 (c) with MRS2500 2, performed by overlapping the adenine moieties. The anhydrohexitol derivative 12 can resemble the bound conformation of the most active compound 2.
As previously observed for 1, the P2Y1 receptor binding pocket was identified within the upper regions of the TMs (transmembrane domains) 3, 6, and 7 and the EL2 (the second extracellular loop), with the phosphate moieties coordinated by the essential cationic residues R128(3.29), K280(6.55), and K310(7.39). As in previous models,20,23 Q307 (7.36) always acted as a hydrogen bond acceptor from the exocyclic amino group, while S314(7.43) donated a hydrogen bond to N-1 of the adenine moiety. Mutagenesis studies outlined the importance of these five residues for the binding of agonists and antagonists at the P2Y1 receptor.25,26 The N6-Me and 2-halo substituents, when present, interacted with Y58(1.39) and Y100(2.53), respectively.
In 2 (Fig. 3a), as in 1, the 2-deoxyribose was substituted by a methanocarba ring system constrained in the (N) conformation by a (bicyclo[3.1.0]hexane) ring system. The bound conformation of 2 showed a pseudorotational angle (P) of −17°, which indicated an almost pure C2′-exo conformation (P = ′18°). This result was in excellent agreement with the crystal structures of various methanocarba nucleosides developed and analyzed by Marquez and co-workers during the past few years.27 According to the biological data, the (N)-methanocarba ring system confers to the nucleotides the ideal conformation for the interaction with the P2Y1 receptor.
The bound conformation of 2 showed only minimal deviation from the free ligand optimized in vacuo, indicating that the ligand required small conformational adjustment to fit into binding site of the P2Y1 receptor and to establish the optimal interactions with the three fundamental cationic residues.
In the case of the cLNA analogue 21, the ribose moiety was replaced by a oxabicyclo[2.2.1]heptane ring system. Like the methanocarba analogues, compound 21 was locked in a (N) conformation by this bicyclic system. However, its bound conformation showed a P of 21°, very close to a pure C3′-endo conformation (P = 18°). Besides having different P angles, the pseudosugar moieties of the two molecules showed other dissimilarities in overall geometry (Fig. 4). The five-membered ring of 2 tended to be relatively flat, with a θm of 31°; In contrast, in the case of 21 it tended to fold more markedly, showing a θm of 60°. Despite these differences, our docking studies suggested that 21 still established interactions with the fundamental cationic residues of TM3, TM6, and TM7 (Fig. 3b). Also in the case of 21, we detected limited conformational changes between the free ligand optimized in vacuo and the ligand docked into the receptor. The superimposition of the bound conformations of 21 and the potent methanocarba derivative 2 showed a good overlapping of the 5′-phosphate moieties, while greater divergence was found in the position of the 3′-phosphates and in the overall shape of the pseudosugars (Fig. 4a). In particular, the steric hindrance exerted by the bulky 2′-cyclic alkoxy group of 21 on the nearby residues of TM3 could be a possible explanation of the diminished affinity of 21 with respect to its (N)-methanocarba analogue 2.
The six-membered pseudosugar moiety of the anhydrohexitol derivative 12, which in the free ligand showed a clear preference for an almost pure chair conformation with a Θ of about 0°, after binding to P2Y1 receptor (Fig. 3c) adopted a O5′-exo/C3′-exo screw–boat conformation (Θ = 77°, P2 = −84°, Q = 57°). The superimposition of the bound conformations of 12 and 2 showed a good overlapping of the phosphates and of the pseudosugar moieties, indicating that this anhydrohexitol derivative has the capability to resemble the conformation of the potent (N)-methanocarba analogues (Fig. 4b). The conformational differences that we detected between the P2Y1 receptor-bound form of 12 and its free form, optimized in vacuo, could be one of the reasons for lower affinity of this compound compared to the (N)-methanocarba analogues.
However, in good agreement with the biological data, the threofuranosyl moiety of 9 did not allow a complete overlapping of the phosphate moieties with those of the most active compounds (1 and 2); thus, the interactions with the key cationic residues were not optimal (Fig. 3d). Being unsubstituted at N6 and 2 positions, the compound lacked also the favorable hydrophobic interactions with the two Y residues in TM1 and TM2.20 With respect to the conformation of the pseudosugar moiety, we detected a substantial difference between the puckering of the free form of 9 optimized in vacuo (P = 215, θmax = 44), and its P2Y1 receptor-bound conformation (P = 260, θmax = 44). Nonetheless, the bound compound 9 did not resemble the bound conformation of 2, but showed significant divergence in the position of the phosphate moieties and in the overall shape of the pseudosugar (Fig. 4c).
