Abstract
Blood vessels course through organs, providing them with essential nutrient and gaseous exchange. However, the vasculature has also been shown to provide non-nutritional signals that play key roles in the control of organ growth, morphogenesis and homeostasis. Here, we examine a decade of work on the contribution of vascular paracrine signals to developing tissues, with a focus on pancreatic β-cells. During the early stages of embryonic development, blood vessels are required for pancreas specification. Later, the vasculature constrains pancreas branching, differentiation and growth. During adult life, capillaries provide a vascular niche for the maintenance of β-cell function and survival. We explore the possibility that the vasculature constitutes a dynamic and regionalized signaling system that carries out multiple and changing functions as it coordinately grows with the pancreatic epithelial tree.
Keywords: Endothelial, Pancreatic epithelium, β-cell, Islet, Signaling, Vascular niche
Introduction
Organ and blood vessel growth and development are inherently coordinated processes. All living cells within the tissues of vertebrates must be in proximity to a blood vessel to allow proper gas exchange, nutrition and elimination of wastes (Folkman, 1971). A distance greater than 100 μm triggers a hypoxic response and the production of angiogenic factors by the local environment to stimulate ingrowth of vessels, thus bridging the distance between cells and vasculature (Folkman, 1971; Fidler et al., 2002). Although it is not trivial to uncouple nutritional from bona fide paracrine vascular signals, several provocative in vivo and in vitro studies of the liver and pancreas (Fig. 1) suggest that blood vessels provide important non-nutritional signals to surrounding tissues. These findings have brought about a conceptual shift in the way we think about the contributions of blood vessels to organogenesis, and have introduced the idea that pancreatic cell lineages are subject to vascular influence (Cleaver and Melton, 2003; Nikolova and Lammert, 2003; Kopp et al., 2005; Crivellato et al., 2007; Tirziu and Simons, 2009). We therefore suggest that an integral aspect of developing regenerative and replacement therapies for diabetes will consist of delineating, and hopefully harnessing, the microenvironment and extrinsic signals that influence the many steps leading to the β-cell fate.
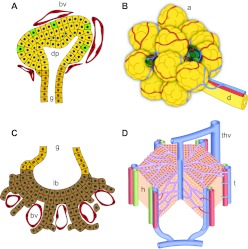
Fig. 1.
Close association of vessels with pancreas and liver epithelium throughout development into adulthood. (A) The early pancreatic bud is encased in capillaries (red) that pervade the gut mesenchyme. Endocrine cells (green) emerge within the bud epithelium (yellow). (B) The developing pancreatic tree grows coordinately with its vasculature, with vessels (red/blue) running along ducts. Islets (green) are embedded within the more abundant exocrine tissue (yellow). (C) The early liver bud shows intercalation of early hepatocytes (brown) and capillaries (red) within the septum transversum. (D) The developing liver contains a dense vascular network – the sinusoidal endothelium (purple). a, acini; bv, blood vessel; d, pancreatic duct; dp, dorsal pancreas; g, gut tube; h, hepatocytes; lb, liver bud; t, triad of hepatic artery, portal vein and bile duct; thv, terminal hepatic vein.
Non-nutritional roles for endothelium in developing organs
To set the stage for understanding how blood vessels influence the developing pancreas, it is crucial to first provide a wider overview of the role of the vasculature in tissue growth and maintenance. We therefore begin by reviewing how blood vessels sustain a variety of vertebrate tissues, and then provide an in-depth analysis of vascular influence on pancreas development, both during development and in adult islets.
Reciprocal interactions between endothelial and organ-specific cell types have been reported in a variety of tissues, including the liver, lung, nervous system, testis, bone, adipose tissue and pancreas (Lammert et al., 2001; Matsumoto et al., 2001; Mukouyama et al., 2002; Rupnick et al., 2002; Shen et al., 2004; Yoshitomi and Zaret, 2004; Jacquemin et al., 2006; Yoshida et al., 2007; Shen et al., 2008; Tang et al., 2008; Tavazoie et al., 2008; Pierreux et al., 2010; Lazarus et al., 2011). In general, signals from tissues to blood vessels consist of angiogenic factors, such as vascular endothelial growth factor A (VEGFA), which attract and stimulate vessel ingrowth. In turn, blood vessels have been found to signal back to surrounding tissues, either via endothelial-, blood- or perivascular cell-derived signals that influence tissue-specific cell types. This crosstalk ensures coordinated growth and tissue maintenance.
Signals from endothelial cells (ECs) to liver cells, for example, have been found to be important for both development and regeneration. At the onset of liver development, ECs communicate with hepatic cells as they intercalate between each other within the septum transversum (Matsumoto et al., 2001) (Fig. 1). The induction of liver genes such as albumin fails in VEGFR2-null embryos that lack ECs. Importantly, these effects were seen in Vegfr–/– explants of foreguts grown in ambient oxygen and rich media, demonstrating that the signals from ECs to the developing liver were uncoupled from nutrients and oxygen. Similarly, in zebrafish, genetic or pharmacological reduction of the vasculature impaired proper liver formation and blocked hepatocyte polarization (Sakaguchi et al., 2008). Specifically, the transmembrane EGF repeat-containing protein Heart of glass (Heg) expressed on ECs was shown to mediate hepatocyte polarization via cell-cell signaling. Increased liver vasculature, however, resulted in adult liver hyperplasia due to paracrine factors, such as hepatocyte growth factor (HGF) (LeCouter et al., 2003). Together, data from both in vivo and in vitro models, and from different species, suggest an essential role for blood vessels during liver development and regeneration.
Studies of the lung vasculature and its impact on lung morphogenesis provide additional evidence of vascular signaling. Studies driven by the clinical need for therapies to ameliorate lung development in premature infants (Northway et al., 1967) have identified endothelial signals in the lung that appear simultaneously to be required for several aspects of lung development, including epithelial development and branching (Zeng et al., 1998; Compernolle et al., 2002; Akeson et al., 2003; Lazarus et al., 2011). Although VEGFA-driven hypervascularization in transgenic mice resulted in increased epithelial branching and proliferation, ex vivo VEGF inhibition and decreased vasculature led to reduction of terminal branches (Del Moral et al., 2006). Similarly, genetic ablation of VEGFA led to loss of primary septae formation during distal lung morphogenesis (Yamamoto et al., 2007). However, careful analysis of lung branching patterns following vascular ablation in explanted lungs revealed that, in addition to loss of orthogonal bifurcation, there was surprising ectopic airway branching, suggesting a role for ECs in determining branching stereotypy (Lazarus et al., 2011). These studies highlight the active role played by blood vessels in controlling branching morphogenesis during lung development.
Evidence from liver and lung studies therefore supports the notion that developing tissues require tight control of blood vessel density and morphology, not only to ensure perfusion, but also to ensure proper organogenesis. Although it might be expected that reduced vasculature hampers normal development (owing to loss of nutrients/gases), excess blood vessels also lead to non-autonomous defects in host tissues. Hence, in this context, loss-of-function studies of the vasculature are better performed in vitro to exclude artifacts or indirect effects due to ischemia, whereas gain-of-function studies (forced hypervascularization) are highly informative, despite the traditional disinclination of developmental biologists for such experiments, as they largely avoid these inherent secondary effects. Although scattered studies in mammals suggest that ECs also play roles in other organs (Eremina and Quaggin, 2004; Gao et al., 2005; Garratt, 2006), it will be of great interest to learn whether other organs, such as the spleen, kidney or heart, or branching organs, such as mammary, salivary and thyroid glands, exhibit similar dependence on ECs.
A vascular ‘niche’ for stem/progenitor cell maintenance
Blood vessels not only signal to budding and branching organs, but they also form specialized ‘vascular niches’ that sustain tissue progenitor cells. A vascular niche is defined as a focal, microanatomic domain in which important cellular events occur in close proximity to blood vessels (Kopp et al., 2005; Nikolova et al., 2007; Butler et al., 2010a). These niches have been shown to provide sustenance and maintenance of various endogenous adult stem cell populations that are essential for tissue homeostasis and/or regeneration.
