Abstract
Embryonic development of the respiratory system is regulated by a series of mesenchymal-epithelial interactions that are only partially understood. Mesenchymal FGF and Wnt2/Wnt2b signaling are implicated in specification of mammalian pulmonary progenitors from the ventral foregut endoderm, but their epistatic relationship and downstream targets are largely unknown. In addition, how wnt2 and wnt2b are regulated in the developing foregut mesenchyme is unknown. We show that the Odd-skipped-related (Osr) zinc-finger transcriptional repressors Osr1 and Osr2 are redundantly required for Xenopus lung specification in a molecular pathway linking foregut pattering by FGFs to Wnt-mediated lung specification and RA-regulated lung bud growth. FGF and RA signals are required for robust osr1 and osr2 expression in the foregut endoderm and surrounding lateral plate mesoderm (lpm) prior to respiratory specification. Depletion of both Osr1 and Osr2 (Osr1/Osr2) results in agenesis of the lungs, trachea and esophagus. The foregut lpm of Osr1/Osr2-depleted embryos fails to express wnt2, wnt2b and raldh2, and consequently Nkx2.1+ progenitors are not specified. Our data suggest that Osr1/Osr2 normally repress bmp4 expression in the lpm, and that BMP signaling negatively regulates the wnt2b domain. These results significantly advance our understanding of early lung development and may impact strategies to differentiate respiratory tissue from stem cells.
Keywords: Lung, Osr, Wnt, BMP, Xenopus, Foregut, Endoderm
INTRODUCTION
The epithelial lining of the vertebrate trachea and lungs is derived from the ventral foregut endoderm. During embryogenesis, the earliest known marker of the respiratory epithelium is the homeodomain transcription factor Nkx2.1 (Ttf1) (Lazzaro et al., 1991). Nkx2.1–/– mutant mice have severely hypoplastic lungs and trachoesophageal fistula (Minoo et al., 1999). Two signaling pathways, FGF and Wnt/β-catenin, have been implicated in specification of the respiratory lineage. In vitro studies using mouse foregut explants suggest that between embryonic days (E) 8 and 8.75 of development, FGF ligands from the cardiac mesoderm pattern the ventral foregut progenitors into lung, liver and pancreas fates in a concentration-dependent manner, with the highest levels of FGF promoting lung and nkx2.1 expression (Serls et al., 2005). It is thought that multiple, redundant FGF ligands are required, although this remains to be validated. Genetic studies in mice have recently demonstrated that between E8.5 and E9.5 Wnt2/Wnt2b signals from the lateral plate mesoderm (lpm) are necessary and sufficient for lung specification. Compound Wnt2–/–;Wnt2b–/– mutants or endoderm-specific conditional β-catenin–/– mutants exhibit lung agenesis and fail to express nkx2.1 (Goss et al., 2009; Harris-Johnson et al., 2009).
After pulmonary specification, further crosstalk between the Wnt, FGF, Shh, TGFβ, BMP and retinoic acid (RA) pathways regulate trachea-esophagus separation, lung bud outgrowth, branching morphogenesis and proximal-distal patterning (reviewed by Cardoso and Lu, 2006; Maeda et al., 2007; Morrisey and Hogan, 2010). For example, analyses of mouse embryos lacking the RA-synthesizing enzyme Raldh2 indicate that RA is required for outgrowth of the specified lung epithelium, in part by suppressing TGFβ and promoting Wnt and FGF10 signaling (Desai et al., 2004; Wang et al., 2006; Chen et al., 2007). Recent evidence suggests that RA may also have an earlier role facilitating Wnt-mediated pulmonary specification by RA-mediated repression of the Wnt antagonist Dkk1 (Chen et al., 2010). BMP signaling also has multiple roles in respiratory development. Analysis of conditional Bmp4–/–, Smad1–/– and compound Bmpr1a–/–;Bmpr1b–/– mutants indicates that BMP signaling is dispensable for pulmonary specification, restricts the location of lung bud formation, promotes tracheal fate, and is required for proper distal differentiation and branching morphogenesis (Eblaghie et al., 2006; Li et al., 2008; Sun et al., 2008; Domyan et al., 2011; Xu et al., 2011). Despite recent progress, there are still a number of important unanswered questions about early lung development. First, the molecular pathways upstream of Wnt2/Wnt2b are undefined. It is unclear if early FGF and Wnt2/Wnt2b act in parallel or in an epistatic fashion, and their specific target genes are largely unknown. Finally, the relationship between early FGF/Wnt-mediated lung specification and the pathways regulating lung bud growth are poorly understood.
Osr zinc-finger transcriptional repressors are homologous to the Odd-skipped family of proteins in Drosophila, which regulate early segmentation and gut development (Green et al., 2002; Johansen et al., 2003; Goldstein et al., 2005). Vertebrates have two odd skipped-related genes, Osr1 and Osr2, which in mice have partially overlapping embryonic expression in the developing heart, kidney, limb and lung, and in craniofacial structures (So and Danielian, 1999; Lan et al., 2001). Analyses of Osr1–/– mutant mice have shown that Osr1 is essential for heart and kidney development (Wang et al., 2005; James et al., 2006; Mudumana et al., 2008), whereas Osr2–/– mutants show defective craniofacial and tooth development (Lan et al., 2004; Zhang et al., 2009). Interestingly, a knock-in of the Osr1 cDNA into the Osr2 locus rescues the Osr2–/– phenotype, suggesting that Osr1 and Osr2 are functionally equivalent (Gao et al., 2009). Compound Osr1–/–;Osr2–/– mutant mice have not been characterized, but in Xenopus and zebrafish both Osr1 and Osr2 are required for kidney development (Tena et al., 2007).
In this study, we show that Osr1 and Osr2 are redundantly required for Xenopus respiratory specification and foregut development. Our data demonstrate that early FGF and RA signaling control osr1/osr2 expression in the foregut epithelium and lpm. Depletion of Osr1/Osr2 results in lung, trachea and esophagus agenesis. We show that the Osr1/Osr2-depleted lpm fails to express wnt2/wnt2b and raldh2 and ectopically expresses bmp4; consequently, Nkx2.1+ respiratory progenitors are not specified in the foregut. Osr1/Osr2 are required to spatially restrict bmp4 expression in the lpm and BMP signaling negatively regulates wnt2b and raldh2. Temporal activation of Wnt/β-catenin signaling can rescue lung specification but not lung bud growth in Osr1/Osr2-depleted embryos, whereas temporal inhibition of BMP signaling can completely rescue lung specification and bud formation. Together, these data indicate that Osr1 and Osr2 are key components of a molecular pathway that connects early foregut pattering by FGFs to Wnt-mediated pulmonary specification and RA-regulated lung bud development.
MATERIALS AND METHODS
Embryo manipulations and microinjections
Culture and injection of X. laevis and X. tropicalis embryos were performed as previously described (Sive et al., 2000; Khokha et al., 2002). The morpholinos Osr1-MO (10 ng), Osr2-MO (10 ng) and Wnt2b-MO (20 ng) have previously been shown to generate specific loss of functions (Tena et al., 2007; Damianitsch et al., 2009). The X. tropicalis Wnt2-MO (20 ng) has the sequence 5′-ATGAGGAGGTAACAACAGCTCCCAT-3′. GR-mOsr1 was constructed by fusing the hormone-binding domain or the human glucocorticoid receptor in-frame with the mouse Osr1 cDNA (Open Biosystems #MMM1013-7512959). GR-mOsr1, GR-Smad1 (Kim and Han, 2011) and GR-DNXTcf3 (McLin et al., 2007) mRNAs were prepared using the Ambion SP6 mMessage mMachine kit. Embryos with clear dorsal-ventral pigmentation were selected for four- to eight-cell stage injections targeting anterior mesendoderm. Small molecules were dissolved in DMSO and working concentrations were as follows: 100 μM PD173074 (Tocris Bioscience), 80 μM SU5402 (Tocris Bioscience), 50 μM XAV939 (Cayman Chemical), 10 μM BMS493 (Tocris Bioscience), 10 μM LDN193189 (Axon MedChem), 10 μM BIO (Cayman Chemical) and 1-5 μM all-trans retinoic acid (Sigma). Dexamethasone (1 μM; Sigma) was used to activate hormone-inducible GR constructs. Culture buffers were refreshed with the inhibitors every 12-16 hours, and embryos were cultured in the dark as some inhibitors are known to be light sensitive. Luciferase assays were performed as described previously (Rankin et al., 2011).
In situ hybridization and Immunohistochemistry
Whole-mount in situ hybridization was performed as previously described (Sive et al., 2000) and details on specific clones and probe production are available upon request. For immunofluorescence, embryos were fixed in MEMFA for 1 hour at 4°C, washed in PBS (pH 7.4) and then transferred into Dent’s post-fixative (80% methanol, 20% DMSO) and stored at –20°C for at least 48 hours. Embryos were rehydrated through a methanol series into PBS+0.1% Triton X-100 (PBSTr), bisected with a razor blade through the foregut region, blocked for 2 hours in PBSTr +10% goat serum + 1% BSA, followed by incubation with primary antibodies [rabbit-anti-phospho-Smad1/5/8, Cell Signaling #9511, 1:350; mouse anti-fibronectin 4H2, kindly provided by D. DeSimone (University of Virginia, Charlottesville, VA, USA), 1:400; mouse anti-Nkx2.1, Thermo Scientific #8G7G3/1, 1:300; rabbit anti-Sox2, Abcam #ab97959, 1:500] in blocking solution overnight at 4°C. After washing in PBSTr, samples were incubated in goat-anti-rabbit DyLight488 and/or goat-anti-mouse DyLight649 (Jackson ImmunoResearch, used at 1:500 in PBSTr + 1%BSA) overnight at 4°C. After extensive washing in PBSTr, samples were dehydrated in 100% methanol, cleared in two parts benzyl benzoate/one part benzyl alcohol and imaged using a Zeiss LSM 510 confocal microscope. Standard immunohistochemical protocols were used to analyze cell proliferation and cell death.
