Abstract
Purpose
Previous studies reported that declines in age-specific lung cancer death rates among women in the United States abruptly slowed in women younger than age 50 years (ie, women born after the 1950s). However, in view of substantial geographic differences in antitobacco measures and sociodemographic factors that affect smoking prevalence, it is unknown whether this change in the trend was similar across all states.
Methods
We examined female age-specific lung cancer death rates (1973 through 2007) by year of death and birth in each state by using age-period-cohort models. Cohort relative risks adjusted for age and period effects were used to compare the lung cancer death rate for a given birth cohort to a referent birth cohort (ie, the 1933 cohort herein).
Results
Age-specific lung cancer death rates declined continuously in white women in California, but the rates declined less quickly or even increased in the remaining states among women younger than age 50 years and women born after the 1950s, especially in several southern and midwestern states. For example, in some southern states (eg, Alabama), lung cancer death rates among women born in the 1960s were approximately double those of women born in the 1930s.
Conclusion
The unfavorable lung cancer trend in white women born after circa 1950 in southern and midwestern states underscores the need for additional interventions to promote smoking cessation in these high-risk populations, which could lead to more favorable future mortality trends for lung cancer and other smoking-related diseases.
INTRODUCTION
Lung cancer death rates in the United States decreased in successive younger birth cohorts after peaking among men and women born in the 1920s and 1930s, respectively.1 However, the decreases abruptly slowed, particularly among women younger than age 50 years who were born circa the 1950s,2–4 which temporally coincided with increased initiation of smoking among girls in the 1960s and 1970s.5,6 However, in view of the large geographic differences in public policies against tobacco use and socioeconomic factors that affect cigarette smoking,7,8 we hypothesized that this overall unfavorable change in the lung cancer mortality rate might vary across states.
California has consistently led the United States in using public policies to reduce cigarette smoking.7,9 It was the first state to establish a comprehensive statewide tobacco control program in 1988 through increased excise taxes on cigarettes.10 California also pioneered local government ordinances for smoke-free work places as early as the mid-1970s.7,8 Likewise, progress in reducing smoking prevalence and death rates from smoking-related diseases, including lung cancer, has been much greater in California than in the rest of the United States.3,9,11 In contrast, public policies against tobacco use have been weaker and progress in reducing smoking prevalence and smoking-related deaths is slower in many southern and midwestern states, particularly among tobacco-growing states such as Kentucky, Tennessee, North Carolina, South Carolina, Virginia, and Georgia.7,12,13 Thus, we examined state-specific lung cancer mortality rates among white women to assess regional differences in lung cancer trends in the United States.
METHODS
We obtained lung and bronchus (defined as lung hereafter) cancer death rates from 1973 through 2007 by age, sex, and race for all 50 states and the District of Columbia from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) mortality database as reported by the National Center for Health Statistics.14 Lung cancer was selected as the underlying cause of death based on death certificate information according to International Classification of Diseases (9th revision; ICD-9) coding and selection rules (codes 162.2-162.9) for deaths occurring from 1973 to 1998 and according to the 10th revision of ICD (ICD-10) coding rules (codes C34) for deaths occurring from 1999 to 2007.15,16
Age-specific lung cancer mortality trends were assessed by year of death and year of birth (birth cohort). We had ten 5-year age groups (35 to 39, 40 to 44, . . ., 80 to 84) and seven 5-year calendar periods (1973 to 1977, 1978 to 1982, . . ., 2003 to 2007), spanning sixteen 10-year partially overlapping birth cohorts referenced by midyear of birth (1893, 1898, . . ., 1968). We used age-period-cohort (APC) models to calculate fitted rates as well as estimable parameters and functions that describe the influences of age, period, and cohort.17,18 These models have been shown to closely track observed rates while providing smoothed estimates of the age-specific mortality rates with narrower 95% CIs.17,18
Cohort relative risks were calculated as rate ratios adjusted for age and period that compared the lung cancer mortality rate for a given cohort to that of a referent cohort (ie, the 1933 cohort in our analysis, which is the cohort with the highest death rates). The major complication of estimating birth cohorts is that cohorts born far apart in time (more than 36 years in this analysis) are never directly compared at the same age. For example, in our analysis, the 1908 birth cohort was observed for ages 65 to 84 years while the 1948 cohort was observed for ages 35 to 59 years. To address this obstacle, one of us (P.S.R.) developed a model-based estimate of the rate ratio for any pair of cohorts that is consistent with the product of the sequential rate ratios between the two cohorts, all of which are directly observable. Briefly, the modeled rate ratio for 1948:1908 is equal to the product of the rate ratios for 1948:1943, 1943: 1938, . . ., 1918:1913, and 1913:1908. The validity of these calculations depends on the assumption that the age-specific rates for any pair of cohorts are proportional after adjusting for period; however, this assumption is integral to the APC model. This model has been widely applied to other cancers and provided good fit to our lung cancer data as confirmed by examining the consistency of the observed to the fitted mortality rates.
