Abstract
Phenylketonuria (PKU), an inborn error in phenylalanine (phe) metabolism, requires lifelong nutrition management with a low-phe diet, which includes a phe-free amino acid-based medical formula to provide the majority of an individual’s protein needs. Compliance with this diet is often difficult for older children, adolescents and adults with PKU. The whey protein glycomacropeptide (GMP) is ideally suited for the PKU diet since it is naturally low in phe. Nutritionally complete, acceptable medical foods and beverages can be made with GMP to increase the variety of protein sources for the PKU diet. As an intact protein, GMP improves protein utilization and increases satiety compared with amino acids. Thus, GMP provides a new, more physiologic source of low-phe dietary protein for those with PKU.
Keywords: Inborn Errors of Metabolism, Whey Proteins, Phenylalanine Metabolism, Glycomacropeptide
INTRODUCTION
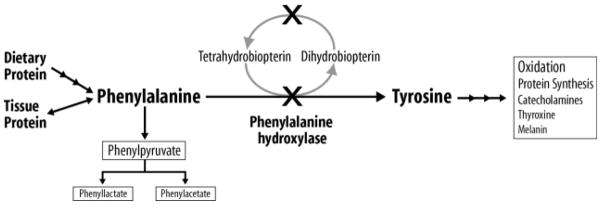
PKU (OMIM# 261600) is an inborn error of phenylalanine (phe) metabolism caused by the deficiency of phenylalanine hydroxylase (PAH, EC 1.14.16.1), which converts phe to tyrosine (Figure 1). Tyrosine is an AA that is indispensable in the diet for those with PKU given the inability to normally hydrolyze phe to tyrosine. PKU is an autosomal recessive disorder with an incidence in the US of 1 in 10,000 to 15,000 births in those of European, Asian and American Indian descent, although PKU can be diagnosed in other ethnic and racial groups and shows a lower incidence in those of African and Hispanic descent (1, 2). There are an estimated 15,000 individuals with treated PKU in the US and 50,000 worldwide (1, 2).
Figure 1.
Phenylalanine (phe) metabolism in phenylketonuria (PKU). As indicated by the “X”, PKU results from mutations (over 600 have been identified) that typically affect the hepatic phe hydroxylase enzyme needed for the hydroxylation of the dietary indispensable amino acid phe to tyrosine. PKU may also result from mutations in the recycling of the essential cofactor tetrahydrobiopterin. Due to these mutations which reduce the conversion of phe to tyrosine, phe accumulates in blood and is transaminated and decarboxylated into many compounds which appear in blood and urine; three of the compounds which are measured clinically are shown. Tyrosine, a precursor for multiple biological products, becomes an indispensable AA and must be provided by the diet for those with PKU.
First described in 1934, the untreated phenotype includes severe mental retardation, seizures, autistic-like behavior and a musty odor caused by elevations in plasma phe and/or its major metabolites including phenylpyruvate, phenyllactate and phenylacetate, Figure 1 (1). The exact mechanism for the neurological damage remains unclear, although poor myelination of the developing central nervous system and disturbances in neurotransmitter production has been suggested (3). PKU is detected presymptomatically by state-mandated population-wide newborn screening programs. Phe concentrations are measured on blood spots collected at 24 to 48 hours of age; those with elevated phe levels are referred to specialized centers for initiation of diet treatment (4).
Use of a phe-restricted diet for treatment of PKU was first described in 1953. Initially, diet was discontinued as early as 3 to 4 years of age; however, the National PKU Collaborative Study (1967 to 1983) clearly demonstrated that those remaining on diet showed greater cognitive functioning skills than those randomized to discontinue the diet (5, 6). This eventually led to the “diet for life” policy now accepted by all US and international clinics as best practice for treatment of PKU (2, 7–9).
This article briefly reviews the physiology of phenylketonuria (PKU) and the current dietary management of this inborn error of metabolism; see Reference 1 for a comprehensive review of PKU (1). The potential for glycomacropeptide (GMP), an intact whey protein, to provide a low-phenylalanine (phe) alternative to amino acids (AA) in medical foods designed to treat this disorder is discussed.
PROCESS FOR FINDING SOURCES
The literature search for this review was conducted using PubMed MEDLINE and Web of Science with the key words - PKU, GMP, and low-phe diet for articles published from 2000 to Feb 1, 2012. Additional articles and peer-reviewed chapters in books were identified from reference lists cited in PhD dissertations and personal communication.
DIETARY MANAGEMENT OF PKU
The primary goal of nutrition management of PKU is to restrict intake of phe to reduce blood and thus, brain concentrations of phe, yet provide sufficient intake of this AA to allow for adequate growth and protein turnover. In infants with classical PKU, minimum phe needs to support protein synthesis range from 200 to 500 mg/d, which can be provided by limited breast-feeding or limited quantities of a standard infant formula (10, 11).
The dietary prescription for phe, expressed as total mg phe/d, changes little from infancy through 10 years of age (11, 12). However, with the onset of the adolescent growth spurt, phe needs increase and reassessment of the dietary prescription for phe is important to provide for optimal growth and control of blood phe levels (11,13).
Using stable isotope methodology, Courtney-Martin et al demonstrated a mean phe requirement for prepubertal children with classical PKU of 14 mg/kg with a safe population intake (upper 95% CI) of 20 mg/kg body weight (14), and Zello et al demonstrated a mean phe requirement for adults of 9.1 mg phe/kg body weight (15). Reassessment of the phe prescription in relation to ideal body weight throughout the life span is needed as clients age and body weight increases (16).
Dietary phe adjustments are typically based on frequent monitoring of phe in blood or plasma to maintain phe levels in the treatment range (11, 17). Current recommendations for optimal metabolic control include maintenance of phe concentrations between 120 and 360 μmol/L (2 to 6 mg/dl) for neonates through age 12 years and 120 and 600 μmol/L (2 to 10 mg/dl) for those over age 12 years (2).
For children, adolescents and adults with PKU, appropriate foods include weighed quantities of fruit and vegetables, although starchy vegetables are particularly high in phe (18, 19). Use of specialty breads and pasta products made from wheat starch is essential for success of the diet since regular grain products often have too much phe to include in the dietary management of classical PKU (18, 19). “Free foods”, which contain no phe, are freely allowed, but these tend to be sugar-based beverages, candy or fat-based foods that are unrealistic to eat in large quantities. Typically, phe intake is “counted” as either mg of phe or phe exchanges (1 exchange = 15 mg of phe). Resources listing the phe content of various foods (18) and recipes for low-phe cookery are available (20).
A diet restricting phe intake is very low in total protein. Thus, to prevent deficiency, a protein source containing synthetic AA must be provided by medical foods to treat this disorder (11). A medical food, as defined by the US Food and Drug Administration (FDA), is a “food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition …” (21). For infants and young children with PKU, over 80% of energy needs and over 90% of protein needs are met by a phe-free AA formula (17).
