Abstract
The dorsoventral (DV) axis of the Drosophila embryo is patterned by a nuclear gradient of the Rel family transcription factor, Dorsal (Dl), that activates or represses numerous target genes in a region-specific manner. Here, we demonstrate that signaling by receptor tyrosine kinases (RTK) reduces nuclear levels and transcriptional activity of Dl, both at the poles and in the mid-body of the embryo. These effects depend on wntD, which encodes a Dl antagonist belonging to the Wingless/Wnt family of secreted factors. Specifically, we show that, via relief of Groucho- and Capicua-mediated repression, the Torso and EGFR RTK pathways induce expression of WntD, which in turn limits Dl nuclear localization at the poles and along the DV axis. Furthermore, this RTK-dependent control of Dl is important for restricting expression of its targets in both contexts. Thus, our results reveal a new mechanism of crosstalk, whereby RTK signals modulate the spatial distribution and activity of a developmental morphogen in vivo.
Keywords: Dorsal, Drosophila, Gene regulation, Negative feedback, RTK signaling, WntD
INTRODUCTION
Dorsoventral (DV) patterning in the Drosophila embryo depends on the ventral-to-dorsal nuclear concentration gradient of Dorsal (Dl) (Rogers and Schier, 2011). Dl is a Rel family transcription factor that regulates the expression of over 50 target genes in a concentration-dependent manner (Reeves and Stathopoulos, 2009). Region-specific transcriptional control by nuclear Dl subdivides the embryo into three germ layers: regions exposed to high, medium and low levels of nuclear Dl ultimately give rise to mesoderm, neuroectoderm and dorsal ectoderm, respectively (Chopra and Levine, 2009; Stathopoulos and Levine, 2002).
Dl is a bi-functional transcription factor that either activates or represses expression of its targets, depending on promoter context. It activates mesoderm-determining genes such as twist and snail (sna) (Ip et al., 1992; Jiang et al., 1991; Pan et al., 1991); yet, in the same nuclei it also represses, together with auxiliary proteins, the expression of dorsalizing genes such as decapentaplegic (dpp) and zerknüllt (zen) (Huang et al., 1993; Jiang et al., 1991). Notably, both modes of Dl-dependent transcriptional regulation appear inactivated at the embryonic poles where, correspondingly, sna is not expressed and dpp and zen are transcribed.
Previous work has suggested that the Torso RTK pathway affects expression of Dl targets at the termini (Casanova, 1991; Goldstein et al., 1999; Häder et al., 2000; Rusch and Levine, 1994). Torso-mediated signaling specifies terminal cell fates by locally activating mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk), which then phosphorylates and downregulates the general repressors Capicua (Cic) and Groucho (Gro) (Astigarraga et al., 2007; Cinnamon et al., 2008; Goff et al., 2001; Häder et al., 2000; Jiménez et al., 2000; Liaw et al., 1995; Paroush et al., 1997). Cic and Gro have been implicated in repression of zen and dpp (Dubnicoff et al., 1997; Jiménez et al., 2000), providing a mechanism by which Torso controls Dl targets. In addition, Torso signaling induces other genes such as tailless (tll) and huckebein (hkb) (Brönner and Jäckle, 1991; Pignoni et al., 1990), and Hkb represses sna transcription at the termini (Reuter and Leptin, 1994; Goldstein et al., 1999). In both cases, the Torso pathway impinges on the transcriptional interpretation of the Dl gradient.
Here, we demonstrate that the Torso pathway also modulates the Dl gradient itself. We find that by downregulating Cic and Gro repression, Torso signaling induces expression of wnt inhibitor of Dorsal (wntD), a gene belonging to the Wingless/Wnt family and encoding a Dl antagonist (Ganguly et al., 2005; Gordon et al., 2005). As wntD is also positively regulated by Dl (Ganguly et al., 2005; Gordon et al., 2005; Zeitlinger et al., 2007), its expression occurs at the intersection between the domains of Dl and activated MAPK/Erk, where it reduces the nuclear levels of Dl. Using loss and gain-of-function assays, we show that Torso signaling acts as a gating mechanism that restricts expression of multiple Dl target genes at the poles. Remarkably, a similar mechanism operates in the trunk region: Dl and EGFR signaling induce WntD, which in turn downregulates Dl and limits expression of its targets along the DV axis. In both contexts, inactivation of wntD results in altered expression patterns of multiple Dl targets. Our results thus identify wntD as a crucial node for crosstalk between RTK signaling and the Dl morphogen.
MATERIALS AND METHODS
Fly culture and stocks
Flies were cultured and crossed on yeast-cornmeal-molasses-malt extract-agar medium at 25°C. The following mutant alleles and Gal4 drivers were used: wntDKO1 (a kind gift from Mark McElwain and Raul Nusse, Stanford University, CA, USA), Egfrf2, rhove vn1, nos-Gal4-VP16, UASp-GroAA (Cinnamon et al., 2008; Helman et al., 2011), CicΔC2 (Astigarraga et al., 2007), cic1, trk1 and torY9. Embryos lacking maternal gro, ras and DSor activities were derived from mosaic groE48; ras1e2f and DSorLH110 (FlyBase) mutant germlines, respectively.
In situ hybridization and antibody staining
Embryos were dechorionated in bleach and fixed in 8% formaldehyde/PBS/heptane for 15-20 minutes. Expression patterns of sog, l’sc and wntD were visualized by whole-mount in situ hybridization using digoxigenin-UTP-labeled antisense RNA probes and anti-digoxigenin antibodies conjugated to alkaline phosphatase (Roche). Fluorescence in situ hybridization for sna and vnd was performed as described elsewhere (Kim et al., 2011).
Fluorescent immunodetection of activated MAPK/Erk in freshly fixed embryos (10% formaldehyde/PBS/Heptane buffer) was attained using rabbit αdpERK (1:100; Cell Signaling) (Helman and Paroush, 2010). Other antibodies used were: mouse αDorsal (1:100; Developmental Studies Hybridoma Bank), rabbit αInd (1:1000; kindly provided by Tonia von Ohlen, Kansas State University, USA), rat αVnd (1:1000) (Helman et al., 2011), rat αOdd (1:200; Asian Distribution Center for Segmentation Antibodies, Mishima, Japan), rabbit αLamin (1:500; kindly provided by Yosef Gruenbaum, The Hebrew University of Jerusalem, Israel), guinea pig αBrk and αSna (1:500 and 1:200, respectively; kindly provided by Jessica Cande (IBDML, Marseille, France) and Mike Levine (UC Berkeley, USA) and sheep anti-DIG (1:200; Roche). Secondary antibodies were FITC- (1:2000), rhodamine- (1:2000) or Cy5-conjugated (1:800) (Jackson Laboratories). Embryos were mounted using DakoCytomation medium.