We applied these methods to assess the evolving patterns in lung cancer death rates among white women in 23 states with sufficient data for APC analysis. In a supplementary analysis, we also examined the trend in 28 states among white men. We restricted our analyses to whites because lung cancer rates vary by race/ethnicity, and data for nonwhite groups are sparse for APC analyses by state.
RESULTS
There were 1,076,613 lung cancer deaths with 1.44 × 109 woman-years of follow-up among white women age 35 to 84 years in the 23 states from 1973 through 2007 considered in this analysis. Observed and fitted rates from the APC model were similar in every age group and state examined in our study among females and males (data available on request); indeed, observed rates were consistently within the confidence bands of the fitted rates. Thus, we present fitted rates in all of the figures.
Figure 1 shows trends in fitted age-specific lung cancer mortality rates by year of death for California, New York, and Alabama (eg, states with favorable, intermediate, and unfavorable age-specific mortality trends, respectively). In California, age-specific lung cancer death rates continued to decrease in all age groups younger than age 75 years beginning in the 1990s, with the decrease in younger women beginning in earlier calendar years. In New York, the age-specific trends were generally similar to those in California except the decreases were much less steep. In Alabama, in contrast, rates continued to increase for those age ≥ 70 years, whereas rates for women age younger than 60 years decreased and then increased in the most recent time periods, especially for those women younger than age 50 years; these women were born after the 1950s (Appendix Fig A1, online only). Lung cancer mortality patterns among white women in the remaining 20 states (Data Supplement) were similar to those in one of the three states shown in Fig 1.
Fig 1.
Trends in age-specific lung cancer death rates by year of death among white women for (A) California, (B) New York, and (C) Alabama. Dots denote the fitted lung cancer death rates, and shaded areas represent 95% point-wise CIs based on age-period-cohort modeling.
Figure 2 summarizes age-specific trends by birth cohort for the three select states with the most favorable (California), least favorable (Alabama), and intermediate (New York) patterns by using birth cohort relative risks. The 1933 birth cohort was designated as the referent group because this cohort had the highest mortality rates in all three states. In California (panel A), cohort relative risks rose 3.6-fold, from 0.28 (95% CI, 0.26 to 0.30) in 1893 to 1.0 in 1933, then fell by a similar magnitude. For New York (panel B), relative risks rose from 1893 to 1930, but then fell more modestly than those for California. In contrast, in Alabama (panel C), cohort relative risks rose more than five-fold from 0.17 (95% CI, 0.14 to 0.22) in 1893 to 1.0 in the 1933 birth cohort, plateaued, and then significantly increased by 50% to 100% from the 1950 cohort forward.
Fig 2.
Rate ratios of lung cancer death rates according to birth cohort among white women for (A) California, (B) New York, and (C) Alabama. The reference group is the 1933 birth cohort, and the shaded areas denote the 95% point-wise CIs of rate ratios.