Invariably, poor consumption of a medical food leads to poor metabolic control in those with PKU as excessive intake of phe-containing foods is common without an adequate intake of energy provided by most medical foods. In addition, nutrient deficiencies can develop when dietary intake of protein is restricted without adequate daily consumption of a complete protein substitute. Specifically, vitamin B6, vitamin B12, calcium, folate, iron and omega-3 essential fatty acids may be inadequate (22–24).
In those with early-treated PKU, IQ and cognitive testing is typically normal, but reductions can be seen in those with poorer lifelong phe control (25, 26). Executive function skills, linked to areas in the mid-dorsolateral prefrontal cortex, include memory, processing tasks, inhibitory control, attention and vigilance skills. Those with PKU can show deficits in these areas and severity of these deficits often correlates with the degree of phe control, both lifelong and short-term (26, 27).
Additionally, a concern for women with PKU of childbearing age is the “maternal PKU syndrome”. As a teratogen, elevated phe can cause microcephaly, mental retardation, growth retardation, and/or congenital heart abnormalities in any offspring born to a woman with PKU (28, 29). The Maternal PKU Collaborative Study (1983 to 2000) clearly demonstrated that the primary determinants of infant outcome are the degree of plasma phe elevation and gestational age at which control of plasma phe is achieved. The best fetal outcomes are achieved by reducing phe concentrations prior to pregnancy and maintaining phe concentrations between 120 and 360 μmol/L (2 and 6 mg/dl) throughout pregnancy (30, 31). Nutrition plays a key role in the outcome of maternal PKU pregnancies. Significant factors associated with abnormalities found in the infant include low prepregnancy weight, poor weight gain during pregnancy and low intake of protein from medical food (32, 33).
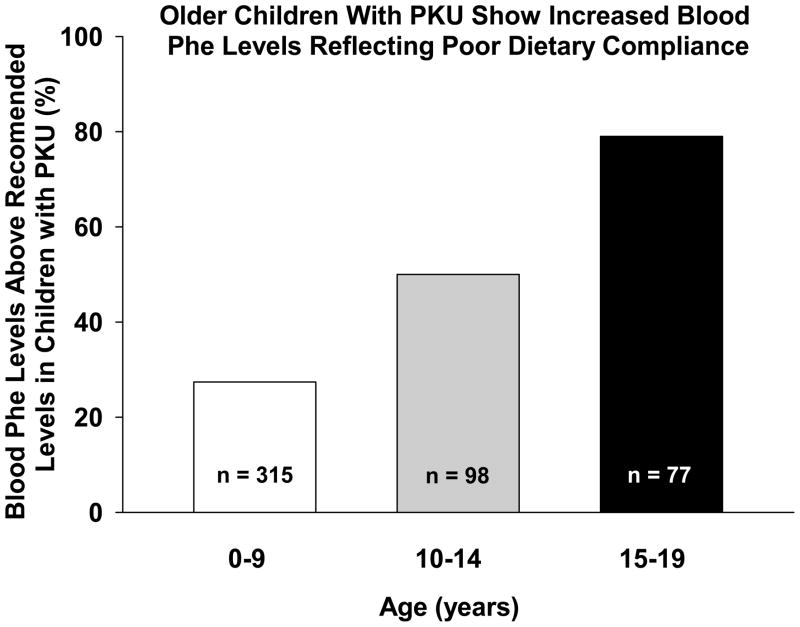
Thus, continuation of a phe-restricted diet and consumption of an adequate amount of medical food are important for long-term health of those with PKU. However, as with many chronic diseases, compliance with dietary treatment for PKU can become a problem for school age children, adolescents and adults (34). For example, in a study conducted in the UK and Australia, approximately 25% of children from birth through 9 years of age had median blood phe levels above the maximum recommended limit and this proportion increased to 50% in the 10–14 year age group; and to more than 75% in the 15–19 year age group (34) (Figure 2). Often, those discontinuing the diet as a teenager will attempt to reinstitute the diet as an adult. Despite strategies such as simplified methods to calculate phe intake (35, 36) and various medical food modifications (37, 38), it is extremely difficult to reestablish the low-phe diet. One study found that only 55% of adults were able to achieve dietary compliance for 3 months after diet reintroduction and only 19% were able to follow the diet for 9 months. However, those who returned to diet showed improved scores in various measures of life skills (39). Other studies have found similar benefits (40, 41).
Figure 2.
Theproportion of children with PKU in different age groups who show median blood phe levels above the maximum recommended limit. As children with PKU enter adolescence, noncompliance with the low-phe diet increases. Data shown were reprinted from The Lancet, volume 360, Walter JH, White FJ, Hall SK, MacDonald A, Rylance G, Boneh A et al, How practical are recommendations for dietary control in phenylketonuria?, pages 55–57, Table 2, 2002, with permission from Elsevier.
New Strategies to Improve Metabolic Control of PKU
Other strategies for treatment of PKU have included dietary supplementation of large neutral amino acids (LNAA), with or without the traditional diet. Phe competes with the other LNAA (arginine, histidine, isoleucine, leucine, lysine, methionine, threonine, tryptophan, tyrosine and valine) for specific carrier proteins that transport LNAA across the intestinal mucosa into the blood and across the blood-brain barrier into the brain (42). The carrier protein responsible for LNAA transport into the brain has a high affinity for phe and this, in combination with high plasma concentrations of phe relative to other AA in poorly controlled PKU, allows for excessive transport of phe into brain. Individuals with PKU given daily supplements of LNAA have shown decreased plasma phe concentrations (43–45) and reduced brain phe concentrations measured by magnetic resonance spectroscopy (46). Supplementation with LNAA is not a substitute for medical food and a low-phe diet and is not recommended for individuals less than 12 years of age. However, for individuals who have difficulty in complying with diet and show elevated plasma phe concentrations, LNAA may offer a cost-effective option to improve metabolic control of PKU.
More recently, supplementation with the cofactor for the PAH enzyme, tetrahydrobiopterin (BH4), has been shown to stabilize enzyme function and thus increase phe tolerance in approximately 40 to 60% of those with PKU (47, 48). BH4 supplementation has been especially effective for those with milder forms of PKU (47, 49). However, BH4 supplementation rarely allows an individual with PKU to completely discontinue diet treatment (50). In addition, clinical trials are currently underway to determine safety and efficacy of injectable PEGylated phenylalanine ammonia lyase to improve metabolic control in those with PKU (51). Even with pharmaceutical advances, diet continues to remain the mainstay of treatment for PKU.