Microscopy and imaging
To minimize nonspecific effects caused by differential antibody or RNA probe concentrations and/or duration of staining reactions, wild-type control embryos expressing Histone-GFP (distinguishable by GFP expression) were mixed together with mutant embryos and simultaneously fixed and processed. Embryos were visualized, at ×20 and ×40 magnification, using a TE2000 inverted confocal laser scanning system (Nikon, Tokyo, Japan). Consecutive Z stakes were taken using a small aperture and converged to create a single image using EZ-C1 software (Nikon).
Imaging for quantification was performed on a Zeiss LSM510 confocal microscope. For lateral imaging of embryos, Zeiss 20×A-plan objective (NA=0.6) was used and images were obtained from a focal plane in the mid-sagittal plan cross section of an embryo. For end-on imaging, Zeiss 40× C-Apo water-immersion objective (NA=1.2) was used and images were collected from a focal plane ∼75 μm from either the anterior or posterior pole of an embryo.
To minimize variability brought about by the dynamics of the Dl gradient, the distribution of nuclear Dl and expression of its targets was quantified in embryos at late nuclear cycle 14, just before gastrulation. This stage was determined based on the appearance of ventrolateral dpERK staining and on the elongated, oval shape of nuclei (revealed by DAPI staining), both of which are observable ∼30 minutes after the onset of cycle 14. Embryos doubly mutant for rho and vn do not stain for dpERK, and were therefore staged only by the shape of their nuclei.
Spatial gradients of nuclear Dl and dpERK, or of wntD and sna mRNA, were extracted from confocal images of stained embryos using the previously described MATLAB image processing program (Kanodia et al., 2011). For imaging nuclear Dl gradients, DAPI staining was used as a nuclear mask to indicate the position of nuclei. The mask was subsequently used to quantify the nuclear concentration of Dl protein along the ventral-to-dorsal axis. For dpERK, wntD and sna expression profiles, cytoplasmic signals were also quantified.
For lateral imaging, images were pre-oriented so that the measurement starts from the mid-ventral point of an embryo. For end-on imaging, the raw nuclear Dl gradient was fitted to a Gaussian curve and the fits were used to find the ventral-most position of the embryo, which corresponds to the maximum of the fit. For each embryo, two values of the nuclear Dl gradient were extracted (from left and right of the ventral-most point, up to the dorsal side).
RESULTS
Torso signaling excludes expression of multiple Dorsal target genes from the embryonic poles
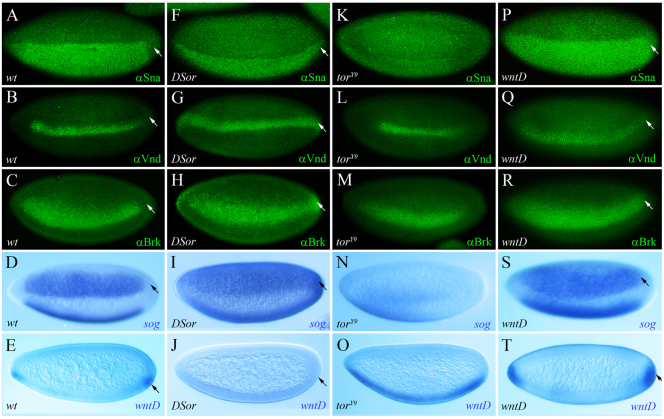
As indicated above, previous studies had shown that expression of two Dl target genes, sna and zen, is controlled by Torso signaling at the level of transcription. Correspondingly, the Sna protein is excluded from the termini of wild-type embryos (particularly from the posterior pole), but is detectable in the termini of DSor (Drosophila MEK) mutant embryos (Fig. 1A,F, respectively). To determine whether the Torso pathway influences Dl-mediated transcriptional activity more broadly, we analyzed the expression profiles of multiple Dl target genes in embryos lacking Ras/MAPK signaling activity. We find that additional primary Dl targets, typically transcribed in the presumptive neuroectoderm, are ectopically expressed at the pole regions of DSor mutant embryos. One example is ventral nervous system defective (vnd), a gene normally activated by Dl in two ventrolateral longitudinal stripes, one on either side, that extend along the trunk region of the embryo (Cowden and Levine, 2003; von Ohlen and Doe, 2000). The Vnd protein is never detected in terminal regions of wild-type embryos (Fig. 1B), but in the absence of MAPK/Erk activity it expands to the termini (Fig. 1G). Similarly, other Dl targets, such as Brinker (Brk) and short gastrulation (sog), the expression of which is normally restricted to medial regions of the embryo at this stage (Fig. 1C,D) (Markstein et al., 2004; Zhang et al., 2001), are detected throughout the anterior and posterior tips of DSor mutants (Fig. 1H,I).
Fig. 1.
Torso RTK signaling restricts expression of multiple Dorsal targets at the embryonic termini. (A-T) Lateral view of stage 5 wild-type (A-E), DSor (F-J), torY9 (K-O) and wntD (P-T) mutant embryos, immunostained for Sna (A,F,K,P), Vnd (B,G,L,Q) and Brk (C,H,M,R), or hybridized using digoxigenin-labeled RNA probes for sog (D,I,N,S) and wntD (E,J,O,T). Torso-dependent activity limits the expression of Dl targets at the poles. (A-D) Expression of four Dl targets, Sna, Vnd, Brk and sog, is confined to the trunk region and is excluded from the termini. (F-I) In DSor mutant embryos, where Torso signaling is blocked, expression of these Dl targets expands into terminal regions. (K-N) In torY9 embryos, where Torso is overactive, expression of these Dl targets retracts towards more central locations. (E,J,O) Torso signaling regulates wntD expression. Expression of wntD, normally observed in ventro-terminal positions (E), is lost in DSor mutants (J) and expands in torY9 embryos throughout the ventral region (O). (P-T) The Dl targets Sna, Vnd, Brk, sog and wntD are ectopically expressed at the poles of wntD mutants, where Dl is nuclear owing to the lack of functional WntD (see Fig. 2). Embryos are oriented with anterior to the left and dorsal side upwards. Arrows point to the posterior pole.
