SUMMARY
The hepatorenal reflex plays an important role in water and salt homeostasis by matching renal excretion to gastrointestinal absorption. This homeostatic mechanism is impaired in nephrotic rats. This study tests the hypothesis that in nephrotic rats, the renal sodium excretion response to hypertonic saline infusion is impaired due to a decreased sensitivity of the hepatoportal sodium-sensing mechanism.
This study was performed in control and adriamycin (ADR)-induced nephrotic syndrome rats. After baseline data collection, urinary sodium (UNaV) and potassium (UKV) excretion responses were tested following continuous infusion of hypertonic NaCl solution (20 μl/min for 30 min) into either femoral or mesenteric vein. A second study tested hepatic and renal nerve responses to continuous mesenteric vein infusion of hypertonic NaCl (10 μl/sec for 30 sec).
Compared to control rats, nephrotic displayed significantly lower baseline UNaV and UKV excretion. In the control rats, mesenteric compared to femoral vein infusion of hypertonic NaCl produced a more rapid and greater increase in UNaV. In contrast, in nephrotic rats, femoral and mesenteric vein infusion caused similar increases in UNaV, and the maximal increases in UNaV to either route of infusion were much lower in nephrotic than control rats. Further, in control compared to nephrotic rats, portal hypertonic saline infusion caused greater increases in hepatic nerve activity and greater decreases in renal nerve activity.
These data suggest that in rats, adriamycin treatment decreases hepatoportal sodium-sensing sensitivity, leading to marked impairment of hepatorenal reflex responses, potentially contributing to salt and water retention.
Keywords: adriamycin, hepatoportal sodium-sensing receptor, hepatorenal reflex, natriuresis, nephrotic syndrome
INTRODUCTION
The liver contains a variety of receptors, including osmotic, glucose, sodium and potassium receptors,1–6 which make the liver very sensitivity to changes of substances that are absorbed from the intestine into the blood. Thus, the receptors enable the liver to contribute significantly to body fluid homeostasis. Several studies indicate that in research animals, infusion of hypertonic NaCl into the portal vein, compared to other veins, results in greater diuresis and natriuresis.1,2,4,5 Mesenteric infusion of hypertonic NaCl stimulates a hepatoportal sodium sensing receptor, which in turn signals the central nervous system (CNS) via hepatic afferent nerves.6 The sensory input to the CNS decreases efferent renal sympathetic nerve activity, leading to increased urinary sodium excretion. Morita et al.7 have shown that hepatoportal NaCl receptors play a physiologically significant role in postprandial natriuresis in conscious dogs. Thus, the hepatorenal reflex contributes importantly to body fluid balance.
In cirrhotic rats, hypertonic saline infusion into the portal versus femoral vein leads to greater diuresis and natriuresis, but the maximal increases are lower in cirrhotic than in control rats, irrespective of the vein infused.8 Moreover, the nephrotic syndrome (NS) is associated with salt and water retention, which can result from both intrinsic and extrinsic effects on renal excretion of sodium and water.9–11 DiBona and colleagues10,11 demonstrated that nephrotic (adriamycin-treated) rats display impaired excretion of salt and water in response to intravenous isotonic saline infusion (5% BW, over 30 min). Bilateral renal denervation returned the renal excretory response to control levels. These findings suggest that in adriamycin-treated rats, dysfunction of hepatic sodium-sensing receptors and their signaling cascade impair natriuretic responses. The current experiments test the hypothesis that nephrotic (adriamycin-treated) rats display impaired sodium and water excretion following hypertonic saline infusion into the hepatoportal circulation (i.e., they retain excess water and salt) due, in part, to impaired sodium-sensing receptor feedback from the hepatoportal system.
MATERIALS AND METHODS
Animals
All experimental procedures were pre-approved by the Khon Kaen University Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health guidelines and the ILAR Guide (2011) and conform to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Studies were performed in male Sprague-Dawley rats (300–350 g) from the animal facility of Faculty of Medicine at Khon Kaen University. Throughout the study, all animals were allowed free access to water and standard laboratory rat chow and were maintained at constant humidity (60 ± 5%), temperature (24 ± 1°C) and light cycle (0600–1800 h). Nephrotic syndrome was induced by a single intravenous injection of adriamycin (ADR 5.0 mg/kg BW) through the tail vein of unanesthetized animals.10–12 The development of nephrotic syndrome was assessed by the appearance of elevated urinary protein in 24-hr samples, using standard metabolic cages for collection. Control rats were injected with the same amount of the isotonic saline vehicle. Twenty days after injection, 24-hr urinary protein excretion was again measured using metabolic cages for collection.
Experimental procedures
Experiment 1: Measurement of renal function
The nephrotic syndrome (NS; ADR injected) and control (C) groups were further divided for femoral versus mesenteric (portal) vein infusion of 5% NaCl, 20 μl/min for 30-min, i.e., control rats received femoral (FV-C) or mesenteric (PV-C) infusion and ADR-treated rats received femoral (FV-NS) or mesenteric (PV-NS) infusion (n= 8/group). On the experimental day, rats were anesthetized with sodium pentobarbital (60 mg/kg i.p.), and a tracheotomy was performed for adequate ventilation. A catheter (PE 10 connected to PE 50) was placed into a femoral artery for measurement of arterial pressure and for collection of blood samples. The catheter was connected to a pressure transducer for continuous measurements of mean arterial pressure (MAP) and heart rate (HR), which were analyzed using Acknowledge software (version 3.5.5, BioPac Systems, CA, USA).
