Abstract
Background
Investigators of homeopathy have proposed that nonlinear dynamical systems (NDS) and complex systems science offer conceptual and analytic tools for evaluating homeopathic remedy effects. Previous animal studies demonstrate that homeopathic medicines alter delta electroencephalographic (EEG) slow wave sleep. The present study extended findings of remedy-related sleep stage alterations in human subjects by testing the feasibility of using two different NDS analytic approaches to assess remedy effects on human slow wave sleep EEG.
Methods
Subjects (N=54) were young adult male and female college students with a history of coffee-related insomnia who participated in a larger 4-week study of the polysomnographic effects of homeopathic medicines on home-based all-night sleep recordings. Subjects took one bedtime dose of a homeopathic remedy (Coffea cruda or Nux vomica 30c). We computed multiscale entropy (MSE) and the correlation dimension (Mekler-D2) for stage 3 and 4 slow wave sleep EEG sampled in artifact-free 2-minute segments during the first two rapid-eye-movement (REM) cycles for remedy and post-remedy nights, controlling for placebo and post-placebo night effects.
Results
MSE results indicate significant, remedy-specific directional effects, especially later in the night (REM cycle 2) (CC: remedy night increases and post-remedy night decreases in MSE at multiple sites for both stages 3 and 4 in both REM cycles; NV: remedy night decreases and post-remedy night increases, mainly in stage 3 REM cycle 2 MSE). D2 analyses yielded more sporadic and inconsistent findings.
Conclusions
Homeopathic medicines Coffea cruda and Nux vomica in 30c potencies alter short-term nonlinear dynamic parameters of slow wave sleep EEG in healthy young adults. MSE may provide a more sensitive NDS analytic method than D2 for evaluating homeopathic remedy effects on human sleep EEG patterns.
Keywords: homeopathy; polysomnography; electroencephalography; Coffea cruda; Nux vomica; nonlinear dynamics, multiscale entropy; correlation dimension; slow wave sleep; complex systems; time-dependent sensitization
Introduction
Homeopathy is a system of complementary and alternative medicine (CAM) that has generated much controversy over potential mechanisms of its purported effects as well as the nature of the animal-, mineral-, or plant-derived medicines.1 Some researchers have even questioned the validity of commonly-held practice theory tenets such as the existence of homeopathic aggravations (transient worsening prior to clinical improvement).2 Finding analytic techniques to detect and monitor subtle fluctuations in the course of remedy effects would improve understanding of the circumstances under which remedy responses are state-dependent, bidirectional, or even oscillatory in living systems.3–6
Time is a major feature of the homeopathic remedy response. Once the “correct” remedy, or simillimum, is administered, clinical responses change over time. The direction of these changes are sometimes reportedly not only multi-system, but also biphasic, i.e., nonlinear. That is, homeopathic patients may exhibit transient worsening prior to improvement in some symptoms and/or gradual reductions in susceptibility to and severity of flares in chronic conditions as other symptoms come to the fore.7 The changes appear to involve multiple subsystems of the person as well as the global state of the person as a whole. Treatment itself is an iterative individualized process using one remedy at a given moment, guided over time by repeated reassessments of the changes in the total clinical presentation.
Over the minutes, hours, days, and weeks following one dose of the remedy in an ill person, the clinical course evolves across many subsystems of the individual, at a pace related in part to the chronicity or acuity of the presenting problems and in a spatial pattern suggestive of self organization in the person as a complex system.8 Hering's Law of Cure in homeopathy predicts an improvement pattern for the “center of gravity of the illness” to shift within the person over time after initiation of remedy treatment from inside out (more important to less important organs), above downward (head toward toes), and from most recent to oldest onset symptoms in time.7,9 In contrast to weekly psychotherapy, homeopathic follow up visits for chronic diseases follow a more medical model, typically spaced 1–2 months or more apart to allow the clinical course to unfold after administration of a single dose.7,10 However, the time course is much faster and compressed in acute conditions1 and perhaps in proving or pathogenetic trials.11,12
Various contemporary scientists who study the nature of the clinical claims in homeopathy have postulated that medicines interact with the patient's state13 by modulating the nonlinear dynamics of the individual as a complex living system.5,6,8,14–24 Nonlinear dynamical systems (NDS) is a branch of the emerging holistic science of complex systems, chaos theory, and network models for physiological and social systems.25–27 NDS appears more applicable to the nature of homeopathic clinical change patterns than conventional medical linear models.18,22,23 For instance, in parallel with the complex nature of homeopathic healing outlined above, experts characterize NDS theory as “the study of how complex processes unfold over time…”.25 A complex system is more than the sum of its parts because of its capacity to generate emergent properties from the interactions of the interconnected parts through their relationships to one another and the larger whole.27–31
It is possible to use complex systems methods to study any level of organizational scale, from biochemical to organ system to organism to societal behaviors.26,31–33 However, these methods usually require sufficiently long time series of data points to evaluate the recurring patterns of change over time in a given behavior. Electroencephalographic (EEG) activity is one type of biological signal to which NDS analytic methods can apply.34–40
For research in a controversial field such as homeopathy, EEG as an outcome measure has advantages.41,42 Neurophysiological assessments enable utilization of more objective and sensitive tools than crude behavioral observations or self-report scales for assessing the real-time dynamics of an organism at integrative levels of functioning such as the central nervous system.19,43 In animal research, for example, Ruiz-Vega et al44 showed that homeopathically prepared medicines such as Coffea cruda at 30c potencies can produce increases in the electroencephalographic delta band power of slow wave sleep (stages 3 and 4) versus placebo. Other placebo-controlled animal studies revealed that (a) homeopathic histamine 30c can mobilize a dynamic biphasic process affecting sleep EEG delta activity that evolves over the course of the night between two locally stationary states according to a power law45,46; and (b) homeopathic Coffea cruda 30c produces different patterns of changes in parietal EEG delta power later versus earlier in the sleep period in a subset of animals pre- or post-treated with caffeine.46 The homeopathic remedy Nux vomica 30c has also produced alterations of sleep in other animal studies.47,48
The purpose of the present study was to explore the feasibility of applying nonlinear dynamical analytic methods to human slow wave sleep EEG from a placebo-controlled study on the effects of two different homeopathic remedies, Coffea cruda 30c and Nux vomica 30c, in relatively healthy young adult human subjects with histories of coffee-induced insomnia (conventional sleep stage findings reported elsewhere49). For the analysis, we adapted previously-published NDS methods for computation of both multiscale entropy (MSE)50–52 and EEG correlation dimension (Mekler method D2).39,53–55
The primary hypothesis was that the verum would exert a pattern of short-term modulatory effects on nonlinear dynamics of the slow wave sleep EEG (stages 3 and 4, which are by definition predominantly, but not exclusively, delta and theta spectral EEG frequencies) different from those seen on baseline nights or with placebo.8,56 The secondary hypothesis, based on homeopathic theory20,42,57 and accumulating basic science data on nanoparticles and succussion-related unique alterations in the dynamic structure of the ethanol-water solvent networks of specific remedies,58–69 was that Coffea cruda and Nux vomica would differ from one another in the pattern of their nonlinear EEG effects. Skeptics would argue the null hypothesis, given the 30c doses (potencies) used of both medicines would have been diluted past Avogadro's number and would, from a diluted bulk form source molecule perspective, contain no active ingredients and hence, effects no different from placebo.70
Methods
Subjects
Subjects were young adult college students of both sexes (N=54, 50% female, mean age 20 years) recruited from an introductory psychology class after completing a screening questionnaire packet. Homeopathic clinical texts suggest that people who benefit from Coffea cruda are sensitive to all types of stimuli, including anxiety; whereas individuals who respond to Nux vomica are intensely driven, competitive, impatient Type A personalities.57 Both remedy types are reportedly associated with insomnia as an adverse effect of coffee. Thus, to increase the possibility of enrolling remedy-responsive subjects71,72, inclusion criteria were not only moderate to good self-rated global health and a history of coffee-induced insomnia in the past, but also an above-mean elevation of scores (using the class member scores of the first 1,036 students screened) for either the Cook-Medley Hostility Scale (high hostility trait, CMHO) or the Anxiety Sensitivity Index (high anxiety sensitivity trait, ASI). To be included in the polysomnography study, subjects with an above-mean value for CMHO or ASI had to score below the sample mean for the other scale (see also49). In order to control for the personality variables in regression analyses, a single combined personality index (CMASI) was created in which higher scores indicate elevated Type A traits and lower scores indicate elevated anxiety sensitivity traits. Specific findings relating to the interaction of personality type with remedy received are also published elsewhere.13
Because of the belief by some homeopaths that beverage coffee (but not caffeine) sometimes antidotes homeopathic medicines,7 subjects had to be willing to eliminate drinking coffee for the full duration of the study, although they were encouraged to change to and stabilize prior to the first baseline sleep recording night for at least one week on non-coffee caffeinated beverages to maintain their customary daily caffeine intake without interruption, if necessary. Exclusion criteria were pregnancy or plan to become pregnant, major psychiatric or serious chronic medical conditions, a history of anaphylactic shock, and/or chronic use of medications other than contraceptive drugs. This study was reviewed and approved by the Institutional Review Board of the University of Arizona. Subjects were paid for participation.
