ABSTRACT
Background
Few predictive indexes for long-term mortality have been developed for community-dwelling elderly populations. Parsimonious predictive indexes are important decision-making tools for clinicians, policy makers, and epidemiologists.
Objective
To develop 1-, 5-, and 10-year mortality predictive indexes for nationally representative community-dwelling elderly people.
Design
Cohort study.
Setting
The Second Longitudinal Study of Aging (LSOA II).
Participants
Nationally representative civilian community-dwelling persons at least 70 years old. We randomly selected 60% of the sample for prediction development and used the remaining 40% for validation.
Main Measures
Sociodemographics, impairments, and medical diagnoses were collected from the LSOA II baseline interviews. Instrumental activities of daily living (IADLs) stages were derived to measure functional status. All-cause mortality was obtained from the LSOA II Linked Mortality Public-use File.
Results
The analyses included 7,373 sample persons with complete data, among which mortality rates were 3.7%, 23.3%, and 49.8% for 1, 5, and 10 years, respectively. Four, eight, and ten predictors were identified for 1-, 5-, and 10-year mortality, respectively, in multiple logistic regression models to create three predictive indexes. Age, sex, coronary artery disease, and IADL stages were the most essential predictors for all three indexes. C-statistics of the three indexes were 0.72, 0.74, and 0.75 in the development cohort and 0.72, 0.72, and 0.74 in the validation cohort for 1-, 5-, and 10-year mortality, respectively. Five risk groups were defined based on the scores.
Conclusions
The 1-, 5-, and 10-year mortality indexes include parsimonious predictor sets maximizing ease of mortality prediction in community settings. Thus, they may provide valuable information for prognosis of elderly patients and guide the comparison of alternative interventions. Including IADL stage as a predictor yields simplified mortality prediction when detailed disease information is not available.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2027-3) contains supplementary material, which is available to authorized users.
KEY WORDS: mortality, prediction, score system, community, instrumental activities of daily living
Clinical prediction rules with better precision and parsimonious predictor sets making them easily and practically implemented in community settings can help us better serve the health needs of elderly people. Such prediction rules are research-based tools that quantify the contributions of relevant patient characteristics to provide numeric indexes in ways that assist with clinical decision-making.1–3 The process of developing clinical prediction rules includes identifying a parsimonious set of the most essential predictors from patients' characteristics to estimate the probability of specific outcomes.1–3
Mortality prediction is particularly important in individualizing care in elderly populations which have great diversity of chronic conditions, functional limitations, and social challenges that can impact health, quality of life, and the benefits and risks of medical interventions. However, most published clinical prediction rules focus on short-term mortality in patients with specific diseases,4–6 with few forecasting intermediate-term mortality in community-dwelling elderly populations.7–10 A newly published systematic review identified 16 indexes that predicted mortality from 6 months to 5 years for older adults in a variety of clinical settings,11 but no long-term mortality index has been developed. The aim of our study is to develop three parsimonious clinical prediction indexes for 1-, 5-, and 10-year all-cause mortality among community-dwelling elderly people based on sociodemographics, impairments, associated morbidity, and activity limitations. The application of these three parsimonious clinical prediction indexes can assist clinicians in determining patient prognosis and help policy makers and epidemiologists monitor survival in older populations.
METHODS
Data Source
We used prospectively collected data from The Second Longitudinal Study of Aging (LSOA II).12 The Second Supplement on Aging (SOA II), conducted in conjunction with the 1994 National Health Interview Survey (NHIS), served as baseline for the study. LSOA II is composed of a nationally representative sample of 9,447 civilian non-institutionalized sample persons (SPs) 70 years of age and over at the time of their SOA II interview.13, 14 Baseline data from the SOA II were merged with the disability supplement follow-back of the 1994 National Health Interview Survey (NHIS-D), which added supplemental details for the subset with disabilities. The overall response rate to the LSOA II was 87.4%.14 Information was collected mainly by self-report; 19.0% of the interviews were answered by proxy. Detailed interviewer instructions and questions are available at the Centers for Disease Control and Prevention (CDC) website.15 This study was approved by the Institutional Review Board of the University of Pennsylvania.
Outcomes
The outcomes for analyses were 1-, 5-, and 10-year all-cause mortality. The updated LSOA II Linked Mortality Public-use File provided mortality follow-up data from the LSOA II baseline interview date through December 31, 2006.16 This allowed us to assess 10-12 year mortality linking the follow-up mortality data with baseline interviews conducted in 1994–1996.
Predictors
When choosing candidate predictors for the predictive models, we considered clinical relevance and generalizability of the variables across different populations. Predictors were expressed in categories rather than as continuous measures to ease calculations, thus enhancing practical applications of the index. We categorized age as ≥70–<75, ≥75–<80, ≥80–<85, and ≥85 years, gender as female and male, race as white, and black/African American, and other. Educational level was dichotomized based on whether or not the SP graduated from high school. We grouped marital status as married and unmarried. Perceived health status was reported as excellent, very good, good, fair, or poor. Diagnoses were captured by asking whether a doctor had ever said that the SP had various illnesses. Illnesses were categorized into the following conditions: hypertension, diabetes, cancer of any kind, chronic bronchitis or emphysema, asthma, coronary artery disease, other heart disease, stroke, and arthritis. Additionally, SPs were asked whether they had major depression lasting two or more weeks in the past 12 months.
To capture impairments, the LSOA II asked whether SPs have blindness in both eyes and deafness in both ears. Individuals with vision from one eye and hearing from one ear may maintain normal lives. SPs were further asked if they ever had a broken hip or fallen in the past 12 months. Proxy use was recorded as yes versus no.