One adult tissue in which stem cells were shown to depend on vascular niches is the central nervous system. Neural stem cells (NSCs) in the subventricular zone (SVZ) of the adult brain have been shown to associate closely with blood vessels (Riquelme et al., 2008) (Fig. 2A). Indeed, these NSCs associate with specialized niches composed of ependymal and ECs that direct self-renewal and initiation of differentiation, respectively. Early studies of explanted NSCs demonstrated that soluble paracrine signals from ECs stimulated proliferation (Shen et al., 2004) and differentiation (Leventhal et al., 1999). The notion that a vascular niche might provide a compartment for stem cells to expand and differentiate was bolstered by in vivo observations. One study showed that neurogenesis occurred within close vicinity to blood vessels in the adult brain (Palmer et al., 2000), whereas another showed that brain-derived neurotrophic factor (BDNF) was secreted by ECs in a seasonal manner in the brains of song birds, stimulating coordinated neurogenesis and angiogenesis in the higher vocal center (Louissaint et al., 2002). Similarly, pigment epithelium-derived factor (PEDF) was shown to be secreted by ECs and ependymal cells in the SVZ and to promote self-renewal of adult NSCs in vitro (Ramirez-Castillejo et al., 2006). In 2008, two studies characterized and quantified the NSC-vascular interface, and showed that soluble circulating factors crossed the blood-brain barrier where ECs directly contacted NSCs (Tavazoie et al., 2008). Furthermore, NSCs were shown to adhere to ECs via the laminin receptor α6β1 integrin, which directed both NSC proliferation and organization (Shen et al., 2008). Building upon these findings, it was demonstrated that homing of activated NSCs occurs via vessel-derived stromal-derived factor 1 (SDF1; CXCL12 – Mouse Genome Informatics) signaling to CXC chemokine receptor 4 (CXCR4), which upregulates α6 integrin and epidermal growth factor receptor in NSCs, enhancing their association with the vascular niche (Kokovay et al., 2010). This work identified different niche microenvironments, with a ‘quiescent’ compartment composed of ependymal cells that support stem cell self-renewal and an ‘activated’ compartment composed of vascular endothelium, that engage early neural progenitor cells (NPCs) as they initiate differentiation. In addition to playing a role in sustaining stem cells or ‘kick-starting’ early progenitors, the vascular niche has also been implicated in SVZ regeneration. Following pharmacological elimination of transit amplifying cells, progenitors were observed to proliferate rapidly in direct association with SVZ blood vessels (Tavazoie et al., 2008). Together, this growing body of work demonstrates that, in the adult brain, blood vessels nurture and guide neuronal stem and progenitor cells.
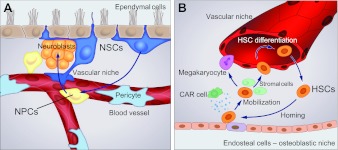
Fig. 2.
Vascular niches in neural and hematopoietic tissues. (A) Neural stem cells (NSCs, dark blue) associate with both ependymal (brown) and endothelial (red) cells as they transition into transit amplifying cells (neural progenitor cells, NPCs, yellow), become neuroblasts (orange) and eventually differentiate into neurons. (B) Hematopoietic stem cell (HSC) mobilization and homing occur in coupled osteoblastic and vascular niches. In response to changes in the levels of stromal-derived factor 1 (SDF1) secreted by stromal CXCL12-abundant reticular (CAR) cells (green), HSCs (orange) leave the bone microniche (osteoblastic niche) where they remain quiescent, passage through the stromal environment and enter the vascular niche, where they proliferate and further differentiate.
Similarly, blood vessel endothelium provides a niche essential for the maintenance of hematopoietic stem cells (HSCs), which ensures blood cell homeostasis (Calvi et al., 2003; Zhang et al., 2003; Sugiyama et al., 2006). Suppression of hematopoeisis and depletion of HSCs, such as that caused by chemotherapy or radiation, are rapidly reversed by HSC expansion within an ‘instructive’ bone marrow niche. In this specialized microenvironment, osteoblasts keep HSCs quiescent, while bone marrow sinusoidal ECs (SECs) drive the regeneration and replenishment of the HSC population (Hooper et al., 2009) (Fig. 2B). It has been proposed that CXCR4-SDF1 signaling regulates HSC shuttling between ‘microniches’, driving either mobilization or homing (Broxmeyer et al., 2005). Bone marrow SECs also provide a cellular platform for the differentiation of megakaryocytes (Avecilla et al., 2004). It has been proposed that SECs regulate HSC differentiation via specific endothelial-derived paracrine trophogens termed ‘angiocrine factors’, which include HGF, Wnt2 and Notch ligands (Butler et al., 2010b; Ding et al., 2010). ECs can also support the maintenance of HSCs in culture, and normal EC function is required for hematopoiesis in vivo (Li et al., 2004; Yao et al., 2005). Unexpectedly, the activation state of SECs non-autonomously influences adjacent cells and determines the balance between HSC self-renewal versus differentiation; Akt-activated SECs drive expansion of HSCs with a long-term repopulating potential, whereas mitogen-activated protein kinase-activated SECs promote differentiation of blood cells (Kobayashi et al., 2010). Therefore, the same ECs can provide different instructions, depending on their own plastic conditions. Bone marrow SECs, therefore, provide yet another example of how blood vessels can actively communicate with neighboring cells.
Indirect evidence suggests the involvement of blood vessels in stem/progenitor cell dynamics in additional tissues. In the testis, for example, undifferentiated spermatogonia were found to be tightly associated with blood vessels (Yoshida et al., 2007), and similarly white fat progenitor cells are associated with the adipose vasculature (Tang et al., 2008). Thus, signals from blood vessels appear poised to play a conserved role in the maintenance of stem/progenitor cells, although the underlying molecular mechanisms remain largely unknown.
There are, however, exceptions to this association of resident tissue stem cells with a vascular niche. For example, intestinal stem cells appear to be maintained by a simple niche composed of Paneth cells, which does not involve blood vessels (Sato et al., 2011). Similarly, stem cell proliferation and differentiation in the skin and hair follicle have not been shown to require blood vessel involvement. The reason for the differential use of a vascular niche in different organs is not known.
The pancreas and its constituent cell types
Blood vessels have also been demonstrated to communicate with the developing pancreas. However, before assessing possible roles for vessels during islet development or the potential existence of vascular niches in the pancreas, we briefly describe below the key processes and cell types involved in pancreas development; for more detailed accounts, we refer readers to a number of recent excellent reviews (Kim and MacDonald, 2002; Jorgensen et al., 2007; Murtaugh, 2007; Gittes, 2009; Pan and Wright, 2011).
The pancreas is a glandular organ that carries out two major functions: exocrine and endocrine. The exocrine pancreas, constituting almost 99% of the total mass of the organ, comprises a ramifying tubular tree of ductal branches, which drain the digestive enzymes secreted by acinar cells to the duodenum (Bonal and Herrera, 2008). The endocrine pancreas comprises the islets of Langerhans, clusters of 100-1000 hormone-secreting cells scattered throughout the exocrine tissue and interconnected via blood vessels. The primary function of islets is to maintain metabolic homeostasis through the production of hormones that regulate blood glucose levels. Five primary endocrine cell types, each responsible for secreting a particular hormone, are found in islets: α-cells (glucagon); β-cells (insulin); δ-cells (somatostatin); ε-cells (ghrelin); and PP-cells (pancreatic polypeptide) (Collombat et al., 2006; Oliver-Krasinski and Stoffers, 2008; Cleaver and MacDonald, 2009). Pancreatic β-cells are destroyed by the immune system in type 1 diabetes and are dysfunctional in type 2 diabetes, and they are thus the long sought after product of intense efforts aimed at replacement therapies for diabetics using in vitro-directed differentiation.
Ontogeny of the β-cell
Given the potential therapeutic applications, it is crucial to understand both the origins of the β-cell and its step-wise differentiation. Both endocrine and exocrine lineages arise from embryonic endodermal epithelium (Fig. 3), which expresses the proteins pancreatic and duodenal homeobox box 1 (PDX1) and pancreas-specific transcription factor 1 (PTF1A) (Offield et al., 1996; Kawaguchi et al., 2002; Gu et al., 2003). This early epithelium undergoes rather dramatic morphogenetic events (Hick et al., 2009; Kesavan et al., 2009; Villasenor et al., 2010), rapidly transforming from a primitive monolayered epithelial sheet to a multilayered stratified epithelial bud and then resolving back to a monolayer as it ramifies into a tubular tree. Branches emerge by remodeling of this epithelium, rather than by classical extension of buds. During morphogenesis, the epithelium segregates into distinct ‘tip’ and ‘trunk’ domains. Cells located in early epithelial ‘tips’ are rapidly proliferating multipotent progenitor cells (MPCs), which fuel branch extension. These progenitors express carboxypeptidase A1 (CPA1), PTF1A and Myc, and have been shown by lineage tracing to give rise to acinar, duct and endocrine cells in mouse until about E14.5, at which point fate potential becomes restricted (Zhou et al., 2007). MPCs are thought to be set aside within the epithelium early during development, between E8.5 and 11.5, with their number at that time ultimately determining the final size of the mature pancreas (Stanger et al., 2007).