Procedures
All participants underwent a total of eight all-night sleep recordings in their home, spaced over 4 weeks in pairs of successive days (same days of each week). The recording nights were as follows: Week 1 Baseline=nights 1,2; Week 2=nights 8,9 (placebo on night 8); Week 3 Baseline=nights15,16; Week 4=nights 22,23 (remedy on night 22). In specific, Weeks 1 and 3 involved baseline recordings. On Week 2, subjects dissolved 3 #30 lactose-sucrose placebo pellets under the tongue at bedtime (night 8 only) in the presence of the sleep recording technician. On Week 4, subjects dissolved 3 #30 lactose-sucrose verum remedy pellets under the tongue at bedtime (night 22 only) in the presence of the sleep recording technician.
Subjects were blinded to the ingredients of the pellets throughout the study. Staff were aware that the Week 2 pellets were placebo but fully blinded as to the identity of the remedy given in a randomly assigned manner on Week 4 (verum remedy: either Coffea cruda 30c or Nux vomica 30c). Medicines and placebos were purchased from an FDA-regulated homeopathic pharmacy, Hahnemann Laboratories, San Rafael, CA USA. The lactose-sucrose pellets used as placebos and on which specific remedy solution was sprayed per usual remedy preparation procedures were tested and certified as pure and contaminant-free by an independent testing laboratory.
Sleep recordings (polysomnograms including bilateral electrooculograms (EOG) and mental/submental electromyography (EMG), with EEG electrode sites at C3, C4, Cz, Pz, O1 and O2, referenced to contralateral mastoids (A1/A2)) were performed using Cadwell Laboratories (Kennewick, WA, USA) Easy Ambulatory 2 equipment. Electrode impedance levels were kept below 5 kiloohms. Data acquisition and data processing for sleep stage scoring involved an EEG sampling rate of 200 Hz, with a low pass filter set at 35 Hz and a high pass filter set at 0.53 Hz (permitting sleep EEG frequency band acquisition in the physiological range between 0.53 and 30 Hz). A 60 cycle notch filter was used to eliminate ambient electrical noise. Sleep stage data were scored for sleep stages using standard criteria (relying on EEG from C3/A2 or C4/A1, EOG, and EMG criteria) from the Rechtschaffen-Kales manual73 by a trained sleep technician blinded as to day and condition. Bell et al (2011) report the details of the conventional sleep stage methodology and findings elsewhere.49
For purposes of the present study, the technician then identified artifact-free 2-minute segments of EEG data within the previously-scored slow wave sleep stages (by definition, Stages 3 and 4, in which delta sleep is more commonly observed) during the first two rapid-eye-movement (REM) cycles of each of the eight recording nights (each REM cycle averages 90 minutes in length and includes some REM sleep stage events) for NDS analysis. Although the sleep stage scoring itself was based on conventional criteria involving in part the EEG C4/A1 or C3/A2 placements together with EOG and EMG,49 the concurrent EEG data from all electrode sites within the selected data segments were used for the current nonlinear dynamical exploratory analyses.
To control for baseline sleep patterns, results of baseline nights 1, 2, 15, and 16 were included for use in the regression models. Because of the animal evidence that the study medicines might have biphasic effects over time on any given biomarker3,46 and findings suggesting changes in normal human sleep EEG complexity over course of a night,39 we examined data from the first two REM cycles of the sleep period for both the night of and the night after taking the remedy, controlling for both baseline and placebo nights.
Analytic Approach
The analysis focused on the findings from nights 22 (verum remedy night) and 23 (post-remedy night). In particular, we examined the findings for night 22 and the change score from night 22 to night 23. We used a random effect regression model for analysis, with person as the random effect. The regression equation used the subject's complexity score (MSE or D2) as the dependent variable, and included dummy coded effects for each week of the study (baseline, placebo, second baseline, remedy), the first and second observed nights of the study week, whether the subject was given pellets or not, and interactions with the personality variables and whether they received the remedy Nux vomica or Coffea cruda. For example, we tested whether a subject's remedy night MSE differed from their placebo night MSE, and whether there was significant change in MSE on the post-remedy night compared to the post-placebo night change. Each comparison adjusted for each subject's own baseline MSE values and personality score. STATA 9.1 or 10.0 were used for all data reduction and analyses. This is an overall multivariate regression-based analytic approach.
NDS Variables
Multiscale entropy (MSE) analysis50,52,74 is a method for computing how a certain entropy measure varies as one successively coarsens a time series over multiple scales, by computing averages over non-overlapping intervals of fixed size (the scale). We applied this to our 2-minute, artifact-free EEG signals, using 20 time scales, following Costa's example analyses. Costa has put her program into the public domain (http://physionet.org), and we translated it into somewhat less ornate code (see Appendix for specifics) for the large number of analyses we needed to perform. Although it may have been preferred to validate the original Costa C-language code involved, funding limitations prevented our doing so. As an alternative for this exploratory study, we have provided the code in the Appendix, for future validation and confirmatory studies.
In addition to computing entropies at 20 scales for an actual series, we then permuted the series at random 5 times, each time re-computing all 20 entropies. The final outcome measure was the area between the actual entropy-scale curve and the average of the 5 entropy-scale curves for the permuted data. Higher values of this measurement are taken to indicate higher scale-free entropy, which in turn may be due either to complex dynamics having effects over long time-scales, or to noise in the data. This duality of interpretation has been remarked by Costa.
Correlation dimension (D2) is considered one of severa key measures in chaotic time series analysis, assessing the degree of similarity between one observation and others later in the time series (http://www.societyforchaostheory.org/tutorials/00006/GlossaryTerms.html, accessed 12/21/11). Prior research indicates that D2 analysis of human EEG can distinguish between normals and patients with neurodegenerative disorders.37 Some recent research indicates that, at least in waking EEG, changes in D2 may relate to change in the theta frequency band (also a feature of slow wave sleep, i.e., stages 3 and 4).38
For computation of the correlation dimension D2 (originally developed by Grassberger and Procaccia 75), we closely followed the EEG analytic approach of Mekler.53 He advocates embedding in 12-dimensional space with relatively large lags, computing the correlation integral for a sequence of small parameter values, and then computing the slope from the middle third of the resulting curve. Mekler acknowledges that the resultant values are not D2 in the strict sense, but still capture nonlinear variability of brain functioning (hereafter, D2 in this paper refers to the calculated Mekler-D2). The advantages of the Mekler technique are that (a) EEG data per se does not meet all of the necessary assumptions or requirements for applying the Grassberger-Procaccia method without requiring subjective evaluation procedures for selecting calculation parameters (leading to difficulties in replication between different laboratories,53 an issue already a known challenge in other types of homeopathic laboratory research76); (b) Mekler's method enables capacity to process large amounts of experimental data.
We confirmed in test cases that his method appeared to apply to our data, in the sense that the correlation integral curves were regular in form, and the slope was a reasonable representation of the middle of the curve. Like Mekler, we also observed high correlation dimensions, near to the dimension of the embedding space. The conventional interpretation of a correlation dimension is that it is the (possibly fractional) dimension of the attractor of the nonlinear dynamic system, in an appropriate representation space. The suggestion is thus that lower correlation dimension represents simpler dynamics or lower complexity.