RESULTS
osr1 and osr2 are dynamically expressed in the developing Xenopus foregut
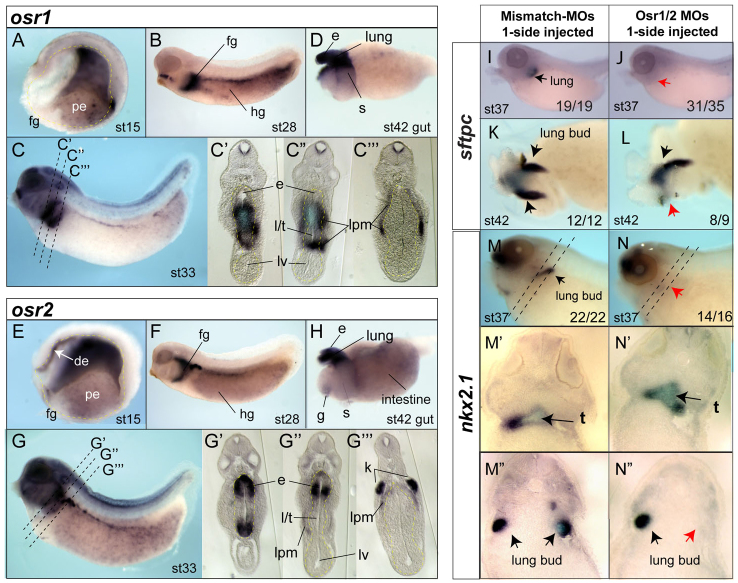
The expression of Osr transcription factors in Xenopus digestive and respiratory system development has not been characterized. In situ hybridization of Xenopus embryos revealed that osr1 and osr2 are dynamically expressed in overlapping domains of the endoderm and surrounding mesoderm during early organogenesis. After gastrulation (stage 15), osr1 and osr2 are expressed in the lpm (Tena et al., 2007) and posterior endoderm but are excluded from the ventral foregut (Fig. 1A,E). They first become expressed in the foregut prior to specification of organ lineages during stages 25-32, at which time they are also expressed in the hindgut, lpm, brain, branchial arches and developing kidney, as previously described (Fig. 1B,F) (Tena et al., 2007). At stage 33, prior to organ bud formation, osr1 and osr2 have overlapping expression patterns in the anterior foregut that gives rise to the esophagus, trachea and lungs (Fig. 1C,G). At this time, the anterior foregut cavity is still a single tube that has not yet separated into a distinct dorsal esophagus and a ventral trachea. osr1 is expressed in both the presumptive pulmonary epithelium and surrounding lpm with lower dorsal expression in the presumptive esophagus (Fig. 1C; supplementary material Fig. S1). By contrast, osr2 is robustly expressed in both the dorsal and ventral aspects of the anterior foregut endoderm and in lpm (Fig. 1G; supplementary material Fig. S1). In isolated stage 42 gut tubes osr1 and osr2 are both expressed in the esophagus, trachea, lungs and proximal stomach (Fig. 1D,H).
Fig. 1.
. osr1 and osr2 are dynamically expressed in the developing Xenopus foregut and are required for lung development. (A-G″′) In situ hybridization of osr1 (A-D) and osr2 (E-H) expression in Xenopus tropicalis embryos and isolated gut tubes (D,H) at the indicated stages (st), anterior leftwards, dorsal upwards. Stage 15 embryos (A,E) are bisected and stage 33 embryos (C′-C[H11630],G′-G[H11630]) are sectioned to show the endoderm (outlined in yellow in A,E). fg, foregut; hg, hindgut; de, dorsal endoderm; pe, posterior endoderm; e, esophagus; l/t, presumptive lung and trachea; lv, liver; lpm, lateral plate mesoderm; k, kidney; s, stomach. (I-N″) surfactant protein c (sftpc) (I-L) and nkx2.1 (M,N″) expression in embryos injected on one side with Osr1/Osr2-MOs (10 ng each) or negative control mutated oligos MM-MO (20 ng) shows lung agenesis on the Osr1/Osr2-depleted side. (M’-N″) Sections. Numbers of embryos with the shown phenotype are indicated. Dorsal view of an isolated gut tube (K,L) shows the lung bud absent on the injected side (red arrow).
Osr1 and Osr2 are redundantly required for respiratory specification and tracheal-esophageal separation
To determine the role of Osr1 and Osr2 in Xenopus foregut development, we injected established translation blocking morpholino oligos (MOs) (Tena et al., 2007) with a fluorescent tracer into vegetal cells of the eight-cell embryo, targeting the anterior mesendoderm. Injection of individual Osr1-MO or Osr2-MO had no detectable effect on early endoderm organogenesis (data not shown). However, co-injection of both the Osr1-MO and Osr2-MO (Osr1/Osr2-MO) into one side of the foregut mesendoderm resulted in lung bud agenesis on the injected side, as indicated by the lack of surfactant protein c (sftpc) and nkx2.1 expression at stage 37, and the absence a lung bud of at stage 42 (Fig. 1I-N). Sectioning of the unilateral injected Osr1/Osr2-MO embryos revealed that the lung bud was missing on the injected side but the nkx2.1-expressing trachea was still present (Fig. 1N-N″). Liver, pancreas, thyroid, heart and intestine development was unaffected by Osr1/Osr2 knockdown (supplementary material Fig. S2). Analysis of early mesendoderm markers also indicated that germ layer formation and anterior-posterior patterning were normal (supplementary material Fig. S2), but kidney development was impaired as described previously (Tena et al., 2007). Comparable results were obtained with Osr1/Osr2-MOs in both Xenopus tropicalis and Xenopus laevis, whereas control mismatch-MOs (MM-MO) with 5/25 nucleotides altered had no effect. We conclude that that Osr1 and Osr2 are redundantly required for lung formation.
The lung bud agenesis could be due to a failure of respiratory specification or impaired lung bud outgrowth after specification. To determine which was the case, we injected Osr1/Osr2-MOs into both sides of the embryo to target the entire foregut (Fig. 2) and examined the expression of nkx2.1, the earliest marker of respiratory progenitors, which is first detectable by in situ hybridization in Xenopus embryos at stage 35 (Small et al., 2000). In over 90% of the Osr1/Osr2-depleted embryos, nkx2.1 transcripts were undetectable (Fig. 2A,B). Confocal immunostaining showed that in stage 35/36 controls, the foregut epithelium is patterned into a dorsal region that upregulates Sox2 and a ventral Nkx2.1+ region with nascent lung buds (Fig. 2D,D′). By contrast, the Osr1/Osr2-MO-depleted foreguts had no detectable Nkx2.1+ cells and only a scattering of cells expressing a low level of Sox2 (Fig. 2E,E′). Analysis of cell proliferation and cell death as this stage revealed no differences between control and Osr1/Osr2-MO embryos (data not shown). We conclude that respiratory fate is not specified in the absence of Osr1/Osr2.
Fig. 2.
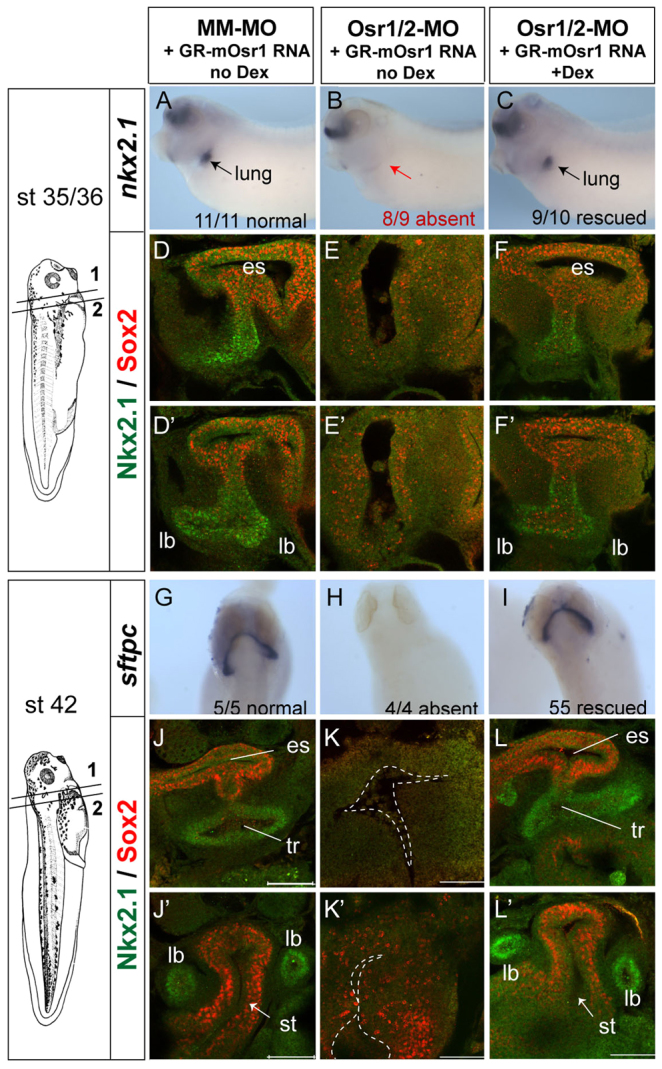
Osr1/Osr2 are required for respiratory specification and tracheal-esophageal separation. (A-L′) Embryos were co-injected with control MM-MOs or Osr1/Osr2-MOs and GR-mOsr1 RNA (250 pg) in both dorsal-vegetal blastomeres at the eight-cell stage (bilateral injection) and then cultured ±dexamethasone from stage 20 onwards. (A-C,G-I) Analysis by in situ hybridization of nkx2.1 at stage 34/35 (A-C) and sftpc at stage 42 (G-I). (D-F′,J-L′) Confocal immunofluorescence of Nkx2.1 (green) and Sox2 (red) at stage 35/36 (D-F) and stage 42 (J-L). es, esophagus; tr, trachea; lb, lung buds; st, stomach, Scale bars: 100 μm.