To confirm that these effects result from loss of Torso pathway activity, we also analyzed expression of the Dl target, Brk, in trunk mutant embryos, where Torso is never activated (Furriols et al., 1996). In this genetic background, Brk is expressed at the embryonic termini (supplementary material Fig. S1B). Conversely, the expression domains of Sna, Vnd, Brk and sog all retract to more central positions in torY9 embryos, where Torso is overactivated (Fig. 1K-N) (Halfar et al., 2001). These results support the idea that Torso signaling restricts expression of Dl-regulated genes at the embryonic poles.
Torso signaling opposes nuclear localization of Dorsal at the termini
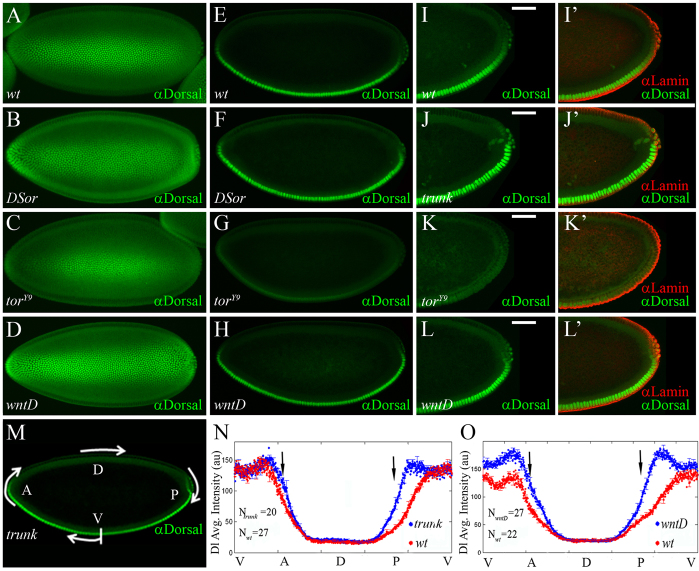
One possible explanation of the above results is that Torso signaling induces a transcriptional repressor that negatively regulates multiple Dl-activated genes at the termini (see Discussion). However, Torso signaling might also act at the level of Dl itself. To test the latter alternative, we compared the subcellular localization of Dl in wild-type and mutant DSor or torY9 embryos. In stage 4 wild-type embryos, a gradient of nuclear Dl forms along the DV axis (Chung et al., 2011; Kanodia et al., 2011), but graded distribution of nuclear Dl is also evident along the anteroposterior axis: Dl is largely nuclear at the center of the embryo, but this accumulation declines towards the termini (Fig. 2A,E,I). We find that in DSor mutants, where Torso signaling is abolished, Dl is nuclear even in terminal regions (Fig. 2B,F), an effect that is also observed in trunk mutants (Fig. 2J,M; see Fig. 2N and supplementary material Fig. S2A for quantification of nuclear Dl in trunk versus wild-type embryos). Reciprocally, torY9 mutant embryos exhibit reduced levels of nuclear Dl, even in ventro-central positions (Fig. 2C,G,K). Collectively, these results indicate that Ras/MAPK signaling negatively regulates nuclear levels of Dl.
Fig. 2.
The Torso pathway antagonizes nuclear localization of Dorsal. (A-M) Stage 5 embryos stained for Dl (green). (A-D) Confocal z-stack images of ventral views, with anterior towards the left. (I-L) High magnification views of posterior poles of embryos stained for Dl (green). (I′-L′) Embryos were also co-stained for Lamin (red), showing that images truly correspond to sagittal cross-sections. Scale bars: 50 μm. (A,E,I,I′) Wild-type embryos. Dl is nuclear on the ventral side and cytoplasmic dorsally. Note the declining nuclear Dl accumulation towards the termini. (B,F) DSor mutants. (J,J′) trunk mutants. Dl is nuclear at the termini. (C,G,K,K′) torY9 embryos. The domain of nuclear Dl retracts towards the center of the embryo. (D,H,L,L′) wntD mutants. Dl is nuclear at the poles, as in DSor and trunk embryos. (M-O) Quantification of nuclear Dl levels. (M) trunk mutant. The arrows indicate the clockwise, left-to-right direction of quantitative measurements of levels of nuclear Dl, presented in the graphs. (E-M) Embryos are oriented with anterior to the left and dorsal side upwards. (N,O) Quantifying nuclear Dl gradients in wild-type and mutant embryos. Solid line designates the average gradient; error bars indicate s.e.m. Levels of nuclear Dl are significantly higher at the anterior and the posterior poles (black arrows) of trunk (N; blue line; n=20 embryos) and wntD (O; blue line; n=27 embryos) mutants, compared with wild-type embryos (red lines; n=27 and 22 embryos, respectively), suggesting that Torso-induced WntD antagonizes nuclear accumulation of Dl at the termini.
Torso signaling induces expression of the Dorsal feedback inhibitor wntD
How could the Torso pathway affect the nuclear localization of Dl? Two reasons led us to consider the possibility that WntD, a novel member of the Wingless/Wnt family of secreted factors, links Torso signaling to Dl. One, the wntD gene, known to be activated by Dl, is transcribed in the ventral part of both embryonic poles at stage 4, when the Torso pathway is active (Fig. 1E) (Ganguly et al., 2005; Gordon et al., 2005; Zeitlinger et al., 2007); its expression, therefore, might also require a positive input by the Torso pathway. Two, wntD encodes an antagonist of Dl nuclear localization, and could thus impinge on the expression of various Dl targets (Ganguly et al., 2005; Gordon et al., 2005). We therefore hypothesized that wntD expression is induced by both Torso signaling and Dl, and that subsequently WntD activity decreases nuclear Dl at the termini.