3. Discussion
The success of conformationally constrained antagonists in enhancing affinity at the P2Y1 receptor and the lack of success so far in applying this approach to other P2Y subtypes,5–8 prompted us to search for other (N)-like ring systems, especially those that may adopt a slightly different conformation according to the pseudorotational cycle and therefore may have an altered pharmacological profile.
These potential advantages encouraged us to synthesize ring-constrained carbocyclic-type analogues for improved ligands for the G protein-coupled P2Y receptors by using both novel cLNA units of the (N) conformation and L-α-threofuranosyl substitution of ribose as a test of the conformational requirements at the putative binding sites.
According to the biological data and our docking experiments, the (N)-methanocarba pseudosugar moiety seemed to be endowed with a puckering that could ensure an optimal fit of the ligand in the P2Y1 binding pocket, together with the ideal orientation of the bisphosphates for the interactions with the cationic residues of the binding site. Also, the cLNA analogue 21 was locked within a preferred conformation range of the pseudoribose for binding to the P2Y1 receptor.
The cLNA analogue 21 (MRS2584) was an antagonist at the hP2Y1 receptor and displayed a high binding affinity indicated by a Ki value of 22.5nM. Compound 21 was roughly equipotent with the 2-iodo ribose analogue 11, which had an IC50 value of 891nM in a functional assay at the turkey P2Y1 receptor. The nucleotide 21 was 3-fold less potent in binding than the corresponding 2-chloro ribose analogue 10, which had a Ki value of 8.6nM. In comparison to the methanocarba analogue 2, the potency of 21 was somewhat diminished. Compound 21 was 29-fold less potent than 2 (MRS2500), which had a Ki value of 0.78nM in binding to the hP2Y1 receptor. Compound 21 was 9-fold less potent than 1 (MRS2279), which had a Ki value of 2.5nM in binding to the hP2Y1 receptor.
Previous data indicated that the presence of a ribose 2′-OMe group decreased antagonist potency at the P2Y1 receptor.35 A 2′-ether group is present in 21, and the effect of this 2′-cyclic alkoxy group on antagonist potency is unknown. It was also suggested from molecular modeling and docking in the human P2Y1 receptor (Fig. 3b) that amino acids from TM3, that is, F131(3.32), H132(3.33), and L135(3.36) may have steric interactions with the 2′-substituents. Furthermore, the substitution of the ribose with the oxabicyclo[2.2.1]heptane ring did not allow the optimal orientation of the 3′-phosphate group for interaction with the essential cationic residues.
The adenosine receptor binding results for 19 showed that a cLNA scaffold does not fit in the binding site of the hA3AR as effectively as does the (N)-methanocarba ring system. Thus, the cLNA, containing the oxabicyclo[ 2.2.1]heptane ring system, apparently is a P2 receptor-selective scaffold. The molecular modeling results of the hA3AR binding site model also indicated a direct interaction of the 2′-OH and 3′-OH groups with Q167(EL2) and H272(7.43). Furthermore, the models suggest that 2′-substitutions may exhibit steric interference with L90(3.32) and L91(3.33) of hA3AR.28,29 On the other hand the (N)-methanocarba moiety exhibited an optimal puckering for interaction with the A3AR (and to a greater degree than other AR subtypes). Compound 22 (precursor of 9) was also inactive at adenosine receptors. However, since TNAs only weakly activated the P2Y receptors, a statement about the receptor selectivity of this ring system would not be justified.
The two threofuranosyl derivatives examined in this study only weakly antagonized (9 at P2Y1) or activated (13 at P2Y2, and P2Y4 receptors) the P2Y receptors. In good agreement with the biological data, our molecular modeling studies emphasized that the threofuranosyl moiety does not confer to the ligands the steric features necessary for recognition by the P2Y1 receptor.
In conclusion, we have synthesized a novel (N)-locked carbocyclic bisphosphate using a cLNA scaffold. The pseudorotational P value of the cLNA compound was calculated to be +21° and its conformation belonged to the (N)–(3E) category. This cLNA bisphosphate derivative displayed potent binding affinity at the human P2Y1 receptor. In contrast, the cLNA nucleoside bound only weakly to the hA3AR receptor and did not bind appreciably to other adenosine receptors. Thus, this ring system contributes to selectivity for the P2Y1 receptor. Thus, the order or suitability of various nonribose ring systems to bind to P2Y1 receptors was: bicyclo[3.1.0]hexane > 2-oxa-bicyclo[2.2.1]heptane > L-α-threofuranose. The L-α-threofuranosyl analogue of UTP maintains a preference for activating the P2Y2 in comparison to the P2Y4 receptor.