We derived instrumental activities of daily living (IADLs) stages to measure functional manifestations of elderly community-dwellers’ cognitive and physical impairments,17 which reflect functional hierarchies consistent with theories of development, loss, recovery, and models and measures of disability.18–20 IADLs, referred to as “domestic life” activities, measure the tasks people must be able to perform if they are to care for themselves in the community.21 The LSOA II asked whether SPs had difficulties performing any IADLs including using the telephone, managing money, doing light housework, preparing meals, shopping, and doing heavy housework.22 Status on each IADL was rated as no difficulty, some difficulty, a lot of difficulty, and unable. IADL stages were developed by observing item responses in the LSOA II baseline data. Based on the hierarchical structure of the IADL activities,18, 23 the IADL stages organize patterns of activity limitation into thresholds, which express the retained ability to perform activities with decreasing complexity as severity of stage increases. We summarize IADL stage assignment in Fig. 1. Additionally, the associations between IADL stages and SPs’ sociodemographics, impairments, and associated morbidity are shown in online Table 1 (available online).
Figure 1.
IADL stage assignment*.
Analysis
The primary analysis was a complete-case analysis. We randomly selected 60% of the sample to develop the prediction score system, and used the remaining 40% as a validation cohort.2, 24 We developed three separate multiple regression models for 1-, 5-, and 10-year mortality in three steps:25 (1) predictor selection and predictive model development; (2) predictive index construction; and (3) internal validation. Specific details are provided below.
Predictor Selection and Predictive Model Development
We first evaluated the univariate associations between each categorical predictor and 1-, 5-, and 10-year mortality separately in the development cohort using Chi-square tests. Predictors with p < 0.20 were entered into the three multiple logistic regression models for 1-, 5-, and 10-year mortality separately. Then, manual backward selections were performed until p-values for all predictors were < 0.05 in the three final models.
Predictive Index Construction
We constructed three separate predictive indexes for 1-, 5-, and 10-year mortality from the three final models. We assigned points to each predictor by dividing each β-coefficient in the final logistic regression models by the lowest significant β-coefficient and rounding up to the nearest integer. A 0 was assigned to a non-significant β-coefficient. A risk score was established for each SP as the sum of points for all predictors present.26
Internal Validation
We applied the three mortality indexes to the validation cohort and calculated the proportion of SPs who died at each sum score. We calculated model discrimination and calibration to assess model predictive accuracy in both the development and validation cohorts. Model discrimination was assessed by calculating the c-statistics which are the areas under the receiver operating characteristic curves.27 Model calibration was assessed by the Hosmer-Lemeshow statistic to test whether the predicted probabilities agree with the actual outcomes. To avoid over-fitting, we removed predictors if removal increased c-statistics in the validation cohorts.
Sensitivity Analysis
We performed sensitivity analyses to assess robustness of findings from the complete-case analysis. To determine if exclusion of SPs with missing data introduced bias, we applied multiple imputation procedures by using the SAS callable IVEware software 0.2 (University of Michigan’s Survey Research Center, Ann Arbor).28 Five multiple imputation datasets were generated. We then repeated all model development steps using imputed data.
We accounted for the multistage sample design of the LSOA II, including clustering, sample weights, and stratification in all analyses to obtain the correct point and variance estimates. We reported unweighted sample sizes and weighted proportions. All analyses (except for multiple imputation as noted above) were performed with Stata 11.0 (Stata Corp, College Station, Texas). The final tests of significance used a 2-sided p < 0.05, except for Hosmer-Lemeshow statistics with p < 0.10 indicating model fit was inadequate.
RESULTS
SPs’ Characteristics
Of the 9,447 SPs in the LSOA II, 7,373 had complete data (78.0%) and were included, among which 3.7%, 23.3%, and 49.8% died within 1, 5, and 10 years, respectively. The development cohort consisted of 4,434 SPs, of whom 3.3%, 22.8%, and 49.5% died within 1, 5, and 10 years, respectively. The validation cohort consisted of 2,939 SPs, of whom 4.1%, 24.0%, and 50.3% died within 1, 5, and 10 years, respectively. Table 1 describes SPs’ characteristics in the development and validation cohorts.
Table 1.