As in the MSE analysis, higher correlation dimension can also represent the presence of noise. These limitations are inherent in the state-of-the-science of various nonlinear dynamical systems computational techniques at present. Nonetheless, our attempt was to replicate methods already in the literature for EEG. The correlation dimension is a well-defined quantity related to certain nonlinear dynamical features of a complex system. We used the Physionet program (http://physionet.org) for computing the correlation integral, and then used the regression slope of the middle third (Mekler used the slope based on only two points, which is less accurate).
Data Management Approach
Of the 54 participants, one had unusable EEG and was dropped from the analysis. Because the analysis, as described above, requires complete data (all 8 nights for the given REM cycle and sleep stage), missing outcomes were linearly interpolated when that was possible, and carried forward or backward when interpolation was not possible. Data completion rates were about 70% for REM cycle 1, 60% for REM cycle 2, stage 3, and 45% for REM cycle 2, stage 4. This was an accumulation of missing nights for some participants, and unavailability of quality signal segments for others. The effect of the imputation of interpolated values should be to suppress any effects on the remedy night 22 or post-remedy night 23. Carrying out analysis without imputation was regarded as infeasible, since then different nights would consist of different subsamples, potentially creating analysis artifacts.
Results
Table 1 gives the demographic information on the participants. Overall there were no baseline differences in age, gender, CMHO, ASI, or CMASI between the Coffea cruda and Nux vomica groups. Twenty-six subjects were randomized to receive Coffea cruda, and 28 subjects received Nux vomica. Conventional sleep stage findings are reported elsewhere.49 Tables 2a,b and 3a,b summarize the findings for the MSE and D2 analyses at each electrode site for each of the two REM cycles of remedy night (night 22) and the change from night 22 to post-remedy night 23, after controlling for factors noted above (including slow wave sleep EEG from baseline nights, placebo night, and change from placebo to post-placebo night, as well as personality trait index).
Table 1.
Demographic and personality trait variables.
| Variable | Nux vomica recipients (n = 28) | Coffea cruda recipients (n = 26) |
|---|---|---|
| Age | 19.6 ± 2.2 | 19.6 ± 2.6 |
| Gender | 11 M | 16 M |
| CMHO | 12.0 ± 2.2 | 11.8 ± 3.1 |
| ASI | 13.1 ± 7.3 | 16.2 ± 7.4 |
| CMASI | 0.67 ± 0.22 | 0.59 ± 0.26 |
CMHO=Cook-Medley Hostility Scale (Type A-like personality traits)
ASI= Anxiety Sensitivity Index
CMASI=single derived personality variable index (higher scores indicate an individual concomitantly higher in CMHO and lower in ASI scores) used in regression analyses (see Methods text)
Table 2a.
Regression Findings for Within-Subject Analyses on Multiscale Entropy for Subjects receiving Coffea cruda
| Stage 3 | Stage 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| REM Cycle 1 | REM Cycle 2 | REM Cycle 1 | REM Cycle 2 | |||||
| Electrode Site | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change |
| C3 | .54(.42) | −.52(.59) | .69(.44) | −1.3(.62)* | .55(.43) | −.11(.61) | .74(.4) † | −.97(.68) |
| C4 | .47(.44) | −.42(.6) | .44(.46) | −1.4(.53)* | .89(.38)* | −.74(.54) | .87(.32)** | −1.7(.62)** |
| CZ | .98(.35)** | −1(.49)* | .69(.49) | −1.5(.51)** | .67(.39) † | −.49(.52) | .95(.38)* | −1.5(.62)* |
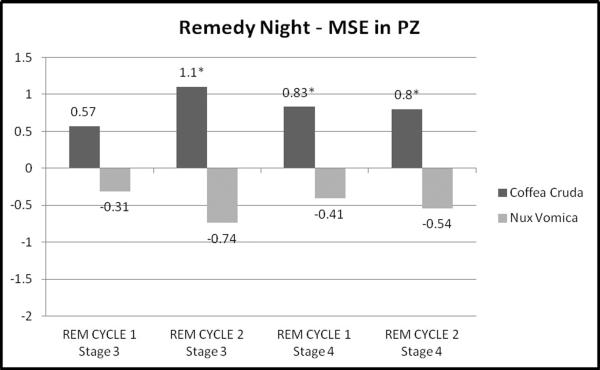
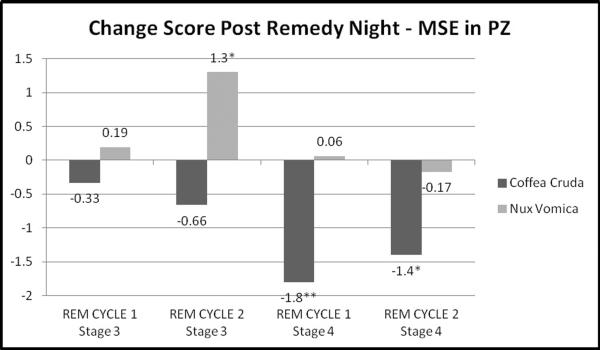
| PZ | .57(.4) | −.33(.56) | 1.1(.46)* | −1.8(.55)** | .83(.42)* | −.66(.56) | .8(.41)* | −1.4(.59)* |
| O1 | .63(.42) | −.53(.61) | .72(.52) | −1.2(.66) † | .03(.44) | .41(.51) | .9(.25)*** | −1.1(.53)* |
| O2 | .61(.4) | −.43(.65) | .97(.46)* | −1.4(.54)* | .84(.35)* | −.55(.5) | .79(.31)* | −1.6(.63)* |
P<0.10,
P<0.05,
P<0.01,
P<0.001.
Reported effects are unstandardized beta coefficients and standard error (in parentheses) for each of the two REM cycles of remedy night (night 22) and the change from night 22 to post-remedy night 23, after controlling for personality, baseline nights, placebo night, and change from placebo to post-placebo night.
Table 2b.
Regression Findings for Within-Subject Analyses on Correlation Dimension (D2) for Subjects receiving Coffea cruda.
| Stage 3 | Stage 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| REM Cycle 1 | REM Cycle 2 | REM Cycle 1 | REM Cycle 2 | |||||
| Electrode Site | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change |
| C3 | −.11(.07) | −.03(.12) | −.22(.14) | .24(.22) | −.03(.07) | .03(.1) | −.2(.12) † | .03(.17) |
| C4 | −.16(.09) † | .27(.15) † | −.1(.12) | .15(.18) | .14(.11) | −.01(.14) | −.09(.12) | .16(.33) |
| CZ | −.06(.11) | .13(.16) | −.15(.19) | .08(.29) | −.07(.09) | .1(.16) | −.19(.13) | .27(.17) |
| PZ | −.15(.11) | .33(.16)* | −.14(.13) | .15(.18) | .23(.15) | −.12(.18) | −.21(.16) | .66(.19)** |
| O1 | −.25(.15) | .22(.11)* | −.18(.14) | .29(.21) | −.16(.18) | .33(.23) | −.3(.18) † | .52(.20)* |
| O2 | −.07(.11) | .29(.16) † | .06(.17) | .15(.19) | −.26(.14) † | .33(.19) † | .02(.17 | .46(.16)** |
P<0.10,
P<0.005,
P<0.01,
P<0.001.
Reported effects are unstandardized beta coefficients and standard error (in parentheses) for each of the two REM cycles of remedy night (night 22) and the change from night 22 to post-remedy night 23, after controlling for personality, baseline nights, placebo night, and change from placebo to post-placebo night.
MSE revealed more extensive patterns of significant changes over multiple electrode sites for both medicines than did D2 analyses. For both remedies, the findings emerged later in the sleep period (REM cycle 2 more than REM cycle 1). For Coffea cruda 30c, MSE in stages 3 and 4 increased on night 22 remedy administration and then changed from night 22 to post-remedy night 23 with a decrease, especially toward the posterior (Pz) and right side (C4, O2) areas of the head (Table 2a). In contrast, for Nux vomica 30c, MSE findings were significant primarily for stage 3 (still REM cycle 2), but not stage 4 sleep (Table 3a). In the Nux vomica MSE data, the directionality was opposite to that of the Coffea effects. That is, Nux vomica led to a decrease in MSE on remedy night 22 and an increase from night 22 to night 23. Figures 1 and 2 provide a graphical presentation of MSE unstandardized beta coefficient values at the central parietal electrode site (Pz) to highlight the unique findings for each remedy. REM cycle 1 to REM cycle 2 shows change over time within the night for each sleep stage.