A catheter (PE 10) was placed either in the femoral or portal vein (via a branch of the mesenteric vein) for infusion of normal or hypertonic saline. Portal infusions were made via PE 10 tubing inserted into a small branch of mesenteric vein that pointed toward the portal vein with no ligation of mesenteric vein. This enabled the infusate to flow into portal vein without severely disturbing mesenteric blood flow. The catheter was connected to an infusion pump (Harvard Apparatus, model 940, USA). A catheter (PE 50) was inserted into the jugular vein and connected to an infusion pump (Harvard model 940, USA), which delivered 0.9% NaCl at 100 μl/min for 50 min to provide adequate urine flow, infusion of inulin and para-aminohippuric acid (PAH) and administration of supplemental doses of anesthetic. A priming dose of 0.5 ml of 5% inulin and 5% PAH in 0.9% NaCl at 200 μl/min was made, followed by a sustaining flow of 20 μl/min that was infused throughout the experiments to measure inulin and PAH clearances. The ureters were cannulated with PE 10 tubing, and urine samples were time collected separately into pre-weighed vials to assess urine flow (calculated gravimetrically).
After surgery, a 60-min stabilization period was followed by two consecutive 20-min control collections (baseline function), followed by four consecutive 20-min experimental collections during which the 5% NaCl was infused via the route selected for each group. Blood samples (0.3 ml) were collected in heparinized syringes from the arterial catheter at the middle of each clearance period. The blood was centrifuged immediately, plasma was separated, and the packed red cells were resuspended with an equivalent volume of normal saline and replaced back to the animal. At the end of the experiment, the animals were killed by an overdose of pentobarbital and the kidneys were removed, blotted dry and weighed.
Experiment 2: Measurement of nerve activity
Hepatic nerve activity (HNA) and renal nerve activity (RNA) were assessed in mesenteric vein infused control (PV-C) and nephrotic (PV-NS) rats (n=8 in each group). The rats were anesthetized, and the renal nerve and periarterial hepatic nerve were carefully isolated through a midline incision, as previously described.13,14 In brief, two stainless-steel electrodes (model 5727, A-M System Inc., WA, USA) were placed in contact with the nerves. The nerve and electrodes were covered and fixed in place with silicone gel (silicon 604A and 604B, Wacker, USA). The recorded nerve activity was amplified using a 50-Hz to 1 kHz band pass filter. The amplifier output was filtered through a gate circuit to extract baseline noise (DAM 80, World Precision Instruments, FL, USA), and the data were processed via a DA 100 and MP 100 analysis system (BioPac Systems, CA, USA). Differences in diameter and/or number of active fibers at the nerve-electrode contact make it difficult to compare absolute frequency of nerve activity between rats. Therefore, to quantitate nerve activity, the values are expressed as a percent of initial baseline (the values immediately before each portal infusion). Thus, HNA and RNA were expressed as the percentage changes from baseline (%Δ HNA, %Δ RNA).13 After a stabilization period (40 min; baseline), the rats were infused with 5% NaCl, 10 μl/sec for 30 sec via mesenteric vein. Both HNA and RNA were recorded simultaneously for two minutes before the infusion and for 3–6 minutes after the infusion.
Chemical analyses
Urinary and plasma albumin, protein and triglyceride concentrations were measured by the Srinagarind Hospital Laboratory Unit. Urine sodium and potassium concentrations were determined by flame photometry (Hitachi, model 775-A, Tokyo, Japan). The urine and plasma concentrations of inulin and PAH were determined by standard colorimetric methods. Glomerular filtration rate (GFR) and renal plasma flow (RPF) were calculated from standard inulin and PAH clearance formulae, respectively. The study calculated sodium and potassium excretion as the product of urine flow and urine sodium and potassium concentrations, respectively.
Statistical analyses
All data are expressed as means ± SEM. The averaged values from the two control clearance periods were compared to each of the experimental periods. Statistical analyses of the data were performed using one-way ANOVA with post-hoc Tukey’s test. Statistical significance was defined as p < 0.05 (2-sided).
RESULTS
Baseline data
After treatment for 20-day, all ADR-treated rats developed significant proteinuria, hypoalbuminemia and hypertriglyceridemia. Compared to control rats, nephrotic rats displayed large increases in urinary protein (8.66 ± 1.12 vs. 77.31 ± 6.17 mg/24 hr; p<0.01) and plasma triglyceride (51.56 ± 5.61 vs. 371.17 ± 20.30 mg/dl; p<0.01) concentrations, and they displayed decreases in plasma albumin (2.56 ± 0.06 vs. 1.87 ± 0.12 g/dl; p <0.01). MAP and HR were not different between groups (Table 1). Compared to control rats, nephrotic rats displayed lower RPF, GFR, urine flow and urinary sodium and potassium excretion (Table 1).