To test this model, we triple stained wild-type embryos for wntD expression, nuclear Dl and doubly phosphorylated MAPK/Erk (dpERK) that serves as readout for Torso signaling activity. This showed that wntD is expressed precisely where nuclear Dl and dpERK intersect (Fig. 3A,A′). Furthermore, quantification of these images showed that, indeed, levels of wntD transcripts inversely correlate with levels of nuclear Dl in ventral positions of both poles, consistent with downregulation of Dl by WntD (Fig. 3B). We also found that wntD expression is absent at the termini of DSor and trunk mutant embryos (Fig. 1J and supplementary material Fig. S1D), whereas ectopic wntD transcription occurs along the ventral side of torY9 embryos, overlapping with the domain of nuclear Dl (Fig. 1O and supplementary material Fig. S3). It is notable that these wntD responses are unique, given that expression of other positively regulated Dl targets expands or retracts when Torso signaling is abrogated or overactivated, respectively (Fig. 1F-I,K-N).
Fig. 3.
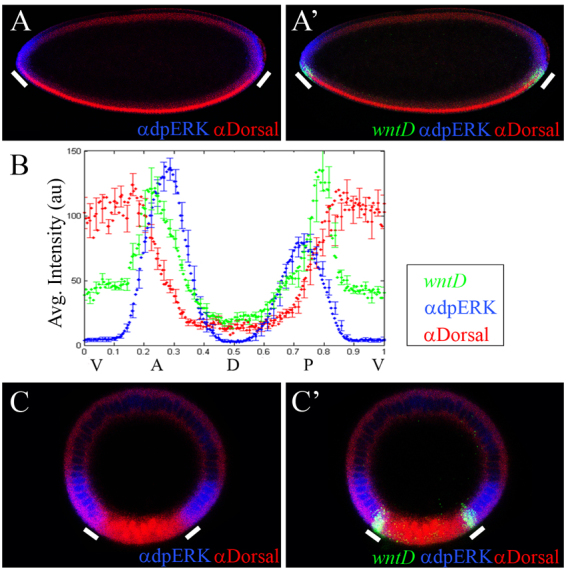
wntD is expressed at the intersection of RTK signaling and Dorsal activity. (A-C′) Wild-type embryos, triple stained for wntD transcripts (green), Dl (red) and dpERK (blue). (A,A′) Sagittal cross-sections of a stage 4 embryo. At this stage, dpERK staining reflects the activity of the Torso pathway. White bars indicate domains of wntD expression. (B) Quantification of wntD expression (green), nuclear Dl (red) and dpERK (blue) in sagittal cross-sections of stage 4 wild-type embryos (n=14 embryos). Error bars indicate s.e.m. wntD is expressed at the junction of the two inputs and there is inverse correlation between the amounts of wntD and nuclear Dorsal. (C,C′) Cross-section views of a stage 6 wild-type embryo. At this stage, dpERK staining reflects MAPK/Erk activation that is dependent on EGFR signaling. wntD is expressed at the point of intersection of the domains of nuclear Dl and activated MAPK/Erk. (A,B) Embryos are oriented with anterior to the left and dorsal side upwards.
Significantly, in wntD mutants the expression of multiple Dl targets expands towards the termini (Fig. 1P-T and supplementary material Fig. S4), consistent with the accumulation of nuclear Dl at this position (Fig. 2D,H,L; see Fig. 2O and supplementary material Fig. S2B for quantification of nuclear Dl in wntD versus wild-type embryos). Thus, our data indicate that Torso signaling, acting via wntD, downregulates Dl nuclear levels and expression of its targets at the embryonic poles. At the same time, these effects are milder than those observed in DSor and trunk mutants (Fig. 1F-I and supplementary material Fig. S1), suggesting the existence of additional mechanism(s) by which Torso negatively regulates Dl targets (see Discussion).
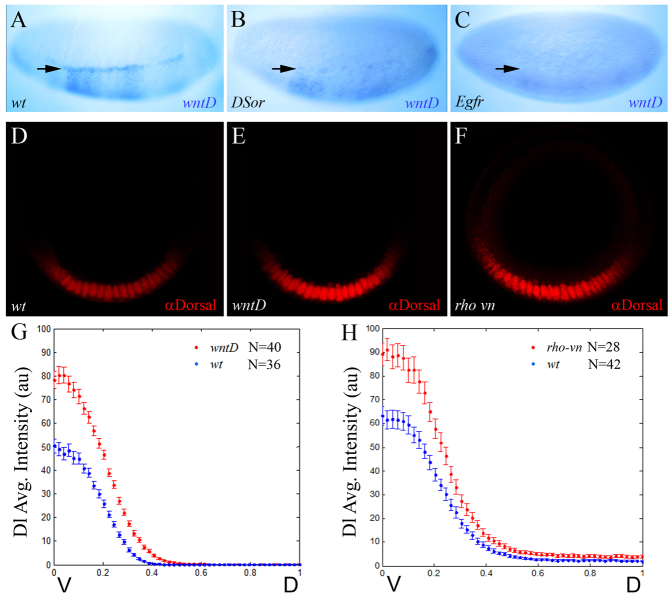
EGFR signaling induces wntD expression and reshapes the gradient of nuclear Dorsal
Later in development, at stage 5/6, expression of wntD delineates the border between the presumptive mesoderm and neuroectoderm, where both Dl and EGFR RTK activities converge (Fig. 3C,C′) (Ganguly et al., 2005; Gordon et al., 2005). Here, too, we find that wntD expression is lost in either DSor or Egfr mutant embryos (Fig. 4A-C), as in dl mutants (Ganguly et al., 2005), indicating that wntD is activated by EGFR and Dl in combination, and that both regulatory inputs are required. We therefore asked whether, similar to the Torso pathway, EGFR signaling also acts through WntD to influence the Dl gradient. To this end, we quantified levels of nuclear Dl in wild-type embryos, and in wntD and rhomboid vein mutants (rho vn; in this genetic background, EGFR ligands are absent and the pathway is inactive). To minimize the influence of dynamic changes in formation of the Dl gradient, the distribution of Dl was quantified specifically in stage 6 embryos at late nuclear cycle 14, right before gastrulation (see Materials and methods). Strikingly, we find that levels of nuclear Dl are significantly higher in wntD and rho vn mutants than in wild-type embryos (Fig. 4D-F; quantification presented in Fig. 4G,H and supplementary material Fig. S2C,D).
Fig. 4.