New Dietary Approaches are Needed for PKU
Although successful in preventing mental impairment when implemented at birth, there is clearly difficulty in following the low-phe, AA-based diet lifelong and evidence of suboptimal growth and nutrition outcomes in patients with PKU treated with diet alone (52). Thus, new approaches are needed for dietary management of PKU. The ideal low-phe diet would contain at least one source of a phe-free or low-phe intact protein providing a complete source of dietary indispensable AAs, and that protein would have functional properties suitable for making a variety of foods, including baked goods, that have acceptable sensory properties including taste, texture and odor. To our knowledge, a phe-free, complete protein that is suitable for making acceptable foods does not exist for individuals with PKU. An ideal phe-free protein could potentially be produced using genetic engineering of microbes or plants, as has been the focus of current investigations in treatment of food allergies (53). However, production of acceptable, palatable foods from such recombinant proteins would be challenging and the cost may prohibit commercialization.
What does exist and is supported by short term research in subjects with PKU is glycomacropeptide (GMP), a protein that contains minimal phe and can be made into nutritionally complete, acceptable low-phe foods for those with PKU (54–57). Compared with the ideal low-phe dietary protein, GMP is not phe-free and requires supplementation with five limiting AA to provide a complete source of protein. Moreover, GMP is not optimal for use in baked products such as bread.
GLYCOMACROPEPTIDE
GMP is a 64 AA glycosylated peptide that occurs naturally in bovine milk within the whey fraction and is released in the newborn and adult human gastrointestinal tract by pepsin mediated proteolysis after milk ingestion (58). Commercial GMP is a by-product of cheese production when bovine κ-casein is cleaved by the action of chymosin into para-κ-casein, which remains with the curd, and κ-caseino glycomacropeptide or GMP, which remains in the whey (59). GMP is an abundant protein as it comprises 15 to 20% of the total protein in sweet cheese whey (59). GMP is currently sold as a food ingredient and has an excellent safety record based on widespread supplementation of foods with whey protein and the use of whey-predominant infant formulas (60). GMP demonstrates a number of interesting biological activities which have been the focus of recent research and commercial activity as summarized in current reviews (61, 62). In vitro studies have shown that GMP inactivates toxins of E. coli and V. Cholerae, inhibits adhesion of cariogenic bacteria with usage in toothpastes (63), promotes growth of bifidobacteria, modulates immune response (64), attenuates colitis in rats (65), increases zinc absorption in rhesus monkeys (66), and may promote satiety in humans (57, 67).
GMP is uniquely suited to the PKU diet as an alternative to AA formula since pure GMP contains no aromatic AA, including phe (59). Isolation of GMP from cheese whey results in contamination from other whey proteins, such as beta-lactoglobulin and alpha-lactalbumin, which contain phe. Thus, commercially available GMP contains 2.0–5.0 mg phe/g of protein (54, 56). GMP contains two to three times the amount of the LNAA isoleucine, threonine and valine compared with other dietary proteins and must be supplemented with indispensable AAs to provide a complete source of protein for individuals with PKU.
Initial estimates for supplementation of GMP with dietary indispensable AA provided 130–150% of the 2002 DRI for tyrosine, histidine, leucine, methionine and tryptophan (68, 69). To evaluate this supplement profile, an inpatient metabolic study was completed to compare AA and GMP diets in 11 subjects with PKU (55). Results from this study suggest that supplementation of GMP-based medical foods needs to include arginine and levels of dietary indispensable AA to provide at least 150% of the most current recommended intake as summarized by the 2007 World Health Organization standards (13). Moreover, studies in the PKU mouse demonstrate that GMP, supplemented with dietary indispensable AA, provides an adequate source of protein to support normal growth and body composition (70).
Acceptability of GMP Medical Foods
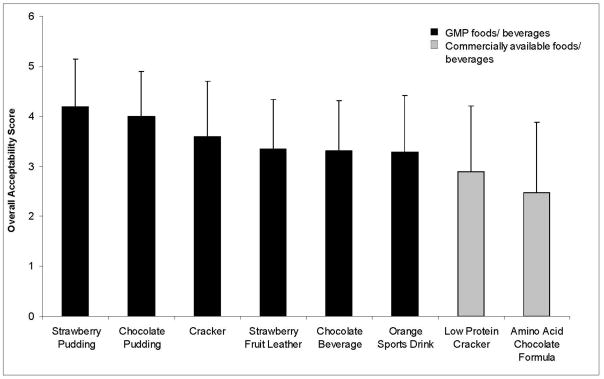
Various low-phe foods and beverages utilizing GMP as the primary protein source have been developed. Unlike synthetic AA, GMP has functional properties suitable for making foods, including good heat stability and solubility in acid (59). To evaluate the acceptability of GMP products, blind sensory studies were performed with PKU subjects attending three PKU summer camps and two family conferences from 2004 to 2007 (n = 18 to 32 subjects/product, age range 7 to 37 years) (54). Products were rated using a five-point hedonic scale (1 = dislike very much, 2 = dislike, 3 = neither like nor dislike, 4 = like, 5 = like very much) to evaluate five sensory categories including appearance, odor, taste, texture and overall acceptability. Overall acceptability scores for six tested products were > 3, indicating a positive response to products made with GMP (Figure 3). Further, at the conclusion of an 8-day inpatient metabolic study establishing short-term safety of GMP, 10 of 11 subjects felt that the GMP foods were better tasting and added variety to the low-phe diet compared to their usual AA formula (55).
Figure 3.
Overall acceptability of foods and beverages made with GMP and amino acid medical foods as tested by subjects with PKU, ages range 7 to 37 years. Acceptability ratings are mean ± standard deviation with 1= dislike very much, 2=dislike, 3= neither like nor dislike, 4 = like and 5 = like very much. Tested products (n = 18 to 32 subjects per product) included 6 products containing GMP: strawberry pudding (4.19 ± 0.95), chocolate pudding (4.0 ± 0.9), snack crackers (3.59 ± 1.11), strawberry fruit leather (3.35 ± 0.98), chocolate beverage (3.31 ± 1.00) and an orange-flavored sport drink (3.28 ± 1.13). Two commercial products, a chocolate-flavored amino acid based formula (Chocolate PhenylAde®, Applied Nutrition Corp., Cedar Knolls, NJ) and a low protein cracker (Loprofin®, Scientific Hospital Supplies, Liverpool, England) were also included in the sensory evaluations.
Since the initial publication with sensory evaluation data of GMP food products (54), additional foods and beverages have been developed and recipes have been modified using a GMP source with a reduced phe content of 2 mg phe/g GMP protein (ARLA Foods, Denmark). These medical foods include beverages, pudding, puffed cereal, crackers, salad dressings and a snack bar. All are nutritionally complete with a nutrient profile similar to commercially available AA medical foods. Each of these GMP products provides 5 to 15 g protein equivalents and only 15 to 25 mg phe/serving (56). Recently, two commercial GMP-based beverages have been released for diet treatment of individuals with PKU (BetterMilk™ and Restore™, Cambrooke Foods, Boston MA) using Glytactin™, a patent pending blend of GMP and dietary indispensable AA.