Table 3a.
Regression Findings for Within-Subject Analyses on Multiscale Entropy for Subjects receiving Nux vomica.
| Stage 3 | Stage 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| REM Cycle 1 | REM Cycle 2 | REM Cycle 1 | REM Cycle 2 | |||||
| Electrode Site | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change |
| C3 | −.32(.28) | .51(.54) | −.56(.41) | 1.28(.55)* | −.29(.36) | .15(.38) | −.11(.52) | −.18(.69) |
| C4 | −.54(.27)* | .24(.36) | −.98(.42)* | .85(.66) | −.71(.34)* | .56(.43) | −.32(.52) | −.24(.45) |
| CZ | −.16(.2) | −.21(.24) | −1.1(.37)** | 1.3(.54)* | −.34(.3) | .02(.33) | −.63(.43) | −.06(.59) |
| PZ | −.31(.24) | .19(.39) | −.74(.45) | 1.3(.56)* | −.41(.36) | .06(.39) | −.54(.67) | −.17(.64) |
| O1 | −.09(.41) | .19(.57) | −.9(.45)* | 1.4(.53)** | −.58(.42) | .21(.46) | −.43(.68) | .29(.6) |
| O2 | −.22(.31) | .34(.65) | −0.4(.37) | .31(.55) | −.38(.39) | .08(.47) | −.25(.49) | −.07(.43) |
P<0.10,
P<0.05,
P<0.01,
P<0.001.
Reported effects are unstandardized beta coefficients and standard error (in parentheses) for each of the two REM cycles of remedy night (night 22) and the change from night 22 to post-remedy night 23, after controlling for personality, baseline nights, placebo night, and change from placebo to post-placebo night.
Figure 1.
Remedy Night MSE for the parietal EEG lead (Pz) - Unstandardized Beta Coefficients by REM Cycle and Stage
*p<0.05
Figure 2.
Post-Remedy Night MSE Change Score for the parietal EEG lead (Pz) Unstandardized Beta Coefficients by REM Cycle and Stage
*p<0.05; **p<0.01
On the other hand, the Mekler-D2 analyses detected fewer, isolated site-specific significant changes than did MSE. With Coffea cruda, for instance, D2 showed a set of significant changes (increases from remedy night 22 to post-remedy night 23) toward the posterior electrode sites in Stage 4 REM cycle 2 (at Pz, O1, and O2), with similar significant effects in Stage 3 REM cycle 1 at Pz and O1 (Table 2b). Nux vomica, however, resulted in more widely scattered, isolated significant changes from remedy night 22 to post-remedy night 23 (increased values) in Stage 3 (REM cycle 1, at C3 and O1) and Stage 4 (REM cycle 1 at Cz and Pz; REM cycle 2 only at Cz)(Table 3b).
Table 3b.
Regression Findings for Within-Subject Analyses on Correlation Dimension (D2) for Subjects receiving Nux vomica.
| Stage 3 | Stage 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| REM Cycle 1 | REM Cycle 2 | REM Cycle 1 | REM Cycle 2 | |||||
| Electrode Site | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change | Remedy Night | Post-Remedy Change |
| C3 | −.15(.08) † | .26(.11)* | .11(.12) | .12(.28) | −.06(.12) | .06(.16) | −.1(.19) | −.04(.18) |
| C4 | −.07(.12) | .28(.21) | −.08(.09) | .18(.18) | .13(.1) | .08(.15) | .07(.22) | .16(.28) |
| CZ | −.09(.13) | .2(.18) | −.08(.11) | .01(.25) | −.03(.13) | .36(.17)* | −.01(.11) | .36(.14)* |
| PZ | .07(.12) | .06(.12) | −.06(.1) | −.05(.24) | .26(.12)* | −.02(.13) | .3(.22) | −.06(.22) |
| O1 | −.03(.11) | .32(.12)* | −.19(.12) | .13(.19) | 0(.11) | .14(.2) | .23(.13) † | .04(.12) |
| O2 | 0(.12) | .04(.16) | .04(.19) | .11(.16) | .09(.1) | −.01(.17) | .12(.2) | .26(.22) |
P<0.10,
P<0.05,
P<0.01,
P<0.001.
Reported effects are unstandardized beta coefficients and standard error (in parentheses) for each of the two REM cycles of remedy night (night 22) and the change from night 22 to post-remedy night 23, after controlling for personality, baseline nights, placebo night, and change from placebo to post-placebo night.
Discussion
The multiscale entropy data showed that two homeopathic remedies in 30c potencies can induce short-term alterations, with carryover effects the next night in the nonlinear dynamics of human slow wave sleep EEG. Coffea cruda had a more extensive effect than did Nux vomica over multiple electrode sites. The direction of the MSE effects was opposite to those seen with Nux vomica. The Mekler-D2 findings were more isolated over scattered electrode sites for both medicines. The direction of changes for MSE and D2 also exhibited opposite patterns from one another within a given remedy.
It might appear that the pattern of significant electrode sites and the direction of changes for MSE and Mekler-D2 suggest differing conclusions for the extent and direction of remedy effects in terms of “complexity ” increasing versus decreasing. However, higher values of MSE and D2 are each considered to reflect different aspects of EEG complexity, i.e., within NDS, MSE evaluates similarity across time scales, whereas Mekler-D2 assesses attractor dimension. Moreover, bifurcations, bipolarity and variability in the direction of change are common and expected features of nonlinear dynamical systems.27,77 In an experimental context, the net direction depends not only on the history, sensitivity to initial conditions, and dynamics of the system itself, but also on the nature of a given nonlinear parameter under consideration (www.SocietyForChaosTheory.org; www.NECSI.org; www.PlexusInstitute.org; www.SantaFe.edu).25
Thus, it is not surprising to find different results using different NDS variables, as in this exploratory study. It will be important in future homeopathy research to choose more than one way to quantify the characteristics of complexity in a given system in order to more fully describe the nature of the treatment effects. “Complexity” of a living system such as a human brain is not a single uniform process or property easily captured with a single variable.78–80 This study is admittedly only a first step toward evaluating the feasibility of using nonlinear dynamical systems approaches to evaluate remedy effects on human sleep EEG.
Because of the inherently changeable nature of nonlinear dynamical systems,27 we also anticipate that reproducibility could be an issue in subsequent investigations. That is, although EEG effects different from those of placebo should still occur in replication studies, the specific affected electrode sites and directionality of remedy-related effects could differ from study to study. Individual differences between study participants in traits and dynamic states, 19,23 as well as possible variations in remedy preparation methods between homeopathic manufacturers 69 and/or batches by the same manufacturer67 could all contribute to inter-experimental variability. More systematic biological and psychological characterization of study participants at rest and under stress, as well as of the medicines themselves could improve reliability of study outcomes. 23,24
Despite these reservations, the findings support the primary and secondary hypotheses that homeopathically-prepared remedies at 30c can affect nonlinear slow wave sleep EEG dynamics beyond effects seen at baseline or with placebo administration. Such data further support the value of examining slow wave sleep EEG parameters in homeopathy. Previous animal studies also observed remedy effects on slow wave sleep EEG patterns.44–48,81,82 The convergent data indicate that additional research in this area is warranted. Because of the controversies surrounding homeopathy, EEG offers a promising objective biomarker for use in preclinical and clinical studies that subjective measures fail to provide.
An underlying question that NDS and other complex systems analytic methods attempt to address is whether or not there are self-organized repeating patterns detectable within a given time series or set of datapoints. That is, if one zooms in and out of different levels of organizational scale,32 does the systemic behavioral pattern repeat? Therefore, NDS analytic methods such as MSE evaluate whether or not the data reflect uncorrelated random signals or noise as opposed to “meaningful structural richness”83 in temporal organization. Other complex systems analytic methods focus more on structural organization32 (http://physionet.org/physiotools/mse/, accessed 02/01/12).