By stage 42, the MM-MO-injected control lung buds express sftpc and the Sox2+ esophagus and stomach are clearly distinct from the Nkx2.1+ trachea and lung buds (Fig. 2G,J,J′). By contrast, Osr1/Osr2-depleted embryos exhibited complete respiratory agenesis, with a single foregut tube and no detectable sftpc and no Nkx2.1+ trachea or lung buds (Fig. 2H,K,K′). The stomach of Osr1/Osr2-MO embryos was also severely hypoplastic with reduced Sox2 levels. Extensive apoptosis and reduced proliferation was detected in both the epithelium and lpm of the Osr1/Osr2-depleted stage 42 foregut (supplementary material Fig. S3; data not shown). To demonstrate the MO phenotype was specifically due to loss of Osr function, we co-injected the Osr1/Osr2-MOs with mRNA encoding an inducible form of mouse Osr1 (GR-mOsr1), which lacks the morpholino target sequence. Addition of dexamethasone (dex) to activate the GR-mOsr1 at stage 20 completely rescued all aspects of the Osr1/Osr2 phenotype (Fig. 2; supplementary material Fig. S3).
We conclude that Osr1/Osr2 are required to specify respiratory fate in the developing foregut and for tracheal-esophageal separation. The remnant foregut appears to be blocked in an immature progenitor state, it does not adopt another foregut lineage (supplementary material Fig. S2) and eventually undergoes programmed cell death.
Osr1/Osr2 are required for the expression of wnt2b and raldh2 in the lateral plate mesoderm
The pathways regulating respiratory development in Xenopus are largely unknown; therefore, we examined the expression of candidate genes required for pulmonary development in mice. We focused on stages 34-35 when nkx2.1+ progenitors can first be detected and used unilateral Osr1/Osr2-MO injections because the uninjected side serves as an internal control that allows the identification of where respiratory tissue should normally form. Consistent with the results of Fig. 2, Osr1/Osr2-depletion resulted in a loss of nkx2.1 transcripts and a reduction of sox2 (Fig. 3D-F), and their expression was rescued by GR-mOsr1 (supplementary material Fig. S3). By contrast, other epithelial markers including shh and foxa1 (Fig. 3G-I; data not shown) were expressed normally, suggesting that endoderm identity was maintained in Osr1/Osr2-MO embryos.
Fig. 3.
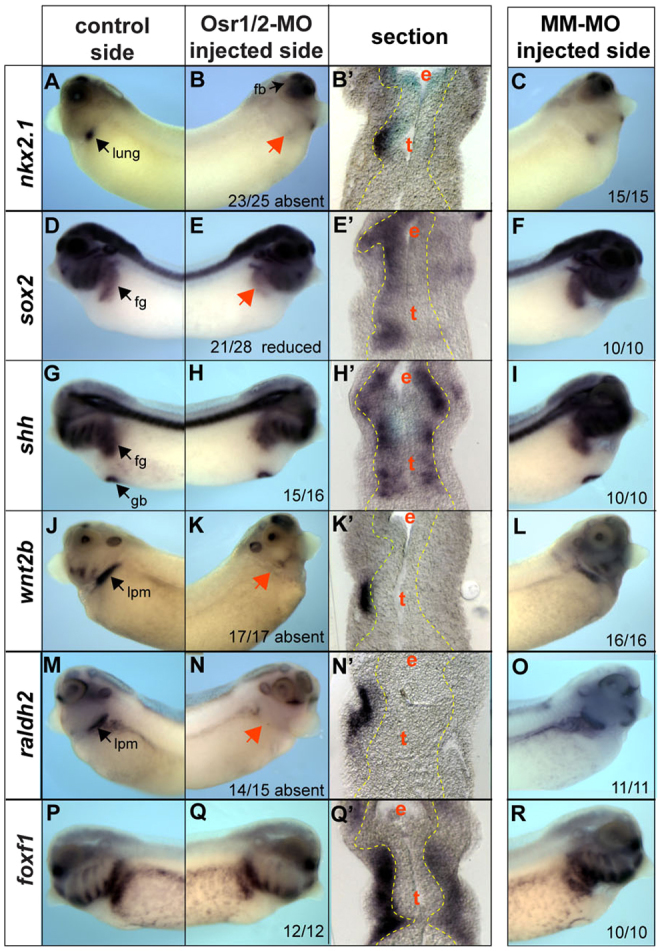
Osr1/Osr2 are required for wnt2b and raldh2 expression in the lpm. (A-X) Analysis of stage 34/35 unilateral-injected embryos shows that Osr1/Osr2 are required for expression of nkx2.1 (A-C) and sox2 (D-F) in the epithelium, whereas shh (G-I) is unaffected. In the lpm, wnt2b (J-L) and raldh2 (M-O) are absent, while foxf1 is unaffected (P-R). Black arrows indicate normal expression on the control side and red arrows indicate lack of expression on the Osr1/Osr2-MO-injected side (right). Endoderm outlined by a yellow line. Numbers of embryos with the shown phenotype are indicated. fg, foregut; hg, hindgut; de, dorsal endoderm; pe, posterior endoderm; e, esophagus; l/t, presumptive lung and trachea; lv, liver; lpm, lateral plate mesoderm; k, kidney; s, stomach.
We next examined the expression of signaling molecules in the lpm. We found that wnt2b, one of the earliest known inducers of respiratory fate in mouse, was absent on the Osr1/Osr2-MO injected side (Fig. 3J-L; supplementary material Fig. S3), as was raldh2 (Fig. 3M-O), which has been shown to regulate RA-mediated lung bud growth. The specific FGF ligands that induce lung fate during foregut patterning in mice are unknown and it is thought that multiple redundant ligands may be involved. Candidates include fgf7 and fgf10, but these were unaffected in Osr1/Osr2-depleted embryos at stage 35, as were the mesenchymal transcription factors foxf1, gli2 and gli3 (Fig. 3P-R; supplementary material Fig. S4; data not shown). These data suggest that loss of respiratory cell fate in Osr1/Osr2-depleted embryos might be due to a loss of Wnt and RA signaling from the lpm.
Osr1/Osr2 regulate foregut development in a molecular pathway with FGF, Wnt and RA
Our analysis suggested that Osr1 and Osr2 might regulate foregut development downstream of FGF signaling but upstream of Wnt and RA signaling. To test this hypothesis, we inhibited the FGF, Wnt and RA pathways, and asked to what extent this could either regulate osr expression or phenocopy Osr1/Osr2 loss of function. Evidence from mouse suggests that multiple redundant FGF/Wnt ligands and RA receptors act over a period of 1-2 days to regulate early lung development. To overcome this complication, we used cell-soluble small molecules to inhibit the FGF, Wnt and RA pathways. We cultured embryos from stages 25-35 because this is after early mesendoderm patterning (McLin et al., 2007; Deimling and Drysdale, 2009; Deimling and Drysdale, 2011), but prior to lung specification. This is also when osr1 and osr2 are first expressed in the foregut and previous studies have shown that during this time mesodermal signals are required for regional specification of the endoderm (Horb and Slack, 2001).
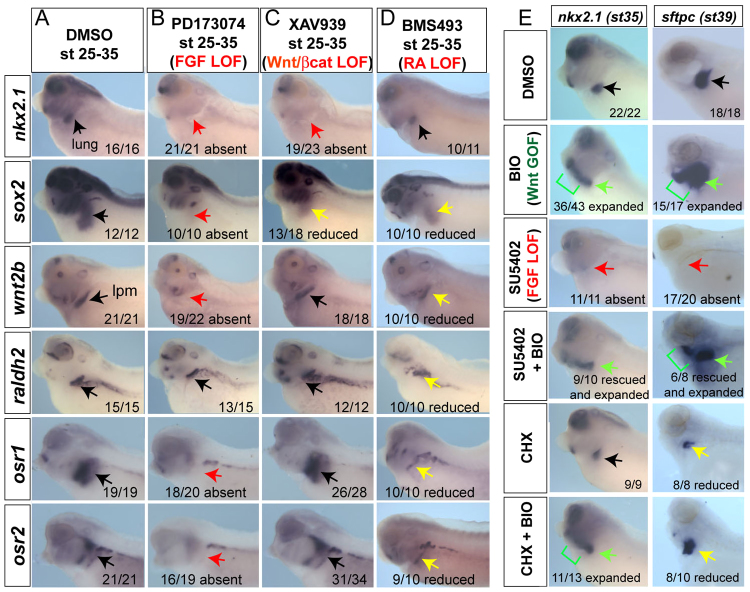
Inhibition of the FGF receptor kinase with PD173074 (or SU5402) (Beenken and Mohammadi, 2009) abolished nkx2.1 expression at stage 35 and embryos completely lacked lung buds at stage 40 (Fig. 4A,B; data not shown). In addition, these embryos failed to express osr1, osr2, sox2 and wnt2b in the foregut region (Fig. 4B). This indicates that FGF signaling between stages 25 and 35 is required for Xenopus respiratory specification, similar to recent findings (Wang et al., 2011) and supports a model in which FGF acts upstream of Osr1/Osr2 and Wnt. Moreover, these data indicate that FGF signaling impacts both the presumptive pulmonary epithelium as well as the pulmonary-inducing lpm.