GMP and protein utilization
The current AA-based low-phe diet provides approximately 80% of protein needs from synthetic AA and 20% from the intact protein found primarily in fruits and vegetables. In contrast, a GMP-based low phe diet provides approximately 70% of protein needs from intact protein found in GMP, fruits and vegetables and approximately 30% of protein needs from synthetic AA needed to supplement GMP with dietary indispensable AA.
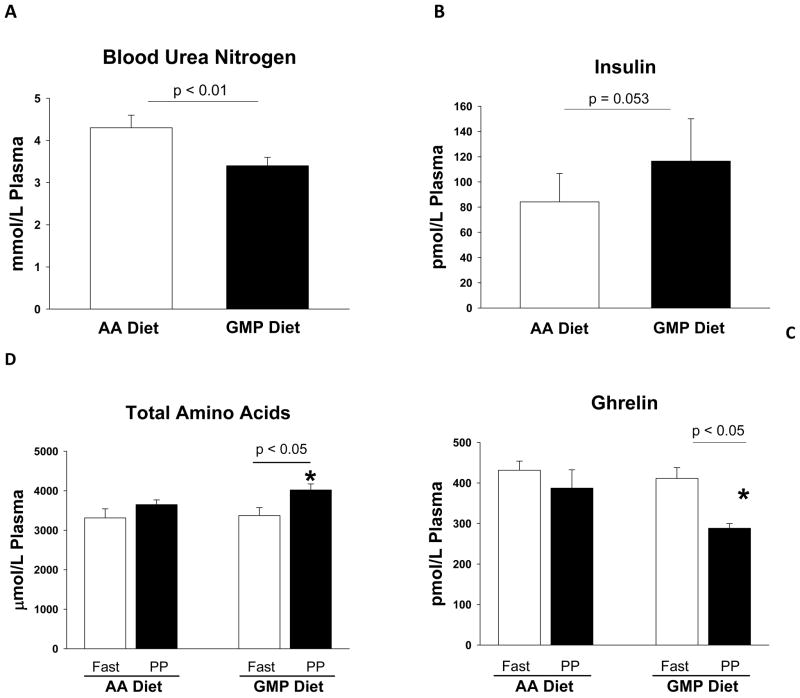
Studies have found slower absorption and improved protein utilization with an intact protein source compared to a mixture of single AA mimicking the intact protein (71, 72). Similar benefits in phe and AA utilization were found based on significantly higher postprandial (PP) plasma concentrations of total AA and significantly lower blood urea nitrogen concentration suggesting decreased ureagenesis (73–75) when PKU subjects replaced their entire protein prescription from AA formula with GMP products for four days, Figure 4 (55). When comparing fasting with PP, fasting phe concentrations were significantly greater than PP concentrations with the AA but not with the GMP diet, suggesting a more constant phe concentration over 24 hours with the GMP compared with the AA diet. Reduced fluctuation in phe levels may be of importance since a recent study suggests that long-term variation in plasma phe concentrations may have significant impact on cognitive outcome (25).
Figure 4.
Plasmaconcentrations of blood ur ea nitrogen (A), insulin (B), total amino acids (C) and ghrelin (D) in subjects with PKU consuming a low-phe diet made with glycomacropeptide (GMP) compared with amino acids (AA) for 4 days in an inpatient study. Consuming a GMP diet resulted in significantly lower postprandial (PP) (2.5 hr after breakfast) blood urea nitrogen concentration and significantly higher PP total AA concentration with moderately higher insulin concentration compared with the AA diet. This response is consistent with improved protein retention and decreased ureagenesis due to ingestion of GMP. With the AA diet, there was no significant difference in concentration of the appetite-stimulating hormone ghrelin after an overnight fast compared with 2.5 hours after breakfast. In contrast, following the GMP diet, PP ghrelin concentration was significantly reduced compared to an overnight fast consistent with the expected response of meal-induced satiety. * indicates significantly different from PP concentrations with the AA diet. Values are means ± SE. Data shown were previously reported as original research findings in references 55 and 57.
GMP and Satiety
Several studies have suggested that GMP may decrease food intake and promote satiety (57, 67). In a recent study, non-PKU subjects ate approximately 10% less at lunch following a breakfast with whey that included GMP compared with a breakfast with whey that did not include GMP (76). Satiety associated with whey protein has been attributed to its rapid digestion and absorption, resulting in rapid increases in plasma AA, insulin, glucagon-like peptide-1 and cholesystokinin compared with more slowly digested proteins such as casein (76–78).
Protein is the most satiating nutrient. Individuals with PKU often experience hunger in association with inadequate distribution of protein equivalents throughout the day and the rapid absorption of AA from AA medical foods. Higher PP plasma AA and insulin levels as noted in subjects fed a GMP-based breakfast compared with an AA-based breakfast are consistent with satiety (Figure 4) (55, 57). In addition, the hormone ghrelin increases during fasting to stimulate hunger and decreases following a meal in proportion to energy intake (79, 80). In the inpatient study with PKU subjects, plasma ghrelin concentrations were measured in a fasting state and after consumption of isocaloric breakfasts containing AA and GMP (57). PP plasma ghrelin concentration measured 2.5 hours after the start of the AA-based breakfast was not different from fasting ghrelin measured prior to eating breakfast (Figure 4). In contrast, the GMP breakfast induced significantly lower PP plasma ghrelin concentrations compared with ghrelin concentrations measured in a fasting state. This suggests that the AA breakfast did not allow for normal meal-induced, sustained suppression of ghrelin and thus, satiety was reduced compared with the meal containing GMP (57). In other words, PKU subjects “felt fuller longer” after ingestion of a GMP breakfast compared with an AA breakfast.
Use of GMP in an outpatient setting
In a recent outpatient trial, a 29-year-old male with classical PKU replaced his usual prescribed AA formula with GMP products for 10 weeks at home (56). This subject’s average phe concentration measured in blood spots was 14% lower with the GMP diet compared with phe concentrations measured on the AA diet when expressed relative to phe intake (56). One explanation for this response is that, because the subject enjoyed the GMP foods, he was able to space them throughout the day, unlike his AA formula which he drank all at once in the morning. Adolescents and adults with PKU often consume their entire daily formula volume divided into only one or two servings (56). Previous studies have shown improved protein utilization with lower plasma phe concentrations when protein equivalents are distributed in smaller, more frequent doses throughout the day (81). Since GMP products improve the taste, convenience and variety of choices in the PKU diet, individuals with this disorder may be more willing to consume these sources throughout the day and thus, improve the daily distribution of protein.