Homeopathic theory7,84,85 suggests that the simillimum should evoke similar themes across global and local levels of scale in the patient.24,43 This feature of repeating patterns in nonlinear systems and networks29 could be particularly relevant to the way in which homeopathic clinicians evaluate their patients. In clinical analysis of a case, for example, the homeopath is often looking for repetitive patterns and similar themes across the patient as a whole (global behavior of the organism at the general level of scale) as well as the more local subsystems of the body (e.g., the descriptive qualities or modalities of the physical symptoms at the organ system level of scale).7,83,84
Given the small sample size and other limitations of the study, the conservative interpretatio of the findings is that it is feasible to use nonlinear dynamical analyses to examine the effects of homeopathic remedies on human sleep patterns. MSE appears more sensitive to revealing homeopathic remedy effects than does the Mekler-method D2 computation for sleep EEG analyses, although both approaches reveal remedy effects beyond placebo. Although it would be desirable to perform explicit between-subject comparisons of the subgroups receiving the two different remedies (Nux vomica and Coffea cruda), the small sample limited power for such a statistical approach. Therefore, we limited our computations to within-subject analyses. Subsequent studies with larger samples should directly evaluate the between-subject effects.
The robust effects of Coffea cruda on MSE, in addition to mood13 and conventional polysomnographic measures49 in this study merit additional research. Many young adult college students have repeated, extensive use of beverage coffee (Coffea as a “tincture”) prior to their participation in the study. In contrast, the single bedtime dose of Nux vomica would likely have been a first-ever exposure to the remedy in any form for the study participants. In the phenomenon of time-dependent sensitization,86 past exposure history can lead to significant amplification of behavioral and physiological responses in susceptible individuals to various agents given intermittently but repeatedly over time (these subjects had a pre-existing history of beverage coffee-induced insomnia).87–89 Future sensitizers and non-sensitizer controls do not necessarily show differences from one another in response patterns to an initial, single exposure to a given agent on the first dose, or at rest, prior to a subsequent exposure.89
Also, for this initial complexity analysis, we controlled for personality and did not attempt to look for NDS sleep EEG effects within subsets of participants whose personality traits might have enhanced or dampened the potential to respond to a given remedy. Future studies will need to look at the adequacy of matching the remedy to the individual, with the prediction that a good match would generate not only net clinical benefit, but also corresponding changes in nonlinear EEG dynamics5 that might help predict or distinguish responders from non-responders to treatment.43,71 Data from this laboratory suggest that individual difference traits and remedy can interact in generating differential mood13 or waking EEG effects.42
Various investigators5,14 previously suggested that the state-dependency of homeopathic remedy effects could relate to effects on the loss of connectivity across the person as a complex network in the diseased state and enhanced system susceptibility to the effects of the signal properties of the remedy. In a recent evidence-based review of biological model systems and homeopathy, Baumgartner76 concluded that (a) remedies have stronger effects on more complex systems such as whole organisms than on simple cell culture model systems; (b) the state dependency of remedy effects involves ceiling/floor limits in the initial condition of individual organisms (e.g., smaller plants at baseline grow better than larger plants with homeopathic treatment)(see also87); (c) homeopathic treatment may produce reduction in the inter-experimental variability of data distribution more than consistent effects on group means.
It is not clear what are the clinical implications, if any, of the present data. In our subjects, the medicines appeared initially to increase total sleep time, but disrupt the quality of the sleep by conventional scoring criteria.49 The self-report data from this study suggest that the medicines also affected mood as a function of the individual personality traits of the participants,13 and that the quality of the dreams recorded in morning diaries may also have changed (unpublished data). Given the history of inter-experimental variability in results for homeopathic basic science and preclinical research findings,76 however, it would be essential to replicate and extend the present study before drawing more speculative conclusions about the type or direction of remedy effects clinically.
Homeopathic remedy effects on complex living systems may be regulatory or adaptogenic overall, rather than unidirectional, in restoring homeostasis.3,6,8,23,24,76,90–92 Hahnemann's Organon (Aphorism 65) discusses the initial action of a stressor, stimulus, drug, or remedy as inducing changes in one direction, followed by the organism's compensatory counter-action or after-action “for the restoration of the normal state.”93 Classical homeopaths refer to a loss of vitality in a less resilient patient as a reduction in “vital force.”94 Using very different terminology to characterize clinical status, contemporary NDS researchers such as Goldberger et al conceptualize most diseases and aging as conditions that reduce overall systemic complexity.52,95,96 Other investigators have postulated that there is an optimum range of dynamical complexity in a living system that allows human well-being, resilience,97 and flourishing to emerge.22,23,98–100 However, EEG complexity assessed by multiscale entropy analysis can change over shorter time scales as well.101 Overall, then, transiently reduced biological complexity might indicate a comparatively adverse effect in the direction of disease, whereas increased complexity might suggest a comparatively beneficial effect in the direction of recovery or healing.22,23
Homeopaths commonly report a biphasic remedy effect phenomenon of initial, short-term clinical exacerbation of symptoms (sometimes termed an “aggravation” or “healing crisis”), prior to gradual resolution of symptoms.7,102 Although our current subjects had histories of coffee-induced insomnia, they were generally healthy. The homeopathic prediction for healthy individuals would be a worsening rather than improvement of sleep quality.7 Exploring these issues from an NDS perspective could contribute a great deal to resolving confusion about the bidirectionality often observed from one homeopathic study to the next.76
Finding ways to document the initial dynamical state of the individual homeopathic subjects' physiology immediately prior to remedy administration may help improve predictions about the nature of any subsequent changes observed over both short- and long-term follow-up. The evolution of change in the dynamics of a complex nonlinear system is highly sensitive to initial starting conditions.25,103 Such research may parallel the issues raised by clinical observations of nonlinear, bidirectional response patterns to isopathic asthma treatment with dust mite 30c5,104 and of sudden worsenings or improvements early in psychotherapy for depression.105,106
As complex organisms further from equilibrium (`sicker' in some sense) may respond more to individually-tailored homeopathic treatment,76 it would also be important to assess the current medicines on patients with primary insomnia evaluated by clinical interview to need one of these specific medicines by an experienced homeopathic provider, rather than the relatively healthy young adults screened by questionnaire and enrolled in this study. At the same time, this study design reflected a modification of a phase 1-like drug development trial (homeopathic pathogenetic trial) in homeopathy. Relatively healthy volunteers usually take the test substance and record subjective effects over a period of days to weeks,12,72,107 with the expectation of a short-term transient increase in some remedy/person-characteristic symptoms.
In conclusion, the homeopathic remedies Coffea cruda and Nux vomica in 30c potencies alter short-term nonlinear dynamic parameters of slow wave sleep EEG in relatively healthy young adults. As an NDS analytic technique, MSE50,52,74 is apparently more sensitive than Mekler-method D253 for detecting these effects. Additional research to explore individual differences in state-dependency, direction and longer term patterns of homeopathic remedy effects on objectively-recorded human sleep in people with and without insomnia is indicated.
Supplementary Material
Acknowledgments
This study was supported by National Center for Complementary and Alternative Medicine Grants R21 AT000388, K24 AT000057, and T32 AT01287 (PI: IRB). The authors would like to thank Keith Fridel, PhD RPSGT for training and supervising the sleep scoring, and Elizabeth Acker, Michael Biuso, Erica Morey, Mallory Taylor, and Alivia Wieseler for their technical assistance in data collection and quality assurance procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Drs. Bell and Brooks serve as consultants to Standard Homeopathic Co./Hyland's Inc., a homeopathic manufacturer whose products were not used in the current study. Standard Homeopathic Co./Hyland's Inc. did not provide any funding for the current study.