The EGFR pathway induces wntD expression and limits the dorsoventral concentration gradient of nuclear Dorsal. (A-C) Stage 6 wild-type (A) and mutant DSor (B) and Egfr (C) embryos were hybridized using a digoxigenin-labeled wntD RNA probe. RTK signaling is impeded in DSor (B) and Egfr (C) mutants, and the expression of wntD is blocked. The weak ventral wntD expression in the mutant embryos (B,C) probably results from ineffective induction by Dl alone. Arrows point to the domain of wntD expression. (D-F) Representative cross-section images of stage 6 wild-type (D), wntD (E) and rho vn (F) embryos, stained for Dl (red). (A-C) Embryos are oriented with anterior to the left and dorsal side upwards. (G,H) Quantification of nuclear Dl gradients along the DV axis in wild-type and mutant embryos. Data are the average gradients±s.e.m. Ventral levels of nuclear Dl are significantly higher in wntD (G; red; n=40 measurements) and rho vn mutants (H; red; n=28 measurements), relative to wild-type controls (blue; n=36 and 42 measurements, respectively), suggesting that EGFR-induced WntD antagonizes nuclear accumulation of Dl along the DV axis. For the nuclear Dl gradient, two values were extracted from each embryo (see Materials and methods).
Statistical significance of this result was established in two different ways. First, by fitting the gradients to the Gaussian profile, we determined that the amplitude of the nuclear Dl gradient, in both of these mutant backgrounds, showed statistically significant increase relative to the amplitude of the wild-type gradient (P<0.001). Second, we performed a pair-wise comparison of nuclear Dl levels between each of these mutant backgrounds and wild-type embryos at multiple points along the DV axis. Based on this analysis, we established that removal of wntD causes expanded nuclear accumulation of Dl in at least the ventral 30% of the DV axis (supplementary material Fig. S5). Taken together, these results strongly indicate that the EGFR pathway induces wntD expression and in this way restricts Dl nuclear localization along the DV axis.
WntD limits Dorsal target gene expression
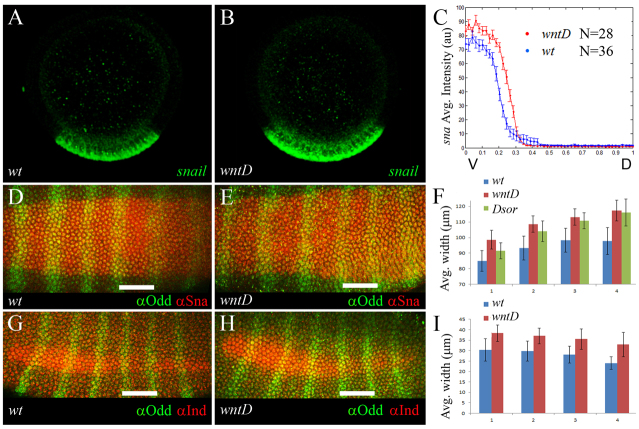
To determine whether WntD-dependent regulation of nuclear Dl affects DV patterning, we quantified the widths of expression domains for several Dl targets in wild-type and mutant embryos (Fig. 5). Thus, we find that the Sna domain expands in stage 6 wntD and DSor mutants, relative to wild-type controls (Fig. 5D-F; note that the effect is more pronounced at the posterior of the embryo). Furthermore, quantification shows that the level of sna expression is significantly higher in wntD mutants, consistent with the increased levels of nuclear Dl in this genetic background (Fig. 5A-C; supplementary material Fig. S2E). Similarly, the expression domains of two additional Dl targets, Intermediate neuroblasts defective (Ind) and lethal of scute (l’sc), expand along the DV axis in wntD mutants (Fig. 5G-I; supplementary material Fig. S6). Noteworthy, wntD and wild-type embryos are of similar size, ruling out the possibility that these effects are simply due to size differences (supplementary material Fig. S7). We therefore conclude that wntD expression, under the control of EGFR signaling, plays an important role in regulating Dl activity and, correspondingly, the expression of multiple Dl targets along the DV axis.
Fig. 5.
WntD restricts the dorsoventral extent of Dorsal target expression. (A,B) Cross-sections of stage 6 wild-type (A) and wntD mutant (B) embryos, hybridized using a fluorescent sna RNA probe (green). (C) Expression levels of sna were quantified along the DV axis (wild-type in blue and wntD in red; n=28 and 36 measurements, respectively). Data are the average gradients±s.e.m. Two values were extracted from each embryo (see Materials and methods). (D,E,G,H) Stage 6 wild-type (D,G) and wntD mutant (E,H) embryos, stained for the Dl targets Sna (red; D,E) or Ind (red; G,H), together with Odd-skipped (Odd; green; D,E,G,H). The DV extent of the Sna and Ind expression domains was measured along Odd stripes 1-4. Scale bars: 50 μm. (D) Embryos are oriented with anterior to the left. (F) The average width (μm) of Sna expression in wild-type, wntD and DSor embryos, along Odd stripes 1-4 (blue, red and green bars, respectively; n=12, 11 and 6 embryos, respectively). Error bars indicate s.d. (I) The average width (μm) of Ind expression in wild-type and wntD embryos, along Odd stripes 1-4 (blue and red, respectively; n=10 embryos for each genotype). Ind is not expressed in DSor mutants. Error bars indicate s.d.
Induction of wntD requires relief of Groucho- and Capicua-mediated repression
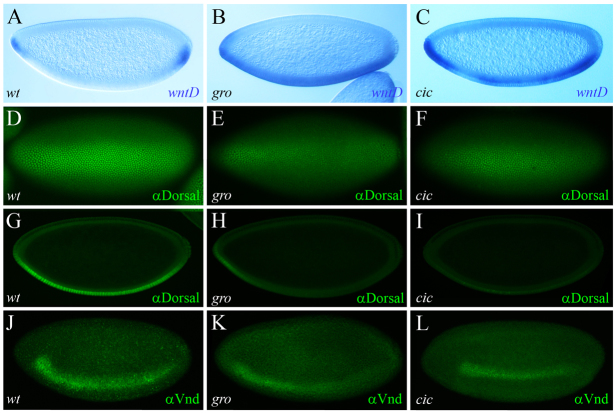
Our work shows that wntD is a novel target of the terminal system, which (together with Dl) induces localized wntD expression. Activation of other known Torso pathway targets, such as hkb and tll, relies on relief of repression. Acting downstream of Torso, MAPK/Erk phosphorylates and downregulates Cic and Gro, enabling localized induction of tll and hkb by broadly distributed activators. We find that, similarly, wntD is subject to repression by Cic and Gro, that is alleviated by the Torso pathway. Thus, wntD expression expands medially in embryos devoid of maternal gro or cic, albeit only in ventral positions where Dl is nuclear (Fig. 6A-C). Accordingly, in both mutant backgrounds the levels of nuclear Dl are significantly reduced (Fig. 6D-I), resembling the effects observed in torY9 embryos (Fig. 1O, Fig. 2C,G,K). Furthermore, the lower Dl nuclear concentration in gro and cic embryos correlates with decreased Dl target gene expression; for example, both mutant backgrounds exhibit attenuated Vnd expression in the lateral ectoderm (Fig. 6J-L).