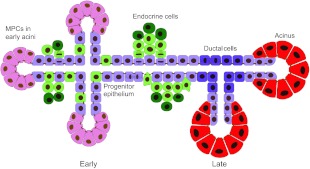
Fig. 3.
The pancreatic progenitor epithelium gives rise to endocrine, exocrine and ductal lineages. Schematic of early (left) and late (right) pancreatic epithelium. Multipotent progenitor cells (MPCs, pink) at epithelial tips proliferate to give rise to more MPC-bearing tips, as well as bipotent ‘trunk’ epithelium (light purple). The latter generates delaminating endocrine cells (green) and differentiates into ductal epithelium (dark purple) following the secondary transition. At this time, MPC potential becomes restricted and branch tips terminally differentiate into acinar cells (red), which form clusters called acini.
During development of the pancreatic epithelium, endocrine cells emerge in two waves of differentiation as they exit the tubular epithelium, differentiate, migrate, coalesce to form islets and proliferate. In mouse, the first wave of endocrine differentiation begins around embryonic day 9.5 (E9.5), after initial budding of both dorsal and ventral pancreatic buds. These ‘early’ endocrine cells are mostly glucagon-expressing α-cells that often co-express multiple hormones and are probably eliminated without significant contribution to adult islets (Herrera, 2000). Following these early events, the epithelium undergoes a massive wave of expansion and differentiation called the ‘secondary transition’, starting around E12.5. Scattered cells located within the ‘trunk’ domain of the ductal epithelium individually take on an endocrine fate (marked by transient expression of neurogenin 3), delaminate and form endocrine cell clusters, while acinar cells at branch tips actively proliferate and increase in size as they accumulate digestive enzyme.
The intrinsic molecular machinery that orchestrates differentiation decisions during the secondary transition is under intense investigation, but it is clear that lateral interactions between epithelial cells, mediated via Delta-Notch signaling, are central to the control of endocrine/exocrine specification (Apelqvist et al., 1999; Jensen et al., 2000; Murtaugh et al., 2003). Secondary wave endocrine cells primarily differentiate into β-cells, along with other endocrine lineages, delaminate from the epithelium and coalesce into small islet-like clusters that progressively form larger islet aggregates during postnatal development. Cell-cell interactions both between β-cells, and between β-cells and ECs are important for islet development and insulin secretion (Nikolova et al., 2006; Parnaud et al., 2011). Interactions between β-cells within islets, occurring via EphA-ephrin A signaling, were shown to reduce insulin secretion under conditions of low glucose and to enhance secretion when blood glucose levels increased (Konstantinova et al., 2007). Thus, the clustering of β-cells in islets serves to improve glucose homeostasis compared with what could be achieved with isolated β-cells.
In adults, the formation of new β-cells gradually ceases, but can be induced under conditions of increased metabolic demand, such as insulin resistance. The extent of β-cell mass plasticity in humans and the reason for the age-related decline in β-cell formation are not clear (Butler et al., 2007; Meier et al., 2008). Although the cellular origins of new β-cells during adult life remain a hotly debated issue, most evidence suggests that rare duplication of pre-existing differentiated β-cells, rather than differentiation of stem or progenitor cells, is the predominant mechanism (Dor et al., 2004; Brennand et al., 2007; Xu et al., 2008; Solar et al., 2009). Unraveling the cell intrinsic versus extrinsic mechanisms that drive β-cell generation, either during development, homeostasis or disease, remains a major and immediate challenge of the field.
Cellular neighbors of the progenitor epithelium
Growth of the pancreatic epithelium, and differentiation of endocrine cells, has long been known to depend on paracrine signals from surrounding mesoderm (Golosow and Grobstein, 1962; Pictet et al., 1972; Gittes et al., 1996). The pancreatic epithelium is sheathed in layers of mesenchyme throughout its development, constituting a greater proportion of the anlagen early on, but becoming less conspicuous and thinner with time. Within that mesenchyme reside a variety of cell types, including neurons, smooth muscle, stroma and ECs. Although roles for many of these cell types remain unclear, the mesenchyme as a whole has long been known to be strictly required not only for epithelial survival, but also for epithelial cell fate choices (Golosow and Grobstein, 1962; Gittes et al., 1996; Miralles et al., 1998). Our understanding of the early mesenchyme has been shaped by decades of classical embryological recombination and explant experiments (Golosow and Grobstein, 1962; Duvillie et al., 2006), as well as by more recent elegant approaches (Landsman et al., 2011). However efforts to decipher potential differential impacts of the different cell types therein still lag. Although the vasculature pervades the early mesenchyme and is intimately woven around the epithelium as it grows and branches, efforts to elucidate signaling between these two tissues continue to this day.
Coordinated growth of vasculature and pancreatic epithelium
The pancreatic epithelial tree grows in coordination with its associated blood vessels, forming a close, lasting and active interface (Fig. 4). Understanding the communication that occurs at this interface, between blood vessel endothelium and the epithelium that gives rise to exocrine and endocrine cells, is likely to elucidate important extrinsic signals required to coax and/or corral β-cell fate, and has therefore become the object of great interest.
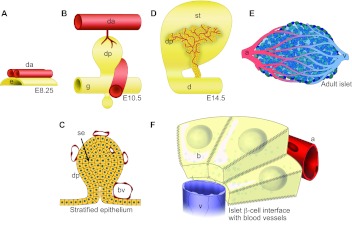
Fig. 4.
Blood vessel and pancreatic tissues interface throughout development and into adulthood. (A,B,D) The developing pancreatic buds and neighboring blood vessels. In A and B, anterior is leftwards, posterior is rightwards. (A) The paired dorsal aortae (red) of mammalian embryos contact the pre-pancreatic endoderm (yellow) during specification, during embryonic day 8. Aortic endothelium has been shown to be required for endocrine specification, PTF1A expression and bud outgrowth. (B) As the pancreatic bud grows, the dorsal aortae fuse into a single vessel. Mesenchyme accumulates around the bud epithelium and displaces the aorta further dorsally. Within the bud mesenchyme, a capillary plexus forms, surrounding the pancreatic epithelium, whereas the portal vein develops and wraps the posterior aspect of the bud. (C) During stratification of the pancreatic progenitor epithelium, it remains strictly avascular. Subsequent vascularization of the bud is coordinated with de-stratification. (D) During pancreatic outgrowth, blood vessels pervade the branching organ, with vessels intercalating among developing branches. There is a particularly high density of blood vessels along the core of the bud, whereas more peripheral acinar-rich regions display lower vascularity. (E) Blood vessels course through islets, forming dense networks of capillaries, with each β-cell contacting more than one vessel. (F) Islet beta cells (yellow) are organized in rosettes around islet capillaries, into which they secrete endocrine hormones (via their lateral surfaces, which are covered with microvilli). a, artery; b, β-cell; bv, blood vessel; d, duodenum; da, dorsal aorta; dp, dorsal pancreas; e, endoderm; g, gut tube; se, stratified epithelium; st, stomach; v, vein.
Although the primary function of the circulatory system in the pancreas is the exchange of nutrients, wastes and gases, additional roles for blood vessels during pancreas development and tissue homeostasis have been discovered in recent years, paralleling discoveries in other organs (as outlined above). The finding that blood vessels may provide non-nutritional cues to the developing and adult pancreas raises questions about when, where and how this signaling takes place? Do blood vessels play a single or multiple roles, at one or more timepoints, during β-cell ontogeny? What is the nature of those signals and what is their impact on pancreatic cells? It is not inconceivable that vessels might provide varying signals as their relationship to pancreatic tissue changes over time. Vessels interface differently during initial pancreas specification, during epithelial stratification or de-stratification, during outgrowth of the pancreatic epithelial tree, during endocrine cell migration and coalescence into islets, and during mature β-cell function. In addition, it is also likely that the underlying competence of pancreatic cells to receive EC signals changes dramatically along their developmental journey.