References
- 1.Owen D. Principles and Practice of Homeopathy: The Therapeutic and Healing Process. Churchill Livingstone; 2007. [Google Scholar]
- 2.Grabia S, Ernst E. Homeopathic aggravations: a systematic review of randomised, placebo-controlled clinical trials. Homeopathy: the Journal of the Faculty of Homeopathy. 2003 Apr;92(2):92–98. doi: 10.1016/s1475-4916(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 3.Bertani S, Lussignoli S, Andrioli G, Bellavite P, Conforti A. Dual effects of a homeopathic mineral complex on carrageenan-induced oedema in rats. British Homoeopathic Journal. 1999;88(3):101–105. doi: 10.1054/homp.1999.0310. [DOI] [PubMed] [Google Scholar]
- 4.Hyland M. Extended network generalized entanglement theory: therapeutic mechanisms, empirical predictions, and investigations. Journal of Alternative & Complementary Medicine. 2003;9(6):919–936. doi: 10.1089/107555303771952262. [DOI] [PubMed] [Google Scholar]
- 5.Hyland ME, Lewith GT. Oscillatory effects in a homeopathic clinical trial: an explanation using complexity theory, and implications for clinical practice. Homeopathy. 2002;91(3):145–149. doi: 10.1054/homp.2002.0025. [DOI] [PubMed] [Google Scholar]
- 6.Bell IR, Koithan M. A model for homeopathic remedy effects: low dose nanoparticles, allostatic cross-adaptation, and time-dependent sensitization in a complex adaptive system. Submitted for publication. 2012 doi: 10.1186/1472-6882-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vithoulkas G. The Science of Homeopathy. Grove Weidenfeld; N.Y.: 1980. [Google Scholar]
- 8.Bell IR, Koithan M. Models for the study of whole systems. Integrative Cancer Therapies. 2006;5(4):293–307. doi: 10.1177/1534735406295293. [DOI] [PubMed] [Google Scholar]
- 9.Brien SB, Harrison H, Daniels J, Lewith G. Monitoring improvement in health during homeopathic intervention. Development of an assessment tool based on Hering's Law of Cure: the Hering's Law Assessment Tool (HELAT) Homeopathy. 2012 Jan;101(1):28–37. doi: 10.1016/j.homp.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Vithoulkas G, van Woensel E. Levels of Health: Practical Applications and Cases. International Academy of Classical Homeopathy; 2010. [Google Scholar]
- 11.Dantas F, Fisher P, Walach H, et al. A systematic review of the quality of homeopathic pathogenetic trials published from 1945 to 1995. Homeopathy. 2007 Jan;96(1):4–16. doi: 10.1016/j.homp.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Sherr J. The Dynamics and Methodology of Homeopathic Provings. 2nd ed Dynamis Books; Malvern, UK: 1994. [Google Scholar]
- 13.Brooks AJ, Bell IR, Howerter A, Jackson N, Aickin M. Effects of homeopathic medicines on mood of adults with histories of coffee-related insomnia. Forsch Komplementarmed Klass Naturheilkd. 2010;17:250–257. doi: 10.1159/000320952. [DOI] [PubMed] [Google Scholar]
- 14.Torres JL. Homeopathic effect: a network perspective. Homeopathy: the Journal of the Faculty of Homeopathy. 2002;91(2):89–94. doi: 10.1054/homp.2002.0007. [DOI] [PubMed] [Google Scholar]
- 15.Torres JL, Ruiz MAG. Stochastic resonance and the homeopathic effect. British Homoeopathic Journal. 1996;85(3):134–140. [Google Scholar]
- 16.Shepperd J. Chaos theory: implications for homeopathy. Journal of the American Institute of Homeopathy. 1994;87(4):22. [Google Scholar]
- 17.Bellavite P, Signorini A. The Emerging Science of Homeopathy. Complexity, Biodynamics, and Nanopharmacology. 2nd ed North Atlantic Books; Berkeley: 2002. [Google Scholar]
- 18.Bellavite P. Complexity science and homeopathy: a synthetic overview. Homeopathy: the Journal of the Faculty of Homeopathy. 2003;92(4):203–212. doi: 10.1016/j.homp.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Bell IR, Baldwin CM, Schwartz GE. Translating a nonlinear systems theory model for homeopathy into empirical tests. Alternative Therapies in Health & Medicine. 2002;8(3):58–66. [PubMed] [Google Scholar]
- 20.Witt C, Albrecht H, editors. New Directions in Homeopathy Research. KVC Verlag; Essen, Germany: 2009. [Google Scholar]
- 21.Milgrom LR. Vitalism, complexity, and the concept of spin. Homeopathy: the Journal of the Faculty of Homeopathy. 2002;91(1):26–31. doi: 10.1054/homp.2001.0013. [DOI] [PubMed] [Google Scholar]
- 22.Koithan M, Bell IR, Niemeyer K, Pincus D. A complex systems science perspective for whole systems of CAM research. Forschende Komplementarmedizin und Klassische Naturheilkunde. 2012;19(Supplement 1):7–14. doi: 10.1159/000335181. [DOI] [PubMed] [Google Scholar]
- 23.Bell IR, Koithan M, Pincus D. Research methodological implications of nonlinear dynamical systems models for whole systems of complementary and alternative medicine. Forschende Komplementarmedizin und Klassische Naturheilkunde. 2012;19(Supplement 1):15–21. doi: 10.1159/000335183. [DOI] [PubMed] [Google Scholar]
- 24.Bell IR, Koithan M, A.J. B. Testing the nanoparticle-allostatic cross-adaptation-sensitization model for homeopathic remedy effects. Submitted for publication. 2012 doi: 10.1016/j.homp.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guastello SJ, Koopmans M, Pincus D. Chaos and Complexity in Psychology: The Theory of Nonlinear Dynamical Systems. Cambridge University Press; New York, NY: 2009. [Google Scholar]
- 26.Bar-Yam Y. Dynamics of Complex Systems. Perseus Books; Reading, MA: 1997. [Google Scholar]
- 27.Mitchell M. Complexity: A Guided Tour. Oxford University Press; NY: 2009. [Google Scholar]
- 28.Burggren WW, Monticino MG. Assessing physiological complexity. J Exp Biol. 2005;208(Pt 17):3221–3232. doi: 10.1242/jeb.01762. [DOI] [PubMed] [Google Scholar]
- 29.Vasquez A, Dobrin R, Sergi D, Eckmann JP, Oltvai ZN, Barabasi AL. The topological relationship between the large-scale attributes and local interaction patterns of complex networks. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):17940–17945. doi: 10.1073/pnas.0406024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barabasi AL. Linked. How everything is connected to everything else and what it means for business, science, and everyday life. Plume; Cambridge, MA: 2003. [Google Scholar]
- 31.Barabasi AL, Bonabeau E. Scale-free networks. Scientific American. 2003;288(5):60–69. doi: 10.1038/scientificamerican0503-60. [DOI] [PubMed] [Google Scholar]
- 32.Bar-Yam Y. Making Things Work: Solving Complex Problems in a Complex World. Knowledge Press; 2004. [Google Scholar]
- 33.Pincus D. Self-organizing biopsychosocial dynamics and the patient-healer relationship. Forsch Komplementarmed. 2012;19(Supplement 1):22–29. doi: 10.1159/000335186. [DOI] [PubMed] [Google Scholar]
- 34.Woyshville MJ, Calabrese JR. Quantification of occipital EEG changes in Alzheimer's disease utilizing a new metric: the fractal dimension. Biological Psychiatry. 1994;35(6):381–387. doi: 10.1016/0006-3223(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 35.Ferri R, Pettinato S, Nobili L, Billiard M, Ferrillo F. Correlation dimension of EEG slow-wave activity during sleep in narcoleptic patients under bed rest conditions. Int J Psychophysiol. 1999 Oct;34(1):37–43. doi: 10.1016/s0167-8760(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 36.Jardanhazy A, Jardanhazy T. Non-linear quantitative electroencephalographic (qEEG) changes during processing of chemo-sensory stimulations: a preliminary study. Behav Brain Res. 2008 Dec 12;194(2):162–168. doi: 10.1016/j.bbr.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 37.Jelles B, Scheltens P, van der Flier WM, Jonkman EJ, da Silva FH, Stam CJ. Global dynamical analysis of the EEG in Alzheimer's disease: frequency-specific changes of functional interactions. Clin Neurophysiol. 2008 Apr;119(4):837–841. doi: 10.1016/j.clinph.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki H, Morinushi T, Yakushiji M, Takigawa M. Nonlinear dynamical analysis of the effect by six stimuli on electroencephalogram. J Clin Neurophysiol. 2009 Feb;26(1):24–38. doi: 10.1097/WNP.0b013e31819862db. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Madokoro S, Wada Y, Misaki K, Nakagawa H. Human sleep EEG analysis using the correlation dimension. Clin Electroencephalogr. 2001 Jul;32(3):112–118. doi: 10.1177/155005940103200305. [DOI] [PubMed] [Google Scholar]
