Three decades ago, Todd (1) postulated that wholesale fission of all “mediocentric” (metacentric, submetacentric, and subtelocentric) chromosomes in a complement plays a major role in chromosome evolution. According to Todd, karyotypic fission produces, as a consequence of a single mutational “event,” dramatic differences in the diploid numbers of closely related species (2, 3). A diversity of karyotypes is generated through the random assortment of parent and fissioned homologous chromosomes. Immediate descendants of the fissioned parent would exhibit identical fundamental numbers of functional chromosomal arms but (potentially) very different diploid numbers. Todd saw karyotypic fission events, followed by the accumulation of pericentric inversions, as the driver for explosive speciation in adaptive radiations. He used the label “Karyotypic Fission Theory” to call attention to his implicit rejection of Darwinian gradualism in chromosomal evolution. Whole karyotypic fissioning can (at least theoretically) generate drastically different karyotypes in far fewer steps than are required according to competing explanations of chromosomal evolution, whether based on reciprocal or nonreciprocal chromosomal fission or fusion.
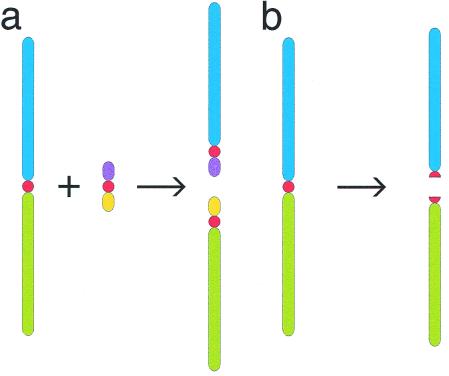
Shortly after Todd's article was published, it was dismissed as preposterous by one of the leading theorists of chromosomal evolution, M. J. D. White (4). If chromosomal fissioning occurs only under unusual circumstances, how could an entire karyotype be expected to fission? A serious problem was the lack of a plausible cellular/molecular mechanism. Indeed, even single chromosomal fissioning appeared difficult to explain (refs. 5 and 6; see ref. 7 for review). Some researchers saw fissioning as requiring a ready supply of extra centromeres, perhaps in the form of vestigial chromosomes (Fig. 1a). But the existence of spare vestigial chromosomes had never been demonstrated. Todd (1) proposed another mechanism—centromeric mis-division and subsequent repair (Fig. 1b). White (4) doubted “whether simple breakage through the centromere of a metacentric can produce two fully functional and stable telocentric chromosomes, capable of persisting indefinitely.” He added, “To suppose that all of the chromosomes of a karyotype would undergo this process simultaneously is equivalent to a belief in miracles, which has no place in science” (ref. 4, p. 401). With rare exceptions (8–11), Todd's theory has been ignored for more than a quarter of a century.
Figure 1.
Two previously posited mechanisms of metacentric chromosomal fissioning. Both are problematic (see text). (a) Spare vestigial chromosomes provide extra centromeres. (b) Centromeric misdivision is followed by repair. Centromeres are shown in red.
In an article published in a recent issue of PNAS, Robin Kolnicki (12) offers a plausible mechanism (Kinetochore Reproduction Theory) for simultaneous chromosomal fissioning. Her argument is largely theoretical, but cellular/molecular evidence is provided for each of its components. Many of the ideas presented are not new; dicentric chromosomes have been known since the 1930s (13), but their linkage to Todd's Karyotypic Fission Theory is original with this paper.
Kolnicki's Kinetochore Reproduction Theory has several components. During DNA replication just before meiotic synapsis and sister chromatid segregation, a mutational agent stimulates the production of extra kinetochores on all (or most) of the mediocentric chromosomes' newly synthesized sister strands. The newly synthesized strands now possess two functional kinetochores instead of one. These supernumerary kinetochores do not disrupt the distribution of chromosomes to daughter cells during meiosis because tension-sensitive mitotic checkpoints operate to prevent errors in chromosome segregation (as illustrated in Fig. 1A of ref. 12). Assuming none of the supernumerary kinetochores is later inactivated, descendants will inherit dicentric chromosomes with two functional kinetochores. When these chromosomes in turn replicate, each pair of sister chromatids will have four functional kinetochores (as illustrated in Fig. 1B of ref. 12). Again, the tension-sensitive checkpoints will prohibit errors in chromosome delivery during meiosis. But now fissioning and nonfissioning are equally probable, and for any pair of dicentric sister chromatids, the outcome will depend on whether spindle attachment is monopolar or bipolar. Under monopolar attachment, there will be no fissioning, and the dicentric chromosomes will be passed on, intact, to the next generation. Under bipolar attachment, chromosomal fissioning occurs. Because monopolar and bipolar attachment are equally probable, 50% of the pairs of dicentric sister strands can be expected to fission during any single meiosis. With such probabilities, it is easy to ascertain that in only 7 successive generations, 99% of the functionally dicentric chromosomes can be expected to have fissioned, and that in 10 generations, only 1 in 1,000 unfissioned dicentric chromosomes will remain. In the absence of strong selection pressure eliminating these variants, they may become fixed in small populations. Thus, we have what must be considered an evolutionarily instantaneous (or macromutational) “event”—“simultaneous” fissioning of all of the mutant mediocentric chromosomes in a complement.