A decade of work on the contribution of vascular paracrine signals to pancreas and β-cell development has been carried out. Understanding and integrating the range of seemingly ‘positive’ and ‘negative’ signals that have been reported is crucial to help move the field forward. In this review, we group blood vessel interactions with pancreatic cells as ‘vessel-derived’ signals; however, a range of vascular signals has been identified, including those derived from endothelium, smooth muscle, vessel ECM and blood-borne plasma or gaseous signals.
Blood vessel signals during pancreatic specification
In 2001, embryonic aortic ECs were shown to play a role during pancreas specification. Between E8.75 and E9.5 in mouse, the fusing aortae are located in immediate contact with the dorsal pancreatic bud (Fig. 4A). Induction of PDX1 and insulin expression in endoderm was shown to depend on signals from ECs in recombination assays using cultured E8.25 isolated embryonic tissues (Lammert et al., 2001). In addition, when the aorta was prevented from forming in frog embryos, β-cells failed to differentiate. Both these experiments suggested an early role for aorta-derived signals during specification of the pancreatic field. Later studies showed that endothelial signals were required for PDX1 maintenance, the induction of PFT1A and dorsal pancreas budding (Yoshitomi and Zaret, 2004), involving a relay pathway in which ECs support survival of FGF10-producing mesenchyme (Jacquemin et al., 2006). Together, these data suggested that the early dorsal aorta, either directly or indirectly, is required for proper specification and subsequent development of the pancreas.
One recent study supported the idea that vascular signals initiated pancreas development but suggested that these signals, at least in the avian system, occurred even earlier (Katsumoto and Kume, 2011). In chick, the pancreatic endoderm was found to attract endothelial progenitor cells (angioblasts) via expression of the secreted chemokine ligand CXCL12/SDF1. Prior to vessel formation, free angioblasts respond via the receptor CXCR4 and migrate into the pre-stomach/pancreas region. Endodermal cells in immediate contact with LIM domain only 2 (LMO2)-expressing angioblasts then initiate expression of PDX1. Authors of the study found that CXCL12 overexpression attracted excess angioblasts and resulted in both an expanded and precocious PDX1-expressing endodermal domain. Conversely, blocking CXCR4 signaling with the inhibitor AMD3100 resulted in disrupted angioblast migration and reduced Pdx1 expression. Although it is suggested that angioblasts might induce PDX1 in a paracrine manner via retinoic acid, as studies in both zebrafish and chicks have proposed (Kumar et al., 2003; Kinkel et al., 2009), the exact nature of the vascular signal in question still remains elusive.
One proposed blood vessel signal required for pancreatic growth is the signaling phospholipid sphingosine-1-phosphate (S1P) (Edsbagge et al., 2005). Whereas earlier studies implicated endothelial factors in pancreas specification, S1P is known to be produced by platelets and to be abundant in blood plasma, suggesting that more than the walls of vessels might be communicating with underlying endoderm. Using N-cadherin null (Cdh2–/–) mouse embryos, which lack both a functional circulatory system and a dorsal pancreas, authors of the study restored circulation via heart-specific expression of N-cadherin and observed a concomitant rescue of pancreas development. These findings underlined how crucial a functional aorta was to the growth of the pancreas. Based on these findings, circulating factors were postulated to be responsible for the rescue. Cultured Cdh2–/– foreguts were therefore treated with either plasma or S1P-soaked beads and pancreas growth restored, presumably via proliferation/survival of pancreatic mesenchyme. S1P thus exhibited the expected traits of a pancreas-promoting blood vessel/plasma-derived molecule. This work confirmed the idea that vessels contributed signals to organ development, but also brought up the possibility that not only endothelium, but also the blood it carries, might constitute sources of signals.
Another proposed blood-borne signal for β-cell differentiation is oxygen (Shah et al., 2011). In this study, authors make the observation that most vessels of the early budding pancreas are not perfused with blood flow, and therefore pancreatic cells therein develop in a hypoxic environment. However, around E14.5-15.0, vessels undergo a relatively sudden perfusion that is coordinated with the rapid proliferative expansion of the second transition. By carrying out intracardiac FITC-tomato lectin injections and monitoring oxidized thiols (oxygenated areas), the authors found a correlation between blood-perfused vessels and differentiated endocrine cells. In support of a positive role for oxygen on endocrine differentiation, ex vivo culture of pancreas rudiments in hypoxic conditions (mimicking the early uterine environment and unperfused state of the budding pancreas) resulted in normal epithelial cell proliferation, but a lack of β-cell differentiation.
In summary, there are several interesting candidates for blood-borne signals that control early pancreas development. However, much more remains to be achieved on this front, in particular with regard to non-nutritional, perfusion-independent signals that control early pancreas formation, such as those originally observed in explant studies (Lammert et al., 2001; Yoshitomi and Zaret, 2004). We still do not know whether the key endothelial signal(s) are secreted or membrane bound, if they are produced equally by ECs from all tissues or just by the ECs that contact the pancreas, and if they function in concert with blood-borne or other local signals from resident cells. Importantly, we still do not fully know the molecular nature of these signals.
Developmental crosstalk between branching pancreatic epithelium and blood vessels
Although early studies showed that blood vessels were required for pancreas specification, more recent work has demonstrated that, surprisingly, they act to restrain outgrowth and morphogenesis of the pancreatic epithelium at later developmental stages. A recent study characterized the distribution of vessels around emerging pancreatic branches and observed a higher density of vessels in central (unbranched) epithelial regions than around distal tips (Pierreux et al., 2010). Higher EC density correlated with high VEGFA expression in proximal epithelial trunks, whereas lower VEGFA expression was observed in epithelial tips. When VEGFA was ablated in mice using a PDX1-Cre driver line, the authors were surprised to observe an increase in the formation of epithelial tips expressing the progenitor marker Cpa1. Similarly, inhibition of vessel development using the VEGF-blocking drug SU5416 resulted in rapid increase in the number of CPA1+ and PTF1A+ tip cells, and upregulation of the exocrine program. Conversely, forced hypervascularization of the developing pancreas using transgenic overexpression of VEGFA in the pancreas resulted in severe downregulation of exocrine differentiation. Together, these data showed that reciprocal signaling results in recruitment of blood vessels via VEGFA to trunk epithelium, and that this endothelium signals back to control acinar cell differentiation. Moreover, this work provocatively suggests that the microenvironmental positioning of ECs relative to pancreatic epithelium controls the fine spatial pattern of acinar differentiation and outgrowth.
Similar observations were made by the Semb group during the course of investigation of the role of S1P signaling during endocrine cell differentiation. Following up on studies showing a requirement for a functional vascular system and aortic S1P in dorsal pancreas development (Edsbagge et al., 2005), the pancreatic bud was examined in mice lacking the function of the S1P receptor (S1P1) (Sand et al., 2011). Because these mutant mice died prior to appreciable pancreatic growth, pancreatic buds were grown in culture to circumvent lethality issues. As expected, the pancreas of these mice exhibited a significant reduction in organ size, whereas endocrine cell mass was relatively unaffected. As the initial idea held that blood vessels (plasma or ECs) were likely to be relaying inductive signals necessary for bud expansion via the S1P1 receptor, the authors used a potent endothelial-ablating agent, quinolin-urea (Ilovich et al., 2008), to assess the role of blood vessels in the growing bud. Their expectation had been that vessels would be needed for pancreas growth; however, to their surprise, expansion and hyperbranching of the pancreatic epithelium was observed, similar to the previous study. Again, contrary to expectation, they found that S1P1-null embryos in fact developed hypervascularized tissues, including the pancreas, suggesting that blood vessels were suppressing epithelial expansion during organogenesis.
Finally, we have recently found, using both genetic and pharmacological tools, that blood vessels restrain pancreas branching, growth and differentiation to both endocrine and exocrine fates (Magenheim et al., 2011). Forced pancreas hypervascularization using VEGFA overexpression resulted in reduced branching and growth, and deficient formation of acinar and endocrine cells; this happened both in vivo and in explants, indicative of paracrine signaling rather than a perfusion-dependent effect. In reciprocal vascular ablation experiments, we found increased branching and growth and excessive differentiation to both endocrine and exocrine fates (Magenheim et al., 2011).