Fig. 4.
FGF, Wnt/β-catenin and RA signaling regulate Xenopus lung development in a pathway with Osr1/Osr2. (A-D) Embryos were treated from stages 25-35 with DMSO vehicle (A), 100 μM PD173074 (B), 50 μM XAV939 (C) or 10 μM BMS493 (D), and assayed at stage 35 with the indicated probes. (E). Embryos were treated between stages 28 and 35 with vehicle (DMSO), 10 μM BIO, 80 μM SU5402, a combination of 80 μM SU5402 + 10 μM BIO, 10 μg/ml cycloheximide (CHX) or CHX +10 μM BIO, and analyzed for nkx2.1 at stage 35 and sftpc at stage 40. Black arrows indicate normal expression, red arrows indicate absent expression, yellow arrows indicate reduced expression and green arrows indicate expanded or rescued expression.
Inhibition of canonical Wnt signaling by XAV939, which promotes β-catenin degradation (Huang et al., 2009; Karner et al., 2010), resulted in loss of nkx2.1 and lung agenesis but had no impact on wnt2b, raldh2, osr1 or osr2 expression (Fig. 4C). To confirm the temporal requirement of Wnt/β-catenin signaling in Xenopus lung specification, we injected RNA encoding a hormone-inducible dominant-negative Tcf3 (GR-dnTcf3) construct, which represses β-catenin/Tcf target genes when activated by dex (Molenaar et al., 1996; McLin et al., 2007). Activation of GR-dnTcf3 from stages 28-35 resulted in a loss of nkx2.1 but had no impact on osr1/osr2 expression (supplementary material Fig. S5A). Finally, we tested the possibility that endogenous Wnt2 and Wnt2b were redundantly required for lung specification in Xenopus as they are in mice. In situ hybridization showed that wnt2 was weakly expressed in a strip of lpm (Garriock et al., 2007), similar to wnt2b; furthermore, neither wnt2 nor wnt2b was expressed in Osr1/Osr2-depleted embryos (supplementary material Fig. S5B). As predicted, co-injection of Wnt2-MO and Wnt2b-MOs resulted in a loss of nkx2.1 at stage 35 and lung bud agenesis at stage 42, whereas liver, thyroid, pancreas and duodenum appeared unaffected (supplementary material Fig. 5C). Knockdown of either Wnt2 or Wnt2b alone did not inhibit nkx2.1 induction (data not shown). Consistent with XAV939-treated or dNXTCF3-injected embryos, the expression of osr1/osr2 was unchanged in Wnt2/Wnt2b-MO embryos. Together, these results suggest that Osr1/Osr2 act upstream of Wnt2/Wnt2b-mediated lung specification in Xenopus.
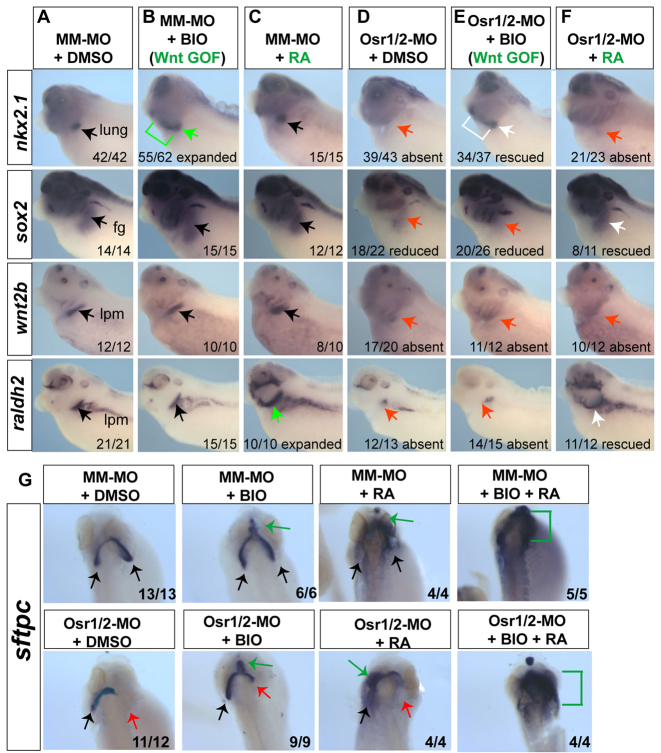
Fig. 5.
Osr1/Osr2 act upstream of Wnt-mediated pulmonary specification and RA-regulated lung bud growth. (A-G) Osr1/Osr2-MO- and MM-MO-injected embryos were treated at stages 25-35 with DMSO vehicle, 10 μM BIO or 1 μM all-trans-retinoic acid (RA), and analyzed for nkx2.1, sox2, wnt2b and raldh2 at stage 35 (A-F) and sftpc at stage 42 (dorsal view) (G). Black arrows indicate normal expression, red arrows show absent expression, green arrows and lines indicate expanded expression and white arrows indicate rescued expression.
RA signaling is well known to regulate lung bud development in mice (Desai et al., 2004) and recent data suggest that RA might facilitate Wnt-mediated specification by repressing expression of the Wnt-antagonist Dkk1 (Chen et al., 2010); these data raise the possibility that the lack of raldh2 might also contribute to the Osr1/Osr2-MO phenotype. To test the role of RA during Xenopus lung development, we treated embryos with the pan-retinoic acid receptor antagonist BMS493 (Desai et al., 2004) during stages 25-35. Specification of nkx2.1+ lung progenitors was unperturbed in these embryos (Fig. 4D), even though the Wnt-antagonist dkk1 was upregulated throughout the embryo (supplementary material Fig. S6A), similar to raldh2-deficient mouse embryos (Chen et al., 2010). Inhibition of RA signaling did, however, reduce osr1, osr2, sox2, wnt2b and raldh2 levels (Fig. 4D), and retarded lung bud growth at later stages (supplementary material Fig. S6B). Therefore, although RA signaling between stages 25 and 35 contributes to maintaining osr1, osr2 and wnt2b levels, it is not absolutely required for their expression and is dispensable for lung specification. However, RA signaling is clearly required for later lung bud growth, and Osr1/Osr2 might contribute to this through regulation of raldh2 expression.
These data demonstrate that Xenopus respiratory development is regulated by FGF, Wnt and RA signaling in a molecular pathway remarkably conserved with mammals, and that Osr factors are novel players in this process.
Wnt/β-catenin signaling is sufficient to specify respiratory fate downstream of FGF
Our results suggest, for the first time in any species, an epistatic relationship where early FGF signaling acts upstream of canonical Wnt/β-catenin to control respiratory specification. To test this, we determined whether activation of Wnt/β-catenin signaling could rescue lung specification in embryos where FGF signaling was inhibited. Treatment of control embryos at stage 28-35 with the small molecule BIO, which inhibits Gsk3 and causes stabilization of β-catenin (Sato et al., 2004), was sufficient to expand nkx2.1 and sftpc expression throughout the pharyngeal region (Fig. 4E), without affecting osr1, osr2, wnt2b, raldh2 or sox2 (data not shown). This confirms that that activation of the canonical Wnt/β-catenin pathway is sufficient to induce pulmonary fate in Xenopus similar to the conditional activation of β-catenin in mouse embryos (Goss et al., 2009; Harris-Johnson et al., 2009). Importantly BIO treatment also rescued (and expanded) nkx2.1 and sftpc expression in embryos where FGF receptor signaling was blocked by PD173074 or SU5402 (Fig. 4E; data not shown), indicating that Wnt/β-catenin is sufficient to control Xenopus lung specification downstream of FGF. We note that although sftpc was expressed in SU5402+BIO embryos, there were no distinct lung buds, consistent with mouse studies demonstrating that FGF10 is required for bud formation after specification (Sekine et al., 1999). BIO was also able to expand nkx2.1 expression in the presence of the translation inhibitor cycloheximide (Fig. 4E), suggesting that nkx2.1 is a direct transcriptional target of β-catenin signaling in Xenopus. Although we cannot exclude the possibility that FGF can act in parallel with Wnt to induce nkx2.1, these data, along with the observation that FGF is required for osr1/osr2 and wnt2b expression, support an FGF→Osr1/Osr2→Wnt2/Wnt2b→Nkx2.1 pathway that specifies respiratory fate.