Table 1.
Baseline renal function parameters during the pre-infusion control periods
| Control (n=10) | NS (n=10) | |
|---|---|---|
| HR (beat/min) | 302 ± 12 | 272 ± 7 |
| MAP (mm Hg) | 110 ± 4 | 117 ± 4 |
| GFR (ml/min/g KW) | 1.11 ± 0.08 | 0.44 ± 0.03* |
| RPF (ml/min/g KW) | 2.97 ± 0.34 | 0.63 ± 0.03* |
| V (μl/min/g KW) | 8.31 ± 0.42 | 7.54 ± 0.14* |
| UNaV(μEq/min/g KW) | 2.75 ± 0.18 | 1.08 ± 0.04* |
| UKV (μEq/min/g KW) | 1.68 ± 0.12 | 1.17 ± 0.06* |
Values are means ± SEM; NS, nephrotic rats; HR, heart rate; MAP, mean arterial pressure; GFR, glomerular filtration rate; RPF, renal plasma flow; V, urine flow; UNaV, urinary sodium excretion; UKV, urinary potassium excretion;
p<0.05 compared to control rats (Tukey’s test).
Control rat responses to hypertonic saline infusion
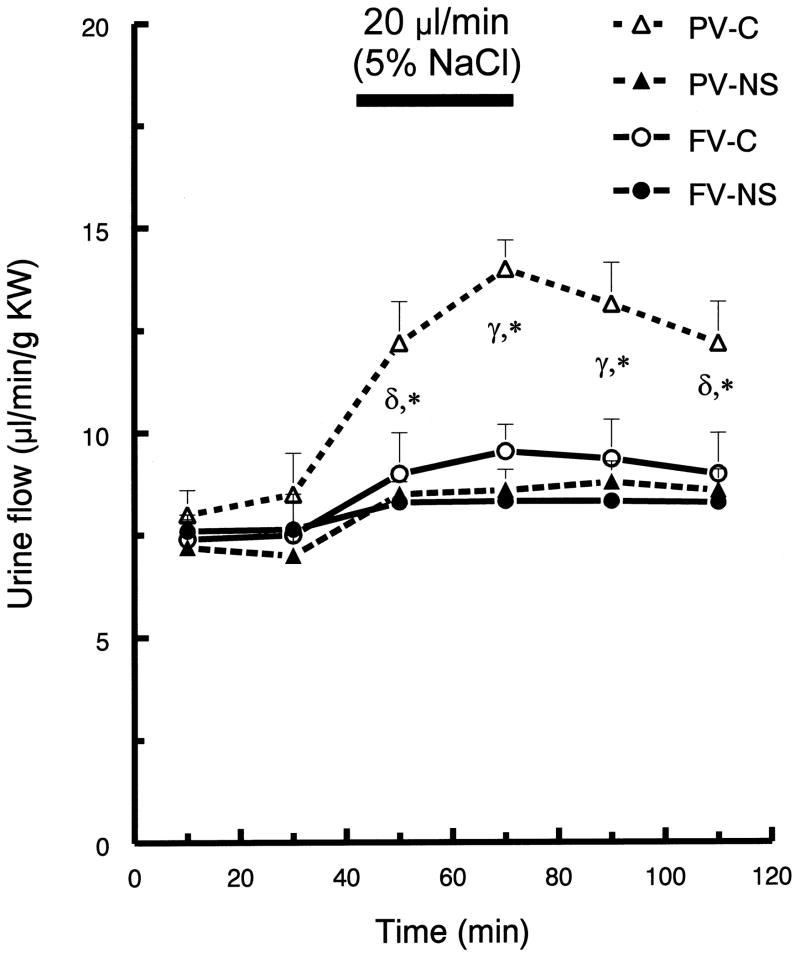
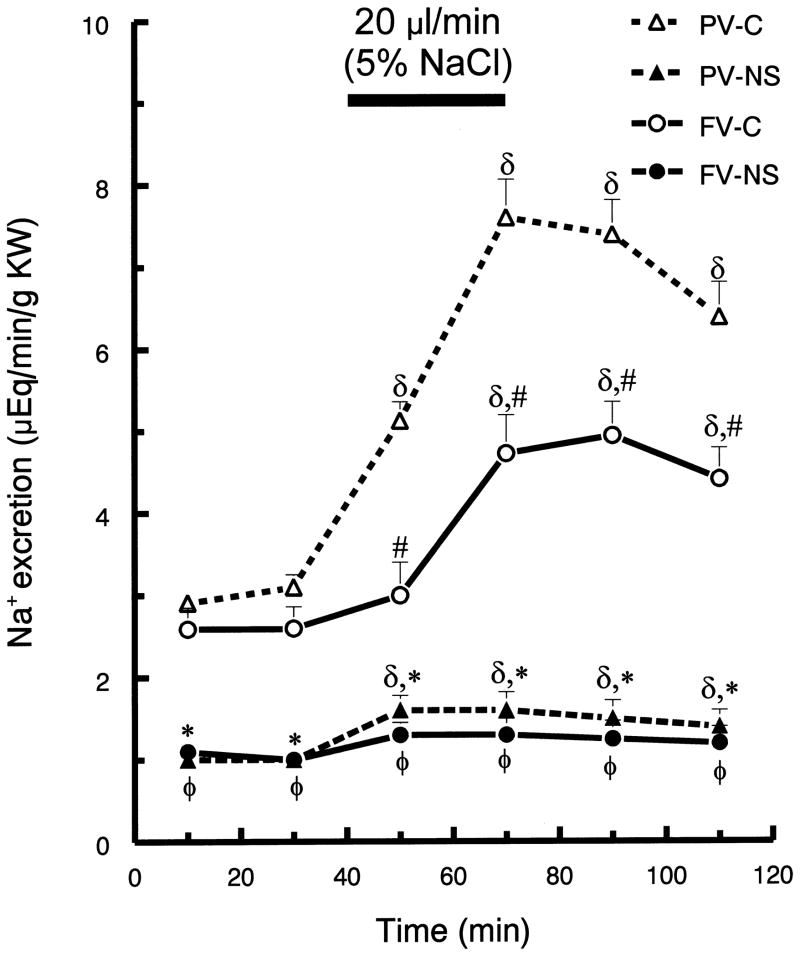
In control rats, infusion of hypertonic saline into the portal (compared to femoral) vein resulted in greater diuresis. Compared to baseline values (40 min prior to infusion), urine flow in the control rats was maximal (~60%) at 10 min after initiation of the portal vein