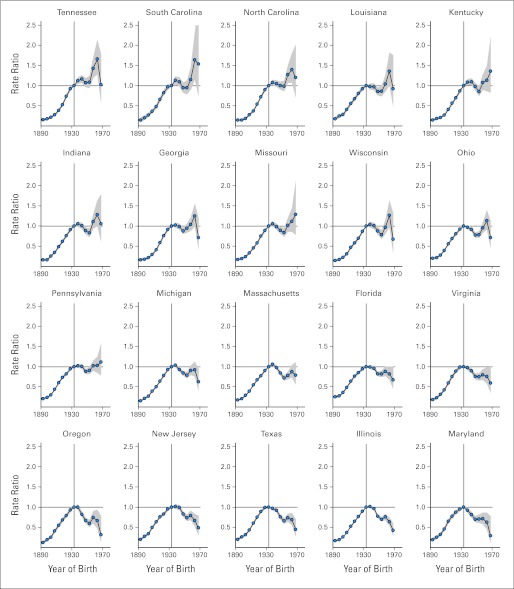
Figure 3 presents birth cohort relative risks of lung cancer death among white women for the 20 remaining states, arranged from the most unfavorable to the least unfavorable pattern based on the state-specific birth cohort rate ratios and their 95% CIs. The decrease in lung cancer death rates that began with the 1933 birth cohort slowed in all states and reversed in many states beginning circa the 1950 birth cohort. In Tennessee, South Carolina, and North Carolina (groups A1 to A3), similar to Alabama, the lung cancer death rates were significantly higher (according to 95% CIs of cohort relative risks) in at least one of the cohorts born circa 1950 and afterward compared with the rates in the 1933 cohort. For the other states, the death rates for cohorts born circa 1950 and afterward compared with the 1933 cohorts were nonsignificantly higher for at least one of the cohorts (groups B1 to B8), or the decrease in the lung cancer death rates markedly slowed in cohorts born after 1950 (groups C1 to C9). In contrast, among white men (Data Supplement) the decrease in lung cancer death rates in successive birth cohorts born since 1950 markedly slowed in some southern states, such as West Virginia, Kentucky, and Alabama, but was not reversed in any state.
Fig 3.
Rate ratios of lung cancer death rates according to birth cohort among white women for selected states. The reference group is the 1933 birth cohort, and the shaded areas denote the 95% point-wise CIs of rate ratios.
DISCUSSION
Our principal finding is that the decrease in age-specific lung cancer death rates among white women continued in younger age groups and birth cohorts in California, but the decline slowed or even reversed among women younger than age 50 years and women born after the 1950s in the remaining 22 states considered in this analysis, especially in several southern and midwestern states. Indeed, in many southern and midwestern states, lung cancer death rates increased rather than decreased among young and middle-aged white women, which could potentially slow or reverse future declines in the overall age-standardized lung cancer mortality rates as these cohorts age.
Factors that may have contributed to the disparate lung cancer trends between California and most parts of the United States for persons born circa 1950 and more recently include differences in initiation of smoking among teenagers in the 1960s and early 1970s and cessation of smoking or consumption among adults since the 1970s. Data on initiation of smoking by state or region are not available before the late 1970s, but initiation rates were not different between California and the rest of the United States from 1978 through 1988.6,19 In contrast, incidence rates of successful smoking cessation during the 1990s in persons age 35 to 49 years were much higher in California than other parts of the United States.20 According to a 2001 to 2002 Tobacco Use Supplement to the Current Population Survey (TUS-CPS), half the ever smokers had quit smoking successfully by age 44 years in California compared with half who quit smoking by age 47 years in New York and New Jersey and half who quit smoking by age 54 years in tobacco-growing states in the South.20 In addition, California smokers also showed the largest decline in intensity of smoking21,22 and in per capita cigarette consumption since the late 1960s.9,23 These favorable changes in smoking behaviors in California have been attributed in part to historical increases in cigarette prices since the mid-1970s and the implementation of the first statewide comprehensive tobacco control program in 1988.20,23–25 Similarly, the continuous declines in lung cancer death rates in New York and New Jersey may partly reflect historically high cigarette tax rates26 and large reductions in smoking prevalence compared with that in other states.27,28
In contrast, most southern and midwestern states historically have had weak public policies on tobacco control, including low state excise taxes on cigarettes and lack of statewide smoking bans in public places to reduce secondhand smoke and to change the norms of cigarette smoking.7,8,27,28 In the tobacco-growing state of South Carolina, the state excise tax on cigarettes was only 7 cents until the early 2000s compared with more than $1 in New York for the same period.26 In addition to weaker public policies in southern states, residents of those states, on average, have a lower socioeconomic status (SES) as measured by education or income,29 and persons with lower SES are less likely to quit smoking than persons with higher SES.12,30 Overall, the striking rise in lung cancer death rates in white women born since circa 1950 in several southern and midwestern states points to a lack of effective policies or interventions by government and public health agencies to deter initiation of smoking among teenagers and to promote smoking cessation among adults.