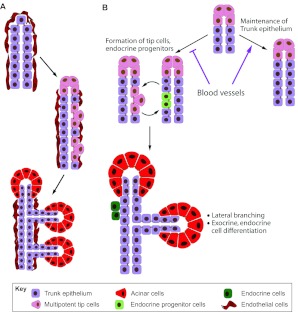
These recent studies strongly argue for a surprising restrictive effect of blood vessels on pancreas branching, growth and differentiation. How can this effect be reconciled with the earlier studies suggesting a positive role of blood vessels in pancreas development, and with the intuitive view of blood vessels as supportive of organ growth? We speculate that blood vessels act to enhance the maintenance of an early, simple and ‘proto-differentiated’ epithelium, perhaps equivalent to their positive effect on self-renewal of stem cells in other systems. The maintenance of this simple epithelial phenotype probably suppresses its evolving 3D architecture and occurs at the expense of alternative choices such as branching and differentiation (Fig. 5). In the context of the early pancreas, the net effect might be expansion of the pool of stem cells from which the organ will develop; in the late-developing pancreas, the same effect of ECs may manifest as reduced branching and growth. Alternatively, the dramatically different consequences of vascular manipulations at different developmental stages may reflect a change in the identity of the paracrine signals produced by ECs. In other words, at different developmental stages the pancreas may receive different EC signals or interpret them differently. At present, these possibilities are yet to be resolved.
Fig. 5.
Blood vessel signals restrain branching and differentiation of the late-developing pancreas. (A) Blood vessels surround the progenitor epithelium prior to tip formation and endocrine/exocrine differentiation. As the multipotent progenitor cell-laden tips develop, they avoid the vascular-rich environment that surrounds the central epithelium, branching out into the surrounding mesenchyme. Acini subsequently display lower vascular density than the central epithelium of the pancreatic tree. (B) Both genetic and explant data identify perfusion-independent signals from the vascular endothelium that inhibit pancreas branching, reduce differentiation into both endocrine and acinar fates, and eventually act to restrict organ size. The endothelium appears to act by interfering with Delta-Notch signaling within the epithelium. We hypothesize that blood vessels promote self-renewal of proto-differentiated trunk epithelium, at the expense of tip formation (branching) and endocrine/exocrine differentiation. Cell types are indicated in the key.
The underlying molecular mechanisms of these proposed functions of blood vessels remain unknown. Preliminary evidence suggests that the effects of blood vessels in this context might be transduced via modulation of Delta-Notch signaling within the epithelium (Pierreux et al., 2010; Magenheim et al., 2011). Identifying and rigorously characterizing this signaling interaction is an important future challenge for the field.
Crosstalk between adult islet endocrine and endothelial cells
In addition to the signals supplied to developing pancreatic β-cells, blood vessels have also been shown to communicate with mature islet endocrine cells. Pancreatic β-cells directly communicate with fenestrated capillaries that pervade the islets at high density (Bonner-Weir and Orci, 1982). This unusual density of blood vessels in islets probably results from constitutive expression of VEGFA by β-cells (Christofori et al., 1995), and presumably serves to ensure efficient communication between the blood and β-cells, for sensing glucose levels and secretion of insulin. β-Cells organize in rosettes of 8-10 cells around a central capillary, clustering their secretory granules towards a vascular lumen, suggesting that vessel endothelium may play a role in establishing endocrine cell polarity (Bonner-Weir, 1988). Deletion of VEGFA in β-cells results in a 90% reduction in capillary density in islets and defects in blood glucose homeostasis, underlining the continued importance of blood vessels in the pancreas (Lammert et al., 2003).
The true paracrine nature of the communication between islet endocrine cells and blood vessels was demonstrated when it was shown that, at least in mouse, islet capillaries supply β-cells with a vascular basement membrane necessary for proper insulin gene expression, as well as survival and proliferative signals (Nikolova et al., 2006). These signals were shown to be transmitted via laminin within the vascular basement membrane, which communicates directly with β1-integrin on β-cells. Similarly, vascular collagen IV was shown to signal via β-cell α1β1 integrin in vitro, enhancing both endocrine cell motility and secretory efficiency (Kaido et al., 2004). In another study of pancreas expansion during pregnancy, islet capillaries were shown to proliferate and express high levels HGF shortly prior to β-cell proliferation, further demonstrating a tangible candidate paracrine cue, suggesting a positive correlation (Johansson et al., 2006).
Together, these results demonstrate the necessary and reciprocal crosstalk that exists between pancreatic β-cells and blood vessels, and suggest the existence of a vascular niche for β-cell survival and function, from their emergence in the progenitor epithelium to their differentiated state in islets. We speculate that blood vessels may have yet additional and unexplored roles in β-cell biology; for example, in shaping the 3D architecture of the pancreatic epithelium, in promoting the developmental coalescence of endocrine cells to form islets (isletogenesis), and in the maintenance of proper islet architecture (e.g. β-cell polarity).
What are the EC signals?
As described in this review, not only have blood vessel signals been identified in a number of tissues over the past 10 years, but ECs have proved beneficial for islet cultures, improving their function and survival (Pan et al., 2011). The nature of their biological effect on host organs is only beginning to be elucidated, and it is possible that the complex phenotypes observed represent an underlying universal principle of the endothelial-tissue interaction, e.g. enhancement of self-renewal. Alternatively, they could represent a more mundane series of tissue- and time-specific communications tailored to local needs. Although progress is being made in understanding the downstream consequences of blood vessel signals, we largely remain ignorant of the nature (e.g. secreted or membrane-bound, acting directly or via a relay) and the molecular identity of endothelial signals, including those within the pancreas.
Given the known regional heterogeneity of the vasculature and its temporally changing character (Gerritsen, 1987; Cleaver and Melton, 2003; Aird, 2007), it is reasonable to speculate that blood vessels provide regionally and temporally distinct signals in different tissues at different times. Indeed, vascular paracrine signals may be as dynamic as the changing vasculature itself. An equally plausible alternative is that the vasculature provides a single homogeneous signal, which is interpreted differently by different tissues (Zaret, 2006; Nikolova et al., 2007; Zaret and Grompe, 2008; Pierreux et al., 2010). It is also possible that at certain developmental timepoints or certain contexts, blood vessels do not provide required signals. For example, in mouse, vascular laminin acts as a signal to β-cells; however, in human islets, β-cells generate their own extracellular matrix to create a ‘double’ basement membrane (Otonkoski et al., 2008; Virtanen et al., 2008), raising the possibility that, here, blood vessel signals are supplanted by analogous signals from adjacent β-cells. Elucidating the spatiotemporal requirements for true, non-nutritional signals provided by the vasculature to growing tissues will therefore without a doubt prove to be a complicated venture.
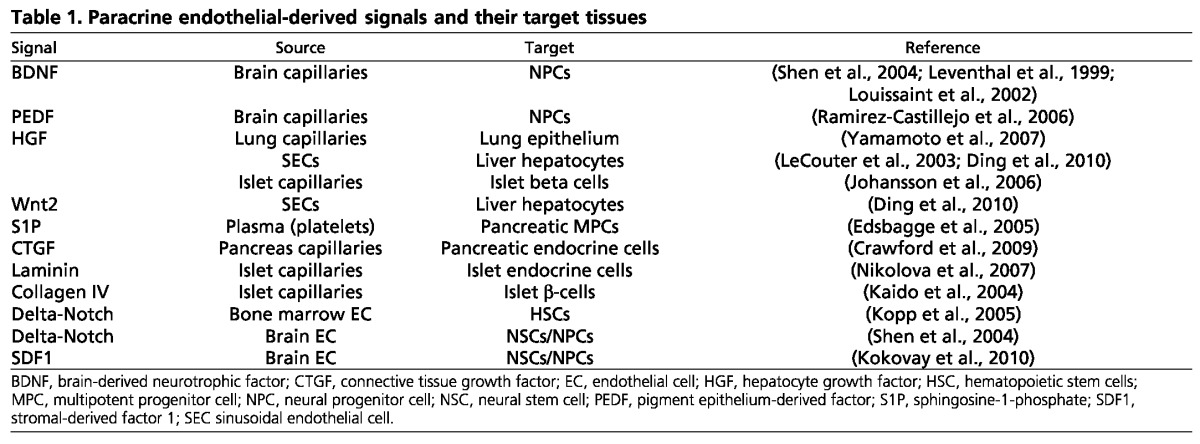
Despite our general lack of understanding of blood vessel signals, a few candidates have been identified (Table 1). What is striking about this list is the variety it represents, both in terms of candidate molecules and their target cell types. Although some have been suggested to constitute circulating signals from blood, such as blood-borne S1P or oxygen, which influence developing β-cells (Edsbagge et al., 2005; Shah et al., 2011), others have been suggested to constitute ECM or derivatives from the vascular basement membrane known as ‘fractones’, which influence mature β-cells or neural progenitors, respectively (Nikolova et al., 2006; Tavazoie et al., 2008). Although many candidates listed here are highly enriched or restricted in endothelium, others, such as connective tissue growth factor are expressed both in pancreatic epithelium and endocrine cells, making their respective signaling contributions more difficult to tease apart (Crawford et al., 2009). It is likely that this list will continue to grow, as methods are developed to improve our ability to separate true paracrine, from nutritional, vascular signals.