4. Experimental
4.1. Chemical synthesis
Nucleosides and synthetic reagents were purchased from Sigma Chemical Co. (St. Louis, MO) and Aldrich (Milwaukee, WI). Compound 12 was synthesized as described7.
1H NMR spectra were obtained with a Varian Gemini-300 spectrometer (300MHz) with D2O, CDCl3, CD3OD, and DMSO-d6 as a solvent. 31P NMR spectra were recorded at room temperature with a Varian XL-300 spectrometer (121.42MHz); orthophosphoric acid (85%) was used as an external standard.
Purity of compounds was checked with a Hewlett–Packard 1090 HPLC apparatus equipped with an SMT OD-5-60 RP-C18 analytical column (250 × 4.6mm; Separation Methods Technologies, Inc., Newark, DE) in two solvent systems. System A: linear gradient solvent system: 0.1M TEAA/CH3CN from 95/5 to 40/60 in 20min; the flow rate was 1mL/min. System B: linear gradient solvent system: 5mM TBAP/CH3CN from 80/20 to 40/60 in 20min; the flow rate was 1mL/min. System C: linear gradient solvent system: H2O/CH3CN from 95/5 to 0/100 in 30 min; the flow rate was 1mL/min. Peaks were detected by UV absorption with a diode array detector. All derivatives tested for biological activity showed ≥97% purity in the HPLC systems. Low-resolution CI-NH3 (chemical ionization) mass spectra were measured with a Finnigan 4600 mass spectrometer and high-resolution EI (electron impact) mass spectrometry was performed with a VG7070F mass spectrometer at 6kV. High-resolution FAB (fast atom bombardment) mass spectrometry was performed with a JEOL SX102 spectrometer with 6kV Xe atoms following desorption from a glycerol matrix. Purification of the nucleotide analogues for biological testing was carried out on Sephadex-DEAE-A-25 resin columns with a linear gradient (0.01–0.5M) of 0.5M ammonium bicarbonate.
4.1.1. (2′R,3′R,4′S)-Phosphoric acid mono-[2-(6-aminopurin-9-yl)-4-phosphonooxy-tetrahydro-furan-3-yl] ester (9)
Compound 23 (10mg, 0.013mmol) was dissolved in a mixture of methanol (2mL) and water (1mL) and hydrogenated over a 10% Pd/C (10mg) at room temperature for 2d. The catalyst was removed by filtration and the methanol was evaporated. The residue was treated with ammonium bicarbonate solution and subsequently frozen and lyophilized. Purification of the obtained residue was performed on an ion-exchange column packed with Sephadex-DEAE A-25 resin, a linear gradient (0.01–0.5M) of 0.5M ammonium bicarbonate was applied as the mobile phase, and UV and HPLC were used to monitor the elution, which furnished 9 (1.8mg, 30%). 1H NMR (D2O) δ 8.41 (s, 1H), 8.31 (s, 1H), 6.33 (s, 1H), 5.10 (d, 1H, J = 8.7 Hz), 4.93–4.90 (br, 1H), 4.51 (d, 1H, J = 10.3 Hz), 4.41(dd, 1H, J = 3.3, 10.3 Hz); 31P NMR (D2O) δ −0.29 (br s); MS (m/e) (negative-FAB) 396 (M+−H). HRMS (negative-FAB) calcd for C9H12N5O9P2 396.0110. Found 396.0119; HPLC 16.0min (99%) (system B), 2.7min (99%) (system C).
4.1.2. 2-Amino-6-chloropurin-9-yl-methyl 2,2-dimethylpropionate (15a)
2-Amino-6-chloropurine 14 (1.00g, 5.90mmol) was dissolved in DMSO (5.0mL) under heating. DMF (20.0mL), chloromethyl pivalate (1.0mL, 6.94mmol) and K2CO3 (990mg, 7.16mmol) were added and the mixture was stirred at room temperature for 3d. The reaction mixture was filtered and the filtrate was evaporated. The obtained residue was purified by flash chromatography (AcOEt/petroleum ether = 2/1) and recrystallized from AcOEt–petroleum ether, which furnished the desired compound 15a (1.33 g, 79 %). 1H NMR (CDCl3) δ 8.01 (s, 1H), 6.00 (s, 2H), 5.20 (br s, 2H), 1.18 (s, 9H); MS (m/e) (positive-FAB) 284, 286 (peak height ratio is 3:1) (M++H).