- 40.Tsirka V, Simos PG, Vakis A, Kanatsouli K, Vourkas M, Erimaki S, Pachou E, Stam CJ, Micheloyannis S. Mild traumatic brain injury: graph-model characterization of brain networks for episodic memory. Int J Psychophysiol. 2011;79(2):89–96. doi: 10.1016/j.ijpsycho.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Dimpfel W. The ultra low dose combination medication ULDCM-310 triggers electroencephalographic patterns in the rat brain in a dose and time dependent manner. European Journal of Integrative Medicine. 2010;2(4):227–228. [Google Scholar]
- 42.Bell IR, Brooks AJ, Howerter A, Jackson N, Schwartz GE. Short term effects of repeated olfactory administration of homeopathic Sulphur or Pulsatilla on electroencephalographic alpha power in healthy young adults. Homeopathy. 2011;100(4):203–211. doi: 10.1016/j.homp.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell IR, Lewis DA, 2nd, Schwartz GE, et al. Electroencephalographic cordance patterns distinguish exceptional clinical responders with fibromyalgia to individualized homeopathic medicines. Journal of Alternative & Complementary Medicine. 2004;10(2):285–299. doi: 10.1089/107555304323062275. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Vega G, Perez-Ordaz L, Leon-Hueramo O, Cruz-Vazquez E, Sanchez-Diaz N. Comparative effect of Coffea cruda potencies on rats. Homeopathy. 2002;91:80–84. doi: 10.1054/homp.2002.0005. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Vega G, Poitevin B, Pérez-Ordaz L. Histamine at high dilution reduces spectral density in delta band in sleeping rats. Homeopathy. 2005;94(2):86–91. doi: 10.1016/j.homp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Vega G, Perez-Ordaz L, Cortes-Galvan L, Juarez-G FM. A kinetic approach to caffeine-Coffea cruda interaction. Homeopathy: the Journal of the Faculty of Homeopathy. 2003;92(1):19–29. doi: 10.1054/homp.2002.0081. [DOI] [PubMed] [Google Scholar]
- 47.Ruiz G, Torres JL. Homeopathic effect on the sleep pattern of rats. British Homoeopathic Journal. 1997;86:201–206. [Google Scholar]
- 48.Sukul A, Sinhabau SP, Sukul NC. Reduction of alcohol induced sleep time in albino mice by potentized Nux vomica prepared with 90% ethanol. British Homoeopathic Journal. 1999;88(2):58–61. doi: 10.1054/homp.1999.0291. [DOI] [PubMed] [Google Scholar]
- 49.Bell IR, Howerter A, Jackson N, Aickin M, Baldwin CM, Bootzin RR. Effects of homeopathic medicines on polysomnographic sleep of young adults with histories of coffee-related insomnia. Sleep Med. 2011 May;12(5):505–511. doi: 10.1016/j.sleep.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102, 068101–068104. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- 51.Costa M, Priplata AA, Lipsitz LA, Wu Z, Huang NE, Goldberger AL, Peng CK. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Europhys Lett. 2007;77:68008. doi: 10.1209/0295-5075/77/68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Physical Review E. 2005;71(021906):1–18. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 53.Mekler A. Calculation of EEG correlation dimension: large massifs of experimental data. Computer Methods and Programs in Biomedicine. 2008;92:154–160. doi: 10.1016/j.cmpb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi T, Madokoro S, Ota T, et al. Analysis of the human sleep electroencephalogram by the correlation dimension. Psychiatry Clin Neurosci. 2000 Jun;54(3):278–279. doi: 10.1046/j.1440-1819.2000.00677.x. [DOI] [PubMed] [Google Scholar]
- 55.Yang H, Wang Y, Wang CJ, Tai HM. Correlation dimensions of EEG changes during mental tasks. Conf Proc IEEE Eng Med Biol Soc. 2004;1:616–619. doi: 10.1109/IEMBS.2004.1403233. [DOI] [PubMed] [Google Scholar]
- 56.Bell IR, Baldwin CM, Schwartz GER. Translating a nonlinear systems theory model for homeopathy into empirical tests. Alternative Therapies in Health & Medicine. 2002;8(3):58–66. [PubMed] [Google Scholar]
- 57.Morrison R. Desktop Guide to Keynotes and Confirmatory Symptoms. Hahnemann Clinic Publishing; Albany, CA: 1993. [Google Scholar]
- 58.Roy R, Tiller W, Bell IR, Hoover MR. The structure of liquid water: novel insights from materials research and potential relevance to homeopathy. Materials Research Innovation. 2005;9(4):557–608. [Google Scholar]
- 59.Rao ML, Roy R, Bell IR. The defining role of structure (including epitaxy) in the plausibility of homeopathy. Homeopathy. 2007;96(3):175–182. doi: 10.1016/j.homp.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Rao M, Roy R. Bell, IR. Characterization of the structure of ultra dilute sols with remarkable biological properties. Materials Letters. 2008;62:1487–1490. doi: 10.1016/j.matlet.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rey L. Thermoluminescence of ultra-high dilutions of lithium chloride and sodium chloride. Physica A: Statistical mechanics and its applications. 2003;323:67–74. [Google Scholar]
- 62.Rey L. Can low-temperature thermoluminescence cast light on the nature of ultra-high dilutions? Homeopathy. 2007;96(3):170–174. doi: 10.1016/j.homp.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Elia V, Niccoli M. Thermodynamics of extremely diluted aqueous solutions. Annals of the New York Academy of Sciences. 1999;879:241–248. doi: 10.1111/j.1749-6632.1999.tb10426.x. [DOI] [PubMed] [Google Scholar]
- 64.Elia V, Niccoli M. New physico-chemical properties of extremely diluted aqueous solutions. Journal of Thermal Analysis and Calorimetry. 2004;75:815–836. [Google Scholar]
- 65.Elia V, Napoli E, Germano R. The 'Memory of Water': an almost deciphered enigma. Dissipative structures in extremely dilute aqueous solutions. Homeopathy. 2007;96(3):163–169. doi: 10.1016/j.homp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 66.van Wijk R, Bosman S, van Wijk EP. Thermoluminescence in ultra-high dilution research. J Altern Complement Med. 2006 Jun;12(5):437–443. doi: 10.1089/acm.2006.12.437. [DOI] [PubMed] [Google Scholar]
- 67.Chikramane PS, Suresh AK, Bellare JR, Kane SG. Extreme homeopathic dilutions retain starting materials: A nanoparticulate perspective. Homeopathy. 2010;99(4):231–242. doi: 10.1016/j.homp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Montagnier L, Aissa J, Ferris S, Montagnier J-L, Lavallee C. Electromagnetic signals are produced by aqueous nanostructures derived from bacterial DNA sequences. Interdisciplinary Sci Comput Life Sci. 2009;1:81–90. doi: 10.1007/s12539-009-0036-7. [DOI] [PubMed] [Google Scholar]
- 69.Bhattacharyya SS, Mandal SK, Biswas R, et al. In vitro studies demonstrate anticancer activity of an alkaloid of the plant Gelsemium sempervirens. Exp Biol Med (Maywood) 2008 Dec;233(12):1591–1601. doi: 10.3181/0805-RM-181. [DOI] [PubMed] [Google Scholar]
- 70.Lancet The end of homeopathy. Lancet. 2005;366:690. [Google Scholar]