Sample Persons' Characteristics
| Characteristics | Total | Development | Validation |
|---|---|---|---|
| No. ( weighted % ) | N = 7373 | N = 4434 | N = 2939 |
| Age | |||
| ≥70–<75 | 3607(49.5) | 2183(49.7) | 1424(49.3) |
| ≥75–<80 | 2016(27.4) | 1202(27.2) | 814(27.7) |
| ≥80–<85 | 1143(15.2) | 685(15.1) | 458(15.2) |
| ≥85 | 607(7.9) | 364(8.0) | 243(7.9) |
| Gender | |||
| Female | 4678(62.9) | 2835(63.3) | 1843(62.4) |
| Male | 2695(37.1) | 1599(36.7) | 1096(37.6) |
| Race | |||
| White | 6340(88.8) | 3822(89.2) | 2518(88.3) |
| Black | 762(7.4) | 463(7.3) | 299(7.5) |
| Other | 271(3.8) | 149(3.5) | 122(4.3) |
| High school graduate | |||
| No | 3030(40.0) | 1820(39.9) | 1210(40.2) |
| Yes | 4343(60.0) | 2614(60.1) | 1729(59.8) |
| Marital status | |||
| Married | 3860(53.0) | 2332(53.3) | 1528(52.5) |
| Unmarried | 3513(47.0) | 2102(46.7) | 1411(47.5) |
| Health status | |||
| Excellent | 1119(15.6) | 672(15.7) | 447(15.3) |
| Very Good | 1762(24.2) | 1086(24.7) | 676(23.5) |
| Good | 2583(35.0) | 1523(34.3) | 1060(36.1) |
| Fair | 1342(17.7) | 816(17.9) | 526(17.3) |
| Poor | 567(7.5) | 337(7.3) | 230(7.8) |
| Hypertension | |||
| No | 4174(57.2) | 2510(57.3) | 1664(57.1) |
| Yes | 3199(42.8) | 1924(42.7) | 1275(42.9) |
| Diabetes | |||
| No | 6502(88.4) | 3890(88.1) | 2612(88.8) |
| Yes | 871(11.6) | 544(11.9) | 327(11.2) |
| Cancer | |||
| No | 6181(83.4) | 3714(83.3) | 2467(83.7) |
| Yes | 1192(16.6) | 720(16.7) | 472(16.3) |
| Chronic bronchitis or emphysema | |||
| No | 6713(90.9) | 4031(90.6) | 2682(91.2) |
| Yes | 660(9.1) | 403(9.4) | 257(8.8) |
| Asthma | |||
| No | 6960(94.5) | 4180(94.3) | 2780(94.8) |
| Yes | 413(5.5) | 254(5.7) | 159(5.2) |
| Coronary artery disease | |||
| No | 5975(80.9) | 3593(80.8) | 2382(81.2) |
| Yes | 1398(19.1) | 841(19.2) | 557(18.8) |
| Other heart disease | |||
| No | 6883(93.3) | 4153(93.6) | 2730(92.9) |
| Yes | 490(6.7) | 281(6.4) | 209(7.1) |
| Stroke | |||
| No | 6826(92.7) | 4104(92.7) | 2722(92.6) |
| Yes | 547(7.3) | 330(7.3) | 217(7.4) |
| Arthritis | |||
| No | 4012(54.5) | 2400(54.0) | 1612(55.2) |
| Yes | 3361(45.5) | 2034(46.0) | 1327(44.8) |
| Major depression* | |||
| No | 7264(99.3) | 4365(99.2) | 2899(99.3) |
| Yes | 52(0.7) | 30(0.8) | 22(0.7) |
| Blindness in both eyes* | |||
| No | 7172(98.5) | 4312(98.6) | 2860(98.5) |
| Yes | 112(1.5) | 63(1.4) | 49(1.5) |
| Deafness in both ears* | |||
| No | 6807(93.4) | 4076(93.0) | 2731(94.0) |
| Yes | 467(6.6) | 297(7.0) | 170(6.0) |
| Ever had broken hip* | |||
| No | 6995(96.0) | 4186(95.6) | 2809(96.5) |
| Yes | 294(4.0) | 191(4.4) | 103(3.5) |
| Fall* | |||
| No | 5864(79.9) | 3530(79.8) | 2334(80.0) |
| Yes | 1463(20.1) | 876(20.2) | 587(20.0) |
| Proxy use | |||
| No | 6226(84.7) | 3745(84.6) | 2481(84.8) |
| Yes | 1147(15.3) | 689(15.4) | 458(15.2) |
| IADL stage | |||
| No limitations | 5134(70.0) | 3090(70.0) | 2044(69.8) |
| Mild limitations | 1232(16.7) | 750(16.9) | 482(16.4) |
| Moderate limitations | 454(6.0) | 275(6.0) | 179(5.9) |
| Severe limitations | 439(5.8) | 255(5.6) | 184(6.2) |
| Complete limitations | 114(1.5) | 64(1.4) | 50(1.7) |
| 1-year mortality | |||
| No | 7103(96.3) | 4285(96.7) | 2818(95.9) |
| Yes | 270(3.7) | 149(3.3) | 121(4.1) |
| 5-year mortality | |||
| No | 5640(76.7) | 3407(77.2) | 2233(76.0) |
| Yes | 1733(23.3) | 1027(22.8) | 706(24.0) |
| 10-year mortality | |||
| No | 3675(50.2) | 2220(50.5) | 1455(49.7) |
| Yes | 3698(49.8) | 2214(49.5) | 1484(50.3) |
*Sample size is slightly less for these variables due to missing data: major depression (N = 7316, 4395, and 2921); blindness in both eyes (N = 7284, 4375, and 2909); deafness in both ears (N = 7274, 4373, and 2901); ever had broken hip (N = 7289, 4377, and 2912); fall (N = 7327, 4406, and 2921)
Predictor Selection and Predictive Model Development
Table 2 shows the unadjusted associations between each predictor and 1-, 5-, and 10-year mortality separately in the development cohort. Table 3 shows the three final multiple logistic regression models for 1-, 5-, and 10-year mortality. The Hosmer-Lemeshow statistic showed a p > 0.1 for all three final models. The c-statistic of the 1-year mortality prediction model with four predictors (age, sex, coronary artery disease, and IADL stage) is 0.74, 0.74 for the 5-year mortality prediction model with 8 predictors (age, sex, perceived health status, cancer, coronary artery disease, other heart disease, diabetes, and IADL stage), and 0.76 for the 10-year mortality prediction model with ten predictors (age, sex, marital status, perceived health status, coronary artery disease, other heart disease, diabetes, chronic bronchitis or emphysema, stroke, and IADL stage).
Table 2.