4.1.3. 6-Chloro-2-iodopurin-9-yl-methyl 2,2-dimethylpropionate (15b)
To a solution of 2-amino-6-chloropurin-9-yl-methy 2,2-dimethylpropionate 15a (704mg, 2.48mmol) in MeCN (2.0mL) was added diiodomethane (8.0mL) and t-butylnitrite (0.90mL, 9.99mmol) and oxygen was purged by N2 bubbling. The tube was sealed and stirred at 80°C for 2.5 h. The solvent was removed under vacuum and the obtained residue was purified by flash chromatography (AcOEt/petroleum ether = 1/2), which furnished 15b (561mg, 57 %). 1H NMR (CDCl3) 8.29 (s, 1H), 6.14 (s, 2H), 1.19 (s, 9H); MS (m/e) (positive-FAB) 395, 397 (peak height ratio is 3:1) (M++H).
4.1.4. 2-Iodo-6-methylamino-9H-purine (16)
2,2-Dimethyl-propionic acid 6-chloro-2-iodopurin-9-ylmethyl ester 15b (148mg, 0.375mmol) was dissolved in THF (2.0mL) and i-PrOH (5.0mL) in sealed tube. 40% MeNH2 in water (1.0mL) was added, and the solution stirred at 60°C for 18h. The precipitate, which formed was filtered and washed with small amount of water to furnish 2-iodo-6-methylaminopurine 16 (102mg, 99%). 1H NMR (CDCl3) δ 7.99 (s, 1H), 7.86 (br s, 1H), 2.88 (br s, 3H); MS (m/e) (positive-FAB) 276 (M++H).
4.1.5. (1′S,4′R,6′S,7′S)-Benzoic acid 4-benzoxymethyl-6-trifluoromethanesulfonyloxy-2-oxa-bicyclo[2.2.1]hept-7-yl ester (17)
To a stirred solution of (1′S,4′R,6′S,7′S)-benzoic acid 4-benzoxymethyl-6-hydroxy-2-oxa-bicyclo[ 2.2.1]hept-7-yl ester16 (54mg, 0.147mmol) and 4-dimethylaminopyridine (195mg, 1.60mmol) in methylene chloride (6.0mL) was added trifluoromethanesulfonic anhydride (75μL, 0.446mmol) at 0°C. After stirring for 10min at 0°C, the reaction mixture was treated with sat NaHCO3 (2mL) and extracted with chloroform. The organic layer was separated and washed with brine, dried over anhydrous sodium sulfate, filtered, and concentrated. The residue was purified by column chromatography (silica gel, AcOEt/petroleum ether = 5/1–3/1) to give triflate 17 (73.6mg, 99%). 1H NMR (CDCl3) δ 8.05–8.01 (m, 2H), 7.99–7.94 (m, 2H), 7.63–7.54 (m, 2H), 7.47–7.39 (m, 4H), 5.33 (ddd, 1H, J = 2.2, 2.7, 9.9 Hz), 5.13 (s, 1H), 4.63 (d, 1H, J = 2.2 Hz), 4.50 (AB quartet, 2H, J = 12.1 Hz), 4.32 (dd, 1H, J = 3.3, 7.4 Hz), 4.05 (d, 1H, J = 7.4 Hz), 2.56 (ddd, 1H, J = 3.3, 10.1, 14.6 Hz), 2.01 (dd, 1H, J = 3.0, 14.6 Hz). MS (m/e) (positive-FAB) 501 (M++H).