Temporal activation of Wnt/β-catenin rescues lung specification but not lung bud growth in Osr1/Osr2-depleted embryos
The observation that Osr1/Osr2-depleted embryos fail to express wnt2b or raldh2 suggested that the lung agenesis might be due to loss of Wnt and/or RA signaling. We tested whether activation of Wnt/β-catenin by BIO or addition of exogenous RA could rescue lung development in Osr1/Osr2-MO unilateral-injected embryos. BIO treatment from stages 25-35 was sufficient to rescue nkx2.1 in Osr1/Osr2-MO embryos (Fig. 5), but did not rescue sox2 nor restore wnt2b or raldh2 expression in the mesoderm. Interestingly, although BIO treatment resulted in ectopic sftpc expression in the rostral foregut at stage 42 (Fig. 5G; green arrow), BIO only partially rescued the absent lung bud on the Osr1/Osr2-depleted side, which remained hypoplastic and failed to grow (Fig. 5G). This suggests that Osr1/Osr2 might also have a later role in lung bud growth that is independent of Wnt activation, which based on the inhibitor experiments (supplementary material Fig. S6B) is likely to be mediated by RA.
RA treatment from stages 25-35 was unable to restore nkx2.1 or wnt2b in Osr1/Osr2-depleted embryos (Fig. 5F). However, exogenous RA did rescue raldh2 (a known RA-target) as well as sox2. At stage 42, RA treatment caused ectopic sftpc expression throughout the anterior foregut and head of both MM-MO and Osr1/Osr2-MO embryos (Fig. 5G). Despite this, ectopic sfptc expression RA did not rescue outgrowth of a discrete lung bud on the Osr1/Osr2-MO injected side. We next tested whether a combination of BIO and RA could rescue both specification and growth. We found that BIO and RA together resulted in a massive upregulation of ectopic sftpc expression throughout the foregut and head tissue; however, we never detected a discreet lung bud on the Osr1/Osr2-MO injected side.
Overall, these results suggest that Osr1/Osr2 regulate both Wnt-mediated lung specification and RA-mediated lung growth, via regulating expression of wnt2/wnt2b and raldh2.
BMP/Smad1 signaling is elevated Osr1/Osr2-depleted embryos
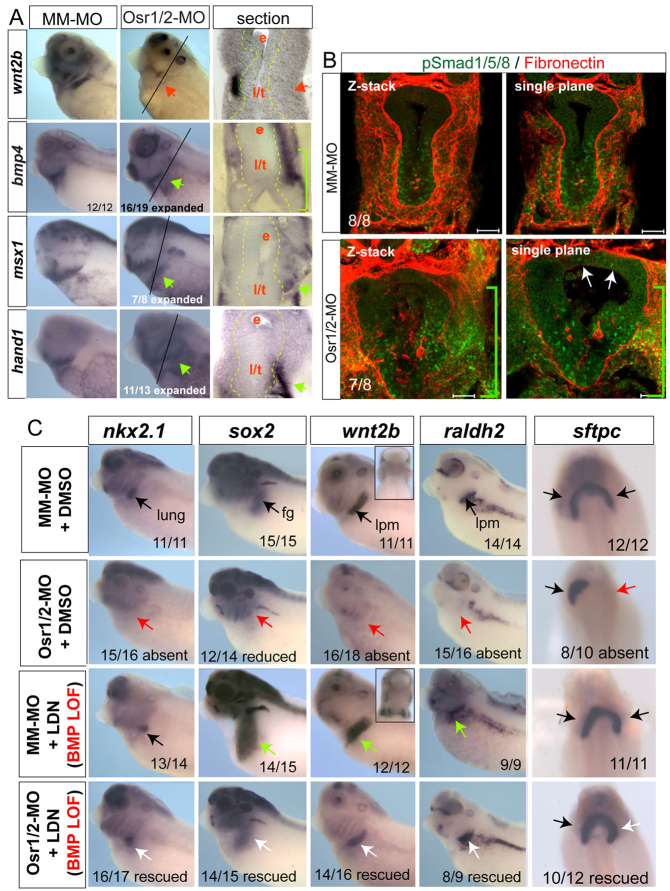
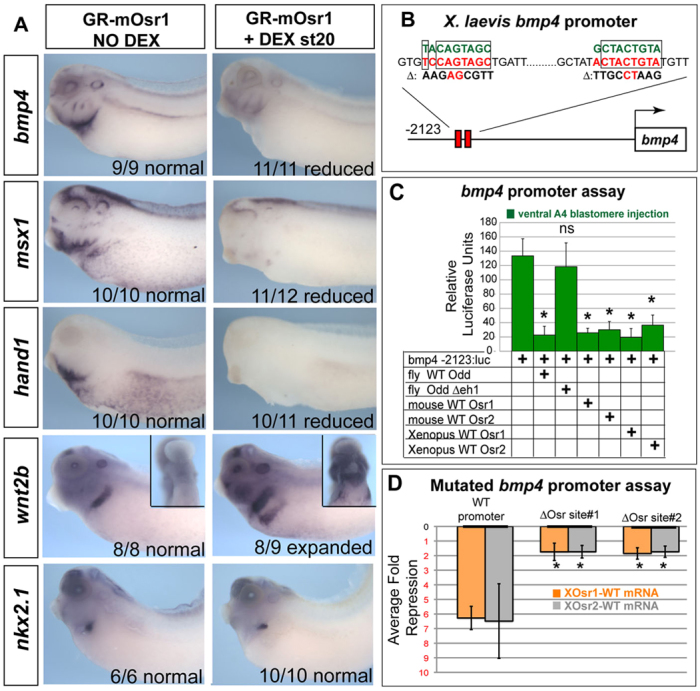
Osr factors are generally thought to act as transcriptional repressors (Goldstein et al., 2005; Tena et al., 2007) so Osr1/Osr2 depletion (loss of a repressor) probably caused the loss of wnt2b and raldh2 transcripts indirectly. A candidate Osr target came from recent mouse studies where Osr2–/– embryos exhibited ectopic bmp4 expression in the developing tooth mesenchyme (Zhang et al., 2009). We found that, in Osr1/Osr2-MO-injected embryos, bmp4 transcripts were ectopically upregulated in the foregut lpm, which should normally express wnt2/wnt2b (Fig. 6A), and the ectopic bmp4 could be abolished by co-injection of GR-mOsr1 RNA (supplementary material Fig. S3). Phospho-Smad1/5/8 (pSmad1) immunostaining and analysis for the BMP-target genes msx1 and hand1 confirmed that BMP signaling was upregulated in both the endoderm and lpm in Osr1/Osr2-MO unilateral- (Fig. 6A,B) and bilateral-injected embryos (supplementary material Fig. S7A). Sox2 is severely downregulated in Osr1/Osr2-MO-injected embryos (Figs 2, 3), and numerous studies have shown BMP signaling to be inhibitory to Sox2 expression (Matsushita, 2008; Domyan et al., 2011; Green et al., 2011). These data led us to hypothesize that, during normal development, Osr1/Osr2 negatively regulate bmp4 expression levels and that BMP signaling in turn restricts wnt2b and raldh2 expression to a thin strip of lpm adjacent to the presumptive pulmonary epithelium. However, in Osr1/Osr2 morphants, elevated BMP signaling would inhibit wnt2b expression, resulting in a failure of lung induction. Consistent with this hypothesis, during stages 25-37, osr1, osr2 and bmp4 have largely exclusive expression patterns, although there is some overlap in the lpm (Fig. 6; supplementary material Fig. S1). Most importantly, bmp4 is clearly not robustly expressed in the wnt2/wnt2b-positive lpm prior to and during lung specification stages.
Fig. 6.
Osr1/Osr2 promotes lung specification and growth by restricting BMP signaling. (A). Analysis of Osr1/Osr2-MO and control MM-MO stage 35 embryos indicated that expression of bmp4 and BMP-target genes msx1 and hand1 are expanded into the ‘wnt2b-domain’ of the lpm on the Osr1/Osr2-depleted side. Black arrows indicate normal expression, red arrows indicate absent expression and green arrows indicate enhanced expression. (B) Confocal immunostaining of stage 35 embryos for phospho-Smad1/5/8 (green) and fibronectin (red). Scale bars: 50 μm. White arrows in B indicate ectopic pSMAD1 expression in dorsal foregut endoderm. (C) Analysis of nxk2.1, sox2, wnt2b and raldh2 at stage 35 or sftpc at stage 41 in embryos injected with Osr1/Osr2-MOs or MM-MOs and treated at stages 25-35 with either DMSO (vehicle control) or 10 μM LDN193189. Insets show ventral views of wnt2b expression. For analysis of sftpc expression at stage 41, embryos were removed from the LDN193189 at stage 35 and further cultured in DMSO vehicle until stage 41. Black arrows indicate normal expression, red arrows indicate absent expression, green arrows indicate expanded expression and white arrows indicate rescued expression.
Osr1/Osr2 promotes lung specification by restricting BMP signaling
To test the hypothesis that BMP signaling negatively regulates wnt2b expression and lung specification, we injected an inducible GR-Smad1 construct (Kim and Han, 2011) into one side of four-cell stage embryos. GR-Smad1 activation by addition of dex between stages 25-35 resulted in a severe reduction of wnt2b and a loss of nkx2.1 and sox2 on the injected side, with no detectable increase in cell death (supplementary material Fig. S7B,C). Therefore, high levels of BMP/Smad1 in the foregut during stages 25-35 are inhibitory to lung specification.