Application of GMP in the PKU Diet
Table 1 demonstrates how GMP products can be incorporated in a typical menu of an adolescent female with classical PKU. Unlike AA-based formulas, food and beverages made with GMP contain some phe from both the GMP itself and the ingredients used to produce these products. This minimal amount of phe needs to be accounted for in each individual’s daily phe prescription. This can be accomplished by either counting the mg phe in each GMP product as part of the daily phe allowance or by determining an average phe intake from GMP products and subtracting this from the amount of phe allowed each day. This latter approach, although less accurate, would be adequate for adolescents and non-pregnant adults for whom a daily variance of < 25 mg/day would likely have little impact on overall average plasma phe concentrations.
Table 1.
| AA Diet | GMP Diet | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Breakfast | Amount | Phe (mg) | Protein (g) | Breakfast | Amount | Phe (mg) | Protein (g) |
| Phenex 2® c | 100 g powder | 0 | 30 | GMP Bettermilk®c | 50 g powder | 23 | 15 |
| Cheerios® cereal | 1 cup (28 g) | 165 | 3.2 | GMP Crisp cereal | 1 1/2 c (66 g) | 30 | 10 |
|
| |||||||
| Subtotal, Breakfast | 165 | 33.2 | Subtotal, Breakfast | 53 | 25 | ||
|
| |||||||
| Lunch | Lunch | ||||||
| GMP sports beverage | 12 oz | 11 | 8 | ||||
| White bread, LPd | 2 slices (100 g) | 9 | 0.2 | White bread, LPd | 2 slices (100 g) | 9 | 0.2 |
| Cheese, LP | 2 slices (40 g) | 42 | 0.8 | Cheese, LP | 2 slices (40 g) | 42 | 0.8 |
| Mayonnaise | 1 Tbsp (13 g) | 8 | 0.2 | Mayonnaise | 1 Tbsp (13 g) | 8 | 0.2 |
| Grapes, green | 20 | 19 | 0.7 | Grapes, green | 20 | 19 | 0.7 |
| Apple juice | 8 oz (240 ml) | 2 | 0 | ||||
|
| |||||||
| Subtotal, Lunch | 80 | 1.9 | Subtotal, Lunch | 89 | 9.9 | ||
|
| |||||||
| Snack | Snack | ||||||
| GMP snack bar | 1 bar (84 g) | 28 | 15 | ||||
| Soda, regular | 12 oz (360 ml) | 0 | 0 | Soda, regular | 12 oz (360 ml) | 0 | 0 |
| Chips, Doritos® | 30 g | 97 | 2.0 | ||||
|
| |||||||
| Subtotal, Snack | 97 | 2.0 | Subtotal, Snack | 28 | 15 | ||
|
| |||||||
| Dinner | Dinner | ||||||
| GMP sports drink | 8 oz (240 ml) | 8 | 5 | ||||
| Spaghetti, LP | 1.5 c. cooked (56 g dry) | 7 | 0.3 | Spaghetti, LP | 1.5 c cooked (56 g dry) | 7 | 0.3 |
| Spaghetti sauce, no meat | 1 c (230 g) | 92 | 3.7 | Spaghetti sauce, no meat | 1 c (230 g) | 92 | 3.7 |
| Carrot sticks | 60 g | 37 | 0.6 | Carrot sticks | 60 g | 37 | 0.6 |
| Italian salad dressing | 1 oz (30 ml) | 0 | 0 | GMP salad dressing | 1 oz (30 ml) | 15 | 5 |
| Apple juice | 12 oz (360 ml) | 3 | 0.2 | ||||
|
| |||||||
| Subtotal, Dinner | 139 | 4.8 | Subtotal, Dinner | 159 | 14.6 | ||
|
| |||||||
| Snack | Snack | ||||||
| Phenex 2® | 67 g, powder | 0 | 20 | GMP strawberry pudding | 1 c (255 g) | 37 | 15 |
| Amino Acid Blend® | 20 g | 0 | 15 | Chips, Doritos® | 30 g | 97 | 2.0 |
|
| |||||||
| Subtotal, Snack | 0 | 35 | Subtotal, Snack | 134 | 17 | ||
|
| |||||||
| TOTAL | 481 | 76.9 | TOTAL | 463 | 81.5 | ||
Diets designed for a 12 year old female with classical PKU; weight = 42 kg, height = 152 cm. Her phe tolerance is 460–480 mg/day, minimum protein needs of 1.0 g/kg = 42 g/day and daily energy needs of 2300–2400 kcals.
Total energy content of AA Diet = 2365 kcals (565 kjoules) and GMP Diet = 2325 kcals (556 kjoules).
GMP and amino acid based medical foods are in bold. Commercial products include Phenex 2® (Abbott Nutrition, Columbus OH), PKU Amino Acid Blend® (Applied Nutrition, Cedar Knolls NJ) and Bettermilk® (Cambrooke Foods, Boston MA). Composition of other GMP products is based on estimates provided by Cambrooke Foods in April 2011.
LP = low protein product made from wheat starch.
Use of GMP-based medical foods as a sole source of protein for young children with PKU needs to be evaluated carefully. Given the phe content of these products, they may be more difficult to incorporate into the diet for those with a daily allowance of < 300 mg phe/day. In addition, the calorie content of currently available GMP medical foods containing Glytactin™ is lower per gram of protein equivalents than AA-based medical foods designed for children. Thus, total energy intake needs to be considered when solely prescribing these products as a protein source to those less than 4 years of age.
Future Research Need for GMP Medical Foods
Consistent with the 2012 NIH PKU Scientific Review Conference (82), further research is needed to extend current understanding of the long-term safety, efficacy and effects on nutritional status of GMP medical foods (55, 56). An ongoing clinical trial in individuals with PKU living at home funded by the FDA Office of Orphan Products Development will address important questions regarding the efficacy of commercially available GMP medical foods (www.clinicaltrials.gov NCT 01428258). Endpoints for the trial include impact on plasma phe concentration and the overall AA profile, nutritional status, neuropsychological function, dietary compliance and acceptability in PKU subjects fed GMP medical foods containing Glytactin™ compared with AA medical foods for 3 weeks in a randomized crossover design. Additional research is needed to assess the ability of GMP to support long term growth in young children and its effects during maternal PKU when provided as the predominant source of dietary protein.
CONCLUSIONS
New dietary options are needed to improve compliance with the low-phe diet and subsequent metabolic control for individuals with PKU. A variety of acceptable, nutritionally complete products can be made from GMP with the potential to replace, or partially replace, the traditional AA based medical foods currently utilized for the PKU diet. GMP-based medical foods represents a new paradigm to move the current PKU diet from synthetic AA as the primary source of protein equivalents to a more physiologically normalized diet based on intact protein, which our research demonstrates improves protein utilization and promotes satiety.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sandra C. Van Calcar, Email: vancalcar@waisman.wisc.edu, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Senior Metabolic Dietitian, Biochemical Genetics Program, Waisman Center, University of Wisconsin-Madison, 1500 Highland Ave., Madison, WI 53705, Phone: 608-263-5981, Fax: 608-263-0530.