- 71.Frei H, Everts R, von Ammon K, et al. Randomised controlled trials of homeopathy in hyperactive children: treatment procedure leads to an unconventional study design Experience with open-label homeopathic treatment preceding the Swiss ADHD placebo controlled, randomised, double-blind, cross-over trial. Homeopathy. 2007 Jan;96(1):35–41. doi: 10.1016/j.homp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Möllinger H, Schneider R, Walach H. Homeopathic pathogenetic trials produce specific symptoms different from placebo. Forsch Komplementmed. 2009;16(2):105–110. doi: 10.1159/000209386. [DOI] [PubMed] [Google Scholar]
- 73.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. UCLA BIS/BRI Publications; Los Angeles, CA: 1968. [DOI] [PubMed] [Google Scholar]
- 74.Costa M, Goldberger AL, Peng CK. Multiscale entropy to distinguish physiologic and synthetic RR time series. Comput Cardiol. 2002;29:137–140. [PubMed] [Google Scholar]
- 75.Grassberger P, Procaccia I. Measuring the strangeness of strange attractors. Physica D: Nonlinear Phenomena. 1983;9(1–2):189–208. [Google Scholar]
- 76.Baumgartner S. The State of Basic Research on Homeopathy. In: Witt C, Albrecht H, editors. New Directions in Homeopathy Research. KVC Verlag; Essen, Germany: 2009. pp. 107–130. [Google Scholar]
- 77.Spiegler A, Kiebel SJ, Atay FM, Knosche TR. Bifurcation analysis of neural mass models: Impact of extrinsic inputs and dendritic time constants. Neuroimage. 2010 Sep;52(3):1041–1058. doi: 10.1016/j.neuroimage.2009.12.081. [DOI] [PubMed] [Google Scholar]
- 78.Whelan R, Conrod PJ, Poline JB, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012 Apr 29; doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 79.Sporns O. The non-random brain: efficiency, economy, and complex dynamics. Front Comput Neurosci. 2011;5:5. doi: 10.3389/fncom.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Priano L, Saccomandi F, Mauro A, Guiot C. Non-linear recurrence analysis of NREM human sleep microstructure discloses deterministic oscillation patterns related to sleep stage transitions and sleep maintenance. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:4934–4937. doi: 10.1109/IEMBS.2010.5627237. [DOI] [PubMed] [Google Scholar]
- 81.Ruiz-Vega G, Perez-Ordaz L, Proa-Flores P, Aguilar-Diaz Y. An evaluation of Coffea cruda effect on rats. British Homoeopathic Journal. 2000;89(3):122–126. doi: 10.1038/sj.bhj.5800417. [DOI] [PubMed] [Google Scholar]
- 82.Sukul NC, Ghosh S, Sinhababu SP, Sukul A. Strychnos nux-vomica extract and its ultra-high dilution reduce voluntary ethanol intake in rats. Journal of Alternative & Complementary Medicine. 2001;7(2):187–193. doi: 10.1089/107555301750164280. [DOI] [PubMed] [Google Scholar]
- 83.Grassberger P. Information and complexity measures in dynamical systems. In: Atmanspacher H, H. S, editors. Information Dynamics. Plenum Press; New York: 1991. pp. 15–33. [Google Scholar]
- 84.Sherr J. Dynamic Materia Medica. Syphilis: A Study of Syphilitic Miasm through Remedies. Dynamis Books; Great Malvern Worcestershire, England: 2002. [Google Scholar]
- 85.Sankaran R. The Synergy in Homoeopathy. Homoeopathic Medical Publishers; Mumbai, India: 2012. [Google Scholar]
- 86.Antelman SM, Levine J, Gershon S. Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Molecular Psychiatry. 2000;5(4):350–356. doi: 10.1038/sj.mp.4000721. [DOI] [PubMed] [Google Scholar]
- 87.Antelman SM, Caggiula AR. Oscillation follows drug sensitization: implications. Critical Reviews in Neurobiology. 1996;10(1):101–117. doi: 10.1615/critrevneurobiol.v10.i1.50. [DOI] [PubMed] [Google Scholar]
- 88.Bell IR, Baldwin CM, Schwartz GE. Sensitization studies in chemically intolerant individuals: implications for individual difference research. Annals of the New York Academy of Sciences. 2001;933:38–47. doi: 10.1111/j.1749-6632.2001.tb05812.x. [DOI] [PubMed] [Google Scholar]
- 89.Antelman SM. Time-dependent sensitization in animals: a possible model of multiple chemical sensitivity in humans. Toxicology & Industrial Health. 1994;10(4–5):335–342. [PubMed] [Google Scholar]
- 90.Van Wijk R, Wiegant FA. Postconditioning hormesis and the similia principle. Front Biosci (Elite Ed) 2011;3:1128–1138. doi: 10.2741/e316. [DOI] [PubMed] [Google Scholar]
- 91.Fisher P. Does homeopathy have anything to contribute to hormesis? Hum Exp Toxicol. 2010 Jul;29(7):555–560. doi: 10.1177/0960327110369776. [DOI] [PubMed] [Google Scholar]
- 92.Calabrese EJ, Jonas WB. Homeopathy: clarifying its relationship to hormesis. Hum Exp Toxicol. 2010 Jul;29(7):531–536. doi: 10.1177/0960327110369857. [DOI] [PubMed] [Google Scholar]
- 93.Hahnemann S. Organon of the Medical Art. 6th ed Birdcage Books; Redmond, WA: 1843. [Google Scholar]
- 94.Bell IR, Lewis DA, 2nd, Lewis SE, Brooks AJ, Schwartz GE, Baldwin CM. Strength of vital force in classical homeopathy: bio-psycho-social-spiritual correlates within a complex systems context. Journal of Alternative & Complementary Medicine. 2004;10(1):123–131. doi: 10.1089/107555304322849048. [DOI] [PubMed] [Google Scholar]
- 95.Lipsitz LA, Goldberger AL. Loss of 'complexity' and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267:1806–1809. [PubMed] [Google Scholar]
- 96.Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002 Jan-Feb;23(1):23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 97.Pincus D, Metten A. Nonlinear dynamics in biopsychosocial resilience. Nonlinear Dynamics Psychol Life Sci. 2010;14(4):353–380. [PubMed] [Google Scholar]
- 98.Fredrickson BL, Losada MF. Positive affect and the complex dynamics of human flourishing. American Psychologist. 2005;60(7):678–686. doi: 10.1037/0003-066X.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Losada M. The complex dynamics of high performance teams. Mathematical Computer Modelling. 1999;30:179–192. [Google Scholar]
- 100.Losada M, Heaphy E. The role of positivity and connectivity in the performance of business teams: a nonlinear dynamics model. American Behavioral Scientist. 2004;47(6):740–765. [Google Scholar]
- 101.Catarino A, Churches O, Baron-Cohen S, Andrade A, Ring H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin Neurophysiol. 2011 Dec;122(12):2375–2383. doi: 10.1016/j.clinph.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 102.Rossi E, Bartoli P, Bianchi A, Endrizzi C, Da Fre M. Homeopathic aggravation with Quinquagintamillesimal potencies. Homeopathy. 2012 Apr;101(2):112–120. doi: 10.1016/j.homp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Good LB, Sabesan S, Marsh ST, Tsakalis K, Treiman DM, Iasemidis LD. Nonlinear dynamics of seizure prediction in a rodent model of epilepsy. Nonlinear Dynamics Psychol Life Sci. 2010;14(4):411–434. [PubMed] [Google Scholar]
- 104.Lewith GT, Watkins AD, Hyland ME, et al. Use of ultramolecular potencies of allergen to treat asthmatic people allergic to house dust mite: double blind randomised controlled clinical trial. BMJ. 2002;324(7336):520–523. doi: 10.1136/bmj.324.7336.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pincus D. Coherence, complexity, and information flow: self-organizing processes in psychotherapy. In: Guastello SJ, Koopmans M, Pincus D, editors. Chaos and Complexity in Psychology. The Theory of Nonlinear Dynamical Systems. Cambridge University Press; NY: 2009. pp. 335–369. [Google Scholar]
- 106.Hayes AM, Laurenceau JP, Feldman G, Strauss JL, Cardaciotto L. Change is not always linear: the study of nonlinear and discontinuous patterns of change in psychotherapy. Clin Psychol Rev. 2007;27(6):715–723. doi: 10.1016/j.cpr.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walach H, Mollinger H, Sherr J, Schneider R. Homeopathic pathogenetic trials produce more specific than non-specific symptoms: results from two double-blind placebo controlled trials. J Psychopharmacol. 2008 Jul;22(5):543–552. doi: 10.1177/0269881108091259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.