Factors in addition to smoking that may contribute to differences in lung cancer mortality trends between states include differences in prevalence of other known risk factors for lung cancer, including occupational and environmental exposures.31 However, the contributions from these are likely to be modest or minimal, since cigarette smoking in the United States accounts for more than 70% of lung cancer deaths in women.32 Further, cigarette smoking and subsequent lung cancer mortality patterns by birth cohorts have shown parallel trends in the United States.2,9,33,34
The strength of our analysis is the use of a nationwide mortality database to identify emerging regional trends in lung cancer death rates. A limitation of our study is its descriptive nature and the speculation about factors contributing to the state differences in lung cancer trends; therefore, the possibility of an ecologic fallacy in interpretation of our findings must be considered.35 However, several studies2,9,34,36 in the United States have documented that trends in lung cancer rates track well with trends in cigarette consumption. We also used a statistical (APC) model to estimate birth cohort effects adjusted for age and period effects. This working model appears reasonable, given the consistency between the observed and modeled rates in every age group, state, and sex examined. Furthermore, inaccuracies in underlying cause of death from death certificates according to ICD criteria are potential limitations in the interpretation of mortality trends. However, according to ICD-9 and ICD-10, more than 93% of lung cancer deaths from death certificates nationally have been matched successfully with a lung cancer diagnosis in medical records.37 The proportion of Hispanics, who generally have lower lung cancer risks than non-Hispanic whites,38 has increased in California, Texas, and many other states. However, the continued decrease in lung cancer death rates in subsequent younger birth cohorts persisted in California, whereas the slowing of the rate of decrease became more prominent in Texas when the analysis was restricted to non-Hispanic whites (data not shown). Therefore, any effect from an increased proportion of Hispanic population in the southern and midwestern states would likely have attenuated the apparent increase in lung cancer death rates in white women born circa 1950 and afterward.
Our findings of higher lung cancer mortality in white women born since circa 1950 compared with white women born before 1950 in several southern and midwestern states have implications for strengthening existing programs or implementing new comprehensive regional programs to control tobacco use and promote smoking cessation among these high-risk women. Women who quit smoking in their 40s and 50s can reduce their lifetime risk of dying from lung cancer by half compared with women who continue to smoke.39 Although the increased lung cancer rates in young middle-aged women are unlikely to substantially influence the overall lung cancer mortality trend in the short term because of their small (< 10%) contributions to the overall age-standardized death rate,4 they could reverse the overall trend in the long term (20 to 40 years) if there are no additional interventions to promote targeted cessation. Clinicians have a unique opportunity to increase the rate of cessation in these high-risk women through the combined use of counseling and medications.40,41 According to the 2005 National Health Interview Survey, only 60% of smokers reported being advised by a physician to quit in the past year, and approximately 35% of smokers tried to quit using treatments for tobacco dependence, including counseling and pharmacotherapy.42
In conclusion, age-specific lung cancer death rates among white women continued to decrease in California, but declines slowed or reversed among women younger than age 50 years and women born since circa 1950 in most other states, especially in the South and Midwest. These findings underscore the need for additional interventions to promote smoking cessation in these high-risk populations, which could lead to more favorable future mortality trends for lung cancer and other smoking-related diseases.
Supplementary Material
Appendix
Fig A1.
Trends in age-specific lung cancer death rates by year of birth among white women for (A) California, (B) New York, and (C) Alabama. Dots denote the fitted lung cancer death rates, and shaded areas represent the 95% point-wise CIs based on age-period-cohort modeling. .