infusion (p <0.05; Fig. 1). In contrast, the maximal urine flow response to the femoral vein infusion only increased ~28% (p <0.05; Fig. 1) in the control rats. Hypertonic saline infusion by both routes increased the rate of sodium excretion in the control rats (Fig. 2). Femoral vein infusion resulted in a 90% increase in sodium excretion, while portal vein infusion resulted in a 160% increase in sodium excretion. Further, the infusion of hypertonic saline into portal (compared to femoral) vein also produced a more rapid response (Fig. 2).
Fig. 1.
Urine flow rate (V) before and after hypertonic saline infusion into either portal vein (PV) or femoral vein (FV) of control (C) and nephrotic (NS) rats. After a 40-min control period all rats were infused with 5% NaCl (20 μl/min for 30-min). Values are means ± SEM (n=8); FV-C, PV-C (control rats with hypertonic saline infusion into femoral vein or portal vein, respectively); FV-NS, PV-NS (nephrotic rats with hypertonic saline infusion into femoral vein or portal vein, respectively); γ p< 0.05 for all groups compared to mean baseline values within group; δ p < 0.05 compared to mean baseline values within group; * p < 0.05 for PV-C vs. all other groups; Tukey’s test.
Fig. 2.
Urinary sodium excretion (UNaV) before and after hypertonic saline infusion into either portal vein (PV) or femoral vein (FV) of control (C) and nephrotic (NS) rats. See Fig. 1 for brief protocol. Values are means ± SEM (n=8);δ p< 0.05 compared to mean baseline values within group; * p < 0.05 PV-C versus PV-NS; # p < 0.05 PV-C versus FV-C; φ p < 0.05 FV-C versus FV-NS; Tukey’s test.
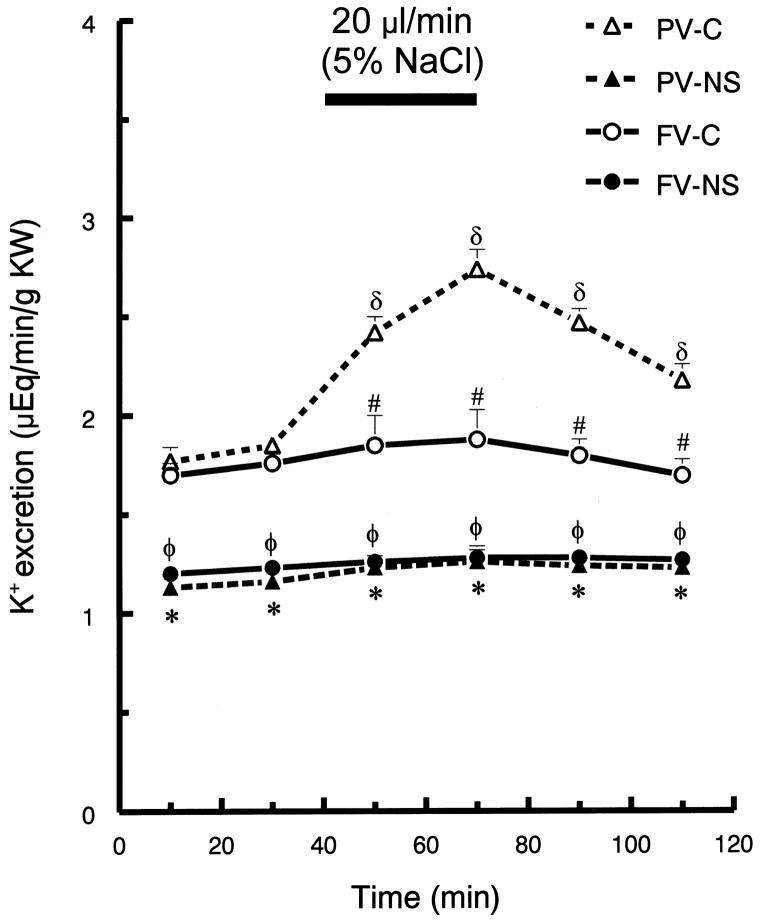
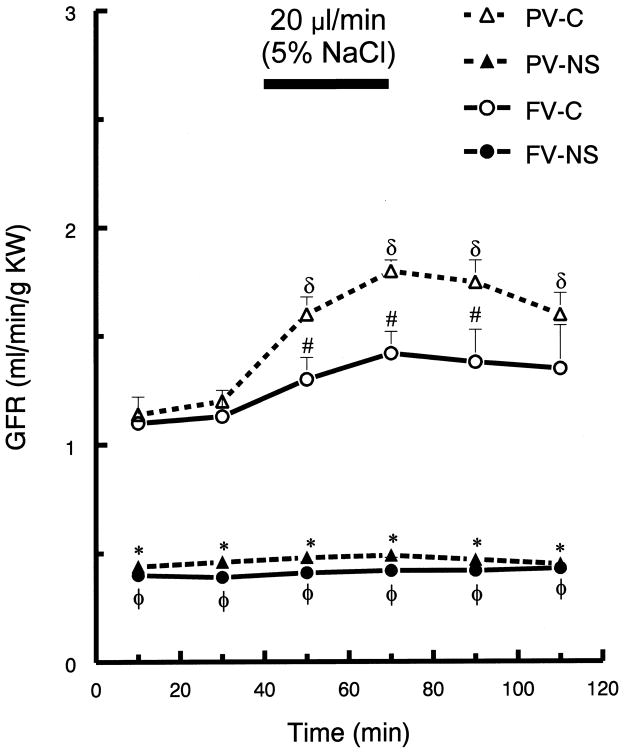
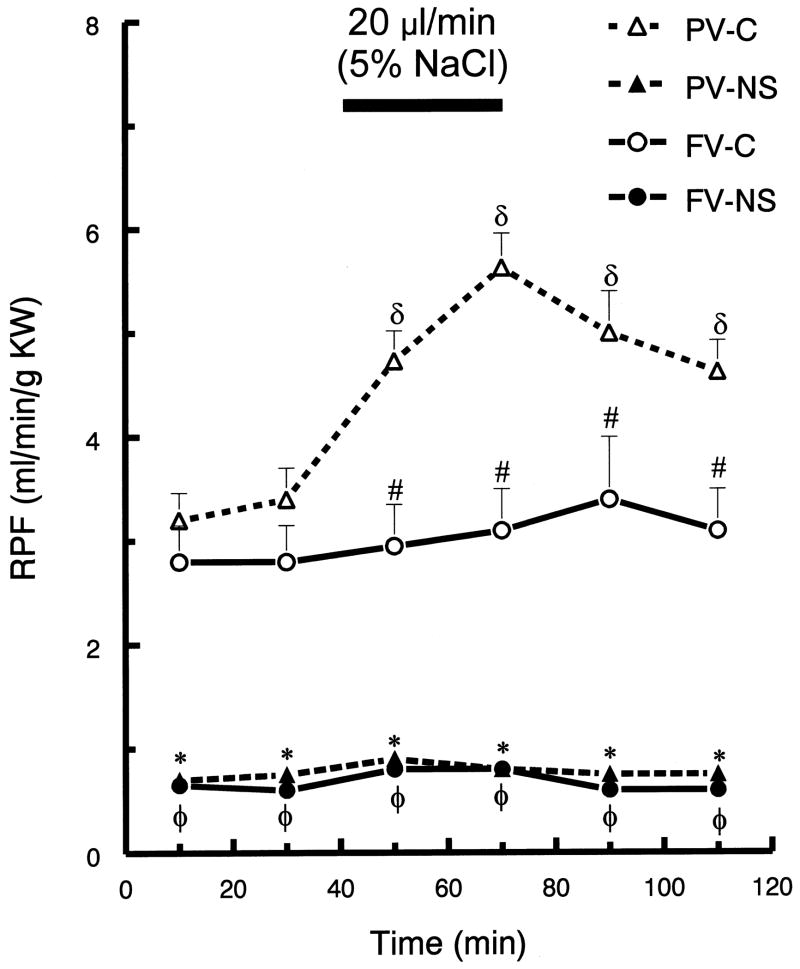
In control rats, portal vein, hypertonic saline infusion significantly increased potassium excretion by ~60%, at 70 min (p <0.05), but there was no change in potassium excretion following femoral infusion (Fig. 3). Portal infusion of hypertonic saline in control rats increased GFR and RPF (p <0.05), but femoral vein infusion resulted in no significant changes in GFR or RPF (Fig. 4 and Fig. 5, respectively). Neither route of hypertonic NaCl infusion significantly altered MAP, HR, plasma Na+ or K+ concentration (data not shown).
Fig. 3.