Unadjusted ORs for 1-, 5-, and 10-Year Mortality in the Development Cohort (N = 4434)
| Characteristics | 1-year mortality | 5-year mortality | 10-year mortality |
|---|---|---|---|
| Unadjusted OR (95% CI) | |||
| Age | |||
| ≥70–<75 | Reference | Reference | Reference |
| ≥75–<80 | 1.16(0.75,1.78) | 1.74(1.46,2.08) | 2.19(1.87,2.55) |
| ≥80–<85 | 2.05(1.31,3.21) | 2.83(2.26,3.53) | 4.01(3.23,4.96) |
| ≥85 | 3.66(2.38,5.61) | 5.73(4.43,7.42) | 11.81(8.44,16.53) |
| Gender | |||
| Female | Reference | Reference | Reference |
| Male | 1.22(0.85,1.77) | 1.45(1.26,1.67) | 1.38(1.21,1.56) |
| Race | |||
| White | Reference | Reference | Reference |
| Black | 0.86(0.45,1.64) | 1.14(0.88,1.47) | 1.29(1.05,1.59) |
| Other | 0.53(0.15,1.89) | 0.92(0.60,1.42) | 0.95(0.64,1.39) |
| High school graduate | |||
| No | Reference | Reference | Reference |
| Yes | 0.76(0.56,1.05) | 0.75(0.65,0.87) | 0.64(0.56,0.72) |
| Marital status | |||
| Married | Reference | Reference | Reference |
| Unmarried | 1.49(1.06,2.09) | 1.29(1.12,1.49) | 1.51(1.33,1.72) |
| Health status | |||
| Excellent | Reference | Reference | Reference |
| Very Good | 0.76(0.40,1.45) | 1.18(0.91,1.54) | 1.21(0.98,1.49) |
| Good | 1.12(0.64,1.96) | 1.65(1.29,2.10) | 1.66(1.34,2.06) |
| Fair | 1.45(0.81,2.59) | 2.58(1.98,3.36) | 2.52(1.99,3.20) |
| Poor | 3.87(2.14,6.99) | 6.42(4.68,8.80) | 6.36(4.70,8.62) |
| Hypertension | |||
| No | Reference | Reference | Reference |
| Yes | 1.12(0.79,1.58) | 1.19(1.02,1.38) | 1.25(1.11,1.40) |
| Diabetes | |||
| No | Reference | Reference | Reference |
| Yes | 1.72(1.11,2.68) | 2.11(1.70,2.62) | 2.10(1.74,2.54) |
| Cancer | |||
| No | Reference | Reference | Reference |
| Yes | 1.87(1.23,2.84) | 1.56(1.29,1.88) | 1.30(1.10,1.54) |
| Chronic bronchitis or emphysema | |||
| No | Reference | Reference | Reference |
| Yes | 1.44(0.89,2.31) | 1.64(1.28,2.10) | 1.73(1.36,2.20) |
| Asthma | |||
| No | Reference | Reference | Reference |
| Yes | 1.40(0.72,2.72) | 1.44(1.08,1.93) | 1.27(0.97,1.66) |
| Coronary artery disease | |||
| No | Reference | Reference | Reference |
| Yes | 2.35(1.62,3.42) | 1.84(1.54,2.19) | 1.99(1.72,2.29) |
| Other heart disease | |||
| No | Reference | Reference | Reference |
| Yes | 2.05(1.17,3.61) | 2.17(1.64,2.88) | 1.83(1.44,2.33) |
| Stroke | |||
| No | Reference | Reference | Reference |
| Yes | 2.74(1.79,4.19) | 2.46(1.90,3.19) | 3.11(2.37,4.06) |
| Arthritis | |||
| No | Reference | Reference | Reference |
| Yes | 1.00(0.71,1.42) | 0.96(0.82,1.11) | 1.15(1.01,1.30) |
| Major depression* | |||
| No | Reference | Reference | Reference |
| Yes | 3.06(0.86,10.87) | 1.65(0.72,3.76) | 1.66(0.70,3.90) |
| Blindness in both eyes* | |||
| No | Reference | Reference | Reference |
| Yes | 2.02(0.70,5.81) | 2.93(1.73,4.98) | 6.22(3.22,12.02) |
| Deafness in both ears* | |||
| No | Reference | Reference | Reference |
| Yes | 1.78(0.98,3.25) | 1.20(0.89,1.63) | 1.51(1.17,1.96) |
| Ever had broken hip* | |||
| No | Reference | Reference | Reference |
| Yes | 1.26(0.63,2.55) | 1.85(1.33,2.58) | 2.24(1.62,3.09) |
| Fall* | |||
| No | Reference | Reference | Reference |
| Yes | 1.54(1.05,2.26) | 1.31(1.11,1.56) | 1.40(1.18,1.67) |
| Proxy use | |||
| No | Reference | Reference | Reference |
| Yes | 3.10(2.16,4.47) | 2.22(1.82,2.70) | 2.00(1.68,2.38) |
| IADL stage | |||
| No limitations | Reference | Reference | Reference |
| Mild limitations | 2.95(1.83,4.78) | 1.91(1.57,2.32) | 2.05(1.75,2.40) |
| Moderate limitations | 4.59(2.80,7.50) | 3.92(2.95,5.19) | 4.16(3.15,5.51) |
| Severe limitations | 6.97(4.20,11.55) | 5.18(3.92,6.85) | 6.89(4.82,9.85) |
| Complete limitations | 18.28(9.98,33.46) | 19.05(10.65,34.08) | 13.75(6.23,30.35) |
*Sample size is slightly less than 4,434 for these variables due to missing data: major depression (N = 4395); blindness in both eyes (N = 4375); deafness in both ears (N = 4373); ever had broken hip (N = 4377); fall (N = 4406)
Table 3.