4.1.6. (1′S,4′R,6′S,7′S)-Benzoic acid 4-benzoyloxymethyl-6-(2-iodo-6-methylaminopurin-9-yl)-2-oxa-bicyclo[2.2.1]-hept-7-yl ester (18)
(1′S,4′R,6′S,7′S)-Benzoic acid 4-benzoyloxymethyl-6-trifluoromethanesulfonyloxy-2-oxa-bicyclo[2.2.1]hept-7-yl ester 17 (20mg, 0.040mmol), 2-iodo-6-methylaminopurine 16 (25mg, 0.091mmol), potassium carbonate (18mg, 0.130mmol) and 18-crown-6 (4mg, 0.015mmol) in DMF (0.30mL) was stirred at 60°C for 3h. The resulting mixture was dried up under vacuum, and the residue was purified by silica gel column chromatography (AcOEt) and preparative TLC (silica gel, AcOEt/petroleum ether = 1/1), to give dibenzoyl adenine derivative 18 (23mg, 91%). 1H NMR (CDCl3) δ 8.06–7.96 (m, 4H), 7.90 (s, 1H), 7.58 (m, 2H), 7.42 (m, 4H), 5.88 (br s, 1H), 5.64 (s, 1H), 4.80 (dd, 1H, J = 5.1, 9.6 Hz), 4.71 (s, 1H), 4.68 (d, 1H, J = 11.7Hz), 4.61 (d, 1H, J = 11.7 Hz), 4.28 (dd, 1H, J = 3.0, 7.2 Hz), 3.97 (d, 1H, J = 7.2 Hz), 3.17 (br s, 3H), 2.70 (dd, 1H, J = 9.6, 13.8 Hz), 2.33 (m, 1H); MS (m/e) (positive-FAB) 626 (M++H).
4.1.7. (1′S,4′R,6′S,7′S)-6-(2-Iodo-6-methylaminopurin-9-yl)-4-hydroxymethyl-2-oxa-bicyclo[2.2.1]hept-7-ol (19)
A mixture of dibenzoyl adenine derivative 18 (21mg, 0.034mmol), potassium carbonate (17mg, 0.123mmol) in THF (0.5mL) and methanol (1.0mL) was stirred at room temperature for 25h. The solvent was removed by nitrogen purging and the residue was purified by silica gel column chromatography (MeOH/CHCl3 = 1/5), to give the cLNA adenine derivative 19 (12.5mg, 89 %). 1H NMR (CD3OD) δ 8.03 (s, 1H), 4.63 (dd, 1H, J = 6.0, 9.3 Hz), 4.32 (s, 1H), 4.15 (s, 1H), 3.90 (dd, 1H, J = 2.1, 6.6 Hz), 3.85 (d, 1H, J = 11.1 Hz), 3.73 (d, 1H, J = 11.1 Hz), 3.68 (d, 1H, J = 6.6 Hz), 3.04 (br s, 3H), 2.41–2.26 (m, 2H); MS (m/e) (positive-FAB) 418 (M++H), HR-MS (positive-FAB) calcd for C13H17N5O3I 418.0376. Found 418.0363.
4.1.8. (1′S,4′R,6′S,7′S)-6-(2-Iodo-6-methylaminopurin-9-yl)-4-[(di-t-butyl-phosphato)-methyl]-7-(di-t-butyl-phosphato)-2-oxa-bicyclo[2.2.1]heptane (20)
The cLNA adenine derivative 19 (8.2mg, 0.020mmol) and 1H-tetrazole (20mg, 0.285mmol) were dissolved in 1.0mL of anhydrous THF. Di-t-butyl diethylphosphoramidite (0.055mL, 0.198mmol) was added, and the mixture was stirred for 3h at room temperature. The reaction mixture was cooled to −78 °C and treated with a solution of m-CPBA (70% max, 55mg) in CH2Cl2 (2.5mL). The resulting mixture was warmed to room temperature and 5% NaHSO3 (2.0mL) was added. The reaction mixture was stirred another 30min at room temperature and extracted with AcOEt. The organic phase was subsequently washed with saturated aqueous NaHCO3 and brine, and then dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue obtained was purified by silica gel column chromatography (MeOH/CHCl3 = 1/5), which furnished 20 (10mg, 64%). 1H NMR (CDCl3) δ 8.06 (s, 1H), 6.20 (br s, 1H), 4.84 (d, 1H, J = 4.8 Hz), 4.67 (m, 2H), 4.21 (m, 2H), 4.00 (m, 1H), 3.82 (d, 1H, J = 6.9 Hz), 3.16 (br s, 3H), 2.60–3.50 (m, 1H), 2.40– 2.30 (m, 1H), 1.48 (s × 2, 18H), 1.47 (s × 2, 18H); MS (m/e) (positive-FAB) 802 (M++H), 824 (M++Na).