Denise M. Ney, Email: ney@nutrisci.wisc.edu, Billings Bascom Professor, Department of Nutritional Sciences and Waisman Center, University of Wisconsin-Madison, 1415 Linden Drive, Madison, WI 53703, Phone: 608-262-4386, Fax: 608-262-5860.
References
- 1.Donlon J, Levy H, Scriver CR. In: Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. Scriver CR, Beaudet AL, Sly WS, et al., editors. New York: McGraw-Hill Medical; 2007. [Accessed April 2011.]. http://www.ommbid.com. [Google Scholar]
- 2.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement. Phenylketonuria: screening and management, October 16–18 2000. Pediatrics. 2001;108(4):972–982. doi: 10.1542/peds.108.4.972. [DOI] [PubMed] [Google Scholar]
- 3.Kölker S, Sauer SW, Hoffmann GF, Müller I, Morath MA, Okun JG. Pathogenesis of CNS involvement in disorders of amino and organic acid metabolism. J Inherit Metab Dis. 2008;31(2):194–204. doi: 10.1007/s10545-008-0823-z. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Puryear MA, Tonniges T, van Dyck PC, et al. American Academy of Pediatrics Newborn Screening Task Force recommendations: how far have we come? Pediatrics. 2006;117(5):S194–S211. doi: 10.1542/peds.2005-2633B. [DOI] [PubMed] [Google Scholar]
- 5.Koch R, Azen CG, Friedman EG, Williamson ML. Paired comparisons between early treated PKU children and their matched sibling controls on intelligence and school achievement test results at eight years of age. J Inherit Metab Dis. 1984;7(2):86–90. doi: 10.1007/BF01805813. [DOI] [PubMed] [Google Scholar]
- 6.Azen CG, Koch R, Friedman EG, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Arch Pediatr Adolesc Med. 1991;145 (1):35–39. doi: 10.1001/archpedi.1991.02160010037012. [DOI] [PubMed] [Google Scholar]
- 7.Recommendations on the dietary management of phenylketonuria: Report of Medical Research Council Working Party on Phenylketonuria. Arch Dis Child. 1993;68(3):426–427. doi: 10.1136/adc.68.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abadie V, Berthelot J, Feillet F, et al. Management of phenylketonuria and hyperphenylalaninemia: the French guidelines (in French) Arch Pediatr. 2005;12(5):594–601. doi: 10.1016/j.arcped.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Burgard P, Bremer HJ, Buhrdel P, et al. Rationale for the German recommendations for phenylalanine level control in phenylketonuria 1997. Eur J Pediatr. 1999;158(1):46–54. doi: 10.1007/s004310051008. [DOI] [PubMed] [Google Scholar]
- 10.Greve LC, Wheeler MD, Green-Burgeson DK, Zorn EM. Breast-feeding in the management of the newborn with phenylketonuria: a practical approach to diet therapy. J Am Diet Assn. 1994;15(3):305–309. doi: 10.1016/0002-8223(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 11.Acosta PB, Michels-Matalon K. Nutrition management of disorders of aromatic amino acids: phenylalanine and tyrosine. In: Acosta PB, editor. Nutrition Management of Patients with Inherited Metabolic Disorders. Sudbury MA: Jones and Bartlett; 2009. pp. 119–174. [Google Scholar]
- 12.Van Spronsen FJ, van Rijn M, Dorgelo B, et al. Phenylalanine tolerance can already reliably be assessed at the age of 2 years in patients with PKU. J Inherit Metab Dis. 2009;32(1):27–31. doi: 10.1007/s10545-008-0937-3. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Protein and Amino Acid Requirements in Human Nutrition. Geneva, Switzerland: United Nations University; 2007. [PubMed] [Google Scholar]
- 14.Courtney-Martin G, Bross R, Raffi M, Clarke JT, Ball RO, Pencharz PB. Phenylalanine requirement in children with classical PKU determined by indicator amino acid oxidation. Am J Physiol Endocrinol Metab. 2002;283(6):E1249–E1256. doi: 10.1152/ajpendo.0319.2001. [DOI] [PubMed] [Google Scholar]
- 15.Zello GA, Pencharz PB, Ball RO. Phenylalanine flux, oxidation, and conversion to tyrosine in humans studied with L-[1-13C]phenylalanine. Am J Physiol. 1990;259(6):E835–E843. doi: 10.1152/ajpendo.1990.259.6.E835. [DOI] [PubMed] [Google Scholar]
- 16.MacLeod EL, Gleason ST, van Calcar SC, Ney DM. Reassessment of phenylalanine tolerance in adults with phenylketonuria is needed as body mass changes. Mol Genet Metab. 2009;98(4):331–337. doi: 10.1016/j.ymgme.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsas LJ, Acosta PB. Inherited metabolic disease: amino acids, organic acids and galactose. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10. Philadelphia PA: Lippincott, Williams & Wilkins; 2006. pp. 909–959. [Google Scholar]
- 18.Schuett VE. Low Protein Food List for PKU. 3. Seattle WA: National PKU News; 2010. [Google Scholar]
- 19.Agriculture Research Center. USDA National Nutrient Database for Standard Reference, Release 23. Washington DC: US Department of Agriculture; 2010. [Accessed April 2011.]. http://www.drs.usda.gov/ba/bhnrc/ndl. [Google Scholar]
- 20.Schuett VE. Low Protein Cookery for Phenylketonuria. 3. Madison WI: The University of Wisconsin Press; 1997. [Google Scholar]
- 21.Food and Drug Administration. Guidance for industry: frequently asked questions about medical foods. Washington DC: US Food and Drug Administration; 2007. [Accessed May 2011.]. http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/Guidance. [Google Scholar]
- 22.Hvas AM, Nexo E, Nielsen JB. Vitamin B12 and vitamin B6 supplementation is needed among adults with phenylketonuria (PKU) J Inherit Metab Dis. 2006;29(1):47–53. doi: 10.1007/s10545-006-0108-3. [DOI] [PubMed] [Google Scholar]
- 23.Hanley WB, Feigenbaum ASJ, Clarke JTR, Schoonheyt WE, Austin VJ. Vitamin B12 deficiency in adolescents and young adults with phenylketonuria. Eur J Pediatr. 1996;155(Suppl 1):S145–S147. doi: 10.1007/pl00014233. [DOI] [PubMed] [Google Scholar]
- 24.Moseley K, Koch R, Moser AB. Lipid status and long-chain polyunsaturated fatty acid concentrations in adults and adolescents with phenylketonuria on phenylalanine restricted diet. J Inherit Metab Dis. 2002;25(1):56–64. doi: 10.1023/a:1015142001578. [DOI] [PubMed] [Google Scholar]