To test whether the Osr1/Osr2-MO phenotype was due to elevated BMP signaling, we treated control and Osr1/Osr2-MO-injected embryos with the BMP type I receptor antagonist LDN193189 (Yu et al., 2008) from stages 25 to 35. Consistent with BMP signaling normally restricting wnt2b and raldh2 expression, LDN193189 treatment of control embryos caused an expansion of the wnt2b, sox2 and ralhd2 expression domains (Fig. 6C, green arrows). However, LDN193189 treatment did not increase nkx2.1 expression, indicating that suppression of BMP signaling is not sufficient to induce lung fate. Importantly, LDN193189 treatment completely rescued expression of wnt2b, raldh2, sox2 and specification of nkx2.1-expressing lung progenitors in Osr1/Osr2 morphants (Fig. 6D, white arrows) and abolished the ectopic pSMAD1 in the foregut (supplementary material Fig. S7D). When rescued embryos were taken out of the BMPR inhibitor at stage 35 and allowed to develop until stage 42, sftpc expression and lung bud growth was also rescued. If embryos were left in the BMPR inhibitor after lung specification between stages 35 and 42, we observed tracheal degeneration and hypoplastic lung buds (supplementary material Fig. S8), consistent with mouse studies demonstrating a role for BMP in maintenance of trachea fate and lung bud morphogenesis (Domyan et al., 2011; Lie et al., 2008). These data indicate that the failure of respiratory specification in Osr1/Osr2-depleted embryos is primarily due to elevated BMP signaling. We conclude that Osr1 and Osr2 are required to restrain BMP/pSmad1 activity in order to allow expression of wnt2b, ralhd2 and nkx2.1, and thus promote lung specification and development.
Osr1/Osr2 can repress bmp4 transcription
To test whether overexpression of Osr1/Osr2 could repress endogenous bmp4 and induce ectopic nkx2.1+ respiratory tissue, we injected a high dose of GR-mOsr1 RNA (750 pg) and induced the construct at stage 20, after gastrulation and early AP patterning are complete. This resulted in a dramatic downregulation of endogenous bmp4, msx1 and hand1 transcripts in the lpm and pharyngeal mesenchyme at stage 35 (Fig. 7A). The induced GR-Osr1 also resulted in upregulated/ectopic wnt2b, similar to LDN treatment and consistent with BMP signaling negatively regulating the wnt2b expression domain. However, similar to LDN-treated embryos, GR-mOsr1 was unable to expand nkx2.1 expression (Fig. 7A).
Fig. 7.
Osr1/Osr2 can repress bmp4 expression. (A) GR-mOsr1 RNA (750 pg) was injected into both dorsal-vegetal cells at the eight-cell stage and embryos were dexamethasone treated at stage 20 to induce the construct and analyzed at stage 34/35 for expression of the indicated genes. Insets show ventral views of wnt2b expression. (B) The X. laevis bmp4 promoter construct, showing two putative odd-skipped-binding sites. Green sequence indicates consensus odd skipped-binding site, red sequence is the exact bmp4 promoter sequence, and mutations to these sites are shown below indicated by ‘Δ’ and in black. (C) RNA (750 pg) encoding Xenopus, mouse or Drosophila Osr factors was co-injected with the –2123 bmp4: luciferase reporter at the 32-cell stage and luciferase activity was assayed at stage 12. Average relative activity ±s.d. (D) Xenopus Osr1 or Osr2 RNA (750 pg) was co-injected with the wild-type bmp4 reporter or the mutated reporter constructs indicated in B, and the resulting average fold repression ±s.d., of the luciferase reporter from three independent experiments was determined. *P<0.005.
An analysis of the Xenopus laevis bmp4 promoter DNA sequence revealed two putative Osr-binding sites (Meng et al., 2005) clustered at approximately –2kb from the transcription start site (Fig. 7B). To test whether Osr1/Osr2 might repress the bmp4 promoter, we injected a –2123bp bmp4:luciferase reporter construct that has previously been shown to drive bmp4 expression (Schuler-Metz et al., 2000) and tested the ability of Osr factors from Xenopus, mouse and Drosophila to repress the reporter. Luciferase assays at late gastrula stage (st12) indicated that the –2123 bmp4:luc construct was robustly expressed in animal caps and that this expression was dramatically downregulated by co-injection of RNA encoding Xenopus or mouse Osr1 and Osr2, as well as by the Drosophila orthlog Odd (Fig. 7C). By contrast, the Drosophila Odd construct lacking the Groucho interaction domain (OddΔeh1) failed to suppress the reporter (Fig. 7C), in agreement with studies showing Osr factors require Groucho co-factors in order to repress their targets (Goldstein et al., 2005). Finally, mutation of either the Osr-binding site sequences dramatically impaired the ability of Xenopus Osr1 or Osr2 to repress the bmp4 reporter (Fig. 7D). These data are consistent with the conclusion that Osr1/Osr2 normally repress bmp4 transcription in the wnt2/wnt2b-expressing lpm during respiratory development.
DISCUSSION
We have identified a novel foregut function for the zinc-finger transcriptional repressors Osr1 and Osr2, and show that they are key components of an epithelial-mesenchymal signaling cascade linking foregut patterning, respiratory specification and lung bud growth. Our data support a model (Fig. 8) in which foregut patterning by early FGF signaling is required to promote osr1 and osr2 expression in the foregut endoderm and surrounding lpm prior to lung specification. Osr1 and Osr2 are redundantly required for the specification of Nkx2.1+ respiratory progenitors and they act primarily by limiting bmp4 expression to the pharyngeal lpm just anterior to the presumptive lung. Our data indicate that in Osr1/Osr2 morphants elevated BMP/pSmad1 activity inhibits wnt2b, raldh2, sox2 and nkx2.1, and results in a failure of respiratory specification with trachea and lung agenesis. Downstream of Osr1/Osr2, canonical Wnt2/Wnt2b signaling is necessary and sufficient to induce pulmonary fate. Finally, our data suggest that Osr1 and/or Osr2 also act after specification to promote RA-regulated lung bud growth by maintaining raldh2.
Fig. 8.
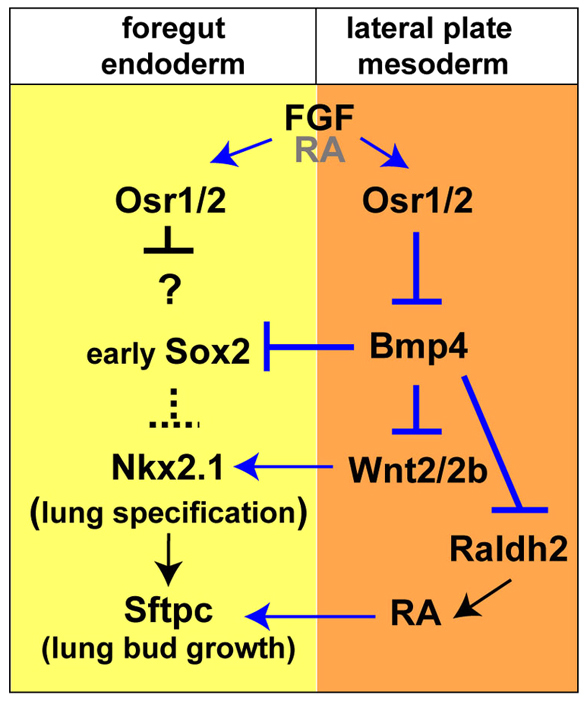
Model of the molecular pathway regulating Xenopus lung development. Osr1/Osr2 are key components of a molecular pathway regulating respiratory specification in Xenopus. Blue lines indicate relationships tested in this study and black lines indicate relationships predicted from published mouse studies.
A novel feature of our model is the importance of transcriptional repression in early lung development. Osr1 and Osr2 are known to interact with groucho co-repressors (Goldstein et al., 2005; Tena et al., 2007) and although future transgenic and ChIP studies will be needed to confirm that Osr1/Osr2 directly repress bmp4 transcription in the lpm, our data support this possibility. It is possible that BMP signaling indirectly suppresses wnt2/wnt2b expression through transcriptional repressors such as Msx1, which is a direct BMP target (Maeda et al., 1997) and is upregulated in Osr1/Osr2 morphants.
It is important to point out that our model focuses on the specification of respiratory fate that we have tested and is not meant to show all possible interactions. For example, the exact FGF ligands involved are unknown and we cannot exclude the possibility that, in addition to acting via Osr1/Osr2, FGF signaling also directly contributes to promoting nkx2.1 or wnt2b expression. Although our data suggest that Osr1/Osr2 act in the mesoderm, they are also expressed in the foregut endoderm and might impact the epithelial response to mesenchymal signals. Preliminary studies preferentially targeting the Osr1/Osr2-MOs to either the endoderm or mesoderm support a function in both tissues, but the mesoderm function of Osr1/Osr2 is essential (S.A.R., unpublished).
FGF, Wnt and BMP signals have multiple roles in later lung bud growth, differentiation and morphogenesis (Cardoso and Lu, 2006; Maeda et al., 2007; Morrisey and Hogan, 2010), and Osr1/Osr2 might similarly have multiple temporally distinct roles. For example, our data suggest that RA may function in a positive regulatory loop acting both upstream and downstream of Osr1/Osr2. Early RA signaling between stages 25-35 is required for robust osr1 and osr2 expression, whereas Osr1/Osr2 are essential for raldh2 expression, and RA signaling is required for later lung bud outgrowth. While our study was in review, Drysdale and colleagues demonstrated that RA inhibition at stage 20 in Xenopus resulted in a more dramatic loss of lung fate than we observed with RA inhibition at stage 25 (Wang et al., 2011). Further studies could define temporally distinct RA-Osr interactions. Similarly FGFs have multiple roles in lung bud growth after specification, which we have not addressed. For example FGF and WNT act in a feedback loop that controls growth of both lung epithelium and mesenchyme with Wnt2/Wnt2b being required for FGF10 expression (Yin et al., 2008; Goss et al., 2009; Goss et al., 2011). Future temporal studies are needed to determine whether Osr factors are involved in this crosstalk after specification.