Adjusted ORs from the Final Multiple Logistic Regression Models for 1-, 5-, and 10- Year Mortality in the Development Cohort (N = 4434)
| Characteristics | 1 year mortality | 5 year mortality | 10 year mortality |
|---|---|---|---|
| Adjusted OR (95% CI) | |||
| C-statistic | 0.74 | 0.74 | 0.76 |
| Age | |||
| ≥70–<75 | Reference | Reference | Reference |
| ≥75–<80 | 0.97 (0.62,1.53) | 1.68 (1.39,2.04) | 2.17 (1.84,2.56) |
| ≥80–<85 | 1.49 (0.92,2.40) | 2.65 (2.08,3.39) | 3.83 (3.04,4.82) |
| ≥85 | 1.99 (1.25,3.17) | 5.23 (3.89,7.05) | 10.58 (7.40,15.12) |
| Gender | |||
| Female | Reference | Reference | Reference |
| Male | 1.56 (1.05,2.32) | 1.87 (1.60,2.19) | 2.01 (1.73,2.34) |
| Marital status | |||
| Married | - | - | Reference |
| Unmarried | - | - | 1.32 (1.12,1.55) |
| Health status | |||
| Excellent | - | Reference | Reference |
| Very Good | - | 1.19 (0.90,1.57) | 1.21 (0.97,1.51) |
| Good | - | 1.55 (1.21,2.00) | 1.60 (1.29,1.99) |
| Fair | - | 1.85 (1.40,2.44) | 1.85 (1.44,2.39) |
| Poor | - | 3.40 (2.31,5.00) | 3.35 (2.30,4.87) |
| Diabetes | |||
| No | - | Reference | Reference |
| Yes | - | 1.67 (1.31,2.13) | 1.69 (1.36,2.10) |
| Cancer | |||
| No | - | Reference | - |
| Yes | - | 1.40 (1.13,1.73) | - |
| Chronic bronchitis or emphysema | |||
| No | - | - | Reference |
| Yes | - | - | 1.48 (1.13,1.95) |
| Coronary artery disease | |||
| No | Reference | Reference | Reference |
| Yes | 1.75 (1.16,2.65) | 1.22 (1.01,1.47) | 1.31 (1.12,1.53) |
| Other heart disease | |||
| No | - | Reference | Reference |
| Yes | - | 1.72 (1.27,2.33) | 1.38 (1.06,1.80) |
| Stroke | |||
| No | - | - | Reference |
| Yes | - | - | 1.68 (1.23,2.31) |
| IADL stage | |||
| No limitations | Reference | Reference | Reference |
| Mild limitations | 2.79 (1.63,4.76) | 1.42 (1.14,1.77) | 1.42 (1.17,1.72) |
| Moderate limitations | 3.92 (2.30,6.67) | 2.23 (1.61,3.11) | 2.16 (1.61,2.90) |
| Severe limitations | 5.59 (3.31,9.44) | 2.58 (1.88,3.53) | 2.88 (1.90,4.37) |
| Complete limitations | 13.55 (7.23,25.41) | 8.18 (4.48,14.94) | 3.88 (1.55,9.72) |
“-” indicates not applicable as a predictor
Predictive Index Construction
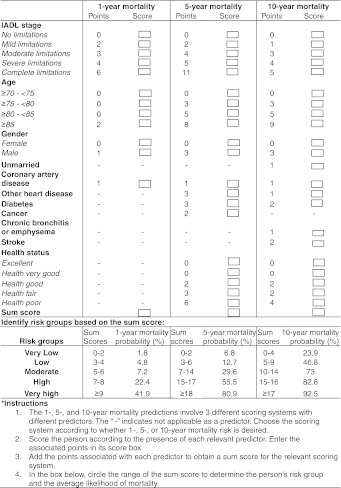
The three indexes for 1-, 5-, and 10-year mortality are shown in Fig. 2. Age, sex, coronary artery disease, and IADL stage were the most essential predictors across all three indexes.
Figure 2.
1-, 5-, and 10-year mortality predictive indexes and associated risk groups*.
Internal Validation
After applying the three indexes in the validation cohort, we compared the 1-, 5-, and 10-year mortality probabilities at each sum score between the development and validation cohorts (available online as online Table 2). C-statistics of the three indexes in the development versus validation cohorts are 0.72 versus 0.72 for 1-year mortality, 0.74 versus 0.72 for 5-year mortality, and 0.75 versus 0.74 for 10-year mortality.
We further combined sum scores with similar mortality probabilities into 5 risk categories in Fig. 2. The 1-year mortality probabilities range from 1.8% to 41.9% across the 5 risk categories, the 5-year mortality probabilities range from 6.8% to 80.9%, and the 10-year mortality probabilities range from 23.9% to 92.5%.
Sensitivity Analysis
The majority of missing data were due to missing IADL stage or health status. Even though SPs with missing data were less healthy than those with complete data, after multiple imputation the results of multiple logistic regression models for 1-, 5-, and 10-year mortality from the imputed datasets were similar to the complete-case analyses.