Table 1.
Paracrine endothelial-derived signals and their target tissues
β-Cells, diabetes and replacement therapies
The consideration given to putative endothelial signals has become increasingly crucial, given the growing efforts to develop replacement therapies for various diseases, such as diabetes. We put forth that understanding and controlling the step-wise differentiation of pancreatic β-cells from stem cells will require a deeper understanding of the developmental interplay between pancreatic progenitor cells and all their cellular neighbors, including EC. In vitro-directed differentiation of naïve cells, such as embryonic stem cells (ES cells) or induced pluripotent stem cells, towards glucose-responsive, insulin-producing β-cells have garnered much attention; however, these efforts have been relatively limited in their success. Although ES cells have been differentiated successfully into definitive endoderm and to a lesser extent into pancreatic progenitors, the generation of mature β-cells from naïve cells remains a major challenge for the field (D’Amour et al., 2006; Kelly et al., 2011). Interestingly, it has been reported that ES cell-derived pancreatic progenitor cells most efficiently mature into β-cells when grafted to mice (Kroon et al., 2008). The reasons for this necessary in vivo ‘incubation’ are unknown. Signals from blood vessels, absent in vitro but abundant in vivo, are tantalizing candidates for this missing link.
On a different front, the roles of blood vessels in β-cell regeneration remain unexplored, largely because regeneration can be studied only in vivo, where it is difficult to uncouple the perfusion function of blood vessels from bona fide paracrine EC signals. Nonetheless, elucidation of the function of EC in β-cell regeneration is an exciting future challenge, given supportive evidence for the role of ECs in liver regeneration (LeCouter et al., 2003).
Finally, a key area in diabetes therapy where blood vessels might serve an underappreciated role is islet transplantation, which has become an effective method of treatment for type 1 diabetes (Robertson, 2010). Importantly, upon preparation of cadaveric islets for transplantation, most associated capillaries are lost. The inherent delay in revascularization of implanted islets is about 1-2 weeks, during which time survival of islets plummets (Mattsson et al., 2002). One proposal is that enhancement of vascularization might be accomplished by manipulation of VEGFA levels, and indeed there have been several examples of beneficial effects of increasing VEGFA at the site of transplantation. However, studies increasing VEGFA within islets have shown that high levels can severely disrupt islets (Lammert et al., 2001), underscoring the importance of controlling therapeutically applied factors to physiological levels. Understanding the delicate balance between blood vessels and pancreatic β-cells, establishing when and where signals are exchanged between them, and elucidating the effects of these signals, together constitute an important challenge to developing useful therapies for type 1 diabetes. We propose that non-nutritional functions of ECs may also contribute to islet survival in this setting.
Conclusions
Growing evidence suggests that blood vessels are essential signaling centers during organogenesis and tissue homeostasis. It is incumbent upon us to decipher their importance. When, where and how do they instruct, support or restrain β-cells or their progenitors? Given their obligatory pervasion of tissues, blood vessels are ideally positioned to provide fine-tuned and localized signals to their host tissues, ensuring proper coordination of growth, morphogenesis and differentiation. They may also represent a bridge between local tissue development and the whole organism. Blood vessels could also play a key role in organ size control, by acting as sensors and relaying global cues to local environments. The emerging view of EC-host signaling is one of increasing complexity, providing a new appreciation for the temporal and spatial diversity that occurs at their interface. We propose that, in many cases, blood vessels may play similar roles in different tissues, including a potential key role during tissue ‘self-renewal’. In different tissues, molecular mediators of EC signals might be different, and the apparent phenotype might appear contradictory (e.g. pancreas early versus late signals), where they in fact embody the same basic principle. We argue that a deep understanding of the molecular nature and the precise dynamics of the EC-pancreas developmental relationships will be essential for the development of effective cell or regenerative therapies for diabetes.
Acknowledgments
O.C. and Y.D. collaboratively conceived and wrote the text of this review. We thank our colleagues for open and useful discussions on this subject, including, but not limited to, Ray MacDonald, Al Powers and Chris Wright.
Footnotes
Funding
The authors research was supported by grants from the Juvenile Diabetes Research Foundation (Y.D. and O.C.); the Helmsley Foundation; European Union Seventh Framework Programme, ERC starting grant and the Dutch Friends of Hebrew University (Y.D.); and the National Institutes of Health and Basil O’Connor March of Dimes Award (O.C.). Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
References
- Aird W. C. (2007). Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 100, 158–173 [DOI] [PubMed] [Google Scholar]
- Akeson A. L., Greenberg J. M., Cameron J. E., Thompson F. Y., Brooks S. K., Wiginton D., Whitsett J. A. (2003). Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev. Biol. 264, 443–455 [DOI] [PubMed] [Google Scholar]
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400, 877–881 [DOI] [PubMed] [Google Scholar]
- Avecilla S. T., Hattori K., Heissig B., Tejada R., Liao F., Shido K., Jin D. K., Dias S., Zhang F., Hartman T. E., et al. (2004). Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10, 64–71 [DOI] [PubMed] [Google Scholar]
- Bonal C., Herrera P. L. (2008). Genes controlling pancreas ontogeny. Int. J. Dev. Biol. 52, 823–835 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. (1988). Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes 37, 616–621 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Orci L. (1982). New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31, 883–889 [DOI] [PubMed] [Google Scholar]
- Brennand K., Huangfu D., Melton D. (2007). All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 5, e163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Orschell C. M., Clapp D. W., Hangoc G., Cooper S., Plett P. A., Liles W. C., Li X., Graham-Evans B., Campbell T. B., et al. (2005). Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 201, 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. C., Meier J. J., Butler A. E., Bhushan A. (2007). The replication of beta cells in normal physiology, in disease and for therapy. Nature clinical practice. Endocrinol. Metab. 3, 758–768 [DOI] [PubMed] [Google Scholar]
- Butler J. M., Kobayashi H., Rafii S. (2010a). Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 10, 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. M., Nolan D. J., Vertes E. L., Varnum-Finney B., Kobayashi H., Hooper A. T., Seandel M., Shido K., White I. A., Kobayashi M., et al. (2010b). Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi L. M., Adams G. B., Weibrecht K. W., Weber J. M., Olson D. P., Knight M. C., Martin R. P., Schipani E., Divieti P., Bringhurst F. R., et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 [DOI] [PubMed] [Google Scholar]
- Christofori G., Naik P., Hanahan D. (1995). Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol. Endocrinol. 9, 1760–1770 [DOI] [PubMed] [Google Scholar]
- Cleaver O., MacDonald R. J. (2009). Developmental molecular biology of the pancreas. In Handbook of Pancreatic Cancer (ed. Neoptolemos J., Abbruzzese J., Buchler M., Urrutia R.). New York, NY: Springer; [Google Scholar]
- Cleaver O., Melton D. A. (2003). Endothelial signaling during development. Nat. Med. 9, 661–668 [DOI] [PubMed] [Google Scholar]
- Collombat P., Hecksher-Sorensen J., Serup P., Mansouri A. (2006). Specifying pancreatic endocrine cell fates. Mech. Dev. 123, 501–512 [DOI] [PubMed] [Google Scholar]
- Compernolle V., Brusselmans K., Acker T., Hoet P., Tjwa M., Beck H., Plaisance S., Dor Y., Keshet E., Lupu F., et al. (2002). Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 8, 702–710 [DOI] [PubMed] [Google Scholar]