Urinary potassium excretion (UKV) before and after hypertonic saline infusion into either portal vein (PV) or femoral vein (FV) of control (C) and nephrotic (NS) rats. See Fig. 1 for brief protocol. Values are means ± SEM (n=8); δ p< 0.05 compared to mean baseline values within group; * p < 0.05 PV-C versus PV-NS; # p < 0.05 PV-C versus FV-C; φ p < 0.05 FV-C versus FV-NS; Tukey’s test.
Fig. 4.
Glomerular filtration rate (GFR) before and after hypertonic saline infusion into either portal vein (PV) or femoral vein (FV) of control (C) and nephrotic (NS) rats. See Fig. 1 for brief protocol. Values are means ± SEM (n=8); δ p< 0.05 compared to mean baseline values within group; * p < 0.05 PV-C versus PV-NS and FV-NS; # p < 0.05 PV-C versus FV-C; φ p < 0.05 FV-C versus FV-NS; Tukey’s test.
Fig. 5.
Renal plasma flow (RPF) before and after hypertonic saline infusion into either portal vein (PV) or femoral vein (FV) of control (C) and nephrotic (NS) rats. See Fig. 1 for brief protocol. Values are means ± SEM (n=8);δ p< 0.05 compared to mean baseline values within group; * p < 0.05 PV-C versus PV-NS; # p < 0.05 PV-C versus FV-C;φ p < 0.05 FV-C versus FV-NS; Tukey’s test.
Response of ADR-treated rat to hypertonic saline infusion
The infusion of hypertonic saline into femoral vein resulted in relatively small diuresis in both control and ADR-treated rats. In contrast, infusion into the portal vein induced much less diuresis in ADR-treated, compared to in control rats (Fig. 1). Compared to their basal values, portal infusion increased maximum urine flow rate by 50% in control rats, but only by 12% in nephrotic rats (p < 0.05). Compared to control rats, ADR-treated rats displayed markedly blunted natriuresis, although following portal vein infusion the nephrotic rats displayed a significant increase in sodium excretion (~48 %). In nephrotic rats, femoral vein hypertonic saline infusion did not induce a natriuretic response. In ADR-treated rats, neither portal nor femoral vein hypertonic saline infusion altered K excretion (Fig. 3).
The nephrotic rats displayed no GFR (Fig. 4) or RPF responses to hypertonic saline infusion via either vein (Fig. 5). Further, hypertonic NaCl infusion via either route did not alter MAP, HR or plasma Na+ or K+ concentration (data not shown).
Measurement of hepatic and renal nerve activity
Portal infusion of hypertonic saline increased hepatic nerve activity and decreased renal nerve activity more in control than in nephrotic rats (Table 2). The infusion resulted in a ~ 8-fold greater increase in hepatic nerve activity in control compared to nephrotic rats and a ~1.7-fold greater decrease in renal nerve activity in control compared to nephrotic rats.
Table 2.
Percent changes from baseline (%Δ) in hepatic and renal nerve activity in control and nephrotic (NS) rats in response to portal infusion of hypertonic saline 10 μl/sec for 30 seconds
Values are means ± SEM; NS, nephrotic rats; HNA, hepatic nerve activity; RNA, renal nerve activity; %ΔHNA and %ΔRNA are the percentage changes from their corresponding control periods;
p<0.05 compared to control rats (Tukey’s test).
DISCUSSION
The present results indicate that the ADR-induced nephrotic syndrome causes decreased GFR responses and blunted hepatorenal reflex responses to portal vein hypertonic saline infusion. Further, compared to control rats, nephrotic rats display reduced hepatic nerve and renal nerve responses to portal vein infusion of hypertonic saline. Together, this indicates that this model of nephrotic syndrome reduces GFR and hepatorenal responses to circulating Na+ concentration, likely contributing to salt and water retention.
While it has been recognized for many years that the ADR-model of nephrotic syndrome is associated with disturbances in renal function, the role of the hepatoportal sodium sensing receptors and the hepatic nerve in this effect have not been directly tested. The current results indicate that ADR-treatment reduces the transmission of Na+ concentration information via the hepatic nerve and that this effect is associated with reduced responsiveness of the renal nerve to portal hypertonic saline. Thus, it is likely that a decrease ability of the liver to inform the CNS about plasma Na+ status contributes to renal dysfunction in this model and perhaps in patients with nephrotic syndrome.