- 25.Anastasoaie V, Kurzius L, Forbes P, Waisbren S. Stability of blood phenylalanine levels and IQ in children with phenylketonuria. Mol Genet Metab. 2008;95(1–2):17–20. doi: 10.1016/j.ymgme.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 26.DeRoche K, Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: intelligence and executive function. Dev Neuropsychol. 2008;33(4):474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- 27.ten Hoedt AE, deSonneville M, Francois B, et al. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomized, double-blind, placebo-controlled, crossover trial. J Inherit Metab Dis. 2011;34(1):165–171. doi: 10.1007/s10545-010-9253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenke RR, Levy HL. Maternal phenylketonuria and hyperphenylalaninemia. An international survey of the outcome of untreated and treated pregnancies. N Engl J Med. 1980;303(21):1202–1208. doi: 10.1056/NEJM198011203032104. [DOI] [PubMed] [Google Scholar]
- 29.Rouse B, Azen CG, Koch R, et al. Maternal phenylketonuria collaborative study (MPKUCS) offspring: facial anomalies, malformations and early neurological sequelae. Am J Med Genet. 1997;69(1):89–95. doi: 10.1002/(sici)1096-8628(19970303)69:1<89::aid-ajmg17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 30.Waisbren SE, Azen CG. Cognitive and behavioral development in maternal phenylketonuria offspring. Pediatrics. 2003;112(6):1544–1547. [PubMed] [Google Scholar]
- 31.Mailot F, Lilburn M, Baudin J, Morley DW, Lee PJ. Factors influencing outcomes in the offspring of mothers with phenylketonuria during pregnancy: The importance of variation in maternal blood phenylalanine. Am J Clin Nutr. 2008;88(3):700–705. doi: 10.1093/ajcn/88.3.700. [DOI] [PubMed] [Google Scholar]
- 32.Matalon K, Acosta PB, Azen C. Role of nutrition in pregnancy in phenylketonuria and birth defects. Pediatrics. 2003;112(6):1534–1536. [PubMed] [Google Scholar]
- 33.Acosta PB, Matalon K, Castiglioni L, et al. Intake of major nutrients by women in the Maternal Phenylketonuria (MPKU) Study and effects on plasma phenylalanine concentrations. Am J Clin Nutr. 2001;73(4):792–796. doi: 10.1093/ajcn/73.4.792. [DOI] [PubMed] [Google Scholar]
- 34.Walter JH, White FJ, Hall SK, et al. How practical are recommendations for dietary control in phenylketonuria? Lancet. 2002;360(9326):55–57. doi: 10.1016/s0140-6736(02)09334-0. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald A. Diet and compliance in phenylketonuria. Eur J Pediatr. 2000;159 (Suppl 2):S136–S141. doi: 10.1007/pl00014375. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald A, Rylance G, Davies P, Asplin D, Hall SK, Booth IW. Free use of fruits and vegetables in phenylketonuria. J Inherit Metab Dis. 2003;26(4):327–338. doi: 10.1023/a:1025150901439. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald A, Ferguson G, Rylance G, et al. Are tablets a practical source of protein substitute in phenylketonuria? Arch Dis Child. 2003;88(4):327–329. doi: 10.1136/adc.88.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald A, Lilburn M, Davies P, et al. ‘Ready to drink’ protein substitute is easier for people with phenylketonuria. J Inherit Metab Dis. 2006;29(4):526–531. doi: 10.1007/s10545-006-0234-y. [DOI] [PubMed] [Google Scholar]
- 39.Bik-Multanowski M, Didycz B, Mozrzymas R, et al. Quality of life in noncompliant adults with phenylketonuria after resumption of the diet. J Inherit Metab Dis. 2008;31(suppl 2):S415–S418. doi: 10.1007/s10545-008-0978-7. [DOI] [PubMed] [Google Scholar]
- 40.Gassio R, Campistol J, Vilaseca MA, Lambruschini N, Cambra FJ, Fuste E. Do adult patients with phenylketonuria improve their quality of life after introduction/resumption of a phenylalanine-restricted diet? Acta Paediatr. 2003;92(12):1474–1478. [PubMed] [Google Scholar]
- 41.Burton BK, Leviton L. Reaching out to the lost generation of adults with early-treated phenylketonuria (PKU) Mol Genet Metab. 2010;101(2–3):146–148. doi: 10.1016/j.ymgme.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88(1):249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 43.Matalon R, Michels-Matalon K, Bhatia G, et al. Double blind placebo control trial of large neutral amino acids in treatment of PKU: Effect on blood phenylalanine. J Inherit Metab Dis. 2007;30(2):153–158. doi: 10.1007/s10545-007-0556-4. [DOI] [PubMed] [Google Scholar]
- 44.Schindeler S, Ghosh-Jerath S, Thompson S, et al. The effects of large neutral amino acid supplements in PKU: An MRS and neuropsychological study. Mol Genet Metab. 2007;91(1):48–54. doi: 10.1016/j.ymgme.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Sanjuro P, Aldamiz I, Georgi G, Jelinek J, Ruiz JI, Boehm G. Dietary threonine reduces plasma phenylalanine levels in patients with hyperphenylalaninemia. J Pediatr Gastroenterol Nutr. 2003;36(1):23–26. doi: 10.1097/00005176-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Moats RA, Moseley KD, Koch R, Nelson M. Brain phenylalanine concentrations in phenylketonuria: research and treatment of adults. Pediatrics. 2003;112(6):1575–1579. [PubMed] [Google Scholar]
- 47.Zurfluh MR, Zshochke J, Lindner M, et al. Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Hum Mutat. 2008;29(1):167–175. doi: 10.1002/humu.20637. [DOI] [PubMed] [Google Scholar]
- 48.Vernon HJ, Koerner CB, Johnson MR, Bergner A, Hamosh A. Introduction of sapropterin dihydrochloride as standard of care in patients with phenylketonuria. Mol Genet Metab. 2010;100(3):229–233. doi: 10.1016/j.ymgme.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy HL, Milanowski A, Chakrapani A, et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomized placebo-controlled study. Lancet. 2007;370(9586):504–510. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 50.Singh RH, Jurecki E, Rohr F. Recommendations for personalized dietary adjustments based on patient response to tetrahydrobiopterin (BH4) in phenylketonuria. Top Clin Nutr. 2008;23:149–157. [Google Scholar]
- 51.Harding CO, Blau N. Advances and challenges in phenylketonuria. J Inherit Metab Dis. 2010;33(6):645–648. doi: 10.1007/s10545-010-9247-7. [DOI] [PubMed] [Google Scholar]