Fig. 6.
RTK signaling promotes wntD expression via relief of Groucho- and Capicua-dependent repression. (A-C) Stage 4 wild-type (A), gro (B) and cic maternal mutant (C) embryos, hybridized using a digoxigenin-labeled wntD RNA probe. There is ventral expansion of wntD expression in the mutants. (D-I) Stage 5 wild-type (D,G), gro (E,H) and cic mutant (F,I) embryos stained for Dl (green). The reduced accumulation of nuclear Dl in the two mutant backgrounds is evident both in ventral views (E,F; compare with D) and in sagittal cross-sections (H,I; compare with G). (J-L) Stage 5 embryos stained for Vnd (green). Note the weaker expression in gro (K) and cic (L) mutant embryos, compared with wild-type control (J). Embryos are oriented with anterior to the left.
In support of these results, we find that expression of unphosphorylatable variants of both Gro (GroAA) and Cic (CicΔC2), which are insensitive to Torso-mediated downregulation (Astigarraga et al., 2007; Cinnamon et al., 2008; Hasson et al., 2005), reduce wntD mRNA levels (supplementary material Fig. S8). This effect is comparable with that caused by mutations in DSor and trunk (Fig. 1J; supplementary material Fig. S1D). Thus, blocking Gro and Cic downregulation mimics the effect caused by the loss of Torso signaling, indicating that wntD expression is induced through derepression.
DISCUSSION
Specification of body axes in all metazoans is initiated by a small number of inductive signals that must be integrated in time and space to control complex and unique patterns of gene expression. It is therefore of utmost importance to unravel the mechanisms underlying crosstalk between different signaling cues that concur during early development. Here, we have elucidated a novel signal integration mechanism that coordinates RTK signaling pathways with the Dl nuclear gradient, and thus with terminal and DV patterning of the Drosophila embryo.
Previous work had identified an input by Torso signaling into specific transcriptional effects of Dl. Our results establish a general mechanism, which involves RTK-dependent control of the nuclear Dl gradient itself, and thus affects a large group of Dl targets. This regulatory input is based on RTK-dependent derepression of wntD, a Dl target that encodes a feedback inhibitor of the Dl gradient. Thus, Dl activates wntD effectively only when accompanied by RTK signaling, enabling region-specific negative-feedback control of the nuclear Dl gradient (Fig. 7). In the absence of RTK signaling, wntD is not expressed and the levels of nuclear Dl are elevated. Consequently, Dl target genes are ectopically expressed, both at the poles and along the DV axis (Figs 1, 5).
Fig. 7.
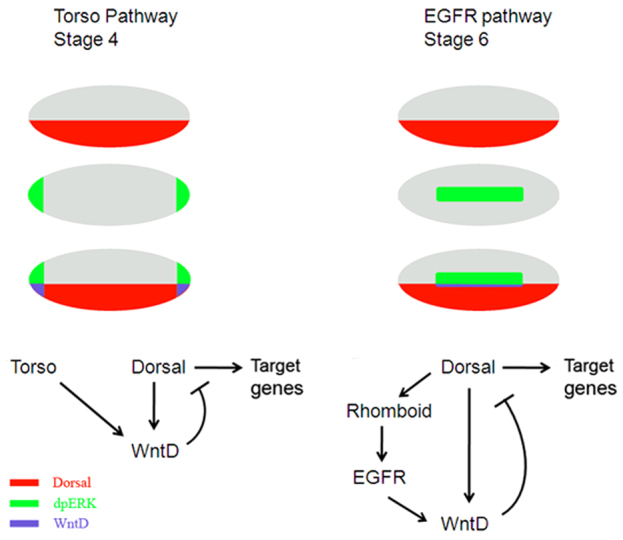
Combinatorial induction of wntD expression by Dorsal and by RTK-mediated signaling. Nuclear Dl (red) and RTK signaling (green) are both required for induction of wntD expression (blue). Accordingly, wntD is transcribed only where the domains of nuclear Dl and activated MAPK/Erk converge, at the termini (stage 4; left) and in the neuroectoderm (stage 6; right). WntD antagonizes nuclear localization of Dl, attenuating Dl function as a transcriptional regulator. Early, at stage 4, the Torso pathway is activated independently of Dl; hence, it acts, via wntD, as a gating mechanism that blocks Dl-mediated activation and repression at the embryonic poles. Later on in development, at stage 6, Dl-dependent EGFR pathway activity provides a negative-feedback regulatory mechanism that restricts Dl target gene expression along the DV axis. Embryos are oriented with anterior to the left and dorsal side upwards.
Torso RTK signaling depends on maternal cues and is independent of the Dl gradient. Thus, it can be viewed as a gating signal that operates only at the embryonic poles, where it controls Dl-dependent gene regulation. However, the activity of the EGFR RTK pathway later on in development crucially depends on Dl, which induces the neuroectodermal expression of rhomboid, a gene encoding a serine protease required for processing of the EGFR ligand Spitz (Bang and Kintner, 2000). In this case, EGFR-dependent induction of WntD represents a negative feedback loop that reduces nuclear levels of Dl laterally and, consequently, limits the expression of multiple Dl targets along the DV axis (Fig. 7).