The present results support the existence of a functional Na+ monitoring system in the hepatoportal system.2,3,5,15–18 This mechanism provides a rapid response to local plasma sodium concentration changes in the splanchnic circulation, which is especially important for the early detection of increased Na+ absorption from the gut. Further, the hepatorenal reflex regulation of renal water and sodium excretion appears to be mediated by intrahepatic adenosine A1 receptors.15,16 In addition, research in humans demonstrates that significantly greater natriuresis occurs after oral versus intravenous sodium administration, and this is not dependent on inhibition of aldosterone secretion.19
In previous studies using an experimental nephrotic syndrome model that was induced by a single intravenous administration of adriamycin,9,10 decreased GFR appeared to significantly contribute to renal sodium and water retention (confirmed in the present study). The decreased GFR appeared primarily due to a marked decline in renal plasma flow. The decreased glomerular capillary filtration coefficient might also play a role as previously reported.9 Moreover, in the present study, hypoalbuminemia reduces plasma oncotic pressure and results in hypovolemia, and reduced effective circulating volume, thus, stimulating the release of renin (via baroreflex) and increased angiotensin II synthesis. The increases in angiotensin II and renal sympathetic nerve activity leading to profound reduction in renal blood flow and decreased glomerular capillary pressure. However, it is important to note that patients with nephrotic syndrome display decreased, normal or increased GFR, and yet they all consistently retain salt and water.20,21
Studies on experimental models of nephrotic syndrome have also shown that basal sodium excretion is decreased in nephrotic compared to control animals.9,10 The present study compared the renal response to hypertonic saline infusions via portal or femoral vein in normal and nephrotic rats. Control rats responded to portal (compared to femoral) vein infusion of hypertonic saline with more rapid and greater magnitude natriuresis and diuresis. The natriuresis appeared within 10 min, was maximal at 30 min after portal infusion initiation and remained higher than pre-infusion baseline for at least 60 min. These results agree with previous observations and support evidence that splanchnic sodium-sensing receptors play an important role in renal Na+ excretion in response to NaCl intake.1,2,5,8
Previous observations demonstrate the role of hepatorenal reflex in sodium and water balance. The decrease in renal nerve activity induced by intravenous infusion of hypertonic saline is abolished by sinoaortic baroreceptor denervation plus vagotomy and hepatic denervation.5 Further, portal infusion of hypertonic saline decreases renal nerve activity, and this response is abolished by hepatic denervation.5 In addition, oral intake of high NaCl decreases renal nerve activity and increases urinary sodium excretion, and this response is abolished by hepatic denervation.7 Thus, the hepatorenal reflex appears to plays a significant role in postprandial natriuresis. The present data indicate that in nephrotic rats, the response to hypertonic saline infusion into the portal vein is markedly blunted. A similar response was observed in cirrhotic rats.8 DiBona et al.10 also found that compared to control rats, rats with nephrotic and hepatic cirrhosis have impaired salt and water excretion following an acute intravenous isotonic saline loading. This response is partially dependent on an elevated basal efferent renal nerve activity, which fails to decrease normally in response to an acute isotonic saline load in nephrotic compared to control rats. Bilateral renal denervation restores the renal response to the acute administration of an intravenous isotonic saline in these models. The present data support these findings and extended them by demonstrating that the increased hepatic nerve activity and the decreased renal nerve activity responses to portal infusion of hypertonic saline (responses that normally occur in control rats) are significantly blunted in nephrotic rats, indicating that in nephrotic rats, the sensitivity of the hepatoportal Na+ monitoring system is reduced. This has also been reported in cirrhotic rats.8,14
The central connection of the hepatic afferent nerves terminates within the medial division of the solitary nucleus and the lateral edge of area postrema.22 Kobashi and Adachi 23 observed that electrical stimulation of the hepatic nerve or infusion of hypertonic saline into the portal vein alters neuronal activity in the nucleus of the solitary tract. Furthermore, the electrical or chemical stimulation of the nucleus of the solitary tract increases renal nerve activity.24
Studies in humans and animal models of nephrotic syndrome and hepatic cirrhosis suggest that elevated basal efferent renal nerve activity is present and may contribute to the renal sodium and water retention and edema formation.25 Increases in efferent renal nerve activity that does not alter renal hemodynamics can increase renal tubular sodium and water reabsorption throughout the nephron.25 Therefore, these studies strongly suggest that in nephrotic syndrome and hepatic cirrhosis, impaired signaling to the CNS by hepatic sodium-sensing receptor responses and impaired inhibition of renal nerve activity contribute to chronic progressive renal sodium and water retention, leading to edema formation.
In conclusion, the present data indicate that in the ADR model of nephrotic syndrome in rats, salt and water retention likely result at least in part from decreased GFR and decreased sensitivity of the hepatoportal sodium-sensing feedback system, leading to decreased hepatorenal feedback.