DISCUSSION
Mortality prediction is often the basis for risk adjustment, and is essential for evaluating medical effectiveness and quality of care, and for informing health policy decisions. Focusing on the rapidly growing elderly population with complex chronic illness which is increasingly recognized as important in internal medicine, we evaluated the effects of multiple co-morbidities and functional status on mortality in efforts to identify parsimonious predictors. Our 10-year mortality index has a 0.75 probability of correctly assigning a higher score to a randomly chosen patient who died than to a randomly chosen patient who did not die, and thus fills a needed gap in the literature regarding a lack of long-term mortality prediction tools for community-dwelling elderly populations.11 Our study adds the development of a long-term predictive mortality index, combined with parsimonious 1- and 5-year mortality indexes to the literature. Our 1-year mortality index applying only 4 predictors has a 0.72 probability of correctly assigning a higher score to a randomly chosen patient who died than to a randomly chosen patient who did not die. All three mortality predictive indexes show internal validity, and are simple to apply in community settings. Our 1-, 5-, and 10-year mortality predictive indexes can maximize the implementation of short-, intermediate- and long-term mortality estimation in community settings assisting clinical decision-making, and may serve as important screening tools for the impact of complex chronic illness on mortality. Thus, our mortality indexes have the potential to inform medical care decisions, identify high-risk persons for interventions, and provide a foundation for discussing care goals with community-dwelling elderly individuals. These rules can further provide surveillance measures to policy makers and epidemiologists when projecting the mortality of older populations.
IADL stage was one of the most important predictors for 1-, 5-, and 10-year mortality. The association between 1-year mortality and IADL stage was particularly strong and only age, sex, and coronary artery disease added explanatory power to the mortality index. Although functional status measured by various methods is known to be associated with mortality in elderly populations,7–10, 29–36 only a few studies applied this knowledge to develop short- or intermediate-term mortality indexes.7–10 Additionally, none expressed IADL as stages.7–10 Unlike individual IADLs,7, 8, 10 IADL stages summarize overall severity of disability across activities. Dissimilar to counts9 where patterns of limitation are obscured, IADL stages define thresholds of function that specify severity but are also transparent to the specific patterns of activities limited reflecting the known hierarchical structure of IADL items.18, 23 We derived IADL stages to capture the persons’ functional status because IADL performance demands higher degrees of integration across individuals’ cognitive and physical capacities, as compared to the self-care ADLs which evaluate personal bodily tasks18, 37–39 IADL limitations can result from physical or cognitive impairments and can be used as a screening tool for cognitive impairment in elderly community-dwellers.37–39 Thus, IADL stage is a strong predictor because it can serve as a proxy for multiple conditions simultaneously contributing to physical and cognitive impairments. The value of IADL stage to the internist is that it is easily determined by self-report and enables a more parsimonious subset of predictors simplifying mortality prediction and enhancing ease of implementation in community-dwelling elderly populations.
Coronary artery disease remained a significant predictor for 1-, 5-, and 10-year mortality, and other heart disease was significantly associated with 5- and 10-year mortality in the final models. Heart disease is known to be the leading cause of death in the US.40, 41 Other leading causes of death, including malignant neoplasms, cerebrovascular diseases, chronic lower respiratory diseases, and diabetes mellitus,42 were all significantly associated with 5- and/or 10-year mortality in the final adjusted models. Most of these conditions were also significantly associated with 1-year mortality in the unadjusted analyses. These factors likely did not enter the final model for 1-year mortality prediction because of their strong correlations with IADL stages as shown in online Table 1. These leading causes of death have both acute and chronic impacts on the subjects’ health although their long-term impact may be more significant.42 An individual’s IADL stage reflects current functional status as resulting from the person’s active cognitive and physical conditions, but functional status as captured by IADL stage will likely change over time due to the progression or regression of various health conditions. Thus, the IADL stage’s association with long-term mortality became attenuated over time while the chronic impact of certain medical diagnoses gained in importance for long-term mortality prediction. Further studies with more detailed clinical disease information are needed to confirm the association between IADL stage and acute and chronic disease burden over time.
We further evaluated the predictive ability of SPs’ perceived health status and various impairments common in the elderly, including blindness, deafness, broken hip, and falls for 1-, 5-, and 10-year mortality. These factors were significantly associated with mortality in unadjusted analyses, but only SPs’ perceived health status remained significantly associated with 5- and 10-year mortality in the final models. Blindness, deafness, broken hip, and falls were highly correlated with IADL stage in our study, and have been shown to have major impacts on functional status in other studies.42–45 Thus, IADL stages likely capture the effects of these conditions on mortality. Other studies showed self-rated health was a strong predictor for long-term mortality, and the association was only partly explained by medical conditions or sociodemographics.46–49 In our study, though SPs’ perceived health status did not contribute much to 1-year mortality, it was a significant predictor for 5- and 10-year mortality. Thus, SPs’ perceived health status appears to be adding health risk information in addition to sociodemographics, medical conditions, and functional status to long-term rather than short-term mortality.
There are several limitations in our study. First, we used prospectively collected self-reported data from the LSOA II, a well-designed national survey. Although the use of self-reported information will reduce the healthcare resources needed for implementation, recall and non-response biases in self-reported data could cause misclassification of our predictors. However, the LSOA II has been standardized and extensively tested.50, 51 Self-reported functional status has been validated,37, 52 and self-reported co-morbidities are commonly used in national surveys17, 23 and have been shown predictive of healthcare resource use and various outcomes.53–55 Second, excluding missing data from our complete-case analysis may have introduced bias. However, it is reassuring that we found similar results when we did multiple imputation as a sensitivity analysis. Third, 19.0% of the original sample used proxy-reports, while 15% of data from our complete-case analyses were reported by proxy due to the high prevalence of missing data in proxy-reports. We included proxy use as a variable in our analyses to adjust for the differences. However, this variable was not significant and hence was not included in the final models. Fourth, there are likely unmeasured predictors (unavailable in the data) that could increase prediction, such as cognitive status which is associated with mortality in the elderly population. 53–55 Although IADL stages37–39 and stroke56, 57 may be capturing some cognitive status information, further studies with directly measured cognition are needed. Finally, our baseline data was from the 1994-1996 national survey with mortality follow-up through 2006. The results may only be generalizable to the US community-dwelling population or developed countries with similar population structure.