It should be noted that the regulatory interactions that we have characterized do not preclude the existence of other mechanisms modulating nuclear Dl concentration or activity. For example, the progressive dilution or degradation of maternal components involved in Toll receptor activation upstream of Dl should cause reduced Dl nuclear accumulation and retraction of its targets as development proceeds. It is also possible that Torso- or EGFR-induced repressors block transcription of Dl target genes directly. Accordingly, the ectopic sna expression observed in embryos mutant for components of the Torso pathway such as DSor and trunk probably reflects both loss of WntD activity on Dl and loss of Hkb-mediated repression of sna. In this context, it is interesting to note that sna expression expands and colocalizes with Hkb at the poles of wntD mutants (Fig. 1P) (Ganguly et al., 2005); perhaps repression of sna by Hkb is not sufficient to override increased Dl activation in this genetic background. Thus, the Torso pathway probably employs more than one mechanism to exclude Dl target expression from the termini. Furthermore, the existence of such additional regulatory mechanisms could explain why wntD mutants do not have a clear developmental phenotype, despite the broad effects on Dl-dependent gene expression patterns caused by the genetic removal of wntD (this study) (Ganguly et al., 2005; Gordon et al., 2005). We propose that corrective mechanisms are present, which make the terminal and DV systems robust with respect to removal of the WntD-based feedback, such as RTK-induced repressors. Understanding the basis of this robustness will require additional studies.
Our work shows that RTK-dependent relief of Gro- and Cic-mediated repression is essential for transcriptional activation of wntD by Dl. Correspondingly, in the absence of cic or gro, the early expression of wntD expands ventrally throughout the domain of nuclear Dl. The early onset of this derepression, and the presence of at least one conserved Cic-binding site in the proximal upstream region of wntD (M.J.A. and G.J., unpublished), indicate that repression of wntD may be direct. Interestingly, it is thought that Gro and Cic are also involved in assisting Dl-mediated repression of other targets such as dpp and zen, as gro and cic mutant embryos show derepression of those targets in ventral regions (Dubnicoff et al., 1997; Jiménez et al., 2000; Ratnaparkhi et al., 2006). However, as ectopic wntD expression in these mutants leads to reduced nuclear localization of Dl along the ventral region, it is conceivable that decreased Dl activity also contributes to the derepression of dpp and zen.
In conclusion, the data presented herein demonstrate RTK-dependent control of nuclear Dl via wntD, based on multiple regulatory inputs, including negative gating, feed-forward loops and negative feedback control. Together, these mechanisms provide additional combinatorial tiers of spatiotemporal regulation to Dl target gene expression. Future studies will show whether other signal transduction cascades and/or additional developmental cues also impinge on the Dl morphogen gradient.
Supplementary Material
Acknowledgments
We thank Maayane Cohen, Rona Grossman, Adi Jacob and Sharon Mezuman for continued help and encouragement during this project. We are grateful to Benny Shilo for his comments on the manuscript and to Jessica Cande, Shari Carmon, Yosef Gruenbaum, Mike Levine, Mark McElwain, Raul Nusse, Benny Shilo, Tonia von Ohlen, the Developmental Studies Hybridoma Bank and the Bloomington Stock Center for antibodies, fly stocks and other reagents.
Footnotes
Funding
This research was supported by grants from the Israel Science Foundation (Center of Excellence; 180/09) and the Król Charitable Foundation to Z.P.; the National Institutes of Health [R01GM086537 from the National Institute of General Medical Sciences] to S.Y.S. and Z.P.; the National Science Foundation [1136913, EFRI-MIKS] to S.Y.S. and H.L.; and by ICREA, Ministerio de Ciencia e Innovación (BFU2008-01875) and Generalitat de Catalunya (2009SGR-1075) to G.J. A.H. was the recipient of a PhD Fellowship by the Rector of the Hebrew University. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.075812/-/DC1
References
- Astigarraga S., Grossman R., Díaz-Delfín J., Caelles C., Paroush Z., Jiménez G. (2007). A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 26, 668–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang A. G., Kintner C. (2000). Rhomboid and Star facilitate presentation and processing of the Drosophila TGF-alpha homolog Spitz. Genes Dev. 14, 177–186 [PMC free article] [PubMed] [Google Scholar]
- Brönner G., Jäckle H. (1991). Control and function of terminal gap gene activity in the posterior pole region of the Drosophila embryo. Mech. Dev. 35, 205–211 [DOI] [PubMed] [Google Scholar]
- Casanova J. (1991). Interaction between torso and dorsal, two elements of different transduction pathways in the Drosophila embryo. Mech. Dev. 36, 41–45 [DOI] [PubMed] [Google Scholar]
- Chopra V. S., Levine M. (2009). Combinatorial patterning mechanisms in the Drosophila embryo. Brief. Funct. Genomics Proteomics 8, 243–249 [DOI] [PubMed] [Google Scholar]
- Chung K., Kim Y., Kanodia J. S., Gong E., Shvartsman S. Y., Lu H. (2011). A microfluidic array for large-scale ordering and orientation of embryos. Nat. Methods 8, 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinnamon E., Helman A., Ben-Haroush Schyr R., Orian A., Jiménez G., Paroush Z. (2008). Multiple RTK pathways downregulate Groucho-mediated repression in Drosophila embryogenesis. Development 135, 829–837 [DOI] [PubMed] [Google Scholar]
- Cowden J., Levine M. (2003). Ventral dominance governs sequential patterns of gene expression across the dorsal-ventral axis of the neuroectoderm in the Drosophila embryo. Dev. Biol. 262, 335–349 [DOI] [PubMed] [Google Scholar]
- Dubnicoff T., Valentine S. A., Chen G., Shi T., Lengyel J. A., Paroush Z., Courey A. J. (1997). Conversion of Dorsal from an activator to a repressor by the global corepressor Groucho. Genes Dev. 11, 2952–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols M., Sprenger F., Casanova J. (1996). Variation in the number of activated torso receptors correlates with differential gene expression. Development 122, 2313–2317 [DOI] [PubMed] [Google Scholar]