Footnotes
Supported by the Intramural Research Department of the American Cancer Society and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Ahmedin Jemal, Jiemin Ma,William F. Anderson
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Devesa SS, Blot WJ, Fraumeni JF., Jr Declining lung cancer rates among young men and women in the United States: A cohort analysis. J Natl Cancer Inst. 1989;81:1568–1571. doi: 10.1093/jnci/81.20.1568. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93:277–283. doi: 10.1093/jnci/93.4.277. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Ward E, Thun MJ, et al. Contemporary lung cancer trends among U.S. women. Cancer Epidemiol Biomarkers Prev. 2005;14:582–585. doi: 10.1158/1055-9965.EPI-04-0554. [DOI] [PubMed] [Google Scholar]
- 5.Pierce JP, Lee L, Gilpin EA. Smoking initiation by adolescent girls, 1944 through 1988: An association with targeted advertising. JAMA. 1994;271:608–611. [PubMed] [Google Scholar]
- 6.Anderson C, Burns DM. Patterns of adolescent smoking initiation rates by ethnicity and sex. Tob Control. 2000;9:II4–II8. doi: 10.1136/tc.9.suppl_2.ii4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. State and Local Legislative Action to Reduce Tobacco Use: Smoking and Tobacco Control Monograph No. 11. Bethesda, MD: NIH publication 00-4804; 2000. [Google Scholar]
- 8.American Lung Association. State of Tobacco Control 2012. Washington, DC: American Lung Association; 2012. [Google Scholar]
- 9.Pierce JP, Messer K, White MM, et al. Forty years of faster decline in cigarette smoking in California explains current lower lung cancer rates. Cancer Epidemiol Biomarkers Prev. 2010;19:2801–2810. doi: 10.1158/1055-9965.EPI-10-0563. [DOI] [PubMed] [Google Scholar]
- 10.Balbach ED, Traynor MP, Glantz SA. The implementation of California's tobacco tax initiative: The critical role of outsider strategies in protecting Proposition 99. J Health Polit Policy Law. 2000;25:689–715. doi: 10.1215/03616878-25-4-689. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) State-specific prevalence and trends in adult cigarette smoking: United States, 1998-2007. MMWR Morb Mortal Wkly Rep. 2009;58:221–226. [PubMed] [Google Scholar]
- 12.Husten CG, Shelton DM, Chrismon JH, et al. Cigarette smoking and smoking cessation among older adults: United States, 1965-94. Tob Control. 1997;6:175–180. doi: 10.1136/tc.6.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finney Rutten LJ, Augustson EM, Moser RP, et al. Smoking knowledge and behavior in the United States: Sociodemographic, smoking status, and geographic patterns. Nicotine Tob Res. 2008;10:1559–1570. doi: 10.1080/14622200802325873. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database. Mortality-All Cause of Death, Aggregated With State, Total U.S. (1969–2008) <Katrina/Rita Population Adjustment>. DCCPS, Surveillance Research Program, Cancer Statistics Branch, released October 2011 ( www.seer.cancer.gov). Underlying mortality data provided by NCHS ( www.cdc.gov/nchs) [Google Scholar]
- 15.WHO. Manual of the international statistical classification of diseases, injuries, and causes of death, based on the recommendations of the Ninth Revision Conference, 1975, and adopted by the Twenty-Ninth World Health Assembly. Geneva, Switzerland: WHO; 1977. [Google Scholar]
- 16.WHO. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Geneva, Switzerland: WHO; 1992. [PubMed] [Google Scholar]
- 17.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39:311–324. [PubMed] [Google Scholar]
- 18.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: Ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20:1263–1268. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson DE, Giovino GA, Shopland DR, et al. Trends in cigarette smoking among US adolescents, 1974 through 1991. Am J Public Health. 1995;85:34–40. doi: 10.2105/ajph.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messer K, Pierce JP, Zhu SH, et al. The California Tobacco Control Program's effect on adult smokers: (1) Smoking cessation. Tob Control. 2007;16:85–90. doi: 10.1136/tc.2006.016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Delaimy WK, Pierce JP, Messer K, et al. The California Tobacco Control Program's effect on adult smokers: (2) Daily cigarette consumption levels. Tob Control. 2007;16:91–95. doi: 10.1136/tc.2006.017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce JP, Messer K, White MM, et al. Prevalence of heavy smoking in California and the United States, 1965-2007. JAMA. 2011;305:1106–1112. doi: 10.1001/jama.2011.334. [DOI] [PubMed] [Google Scholar]