In conclusion, the 1-, 5-, and 10-year mortality indexes developed from the LSOA II are practical for use in the community setting and can estimate prognosis for short-, intermediate-, and long-term mortality to assist with decision-making of clinicians, researchers, and policy makers. The use of IADL stage, which captures the cognitive and physical disease burden of the elderly population, can simplify mortality prediction in community settings when specific diagnostic information is lacking. If further studies demonstrate external validity of these mortality indexes in various community-dwelling elderly populations, these three mortality predictive indexes could become widely used tools for providing prognostic information and guiding therapeutic interventions among elderly community-dwellers.
Electronic Supplemental Materials
(DOC 163 kb)
ACKNOWLEDGEMENTS
Funders:
The research for this manuscript was supported by the National Institutes of Health (AG032420-01A1). There are no personal conflicts of interest of any of the authors, and no authors reported disclosures beyond the funding source. The opinions and conclusions of the authors are not necessarily those of the sponsoring agency or of the NCHS who is responsible only for provision of the data.
Prior Presentations:
None.
Conflict of Interest:
There are no personal conflicts of interest of any of the authors, and no authors reported disclosures beyond the funding source. The opinions and conclusions of the authors are not necessarily those of the sponsoring agencies.
References
- 1.Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793–799. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277(6):488–494. doi: 10.1001/jama.1997.03540300056034. [DOI] [PubMed] [Google Scholar]
- 3.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users' guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 4.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26–35. doi: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929–1935. doi: 10.1001/jama.2010.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine SK, Sachs GA, Jin L, Meltzer D. A prognostic model for 1-year mortality in older adults after hospital discharge. Am J Med. 2007;120(5):455–460. doi: 10.1016/j.amjmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 8.Carey EC, Covinsky KE, Lui LY, Eng C, Sands LP, Walter LC. Prediction of mortality in community-living frail elderly people with long-term care needs. J Am Geriatr Soc. 2008;56(1):68–75. doi: 10.1111/j.1532-5415.2007.01496.x. [DOI] [PubMed] [Google Scholar]
- 9.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24(10):1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19(10):1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. The Second Longitudinal Study on Aging, 1994-2000. 2002; http://www.cdc.gov/nchs/lsoa/lsoa2.htm. Accessed 2/9/12.
- 13.National Center for Health Statistics. Data File Documentation, National Health Interview Survey on Disability, Phase I, Adult File 1994 Hayattsville, MD: National Center for Health Statistics; 1996
- 14.National Center for Health Statistics: Data file Documentation. National Health Interview Survey on Disability, Phase II, Adult File 1995. Hayattsville, MD: Center for Disease Control and Prevention; 1998.
- 15.Center for Disease Control and Prevention. Survey Instruments. 2009; http://www.cdc.gov/nchs/data/nhis/dfs_100_1994.pdf Accessed 2/9/12.
- 16.National Center for Health Statistics. Office of Analysis and Epidemiology. Public-use Second Longitudinal Study of Aging (LSOA II) Linked Mortality File. 2010; http://www.cdc.gov/nchs/data_access/data_linkage/mortality/lsoaii_linkage_public_use.htm Accessed 2/9/12.
- 17.Stineman MG, Henry-Sánchez JT, Kurichi JE, et al. Staging sctivity limitation and participation restriction in elderly community-dwelling persons according to difficulties in self-care and domestic life functioning. Am J Phys Med Rehabil. 2012;91:126–140. doi: 10.1097/PHM.0b013e318241200d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist Autumn. 1969;9(3):179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 19.Kempen GI, Myers AM, Powell LE. Hierarchical structure in ADL and IADL: analytical assumptions and applications for clinicians and researchers. J Clin Epidemiol. 1995;48(11):1299–1305. doi: 10.1016/0895-4356(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 20.Siu AL, Reuben DB, Hays RD. Hierarchical measures of physical function in ambulatory geriatrics. J Am Geriatr Soc. 1990;38(10):1113–1119. doi: 10.1111/j.1532-5415.1990.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 21.Ustun TB, Chatterji S, Bickenbach J, Kostanjsek N, Schneider M. The International Classification of Functioning, Disability and Health: a new tool for understanding disability and health. Disabil Rehabil. 2003;25(11-12):565–571. doi: 10.1080/0963828031000137063. [DOI] [PubMed] [Google Scholar]
- 22.Stineman M, Ross R, Maislin G. Functional status measures for integrating medical and social care. International Journal of Integrated Care. 2005;5:e07. doi: 10.5334/ijic.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saliba D, Orlando M, Wenger NS, Hays RD, Rubenstein LZ. Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci. 2000;55(12):M750–M756. doi: 10.1093/gerona/55.12.M750. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–781. doi: 10.1016/S0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 25.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118(3):201–210. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Toll DB, Janssen KJ, Vergouwe Y, Moons KG. Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol. 2008;61(11):1085–1094. doi: 10.1016/j.jclinepi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 28.Raghunathan, Solenberger, Hoewyk . IVEware: Imputation and Variance Estimation Software Users Guide. University of Michigan: Ann Arbor: Institute for Social Research; 2002. [Google Scholar]