- Ganguly A., Jiang J., Ip Y. T. (2005). Drosophila WntD is a target and an inhibitor of the Dorsal/Twist/Snail network in the gastrulating embryo. Development 132, 3419–3429 [DOI] [PubMed] [Google Scholar]
- Goff D. J., Nilson L. A., Morisato D. (2001). Establishment of dorsal-ventral polarity of the Drosophila egg requires capicua action in ovarian follicle cells. Development 128, 4553–4562 [DOI] [PubMed] [Google Scholar]
- Goldstein R. E., Jiménez G., Cook O., Gur D., Paroush Z. (1999). Huckebein repressor activity in Drosophila terminal patterning is mediated by Groucho. Development 126, 3747–3755 [DOI] [PubMed] [Google Scholar]
- Gordon M. D., Dionne M. S., Schneider D. S., Nusse R. (2005). WntD is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature 437, 746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häder T., Wainwright D., Shandala T., Saint R., Taubert H., Brönner G., Jäckle H. (2000). Receptor tyrosine kinase signaling regulates different modes of Groucho-dependent control of Dorsal. Curr. Biol. 10, 51–54 [DOI] [PubMed] [Google Scholar]
- Halfar K., Rommel C., Stocker H., Hafen E. (2001). Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development 128, 1687–1696 [DOI] [PubMed] [Google Scholar]
- Hasson P., Egoz N., Winkler C., Volohonsky G., Jia S., Dinur T., Volk T., Courey A. J., Paroush Z. (2005). EGFR signaling attenuates Groucho-dependent repression to antagonize Notch transcriptional output. Nat. Genet. 37, 101–105 [DOI] [PubMed] [Google Scholar]
- Helman A., Paroush Z. (2010). Detection of RTK pathway activation in Drosophila using anti-dpERK immunofluorescence staining. Methods Mol. Biol. 661, 401–408 [DOI] [PubMed] [Google Scholar]
- Helman A., Cinnamon E., Mezuman S., Hayouka Z., Von Ohlen T., Orian A., Jiménez G., Paroush Z. (2011). Phosphorylation of Groucho mediates RTK feedback inhibition and prolonged pathway target gene expression. Curr. Biol. 21, 1102–1110 [DOI] [PubMed] [Google Scholar]
- Huang J. D., Schwyter D. H., Shirokawa J. M., Courey A. J. (1993). The interplay between multiple enhancer and silencer elements defines the pattern of decapentaplegic expression. Genes Dev. 7, 694–704 [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Park R. E., Kosman D., Yazdanbakhsh K., Levine M. (1992). dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 6, 1518–1530 [DOI] [PubMed] [Google Scholar]
- Jiang J., Kosman D., Ip Y. T., Levine M. (1991). The dorsal morphogen gradient regulates the mesoderm determinant twist in early Drosophila embryos. Genes Dev. 5, 1881–1891 [DOI] [PubMed] [Google Scholar]
- Jiménez G., Guichet A., Ephrussi A., Casanova J. (2000). Relief of gene repression by torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes Dev. 14, 224–231 [PMC free article] [PubMed] [Google Scholar]
- Kanodia J. S., Kim Y., Tomer R., Khan Z., Chung K., Storey J. D., Lu H., Keller P. J., Shvartsman S. Y. (2011). A computational statistics approach for estimating the spatial range of morphogen gradients. Development 138, 4867–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Andreu M. J., Lim B., Chung K., Terayama M., Jiménez G., Berg C. A., Lu H., Shvartsman S. Y. (2011). Gene regulation by MAPK substrate competition. Dev. Cell 20, 880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw G. J., Rudolph K. M., Huang J. D., Dubnicoff T., Courey A. J., Lengyel J. A. (1995). The torso response element binds GAGA and NTF-1/Elf-1, and regulates tailless by relief of repression. Genes Dev. 9, 3163–3176 [DOI] [PubMed] [Google Scholar]
- Markstein M., Zinzen R., Markstein P., Yee K. P., Erives A., Stathopoulos A., Levine M. (2004). A regulatory code for neurogenic gene expression in the Drosophila embryo. Development 131, 2387–2394 [DOI] [PubMed] [Google Scholar]
- Pan D. J., Huang J. D., Courey A. J. (1991). Functional analysis of the Drosophila twist promoter reveals a dorsal-binding ventral activator region. Genes Dev. 5, 1892–1901 [DOI] [PubMed] [Google Scholar]
- Paroush Z., Wainwright S. M., Ish-Horowicz D. (1997). Torso signalling regulates terminal patterning in Drosophila by antagonising Groucho-mediated repression. Development 124, 3827–3834 [DOI] [PubMed] [Google Scholar]
- Pignoni F., Baldarelli R. M., Steingrímsson E., Diaz R. J., Patapoutian A., Merriam J. R., Lengyel J. A. (1990). The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell 62, 151–163 [DOI] [PubMed] [Google Scholar]
- Ratnaparkhi G. S., Jia S., Courey A. J. (2006). Uncoupling dorsal-mediated activation from dorsal-mediated repression in the Drosophila embryo. Development 133, 4409–4414 [DOI] [PubMed] [Google Scholar]
- Reeves G. T., Stathopoulos A. (2009). Graded dorsal and differential gene regulation in the Drosophila embryo. Cold Spring Harb. Perspect. Biol. 1, a000836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter R., Leptin M. (1994). Interacting functions of snail, twist and huckebein during the early development of germ layers in Drosophila. Development 120, 1137–1150 [DOI] [PubMed] [Google Scholar]
- Rogers K. W., Schier A. F. (2011). Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 27, 377–407 [DOI] [PubMed] [Google Scholar]
- Rusch J., Levine M. (1994). Regulation of the dorsal morphogen by the Toll and torso signaling pathways: a receptor tyrosine kinase selectively masks transcriptional repression. Genes Dev. 8, 1247–1257 [DOI] [PubMed] [Google Scholar]
- Stathopoulos A., Levine M. (2002). Dorsal gradient networks in the Drosophila embryo. Dev. Biol. 246, 57–67 [DOI] [PubMed] [Google Scholar]
- von Ohlen T., Doe C. Q. (2000). Convergence of dorsal, dpp, and egfr signaling pathways subdivides the drosophila neuroectoderm into three dorsal-ventral columns. Dev. Biol. 224, 362–372 [DOI] [PubMed] [Google Scholar]
- Zeitlinger J., Zinzen R. P., Stark A., Kellis M., Zhang H., Young R. A., Levine M. (2007). Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev. 21, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Levine M., Ashe H. L. (2001). Brinker is a sequence-specific transcriptional repressor in the Drosophila embryo. Genes Dev. 15, 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.