Finally, there are almost certainly other players not yet incorporated into the model. For example, the winged-helix DNA-binding factor Rfx6 is required for Xenopus lung bud development, but how this fits into the known paradigms is unknown (Pearl et al., 2011). Shh signaling is also crucial in early mouse foregut development (Litingtung et al., 1998) and Gli2–/–;Gli3–/– double mutants have agenesis of the lungs, trachea and esophagus (Motoyama et al., 1998) similar to depletion of Osr1/Osr2. We found that shh, gli2 and gli3 expression were unchanged in Osr1/Osr2 morphants, suggesting that perhaps Shh also acts up stream of Osr. Consistent with this possibility, Hedgehog signaling is required for Osr2 expression in the embryonic mouse palate (Lan and Jiang, 2009), and inhibition of hedgehog signaling in Xenopus downregulates osr1/osr2 expression (S.A.R., unpublished).
A number of lines of evidence suggest that the model we propose is conserved in mammals: (1) key molecules in lung development (Hyatt et al., 2007; Yin et al., 2010) and the roles of FGF, Wnt and RA are remarkably conserved between Xenopus and mice; (2) mouse Osr1 and Osr2 are expressed at the right time and place to have a similar role. Osr1 is expressed in the lpm at E7.5 and in the foregut epithelium and surrounding splanchnic mesoderm at E9.5 (So and Danielian, 1999; Lan et al., 2001; Wang et al., 2005; Grieshammer et al., 2008). Osr2 is also weakly expressed is the foregut beginning E9.5 and later in the lung bud mesenchyme (Lan et al., 2001; Lan et al., 2004; Lan et al., 2007); (3) although Osr1–/– and Osr2–/– mutant embryos have lungs, the Osr1 mutants do exhibit lung hypoplasia (Rulang Jiang, personal communication), which has not been characterized, suggesting that Osr1 and Osr2 might redundantly regulate lung specification in mice. Consistent with this possibility, murine Osr1 and Osr2 are partially redundant in joint formation where they regulate Wnt4 and Wnt9b expression (Gao et al., 2011), and Osr2 is required to negatively regulate Bmp4 and Msx1 during tooth development (Zhang et al., 2009). Furthermore, we have recently found that, in zebrafish, Osr1/Osr2 also regulates wnt2b expression in lpm during fin development (Neto et al., 2012), suggesting that this regulatory cassette is employed in many tissues.
Our finding that FGF signaling is required for Xenopus lung specification in vivo is consistent with in vitro mouse embryonic foregut explants where exogenous FGF ligands induce pulmonary fate and FGF antagonists inhibit lung development (Serls et al., 2005). Importantly, we show that early FGF acts not only in the epithelium but also in the mesoderm and that FGF functions upstream of Osr1/Osr2 and Wnt-mediated lung specification. Our observation that β-catenin signaling is necessary and sufficient to induce nkx2.1+ lung progenitors in Xenopus is identical to reports in mice in which β-catenin is genetically deleted or activated (Goss et al., 2009; Harris-Johnson et al., 2009). Moreover, our data suggesting that nkx2.1 is a direct transcriptional target of canonical Wnt signaling are consistent with a recent report that β-catenin/Tcf directly stimulated Nkx2.1 transcription in human thyroid carcinoma cells (Gilbert-Sirieix et al., 2011).
An important novel finding in our study is the need for BMP repression to allow Wnt-mediated respiratory specification. Genetic studies in mice agree with this and show that BMP signaling is dispensable for lung specification, promotes later tracheal development and separation of the foregut tube (Eblaghie et al., 2006; Que et al., 2006; Li et al., 2008; Sun et al., 2008; Xu et al., 2011). During this process in mice, Sox2 is initially expressed throughout the foregut epithelium and then around E9.5 BMP signaling suppresses Sox2 and esophagus fate in the ventral foregut. Mutual repression between Sox2 and Nkx2.1 then resolves the two lineages (Que et al., 2006; Li et al., 2008; Domyan et al., 2011). Our data suggest that BMP has a similar role in Xenopus; BMP signaling was not required for Xenopus lung specification but is necessary to maintain later tracheal fate (supplementary material Fig. S8). Most importantly, we found that excessive BMP signaling in Osr1/Osr2 morphants (or overexpression of Smad1) reduced wnt2b, nkx2.1 and early pan-foregut sox2 expression. Interestingly, we found that BMP inhibition or Osr overexpression could expand wnt2b; however, this was not sufficient to induce ectopic nkx2.1+ respiratory tissue. In both these conditions, we found that Sox2 was also highly upregulated (S.A.R., unpublished), and this may explain why nkx2.1 was unchanged, as Sox2 is known to be inhibitory to Nkx2.1 (Que et al., 2007; Domyan et al., 2011).
Finally, the results of this study may impact efforts to direct the differentiation of respiratory lineages from human stem cells. The best successes in making human tissue in vitro have come from recapitulating embryonic paradigms in culture (Spence and Wells, 2007; Green et al., 2011), which is an approach that requires a detailed understanding of the temporal-spatial dynamics of cell signaling during normal organogenesis. Our results suggest that co-culture of Osr-expressing mesenchymal cells and suppression of BMP in addition to Wnt at the right time may facilitate induction of pulmonary progenitors.
Supplementary Material
Acknowledgments
We are grateful to Drs DeSimone, Han and Krieg for reagents. We thank the members of the Zorn and Wells labs, and the endoderm club for discussion, particularly Jeff Whitsett, John Shannon, Rulang Jiang and Jim Wells.
Footnotes
Funding
This project was supported by National Institutes of Health (NIH) [DK70858 to A.M.Z.] and by the Spanish and Andalusian Governments [BFU2010-14839, CSD2007-00008 and Proyecto de Excelencia CVI-3488 to J.L.G-S.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.078220/-/DC1
References
- Beenken A., Mohammadi M. (2009). The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso W. V., Lü J. (2006). Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611–1624 [DOI] [PubMed] [Google Scholar]
- Chen F., Desai T. J., Qian J., Niederreither K., Lü J., Cardoso W. V. (2007). Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134, 2969–2979 [DOI] [PubMed] [Google Scholar]
- Chen F., Cao Y., Qian J., Shao F., Niederreither K., Cardoso W. V. (2010). A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Invest. 120, 2040–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimling S. J., Drysdale T. A. (2009). Retinoic acid regulates anterior-posterior patterning within the lateral plate mesoderm of Xenopus. Mech. Dev. 126, 913–923 [DOI] [PubMed] [Google Scholar]
- Deimling S. J., Drysdale T. A. (2011). Fgf is required to regulate anterior-posterior patterning in the Xenopus lateral plate mesoderm. Mech. Dev. 128, 327–341 [DOI] [PubMed] [Google Scholar]
- Desai T. J., Malpel S., Flentke G. R., Smith S. M., Cardoso W. V. (2004). Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev. Biol. 273, 402–415 [DOI] [PubMed] [Google Scholar]
- Domyan E. T., Ferretti E., Throckmorton K., Mishina Y., Nicolis S. K., Sun X. (2011). Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 138, 971–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblaghie M. C., Reedy M., Oliver T., Mishina Y., Hogan B. L. (2006). Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev. Biol. 291, 67–82 [DOI] [PubMed] [Google Scholar]
- Gao Y., Lan Y., Ovitt C. E., Jiang R. (2009). Functional equivalence of the zinc finger transcription factors Osr1 and Osr2 in mouse development. Dev. Biol. 328, 200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Lan Y., Liu H., Jiang R. (2011). The zinc finger transcription factors Osr1 and Osr2 control synovial joint formation. Dev. Biol. 352, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock R. J., Warkman A. S., Meadows S. M., D’Agostino S., Krieg P. A. (2007) Census of vertebrate Wnt genes: isolation and developmental expression of Xenopus Wnt2, Wnt3, Wnt9a, Wnt9b, Wnt10a, and Wnt16. Dev. Dyn. 236, 1249–1258 [DOI] [PubMed] [Google Scholar]
- Gilbert-Sirieix M., Makoukji J., Kimura S., Talbot M., Caillou B., Massaad C., Massaad-Massade L. (2011). Wnt/β-catenin signaling pathway is a direct enhancer of thyroid transcription factor-1 in human papillary thyroid carcinoma cells. PLoS ONE 6, e22280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. E., Cook O., Dinur T., Pisanté A., Karandikar U. C., Bidwai A., Paroush Z. (2005). An eh1-like motif in odd-skipped mediates recruitment of Groucho and repression in vivo. Mol. Cell. Biol. 25, 10711–10720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A. M., Tian Y., Tsukiyama T., Cohen E. D., Zhou D., Lu M. M., Yamaguchi T. P., Morrisey E. E. (2009). Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 17, 290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. D., Chen A., Nostro M. C., d’Souza S. L., Schaniel C., Lemischka I. R., Gouon-Evans V., Keller G., Snoeck H. W. (2011). Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 29, 267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. B., Hatini V., Johansen K. A., Liu X. J., Lengyel J. A. (2002). Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development 129, 3645–3656 [DOI] [PubMed] [Google Scholar]