- 29.Klijs B, Mackenbach JP, Kunst AE. Disability occurrence and proximity to death. Disabil Rehabil. 2010;32(21):1733–1741. doi: 10.3109/09638281003746049. [DOI] [PubMed] [Google Scholar]
- 30.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66(1):89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turvey CL, Schultz SK, Beglinger L, Klein DM. A longitudinal community-based study of chronic illness, cognitive and physical function, and depression. Am J Geriatr Psychiatry. 2009;17(8):632–641. doi: 10.1097/JGP.0b013e31819c498c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landi F, Liperoti R, Russo A, et al. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80years and older. J Clin Epidemiol. 2010;63(7):752–759. doi: 10.1016/j.jclinepi.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070–2076. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keeler E, Guralnik JM, Tian H, Wallace RB, Reuben DB. The impact of functional status on life expectancy in older persons. J Gerontol A Biol Sci Med Sci. 2010;65(7):727–733. doi: 10.1093/gerona/glq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26(2):130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng TP, Niti M, Chiam PC, Kua EH. Physical and cognitive domains of the Instrumental Activities of Daily Living: validation in a multiethnic population of Asian older adults. J Gerontol A Biol Sci Med Sci. 2006;61(7):726–735. doi: 10.1093/gerona/61.7.726. [DOI] [PubMed] [Google Scholar]
- 38.Wang CY, Hu MH, Chen HY, Li RH. Self-Reported Mobility and Instrumental Activities of Daily Living Disability Status in Community-Dwelling Older Asian Adults: Test-Retest Reliability and Criterion Validity. J Aging Phys Act. Oct 19 2011. [DOI] [PubMed]
- 39.Barberger-Gateau P, Commenges D, Gagnon M, Letenneur L, Sauvel C, Dartigues JF. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc. 1992;40(11):1129–1134. doi: 10.1111/j.1532-5415.1992.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell SL, Kiely DK, Hamel MB, Park PS, Morris JN, Fries BE. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291(22):2734–2740. doi: 10.1001/jama.291.22.2734. [DOI] [PubMed] [Google Scholar]
- 42.Heron M, Tejada-Vera B. Deaths: leading causes for 2005. Natl Vital Stat Rep. 2009;58(8):1–97. [PubMed] [Google Scholar]
- 43.Zhou H, Isaman DJ, Messinger S, et al. A computer simulation model of diabetes progression, quality of life, and cost. Diabetes Care. 2005;28(12):2856–2863. doi: 10.2337/diacare.28.12.2856. [DOI] [PubMed] [Google Scholar]
- 44.Emond M, Mock MB, Davis KB, et al. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1994;90(6):2645–2657. doi: 10.1161/01.CIR.90.6.2645. [DOI] [PubMed] [Google Scholar]
- 45.National Cancer Institute. Cancer survival statistics. http://surveillance.cancer.gov/statistics/types/survival.html Accessed 2/9/12.
- 46.Sloan FA, Ostermann J, Brown DS, Lee PP. Effects of changes in self-reported vision on cognitive, affective, and functional status and living arrangements among the elderly. Am J Ophthalmol. 2005;140(4):618–627. doi: 10.1016/j.ajo.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- 48.Shyu YI, Chen MC, Liang J, Wu CC, Su JY. Predictors of functional recovery for hip fractured elders during 12months following hospital discharge: a prospective study on a Taiwanese sample. Osteoporos Int. 2004;15(6):475–482. doi: 10.1007/s00198-003-1557-2. [DOI] [PubMed] [Google Scholar]
- 49.Overcash J. Prediction of falls in older adults with cancer: a preliminary study. Oncol Nurs Forum. 2007;34(2):341–346. doi: 10.1188/07.ONF.341-346. [DOI] [PubMed] [Google Scholar]
- 50.Heistaro S, Jousilahti P, Lahelma E, Vartiainen E, Puska P. Self rated health and mortality: a long term prospective study in eastern Finland. J Epidemiol Community Health. 2001;55(4):227–232. doi: 10.1136/jech.55.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gander J, Lee DC, Sui X, Hebert JR, Hooker SP, Blair SN. Self-rated health status and cardiorespiratory fitness as predictors of mortality in men. Br J Sports Med. 45:1095–1100. [DOI] [PMC free article] [PubMed]
- 52.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 53.Banerjee D, Perry M, Tran D, Arafat R. Self-reported health, functional status and chronic disease in community dwelling older adults: untangling the role of demographics. J Community Health. 2010;35(2):135–141. doi: 10.1007/s10900-009-9208-y. [DOI] [PubMed] [Google Scholar]
- 54.Fan VS, Au D, Heagerty P, Deyo RA, McDonell MB, Fihn SD. Validation of case-mix measures derived from self-reports of diagnoses and health. J Clin Epidemiol. 2002;55(4):371–380. doi: 10.1016/S0895-4356(01)00493-0. [DOI] [PubMed] [Google Scholar]
- 55.Hornbrook MC, Goodman MJ. Chronic disease, functional health status, and demographics: a multi-dimensional approach to risk adjustment. Health Serv Res. 1996;31(3):283–307. [PMC free article] [PubMed] [Google Scholar]
- 56.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57(2):202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mok VC, Wong A, Lam WW, et al. Cognitive impairment and functional outcome after stroke associated with small vessel disease. J Neurol Neurosurg Psychiatry. 2004;75(4):560–566. doi: 10.1136/jnnp.2003.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 163 kb)