- 52.Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: Revisiting the evidence. Mol Genet Metab. 2010;101(2–3):99–109. doi: 10.1016/j.ymgme.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 53.Li XM, Srivastova K, Grishin A, et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112(1):159–167. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- 54.Lim K, van Calcar SC, Nelson KL, Gleason ST, Ney DM. Acceptable low-phenylalanine foods and beverages can be made from glycomacropeptide from cheese whey for individuals with PKU. Mol Genet Metab. 2007;92(1–2):176–178. doi: 10.1016/j.ymgme.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Calcar SC, MacLeod EL, Gleason ST, et al. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am J Clin Nutr. 2009;89(4):1068–1077. doi: 10.3945/ajcn.2008.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ney DM, Gleason ST, van Calcar SC, et al. Nutritional management of PKU with glycomacropeptide from cheese whey. J Inherit Metab Dis. 2009;32(1):32–39. doi: 10.1007/s10545-008-0952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacLeod EL, Clayton M, Van Calcar SC, Ney DM. Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria. Mol Genet Metab. 2010;100(4):303–308. doi: 10.1016/j.ymgme.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chabance B, Marteau P, Rambaud JC, et al. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie. 1998;80(2):1551–65. doi: 10.1016/s0300-9084(98)80022-9. [DOI] [PubMed] [Google Scholar]
- 59.Etzel MR. Manufacture and use of dairy protein fractions. J Nutr. 2004;134(4):S996–S1002. doi: 10.1093/jn/134.4.996S. [DOI] [PubMed] [Google Scholar]
- 60.Bruck WM, Redgrave M, Tuohy KM, et al. Effects of bovine alpha-lactalbumin and casein glycomacropeptide-enriched infant formulae on faecal microbiota in healthy term infants. J Pediatr Gastroenterol Nutr. 2006;43(5):673–679. doi: 10.1097/01.mpg.0000232019.79025.8f. [DOI] [PubMed] [Google Scholar]
- 61.Brody EP. Biological activities of bovine glycomacropeptide. Br J Nutr. 2000;84(suppl 1):S39–S46. doi: 10.1017/s0007114500002233. [DOI] [PubMed] [Google Scholar]
- 62.Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr. 2007;26(6):S713–S723. doi: 10.1080/07315724.2007.10719652. [DOI] [PubMed] [Google Scholar]
- 63.Aimulis WR. Bioactive properties of milk proteins with particular focus on anticariogenesis. J Nutr. 2004;134(4):S989–S995. doi: 10.1093/jn/134.4.989S. [DOI] [PubMed] [Google Scholar]
- 64.Requena P, Gonzales R, Lopez-Posadas R, et al. The intestinal antiinflammatory agent glycomacropeptide has immunomodulatory actions on rat splenocytes. Biochem Pharmacol. 2010;79(12):1797–1804. doi: 10.1016/j.bcp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Posadas R, Requena P, Gonzales R, et al. Bovine glycomacropeptide has intestinal antiinflammatory effects in rats with dextran sulfate-induced colitis. J Nutr. 2010;140(11):2014–2019. doi: 10.3945/jn.109.118448. [DOI] [PubMed] [Google Scholar]
- 66.Kelleher SR, Chatterton D, Nielsen K, Lonnerdal B. Glycomacropeptide and alpha-lactalbumin supplementation of infant formula affects growth and nutritional status in infant rhesus monkeys. Am J Clin Nutr. 2003;77(5):1261–1268. doi: 10.1093/ajcn/77.5.1261. [DOI] [PubMed] [Google Scholar]
- 67.Burton-Freeman BM. Glycomacropeptide (GMP) is not critical to whey-induced satiety, but may have a unique role in energy intake regulation through cholecystokinin (CCK) Physiol Behav. 2008;93(1–2):379–387. doi: 10.1016/j.physbeh.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 68.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein and Amino Acids. Washington DC: National Academy Press; 2002. Protein and amino acids; pp. 589–768. [Google Scholar]
- 69.LaClair CE, Ney DM, MacLeod EL, Etzel MR. Purification and use of glycomacropeptide for nutritional management of phenylketonuria. J Food Sci. 2009;74(4):E199–E206. doi: 10.1111/j.1750-3841.2009.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solverson P, Murali SG, Brinkman AS, et al. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am J Physiol Endocrinol Metab. 2012;302(7):E885–E895. doi: 10.1152/ajpendo.00647.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gropper SS, Acosta PB. Effect of simultaneous ingestion of L-amino acids and whole protein on plasma amino acid and urea nitrogen concentrations in humans. JPEN J Parenter Enteral Nutr. 1991;15(1):48–53. doi: 10.1177/014860719101500148. [DOI] [PubMed] [Google Scholar]
- 72.Metges CC, El-Khoury A, Selvaraj AB, et al. Kinetics of L-[1-13C]leucine when ingested with free amino acids, unlabeled or intrinsically labeled casein. Am J Physiol Endocrinol Metab. 2000;278(6):E1000–E1009. doi: 10.1152/ajpendo.2000.278.6.E1000. [DOI] [PubMed] [Google Scholar]
- 73.Dangin M, Boirie Y, Garcia-Rodenas C, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280(2):E340–E348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- 74.Young VR, El-Khoury AE, Raguso CA, Forslund AH, Hambraeus L. Rates of urea production and hydrolysis and leucine oxidation change linearly over widely varying protein intakes in healthy adults. J Nutr. 2000;130(4):761–766. doi: 10.1093/jn/130.4.761. [DOI] [PubMed] [Google Scholar]
- 75.Calbet JA, MacLean DA. Plasma glucagon and insulin responses depend on rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr. 2002;132(8):2174–2182. doi: 10.1093/jn/132.8.2174. [DOI] [PubMed] [Google Scholar]
- 76.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, et al. Effects of complete whey-protein breakfasts versus whey without GMP-breakfast on energy intake and satiety. Appetite. 2009;52(2):388–395. doi: 10.1016/j.appet.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Baer DJ, Slate K, Paul DR, Harris GK, Rumpler WW, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr. 2011;141(8):1489–1494. doi: 10.3945/jn.111.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hall WL, Mullward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89(2):239–248. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 79.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 80.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89(3):1319–1324. doi: 10.1210/jc.2003-031267. [DOI] [PubMed] [Google Scholar]
- 81.MacDonald A, Rylance G, Davies P, Asplin D, Hall SK, Booth IW. Administration of protein substitute and quality of control in phenylketonuria: A randomized study. J Inherit Metab Dis. 2003;26(4):319–326. doi: 10.1023/a:1025186217369. [DOI] [PubMed] [Google Scholar]
- 82.Phenylketonuria Scientific Review Conference: State of the Science and Future Research Needs. [Accessed April 2012.];NIH campus. 2012 Feb; doi: 10.1016/j.ymgme.2014.02.013. https://www.team-share.net/Phenylketonuria_Scientific_Review_Conference/Overview.aspx. [DOI] [PubMed]