4.1.9. (1′S,4′R,6′S,7′S)-Phosphoric acid mono-[6-(2-iodo-6-methylaminopurin-9-yl)-4-phosphonooxymethyl-2-oxabicyclo[2.2.1]hept-7-yl] ester (21)
A solution of 20 (8.0mg, 0.011mmol) in 5% TFA/CH2Cl2 (1.0mL) was stirred for 2h at 25°C. The solvent was removed under reduced pressure and the residue was quenched by addition of 5.0mL of triethylammonium bicarbonate buffer (1.0M). The mixture was subsequently frozen and lyophilized. Purification of the obtained residue was performed on an ion-exchange column packed with Sephadex-DEAE A-25 resin, a linear gradient (0.01–0.5M) of 0.5M ammonium bicarbonate was applied as the mobile phase, and UV and HPLC were used to monitor the elution, which furnished 21 (5.3mg, 73%). 1H NMR (D2O) δ 8.23 (s, 1H), 4.64 (m, 1H), 4.47 (br s, 2H), 4.12 (m, 1H), 3.96 (m, 2H), 3.83 (d, 1H, J = 6.9 Hz), 3.09 (br s, 3H), 2.53–2.32 (m, 2H); 31P NMR (D2O) δ 2.22, 1.81 (2s); MS (m/e) (negative-FAB) 576 (M+−H); HRMS (negative-FAB) calcd for C13H17N5O9P2I 575.9546. Found 575.9557; HPLC 9.1min (99%) (system A), 15.8min (99%) (system B).
4.1.10. (2′R,3′R,4′S)-Phosphoric acid 2-(6-aminopurin-9-yl)-4-(bis-benzyloxy-phosphoryloxy)-tetrahydro-furan-3-yl ester dibenzyl ester (23)
To a stirred solution of (2′R,3′R,4′S)-2-(6-aminopurin-9-yl)-tetrahydro-furan-3,4-diol 22 (5mg, 0.021mmol) and 1H-tetrazole (13mg, 0.186mmol) in anhydrous THF (2.0mL) was added dibenzyl diisopropylphosphoramidite (22mg, 0.069mmol). After stirring for 3h at room temperature, the reaction mixture was cooled to −78 °C and was treated with a solution of m-CPBA (70% max, 25mg) in CH2Cl2 (1mL). The resulting mixture was warmed to room temperature, treated with 5% NaHSO3 (2.0mL) for another 30min at room temperature and extracted with AcOEt. The organic phase was washed with satd NaHCO3 aq and brine and dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue obtained was purified by column chromatography (silica gel, eluent: CHCl3/MeOH = 10/1), to give 23 (10mg, 63%). 1H NMR (CDCl3) δ 8.18 (s, 1H), 7.87 (s, 1H), 7.36–7.19 (m, 20H), 6.51 (br s, 2H), 6.18 (d, 1H, J = 1.5 Hz), 5.33 (d, 1H, J = 7.5 Hz), 5.07 (m, 4H), 5.00 (br, 1H), 4.91 (m, 4H), 4.31 (d, 1H, J = 10.8 Hz), 4.10(dd, 1H, J = 3.9, 10.8 Hz); MS (m/e) (negative-CI) 756 (M+−H).
4.2. Pharmacology
2-MeSADP and GTP were purchased from Sigma (St. Louis, MO). Myo-[3H]inositol (20Ci/mmol) was obtained from American Radiolabeled Chemicals (St. Louis, MO).
P2Y receptor-promoted stimulation of inositol phosphate formation was measured at human P2Y1, P2Y2, P2Y4, or P2Y6 receptors stably expressed in 1321N1 human astrocytoma cells as previously described.5,30,31 The IC50 values were averaged from 3 to 8 independently determined concentration–effect curves for each compound. Briefly, cells plated in 24-well dishes were labeled in inositol-free medium (DMEM; Gibco, Gaithersburg MD) containing 1.0μCi of 2-[3H]myo-inositol (20 Ci/mmol; American Radiolabeled Chemicals, Inc., St. Louis MO) for 18–24h in a humidified atmosphere of 95% air/5% CO2 at 37°C. PLC activity was measured the following day by quantitating [3H]inositol phosphate accumulation after a 10min incubation at 37 °C in the presence of 10mM LiCl. Total [3H]inositol phosphates were quantified by anion exchange chromatography as previously described.30,31 The affinities of bisphosphate analogues for the human P2Y1 receptor were directly determined by using [3H]1 in a radioligand binding assay, as we recently described in detail.9 Binding and functional parameters were estimated using GRAPHPAD PRISM software (GraphPAD, San Diego, CA, USA).