- 23.Gilpin EA, Messer K, White MM, et al. What contributed to the major decline in per capita cigarette consumption during California's comprehensive tobacco control programme? Tob Control. 2006;15:308–316. doi: 10.1136/tc.2005.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinidad DR, Messer K, Gilpin EA, et al. The California Tobacco Control Program's effect on adult smokers: (3) Similar effects for African Americans across states. Tob Control. 2007;16:96–100. doi: 10.1136/tc.2006.017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce JP, Gilpin EA, Emery SL, et al. Has the California tobacco control program reduced smoking? JAMA. 1998;280:893–899. doi: 10.1001/jama.280.10.893. [DOI] [PubMed] [Google Scholar]
- 26.Orzechowski W, Walker R. Arlington, VA: Orzechowski and Walker; 2008. The Tax Burden on Tobacco: Historical compilation. [Google Scholar]
- 27.Shopland DR, Hartman AM, Gibson JT, et al. Cigarette smoking among U.S. adults by state and region: Estimates from the Current Population Survey. J Natl Cancer Inst. 1996;88:1748–1758. doi: 10.1093/jnci/88.23.1748. [DOI] [PubMed] [Google Scholar]
- 28.Jemal A, Thun M, Yu XQ, et al. Changes in smoking prevalence among U.S. adults by state and region: Estimates from the Tobacco Use Supplement to the Current Population Survey, 1992-2007. BMC Public Health. 2011;11:512. doi: 10.1186/1471-2458-11-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Census Bureau: The 2011 Statistical Abstract. The National Data Book. http://www.census.gov/compendia/statab/
- 30.Gilpin EA, Pierce JP. Demographic differences in patterns in the incidence of smoking cessation: United States 1950-1990. Ann Epidemiol. 2002;12:141–150. doi: 10.1016/s1047-2797(01)00266-6. [DOI] [PubMed] [Google Scholar]
- 31.Spitz MR, Wu X, Wilkinson A, et al. Cancer of the lung. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention (ed 3) New York, NY: Oxford University Press; 2006. pp. 628–658. [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses: United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 33.Jemal A, Travis WD, Tarone RE, et al. Lung cancer rates convergence in young men and women in the United States: Analysis by birth cohort and histologic type. Int J Cancer. 2003;105:101–107. doi: 10.1002/ijc.11020. [DOI] [PubMed] [Google Scholar]
- 34.Shopland DR. Tobacco use and its contribution to early cancer mortality with a special emphasis on cigarette smoking. Environ Health Perspect. 1995;103:131–142. doi: 10.1289/ehp.95103s8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piantadosi S, Byar DP, Green SB. The ecological fallacy. Am J Epidemiol. 1988;127:893–904. doi: 10.1093/oxfordjournals.aje.a114892. [DOI] [PubMed] [Google Scholar]
- 36.Jemal A, Center MM, Ward E. The convergence of lung cancer rates between blacks and whites under the age of 40, United States. Cancer Epidemiol Biomarkers Prev. 2009;18:3349–3352. doi: 10.1158/1055-9965.EPI-09-0740. [DOI] [PubMed] [Google Scholar]
- 37.German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35:126–131. doi: 10.1016/j.canep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107:1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 39.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: An epidemiologic perspective for geneticists. Oncogene. 2002;21:7307–7325. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
- 40.Curry SJ, Orleans CT, Keller P, et al. Promoting smoking cessation in the healthcare environment: 10 years later. Am J Prev Med. 2006;31:269–272. doi: 10.1016/j.amepre.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update—A U.S. Public Health Service report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cokkinides VE, Halpern MT, Barbeau EM, et al. Racial and ethnic disparities in smoking-cessation interventions: Analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34:404–412. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.