- Crawford L. A., Guney M. A., Oh Y. A., Deyoung R. A., Valenzuela D. M., Murphy A. J., Yancopoulos G. D., Lyons K. M., Brigstock D. R., Economides A., et al. (2009). Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol. Endocrinol. 23, 324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E., Nico B., Ribatti D. (2007). Contribution of endothelial cells to organogenesis: a modern reappraisal of an old Aristotelian concept. J. Anat. 211, 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour K. A., Bang A. G., Eliazer S., Kelly O. G., Agulnick A. D., Smart N. G., Moorman M. A., Kroon E., Carpenter M. K., Baetge E. E. (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 [DOI] [PubMed] [Google Scholar]
- Del Moral P. M., Sala F. G., Tefft D., Shi W., Keshet E., Bellusci S., Warburton D. (2006). VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev. Biol. 290, 177–188 [DOI] [PubMed] [Google Scholar]
- Ding B. S., Nolan D. J., Butler J. M., James D., Babazadeh A. O., Rosenwaks Z., Mittal V., Kobayashi H., Shido K., Lyden D., et al. (2010). Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468, 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O. I., Melton D. A. (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 [DOI] [PubMed] [Google Scholar]
- Duvillie B., Attali M., Bounacer A., Ravassard P., Basmaciogullari A., Scharfmann R. (2006). The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes 55, 582–589 [DOI] [PubMed] [Google Scholar]
- Edsbagge J., Johansson J. K., Esni F., Luo Y., Radice G. L., Semb H. (2005). Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development 132, 1085–1092 [DOI] [PubMed] [Google Scholar]
- Eremina V., Quaggin S. E. (2004). The role of VEGF-A in glomerular development and function. Curr. Opin. Nephrol. Hypertens. 13, 9–15 [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Yano S., Zhang R. D., Fujimaki T., Bucana C. D. (2002). The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 3, 53–57 [DOI] [PubMed] [Google Scholar]
- Folkman J. (1971). Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 [DOI] [PubMed] [Google Scholar]
- Gao X., Chen X., Taglienti M., Rumballe B., Little M. H., Kreidberg J. A. (2005). Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development 132, 5437–5449 [DOI] [PubMed] [Google Scholar]
- Garratt A. N. (2006). “To erb-B or not to erb-B...” Neuregulin-1/ErbB signaling in heart development and function. J. Mol. Cell. Cardiol. 41, 215–218 [DOI] [PubMed] [Google Scholar]
- Gerritsen M. E. (1987). Functional heterogeneity of vascular endothelial cells. Biochem. Pharmacol. 36, 2701–2711 [DOI] [PubMed] [Google Scholar]
- Gittes G. K. (2009). Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 326, 4–35 [DOI] [PubMed] [Google Scholar]
- Gittes G. K., Galante P. E., Hanahan D., Rutter W. J., Debase H. T. (1996). Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development 122, 439–447 [DOI] [PubMed] [Google Scholar]
- Golosow N., Grobstein C. (1962). Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev. Biol. 4, 242–255 [DOI] [PubMed] [Google Scholar]
- Gu G., Brown J. R., Melton D. A. (2003). Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech. Dev. 120, 35–43 [DOI] [PubMed] [Google Scholar]
- Herrera P. L. (2000). Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127, 2317–2322 [DOI] [PubMed] [Google Scholar]
- Hick A. C., van Eyll J. M., Cordi S., Forez C., Passante L., Kohara H., Nagasawa T., Vanderhaeghen P., Courtoy P. J., Rousseau G. G., et al. (2009). Mechanism of primitive duct formation in the pancreas and submandibular glands: a role for SDF-1. BMC Dev. Biol. 9, 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. T., Butler J. M., Nolan D. J., Kranz A., Iida K., Kobayashi M., Kopp H. G., Shido K., Petit I., Yanger K., et al. (2009). Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilovich O., Jacobson O., Aviv Y., Litchi A., Chisin R., Mishani E. (2008). Formation of fluorine-18 labeled diaryl ureas-labeled VEGFR-2/PDGFR dual inhibitors as molecular imaging agents for angiogenesis. Bioorg. Med. Chem. 16, 4242–4251 [DOI] [PubMed] [Google Scholar]
- Jacquemin P., Yoshitomi H., Kashima Y., Rousseau G. G., Lemaigre F. P., Zaret K. S. (2006). An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev. Biol. 290, 189–199 [DOI] [PubMed] [Google Scholar]
- Jensen J., Pedersen E. E., Galante P., Hald J., Heller R. S., Ishibashi M., Kageyama R., Guillemot F., Serup P., Madsen O. D. (2000). Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36–44 [DOI] [PubMed] [Google Scholar]
- Johansson M., Mattsson G., Andersson A., Jansson L., Carlsson P. O. (2006). Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 147, 2315–2324 [DOI] [PubMed] [Google Scholar]
- Jorgensen M. C., Ahnfelt-Ronne J., Hald J., Madsen O. D., Serup P., Hecksher-Sorensen J. (2007). An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28, 685–705 [DOI] [PubMed] [Google Scholar]
- Kaido T., Yebra M., Cirulli V., Montgomery A. M. (2004). Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J. Biol. Chem. 279, 53762–53769 [DOI] [PubMed] [Google Scholar]
- Katsumoto K., Kume S. (2011). Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development 138, 1947–1955 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Cooper B., Gannon M., Ray M., MacDonald R. J., Wright C. V. (2002). The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32, 128–134 [DOI] [PubMed] [Google Scholar]
- Kelly O. G., Chan M. Y., Martinson L. A., Kadoya K., Ostertag T. M., Ross K. G., Richardson M., Carpenter M. K., D’Amour K. A., Kroon E., et al. (2011). Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol. 29, 750–756 [DOI] [PubMed] [Google Scholar]
- Kesavan G., Sand F. W., Greiner T. U., Johansson J. K., Kobberup S., Wu X., Brakebusch C., Semb H. (2009). Cdc42-mediated tubulogenesis controls cell specification. Cell 139, 791–801 [DOI] [PubMed] [Google Scholar]
- Kim S. K., MacDonald R. J. (2002). Signaling and transcriptional control of pancreatic organogenesis. Curr. Opin. Genet. Dev. 12, 540–547 [DOI] [PubMed] [Google Scholar]
- Kinkel M. D., Sefton E. M., Kikuchi Y., Mizoguchi T., Ward A. B., Prince V. E. (2009). Cyp26 enzymes function in endoderm to regulate pancreatic field size. Proc. Natl. Acad. Sci. USA 106, 7864–7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Butler J. M., O’Donnell R., Kobayashi M., Ding B. S., Bonner B., Chiu V. K., Nolan D. J., Shido K., Benjamin L., et al. (2010). Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12, 1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E., Goderie S., Wang Y., Lotz S., Lin G., Sun Y., Roysam B., Shen Q., Temple S. (2010). Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 7, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova I., Nikolova G., Ohara-Imaizumi M., Meda P., Kucera T., Zarbalis K., Wurst W., Nagamatsu S., Lammert E. (2007). EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129, 359–370 [DOI] [PubMed] [Google Scholar]
- Kopp H. G., Avecilla S. T., Hooper A. T., Rafii S. (2005). The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 20, 349–356 [DOI] [PubMed] [Google Scholar]
- Kroon E., Martinson L. A., Kadoya K., Bang A. G., Kelly O. G., Eliazer S., Young H., Richardson M., Smart N. G., Cunningham J., et al. (2008). Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 [DOI] [PubMed] [Google Scholar]
- Kumar M., Jordan N., Melton D., Grapin-Botton A. (2003). Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 259, 109–122 [DOI] [PubMed] [Google Scholar]
- Lammert E., Cleaver O., Melton D. (2001). Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 [DOI] [PubMed] [Google Scholar]
- Lammert E., Gu G., McLaughlin M., Brown D., Brekken R., Murtaugh L. C., Gerber H. P., Ferrara N., Melton D. A. (2003). Role of VEGF-A in vascularization of pancreatic islets. Curr. Biol. 13, 1070–1074 [DOI] [PubMed] [Google Scholar]
- Landsman L., Nijagal A., Whitchurch T. J., Vanderlaan R. L., Zimmer W. E., Mackenzie T. C., Hebrok M. (2011). Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol. 9, e1001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus A., Del-Moral P. M., Ilovich O., Mishani E., Warburton D., Keshet E. (2011). A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development 138, 2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter J., Moritz D. R., Li B., Phillips G. L., Liang X. H., Gerber H. P., Hillan K. J., Ferrara N. (2003). Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science 299, 890–893 [DOI] [PubMed] [Google Scholar]
- Leventhal C., Rafii S., Rafii D., Shahar A., Goldman S. A. (1999). Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell. Neurosci. 13, 450–464 [DOI] [PubMed] [Google Scholar]
- Li W., Johnson S. A., Shelley W. C., Yoder M. C. (2004). Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp. Hematol. 32, 1226–1237 [DOI] [PubMed] [Google Scholar]
- Louissaint A., Jr, Rao S., Leventhal C., Goldman S. A. (2002). Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34, 945–960 [DOI] [PubMed] [Google Scholar]