4.3. Molecular modeling
Molecular mechanics calculations have been carried out by means of the Discover3 module of INSIGHTII,32 using the CFF91 forcefield.33 The P2Y1 model used for the docking experiments was the rhodopsin based homology model previously constructed by us.20 Before performing the docking experiments, the ligands have been optimized by means of a quenched molecular dynamics simulation followed by energy minimization.
An NVT (constant-volume/constant-temperature) molecular dynamic simulation was carried out at constant temperature of 300K for 10ps, using a time step of 1 fs. During the simulation, snapshots of the system were taken at regular intervals of 1ps. The structures in each snapshot were energy minimized (BFGS Newton method) until an RMS (root mean squared) of 0.00001kcal/mol/Å was reached and the one showing the lowest energy was used as starting point for the docking experiments.
The flexible superimpositions have been carried out by means of the FIELDFIT program of Search Compare module of INSIGHTII,32 giving equal weight to the steric and the electrostatic factors. The bound conformation of MRS2279 was kept rigid during the calculation, while the structures to be superimposed were fully flexible. An alignment of the molecules based on their dipole and quadripole moments was used as the starting position.
The automatic docking experiments were carried out by means of the Monte Carlo minimization/simulated annealing approach implemented in the Affinity module of INSIGHTII. The binding site was defined as all the residues within 6Å distance from the ligands. Full flexibility was granted to the ligands and to the residues of the binding site. All the atoms of the ligands and the key residues of the binding pocket (R128, K280, R310, Q307, S314) capable of donating or accepting hydrogen bonds were manually marked. During the Monte Carlo simulation the scaling factor for the van der Waals term was set at 0.1, while the coulombic and hydrogen bond terms were set at 1. The maximum movement in each random translation and rotation of the ligand were set at 0.1Å and 1°, respectively. Fifty stages of simulated annealing were performed after the Monte Carlo simulation, setting the initial temperature at 500K, the final temperature at 300K, the final van der Waals and coulombic scaling factors at 1.0 and the final hydrogen bonds scaling factor at 0.0. In other words the van der Waals and coulombic factors were fully considered during the simulated annealing and the hydrogen bond external biases were gradually removed. After the docking procedure, the ligand/receptor complexes were energy minimized until reaching an RMS of 0.05 kcal/mol/Å.
The puckering coordinates P, θm, P2, Q, and Θ were calculated by means of the formulae:
which we derived from the works of Altona and Sundaralingham1 and of Haasnoot.24 The pseudosugar endocyclic torsion angles νj are numbered according to the IUPAC-IUB recommendations for the conformation of polysaccharide chains (www.chem.qmul.ac.uk/iupac/misc/psac.html). All the angles are expressed in radians.
Acknowledgments
MO is on sabbatical from Toray Industries (Kamakura, Japan) and thanks them for financial support. Mass spectral measurements were carried out by Dr. Victor Livengood and NMR by Wesley White (NIDDK). We thank Prof. A. Eschenmoser and Dr. R. Krishnamurthy of the Scripps Res. Inst. (San Diego, CA) for helpful discussions and for the gift of L-α-threofuranosyl-adenine.
References and notes
- 1.Altona C, Sundaralingham M. J Am Chem Soc. 1972;94:8205. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- 2.Marquez VE, Siddiqui MA, Ezzitouni A, Russ P, Wang J, Wagner RW, Matteucci MD. J Med Chem. 1996;39:3739. doi: 10.1021/jm960306+. [DOI] [PubMed] [Google Scholar]