- Grieshammer U., Agarwal P., Martin G. R. (2008). A Cre transgene active in developing endodermal organs, heart, limb, and extra-ocular muscle. Genesis 46, 69–73 [DOI] [PubMed] [Google Scholar]
- Harris-Johnson K. S., Domyan E. T., Vezina C. M., Sun X. (2009). beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA 106, 16287–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horb M. E., Slack J. M. (2001). Endoderm specification and differentiation in Xenopus embryos. Dev. Biol. 236, 330–343 [DOI] [PubMed] [Google Scholar]
- Huang S. M., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., et al. (2009). Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 [DOI] [PubMed] [Google Scholar]
- Hyatt B. A., Resnik E. R., Johnson N. S., Lohr J. L., Cornfield D. N. (2007). Lung specific developmental expression of the Xenopus laevis surfactant protein C and B genes. Gene Expr. Patterns 7, 8–14 [DOI] [PubMed] [Google Scholar]
- James R. G., Kamei C. N., Wang Q., Jiang R., Schultheiss T. M. (2006). Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133, 2995–3004 [DOI] [PubMed] [Google Scholar]
- Johansen K. A., Green R. B., Iwaki D. D., Hernandez J. B., Lengyel J. A. (2003). The Drm-Bowl-Lin relief-of-repression hierarchy controls fore- and hindgut patterning and morphogenesis. Mech. Dev. 120, 1139–1151 [DOI] [PubMed] [Google Scholar]
- Karner C. M., Merkel C. E., Dodge M., Ma Z., Lu J., Chen C., Lum L., Carroll T. J. (2010). Tankyrase is necessary for canonical Wnt signaling during kidney development. Dev. Dyn. 239, 2014–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha M. K., Chung C., Bustamante E. L., Gaw L. W., Trott K. A., Yeh J., Lim N., Lin J. C., Taverner N., Amaya E., et al. (2002). Techniques and probes for the study of Xenopus tropicalis development. Dev. Dyn. 225, 499–510 [DOI] [PubMed] [Google Scholar]
- Kim H., Han J. K. (2011) Rab3d is required for Xenopus anterior neurulation by regulating Noggin secretion. Dev. Dyn. 240, 1430–1439 [DOI] [PubMed] [Google Scholar]
- Lan Y., Jiang R. (2009). Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development 136, 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Kingsley P. D., Cho E. S., Jiang R. (2001). Osr2, a new mouse gene related to Drosophila odd-skipped, exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech. Dev. 107, 175–179 [DOI] [PubMed] [Google Scholar]
- Lan Y., Ovitt C. E., Cho E. S., Maltby K. M., Wang Q., Jiang R. (2004). Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development 131, 3207–3216 [DOI] [PubMed] [Google Scholar]
- Lan Y., Wang Q., Ovitt C. E., Jiang R. (2007). A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis 45, 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro D., Price M., de Felice M., Di Lauro R. (1991). The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113, 1093–1104 [DOI] [PubMed] [Google Scholar]
- Li Y., Gordon J., Manley N. R., Litingtung Y., Chiang C. (2008). Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev. Biol. 322, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y., Lei L., Westphal H., Chiang C. (1998). Sonic hedgehog is essential to foregut development. Nat. Genet. 20, 58–61 [DOI] [PubMed] [Google Scholar]
- Maeda R., Kobayashi A., Sekine R., Lin J. J., Kung H., Maéno M. (1997). Xmsx-1 modifies mesodermal tissue pattern along dorsoventral axis in Xenopus laevis embryo. Development 124, 2553–2560 [DOI] [PubMed] [Google Scholar]
- Maeda Y., Davé V., Whitsett J. A. (2007). Transcriptional control of lung morphogenesis. Physiol. Rev. 87, 219–244 [DOI] [PubMed] [Google Scholar]
- Matsushita S., Urase K., Komatsu A., Scotting P. J., Kuroiwa A., Yasugi S. (2008). Foregut endoderm is specified early in avian development through signal(s) emanating from Hensen’s node or its derivatives. Mech. Dev. 125, 377–395 [DOI] [PubMed] [Google Scholar]
- McLin V. A., Rankin S. A., Zorn A. M. (2007). Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 134, 2207–2217 [DOI] [PubMed] [Google Scholar]
- Meng X., Brodsky M. H., Wolfe S. A. (2005). A bacterial one-hybrid system for determining the DNA-binding specificity of transcription factors. Nat. Biotechnol. 23, 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P., Su G., Drum H., Bringas P., Kimura S. (1999). Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(–/–) mouse embryos. Dev. Biol. 209, 60–71 [DOI] [PubMed] [Google Scholar]
- Molenaar M., van de Wetering M., Oosterwegel M., Peterson-Maduro J., Godsave S., Korinek V., Roose J., Destrée O., Clevers H. (1996). XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 [DOI] [PubMed] [Google Scholar]
- Morrisey E. E., Hogan B. L. (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudumana S. P., Hentschel D., Liu Y., Vasilyev A., Drummond I. A. (2008). odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development 135, 3355–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto A., Mercader N., Gomez-Skarmeta J. L. (2012). The Osr1 and Osr2 genes act in the pronephric anlage downstream of retinoic acid signaling and upstream of Wnt2b to maintain pectoral fin development. Development 139, 301–311 [DOI] [PubMed] [Google Scholar]
- Pearl E. J., Jarikji Z., Horb M. E. (2011). Functional analysis of Rfx6 and mutant variants associated with neonatal diabetes. Dev. Biol. 351, 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Choi M., Ziel J. W., Klingensmith J., Hogan B. L. (2006). Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation 74, 422–437 [DOI] [PubMed] [Google Scholar]
- Que J., Okubo T., Goldenring J. R., Nam K. T., Kurotani R., Morrisey E. E., Taranova O., Pevny L. H., Hogan B. L. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S. A., Kormish J., Kofron M., Jegga A., Zorn A. M. (2011). A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev. Biol. 351, 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A. H. (2004). Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 [DOI] [PubMed] [Google Scholar]
- Schuler-Metz A., Knöchel S., Kaufmann E., Knöchel W. (2000). The homeodomain transcription factor Xvent-2 mediates autocatalytic regulation of BMP-4 expression in Xenopus embryos. J. Biol. Chem. 275, 34365–34374 [DOI] [PubMed] [Google Scholar]
- Sekine K., Ohuchi H., Fujiwara M., Yamasaki M., Yoshizawa T., Sato T., Yagishita N., Matsui D., Koga Y., Itoh N., et al. (1999). Fgf10 is essential for limb and lung formation. Nat. Genet. 21, 138–141 [DOI] [PubMed] [Google Scholar]
- Serls A. E., Doherty S., Parvatiyar P., Wells J. M., Deutsch G. H. (2005). Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development 132, 35–47 [DOI] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M., Harland R. M. (2000) Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Small E. M., Vokes S. A., Garriock R. J., Li D., Krieg P. A. (2000). Developmental expression of the Xenopus Nkx2-1 and Nkx2-4 genes. Mech. Dev. 96, 259–262 [DOI] [PubMed] [Google Scholar]
- So P. L., Danielian P. S. (1999). Cloning and expression analysis of a mouse gene related to Drosophila odd-skipped. Mech. Dev. 84, 157–160 [DOI] [PubMed] [Google Scholar]
- Spence J. R., Wells J. M. (2007). Translational embryology: using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Dev. Dyn. 236, 3218–3227 [DOI] [PubMed] [Google Scholar]
- Sun J., Chen H., Chen C., Whitsett J. A., Mishina Y., Bringas P., Jr, Ma J. C., Warburton D., Shi W. (2008). Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am. J. Pathol. 172, 571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena J. J., Neto A., de la Calle-Mustienes E., Bras-Pereira C., Casares F., Gómez-Skarmeta J. L. (2007). Odd-skipped genes encode repressors that control kidney development. Dev. Biol. 301, 518–531 [DOI] [PubMed] [Google Scholar]
- Wang J. H., Deimling S. J., D’Alessandro N. E., Zhao L., Possmayer F., Drysdale T. A. (2011). Retinoic acid is a key regulatory switch determining the difference between lung and thyroid fates in Xenopus laevis. BMC Dev. Biol. 11, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Lan Y., Cho E. S., Maltby K. M., Jiang R. (2005). Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev. Biol. 288, 582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Dollé P., Cardoso W. V., Niederreither K. (2006). Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 297, 433–445 [DOI] [PubMed] [Google Scholar]
- Xu B., Chen C., Chen H., Zheng S. G., Bringas P., Jr, Xu M., Zhou X., Chen D., Umans L., Zwijsen A., et al. (2011). Smad1 and its target gene Wif1 coordinate BMP and Wnt signaling activities to regulate fetal lung development. Development 138, 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin A., Winata C. L., Korzh S., Korzh V., Gong Z. (2010). Expression of components of Wnt and Hedgehog pathways in different tissue layers during lung development in Xenopus laevis. Gene Expr. Patterns 10, 338–344 [DOI] [PubMed] [Google Scholar]
- Yu P. B., Deng D. Y., Lai C. S., Hong C. C., Cuny G. D., Bouxsein M. L., Hong D. W., McManus P. M., Katagiri T., Sachidanandan C., et al. (2008). BMP type I receptor inhibition reduces heterotopic ossification. Nat. Med. 14, 1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lan Y., Chai Y., Jiang R. (2009). Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science 323, 1232–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.