- Magenheim J., Ilovich O., Lazarus A., Klochendler A., Ziv O., Werman R., Hija A., Cleaver O., Mishani E., Keshet E., et al. (2011). Blood vessels restrain pancreas branching, differentiation and growth. Development 138, 4743–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yoshitomi H., Rossant J., Zaret K. S. (2001). Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559–563 [DOI] [PubMed] [Google Scholar]
- Mattsson G., Jansson L., Carlsson P. O. (2002). Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 51, 1362–1366 [DOI] [PubMed] [Google Scholar]
- Meier J. J., Butler A. E., Saisho Y., Monchamp T., Galasso R., Bhushan A., Rizza R. A., Butler P. C. (2008). Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57, 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F., Czernichow P., Scharfmann R. (1998). Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development 125, 1017–1024 [DOI] [PubMed] [Google Scholar]
- Mukouyama Y. S., Shin D., Britsch S., Taniguchi M., Anderson D. J. (2002). Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705 [DOI] [PubMed] [Google Scholar]
- Murtaugh L. C. (2007). Pancreas and beta-cell development: from the actual to the possible. Development 134, 427–438 [DOI] [PubMed] [Google Scholar]
- Murtaugh L. C., Stanger B. Z., Kwan K. M., Melton D. A. (2003). Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 100, 14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova G., Lammert E. (2003). Interdependent development of blood vessels and organs. Cell Tissue Res. 314, 33–42 [DOI] [PubMed] [Google Scholar]
- Nikolova G., Jabs N., Konstantinova I., Domogatskaya A., Tryggvason K., Sorokin L., Fassler R., Gu G., Gerber H. P., Ferrara N., et al. (2006). The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev. Cell 10, 397–405 [DOI] [PubMed] [Google Scholar]
- Nikolova G., Strilic B., Lammert E. (2007). The vascular niche and its basement membrane. Trends Cell Biol. 17, 19–25 [DOI] [PubMed] [Google Scholar]
- Northway W. H., Jr, Rosan R. C., Porter D. Y. (1967). Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 276, 357–368 [DOI] [PubMed] [Google Scholar]
- Offield M. F., Jetton T. L., Labosky P. A., Ray M., Stein R. W., Magnuson M. A., Hogan B. L., Wright C. V. (1996). PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122, 983–995 [DOI] [PubMed] [Google Scholar]
- Oliver-Krasinski J. M., Stoffers D. A. (2008). On the origin of the beta cell. Genes Dev. 22, 1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otonkoski T., Banerjee M., Korsgren O., Thornell L. E., Virtanen I. (2008). Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes. Metab. 10 Suppl. 4, 119–127 [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Willhoite A. R., Gage F. H. (2000). Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479–494 [DOI] [PubMed] [Google Scholar]
- Pan F. C., Wright C. (2011). Pancreas organogenesis: from bud to plexus to gland. Dev. Dyn. 240, 530–565 [DOI] [PubMed] [Google Scholar]
- Pan X., Xue W., Li Y., Feng X., Tian X., Ding C. (2011). Islet graft survival and function: concomitant culture and transplantation with vascular endothelial cells in diabetic rats. Transplantation 92, 1208–1214 [DOI] [PubMed] [Google Scholar]
- Parnaud G., Gonelle-Gispert C., Morel P., Giovannoni L., Muller Y. D., Meier R., Borot S., Berney T., Bosco D. (2011). Cadherin engagement protects human beta-cells from apoptosis. Endocrinology 152, 4601–4609 [DOI] [PubMed] [Google Scholar]
- Pictet R. L., Clark W. R., Williams R. H., Rutter W. J. (1972). An ultrastructural analysis of the developing embryonic pancreas. Dev. Biol. 29, 436–467 [DOI] [PubMed] [Google Scholar]
- Pierreux C. E., Cordi S., Hick A. C., Achouri Y., Ruiz de Almodovar C., Prevot P. P., Courtoy P. J., Carmeliet P., Lemaigre F. P. (2010). Epithelial: Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev. Biol. 347, 216–227 [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillejo C., Sanchez-Sanchez F., Andreu-Agullo C., Ferron S. R., Aroca-Aguilar J. D., Sanchez P., Mira H., Escribano J., Farinas I. (2006). Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat. Neurosci. 9, 331–339 [DOI] [PubMed] [Google Scholar]
- Riquelme P. A., Drapeau E., Doetsch F. (2008). Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P. (2010). Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes 59, 1285–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnick M. A., Panigrahy D., Zhang C. Y., Dallabrida S. M., Lowell B. B., Langer R., Folkman M. J. (2002). Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. USA 99, 10730–10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T. F., Sadler K. C., Crosnier C., Stainier D. Y. (2008). Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr. Biol. 18, 1565–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand F. W., Hornblad A., Johansson J. K., Loren C., Edsbagge J., Stahlberg A., Magenheim J., Ilovich O., Mishani E., Dor Y., et al. (2011). Growth-limiting role of endothelial cells in endoderm development. Dev. Biol. 352, 267–277 [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M., Clevers H. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. R., Esni F., Jakub A., Paredes J., Lath N., Malek M., Potoka D. A., Prasadan K., Mastroberardino P. G., Shiota C., et al. (2011). Embryonic mouse blood flow and oxygen correlate with early pancreatic differentiation. Dev. Biol. 349, 342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q., Goderie S. K., Jin L., Karanth N., Sun Y., Abramova N., Vincent P., Pumiglia K., Temple S. (2004). Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 [DOI] [PubMed] [Google Scholar]
- Shen Q., Wang Y., Kokovay E., Lin G., Chuang S. M., Goderie S. K., Roysam B., Temple S. (2008). Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar M., Cardalda C., Houbracken I., Martin M., Maestro M. A., De Medts N., Xu X., Grau V., Heimberg H., Bouwens L., et al. (2009). Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 [DOI] [PubMed] [Google Scholar]
- Stanger B. Z., Tanaka A. J., Melton D. A. (2007). Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445, 886–891 [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kohara H., Noda M., Nagasawa T. (2006). Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 [DOI] [PubMed] [Google Scholar]
- Tang W., Zeve D., Suh J. M., Bosnakovski D., Kyba M., Hammer R. E., Tallquist M. D., Graff J. M. (2008). White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M., Van der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J. M., Doetsch F. (2008). A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirziu D., Simons M. (2009). Endothelium as master regulator of organ development and growth. Vascul. Pharmacol. 50, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasenor A., Chong D. C., Henkemeyer M., Cleaver O. (2010). Epithelial dynamics of pancreatic branching morphogenesis. Development 137, 4295–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I., Banerjee M., Palgi J., Korsgren O., Lukinius A., Thornell L. E., Kikkawa Y., Sekiguchi K., Hukkanen M., Konttinen Y. T., et al. (2008). Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia 51, 1181–1191 [DOI] [PubMed] [Google Scholar]
- Weir G. C., Bonner-Weir S. (1990). Islets of Langerhans: the puzzle of intraislet interactions and their relevance to diabetes. J. Clin. Invest. 85, 983–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., D’Hoker J., Stange G., Bonne S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D., et al. (2008). Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Yun E. J., Gerber H. P., Ferrara N., Whitsett J. A., Vu T. H. (2007). Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev. Biol. 308, 44–53 [DOI] [PubMed] [Google Scholar]
- Yao L., Yokota T., Xia L., Kincade P. W., McEver R. P. (2005). Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood 106, 4093–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Sukeno M., Nabeshima Y. (2007). A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317, 1722–1726 [DOI] [PubMed] [Google Scholar]
- Yoshitomi H., Zaret K. S. (2004). Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development 131, 807–817 [DOI] [PubMed] [Google Scholar]
- Zaret K. S. (2006). Pancreatic beta cells: responding to the matrix. Cell Metab. 3, 148–150 [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Grompe M. (2008). Generation and regeneration of cells of the liver and pancreas. Science 322, 1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Wert S. E., Federici R., Peters K. G., Whitsett J. A. (1998). VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev. Dyn. 211, 215–227 [DOI] [PubMed] [Google Scholar]
- Zhang J., Niu C., Ye L., Huang H., He X., Tong W. G., Ross J., Haug J., Johnson T., Feng J. Q., et al. (2003). Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Law A. C., Rajagopal J., Anderson W. J., Gray P. A., Melton D. A. (2007). A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell 13, 103–114 [DOI] [PubMed] [Google Scholar]