- 3.Shin KJ, Moon HR, George C, Marquez VE. J Org Chem. 2000;65:2172. doi: 10.1021/jo9917691. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson KA, Ji X-D, Li AH, Melman N, Siddiqi MA, Shin KJ, Marquez VE, Ravi RG. J Med Chem. 2000;43:2196. doi: 10.1021/jm9905965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg A-K, Erlinge D, Malmsjö M, Harden TK, Boyer JL, Jacobson KA. J Med Chem. 2002;45:208. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. Br J Pharmacol. 2002;135:2004. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nandanan E, Jang SY, Moro S, Kim H, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA. J Med Chem. 2000;43:829. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. J Med Chem. 2003;46:4974. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji X-D, Lacy J, Jacobson KA, Harden TK. Mol Pharmacol. 2002;62:1249. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obika S, Nanbu D, Fari Y, Morio K-I, In Y, Ishida T, Imanishi T. Tetrahedron Lett. 1997;38:8735. [Google Scholar]
- 11.(a) Petersen M, Bondensgaard K, Wengel J, Jacobsen JP. J Am Chem Soc. 2002;124:5974. doi: 10.1021/ja012288d. [DOI] [PubMed] [Google Scholar]; (b) Petersen M, Wengel J. Trends Biotechnol. 2003;21:74. doi: 10.1016/S0167-7799(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 12.Koshkin AA, Fensholdt J, Pfundheller HM, Lomholt C. J Org Chem. 2001;66:8504. doi: 10.1021/jo010732p. [DOI] [PubMed] [Google Scholar]
- 13.Hodgson DM, Gibbs AR, Drew MGB. J Chem Soc, Perkin Trans 1. 1999;24:3579. [Google Scholar]
- 14.(a) Alex G, Kruger AW, Meyers AI. Tetrahedron Lett. 2001;42:4305. [Google Scholar]; (b) Arrington MP, Meyers AI. Chem Commun. 1999;15:1371. [Google Scholar]
- 15.Obike S, Morio K-I, Nanbu D, Hari Y, Itoh H, Imanishi T. Tetrahedron. 2002;58:3039. [Google Scholar]
- 16.Kim HS, Jacobson KA. Org Lett. 2003;5:1665. doi: 10.1021/ol034326z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. J Med Chem. 2002;45:2090. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schöning K, Scholz P, Guntha S, Wu X, Krishnamurthy R, Eschenmoser A. Science. 2000;290:1347. doi: 10.1126/science.290.5495.1347. [DOI] [PubMed] [Google Scholar]
- 19.Schöning K, Scholz P, Wu X, Guntha S, Delgado G, Krishnamurthy R, Eschenmoser A. Helv Chim Acta. 2002;85:4111. [Google Scholar]
- 20.Costanzi S, Mamedova L, Gao ZG, Jacobson KA. J Med Chem. doi: 10.1021/jm049914c. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Nair V, Fasbender AJ. Tetrahedron. 1993;49:2169–2184. [Google Scholar]; (b) Nair V, Richardson SG. J Org Chem. 1980;45:3969. [Google Scholar]; (c) Nair V, Richardson SG. Synthesis. 1982:670. [Google Scholar]
- 22.Lee K, Ravi RG, Ji X-d, Marquez VE, Jacobson KA. Bioorg Med Chem Lett. 2001;11:1333. doi: 10.1016/s0960-894x(01)00213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HS, Barak D, Harden TK, Boyer JL, Jacobson KA. J Med Chem. 2001;44:3092. doi: 10.1021/jm010082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haasnoot CAG. J Am Chem Soc. 1993;115:1460. [Google Scholar]
- 25.Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. J Med Chem. 1998;41:1456. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Q, Guo D, Lee BX, van Rhee AM, Kim Y-C, Nicholas RA, Schachter J, Harden TK, Jacobson KA. Mol Pharmacol. 1997;52:499. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi Y, George C, Comin MJ, Barchi JJ, Jr, Kim HS, Jacobson KA, Balzarini J, Mitsuya H, Boyer PL, Hughes SH, Marquez VE. J Med Chem. 2003;46:3292. doi: 10.1021/jm030116g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costanzi S, Lambertucci C, Vittori S, Volpini R, Cristalli G. J Mol Graph Model. 2003;21:253. doi: 10.1016/s1093-3263(02)00161-4. [DOI] [PubMed] [Google Scholar]
- 29.Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. J Med Chem. 2002;45:4471. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harden TK, Hawkins PT, Stephens L, Boyer JL, Downes P. Biochem J. 1988;252:583. doi: 10.1042/bj2520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer JL, Downes CP, Harden TK. J Biol Chem. 1989;264:884. [PubMed] [Google Scholar]
- 32.InsightII version 2000.1, Accelrys (former MSI) San Diego, CA: [Google Scholar]
- 33.Maple JR, Hwang MJ, Stockfisch TP, Dinur U, Waldman M, Ewig CS, Hagler AT. J Comput Chem. 1994;15:162. [Google Scholar]
- 34.Kempeneers V, Froeyen M, Vastmans K, Herdewijn P. Chem Biodiver. 2004;1:112. doi: 10.1002/cbdv.200490002. [DOI] [PubMed] [Google Scholar]
- 35.Nandanan E, Camaioni E, Jang SY, Kim YC, Cristalli G, Herdewijn P, Secrist JA, Tiwari KN, Mohanram A, Harden TK, Boyer JL, Jacobson KA. J Med Chem. 1999;42:1625. doi: 10.1021/jm980657j. [DOI] [PMC free article] [PubMed] [Google Scholar]