Acknowledgments
The study was supported by a research grant from the Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
References
- 1.Daly JJ, Roe JW, Horrocks P. A comparison of sodium excretion following the infusion of saline into systemic and portal veins in the dog: evidence for a hepatic role in the control of sodium excretion. Clin Sci. 1967;33:481–7. [PubMed] [Google Scholar]
- 2.Strandhoy JW, Williamson HE. Evidence for an hepatic role in the control of sodium excretion. Proc Soc Exp Biol Med. 1970;133:419–22. doi: 10.3181/00379727-133-34487. [DOI] [PubMed] [Google Scholar]
- 3.Sawchenko PE, Friedman MI. Sensory functions of the liver--a review. Am J Physiol. 1979;236:R5–20. doi: 10.1152/ajpregu.1979.236.1.R5. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Novoa JM, Zubiaur M. Contribution of splanchnic area to the control of renal electrolyte excretion in conscious rats. Miner Electrolyte Metab. 1982;8:52–60. [PubMed] [Google Scholar]
- 5.Morita H, Nishida Y, Hosomi H. Neural control of urinary sodium excretion during hypertonic NaCl load in conscious rabbits: role of renal and hepatic nerves and baroreceptors. J Auton Nerv Syst. 1991;34:157–69. doi: 10.1016/0165-1838(91)90082-e. [DOI] [PubMed] [Google Scholar]
- 6.Morita H, Ishiki K, Hosomi H. Effects of hepatic NaCl receptor stimulation on renal nerve activity in conscious rabbits. Neurosci Lett. 1991;123:1–3. doi: 10.1016/0304-3940(91)90143-h. [DOI] [PubMed] [Google Scholar]
- 7.Morita H, Matsuda T, Furuya F, Khanchowdhury MR, Hosomi H. Hepatorenal reflex plays an important role in natriuresis after high-NaCl food intake in conscious dogs. Circ Res. 1993;72:552–9. doi: 10.1161/01.res.72.3.552. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Novoa JM, Martinez-Maldonado M. Impaired renal response to splanchnic infusion of hypertonic saline in conscious cirrhotic rats. Am J Physiol. 1982;242:F390–F394. doi: 10.1152/ajprenal.1982.242.4.F390. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa I, Rennke HG, Hoyer JR, Badr KF, Schor N, Troy JL, et al. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983;71:91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiBona GF, Herman PJ, Sawin LL. Neural control of renal function in edema-forming states. Am J Physiol. 1988;254:R1017–R1024. doi: 10.1152/ajpregu.1988.254.6.R1017. [DOI] [PubMed] [Google Scholar]
- 11.Perico N, Delaini F, Lupini C, Benigni A, Galbusera M, Boccardo P, et al. Blunted excretory response to atrial natriuretic peptide in experimental nephrosis. Kidney Int. 1989;36:57–64. doi: 10.1038/ki.1989.161. [DOI] [PubMed] [Google Scholar]
- 12.Bertani T, Poggi A, Pozzoni R, Delaini F, Sacchi G, Thoua Y, et al. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest. 1982;46:16–23. [PubMed] [Google Scholar]
- 13.DiBona GF, Sawin LL. Hepatorenal baroreflex in cirrhotic rats. Am J Physiol. 1995;269:G29–G33. doi: 10.1152/ajpgi.1995.269.1.G29. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Matsuda T, Morita H, Hosomi H. Depressed sensitivity of the hepatoportal NaCl receptors in rats with carbon tetrachloride-induced liver cirrhosis. Am J Physiol. 1995;269:R1390–R1395. doi: 10.1152/ajpregu.1995.269.6.R1390. [DOI] [PubMed] [Google Scholar]
- 15.Ming Z, Lautt WW. Intrahepatic adenosine-mediated activation of hepatorenal reflex is via A1 receptors in rats. Can J Physiol Pharmacol. 2006;84:1177–84. doi: 10.1139/y06-063. [DOI] [PubMed] [Google Scholar]
- 16.Ming Z, Smyth DD, Lautt WW. Decreases in portal flow trigger a hepatorenal reflex to inhibit renal sodium and water excretion in rats: role of adenosine. Hepatology. 2002;35:167–75. doi: 10.1053/jhep.2002.30425. [DOI] [PubMed] [Google Scholar]
- 17.Lo R, Austin A, Freeman J. Vasopressin in liver disease--should we turn on or off? Curr Clin Pharmacol. 2008;3:156–65. doi: 10.2174/157488408785747728. [DOI] [PubMed] [Google Scholar]
- 18.Osborn JW, Collister JP, Carlson SH. Angiotensin and osmoreceptor inputs to the area postrema: role in long-term control of fluid homeostasis and arterial pressure. Clin Exp Pharmacol Physiol. 2000;27:443–9. doi: 10.1046/j.1440-1681.2000.03263.x. [DOI] [PubMed] [Google Scholar]
- 19.Carey RM. Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ Res. 1978;43:19–23. doi: 10.1161/01.res.43.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Zacchia M, Trepiccione F, Morelli F, Pani A, Capasso G. Nephrotic syndrome: new concepts in the pathophysiology of sodium retention. J Nephrol. 2008;21:836–42. [PubMed] [Google Scholar]
- 21.Palmer BF, Alpern RJ. Pathogenesis of edema formation in the nephrotic syndrome. Kidney Int Suppl. 1997;59:S21–S27. [PubMed] [Google Scholar]
- 22.Rogers RC, Hermann GE. Central connections of the hepatic branch of the vagus nerve: a horseradish peroxidase histochemical study. J Auton Nerv Syst. 1983;7:165–74. doi: 10.1016/0165-1838(83)90044-9. [DOI] [PubMed] [Google Scholar]
- 23.Kobashi M, Adachi A. Convergence of hepatic osmoreceptive inputs on sodium-responsive units within the nucleus of the solitary tract of the rat. J Neurophysiol. 1985;54:212–9. doi: 10.1152/jn.1985.54.2.212. [DOI] [PubMed] [Google Scholar]
- 24.Paton JF, Silva-Carvalho L, Thompson CS, Spyer KM. Nucleus tractus solitarius as mediator of evoked parabrachial cardiovascular responses in the decerebrate rabbit. J Physiol. 1990;428:693–705. doi: 10.1113/jphysiol.1990.sp018235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]