This theory is attractive for a number of reasons. First, none of the posited prefission steps (i.e., the production of supernumerary kinetochores, the retention and inheritance of chromosomes with two functional kinetochores) should have deleterious effects on cell division or phenotypes. In addition, unstable telocentric chromosomes produced by fissioning are likely to be repaired by the high amounts of telomerase in embryonic cells, so the fissioned chromosomes themselves should function normally. Fissioning should have no negative consequences for meiotic synapsis in succeeding generations, as the two fissioned autosomal acrocentrics pair easily with their homologous mediocentrics. Indeed, selection might favor the retention of fissioned acrocentrics over homologous mediocentrics, if smaller chromosomes are more likely than larger ones to segregate without error during cell division (14). The only chromosomes that would not be expected to synapse properly after fissioning are the sex chromosomes (1); strong selection pressure should promote the retention of unfissioned X-chromosomes. This might well help to explain the relative conservatism of X-chromosomes across a wide variety of organisms (15).
Karyotypic Fission Theory embraces the well-substantiated general predictions of reciprocal (“Robertsonian”) translocation theory (16–18). These predictions are that redistribution of DNA (with pairs of acrocentrics demonstrably homologous to single mediocentric chromosomes) should occur without changes in the amount of DNA, and that closely related taxa will share commonly the same fundamental number of chromosome arms but not the same diploid number (because their karyotypes differ only by reciprocal translocations). These sorts of differences should (and do) occur at an intraspecific level. (Other types of meiotic errors, such as pericentric inversions, alter the fundamental number without affecting the diploid number.)
Specific predictions of karyotypic fission theory include the occurrence of very different diploid numbers in closely related species with the same fundamental number and of distributions among closely related species of karyotypes that might be generated through a single karyotype fission event. Finally, Karyotypic Fission Theory predicts that low diploid numbers will be primitive for clades.
Whereas Kolnicki offers a plausible mechanism for simultaneous chromosomal fissioning, the jury is still out on the generality of this occurrence. Even if Todd and Kolnicki are correct about the feasibility of wholesale karyotypic fissioning, it would simply join a battery of known mechanisms of chromosomal evolution, the relative frequency of which has yet to be determined. That relative frequency must be assessed by using studies of chromosomal banding and painting as well as proper (cladistic outgroup) analysis of the probable ancestral karyotypes of particular phylogenetic groups. There are, of course, many examples of karyotypic differences among closely related species that simply cannot be explained via karyotypic fissioning. For example, the differences between the Indian (2n = 6 or 7) and Chinese (2n = 24) muntjac karyotypes cannot be explained in this manner (19). Nevertheless, Karyotypic Fission Theory owes its relative obscurity more to the lack of a plausible cellular/molecular mechanism than to perceived weaknesses in its explanatory power. Kolnicki offers such a mechanism, and her work should stimulate further research on the plausibility as well as the potential explanatory power of karyotypic fission events. Kolnicki's Kinetochore Reproduction Theory may even explain empirical observations that appear to contravene karyotypic fissioning in particular clades (e.g., refs. 9 and 11). For example, Finelli et al. (11) embrace fission as the primary vehicle for differences between the karyotypes of green monkeys and humans, but they dismiss Karyotypic Fission Theory because reciprocal chromosome painting suggests that most break points lie outside the centromere regions. If, as Kolnicki suggests, one of the possible mechanisms of kinetochore reproduction involves the epigenetic formation of neokinetochores in regions previously devoid of centromeric activity, and if such synthesis is followed by chromosome breakage between kinetochores, this objection may be moot.
Kolnicki's attempt to link karyotype fissioning and speciation is weak and unnecessary. Although it is true that karyotypic rearrangements may become fixed by genetic drift in small populations during the course of speciation, assigning these rearrangements a causative role in the process flies in the face of the extensive literature that argues otherwise (20–24). Todd and Kolnicki have argued that difficulties in the meiotic pairing of fissioned and unfissioned homologous chromosomes can be expected to arise through the accumulation of pericentric inversions on fissioned chromosomes, thereby generating immediate reproductive isolation. However, for deleterious inversions to trigger speciation, they would need to spread through the population. Rearrangements appear first in populations as heterozygotes, and inviable or sterile heterozygotes will be eliminated by normalizing natural selection, regardless of how fit the corresponding homozygotes might be (20). The chances of fixing a deleterious rearrangement, the effects of which are strong enough to present a significant barrier to gene flow, are extremely low without the aid of a prolonged and severe population bottleneck (21, 23).
It is nevertheless possible that karyotypic fissioning explains major evolutionary changes in karyotypes. Certainly it poses a welcome challenge to the hegemony that Robertsonian fusion has exercised over interpretations of chromosomal evolution during the past 50 years. In our view, it is unlikely that one process or the other can independently account for the wide range of karyotype structures that are observed, or that the derived or ancestral nature of a taxon can be inferred from its diploid and/or fundamental numbers. For example, few lemur specialists would embrace the suggestion that the 2n = 66 largely acrocentric karyotype shared by the dwarf lemur genera Microcebus, Mirza, and Cheirogaleus was evidence of a recent radiation of this group. But used in conjunction with other phylogenetic data, karyotypic fissioning may help to explain dramatic differences in diploid numbers between closely related species, which were previously inexplicable.
Footnotes
See companion article on page 9493 in issue 17 of volume 97.
References
- 1.Todd N B. J Theor Biol. 1970;26:445–480. doi: 10.1016/0022-5193(70)90096-2. [DOI] [PubMed] [Google Scholar]
- 2.Todd N B. Paleobiology. 1975;1:175–188. [Google Scholar]
- 3.Todd N B. In: Environmental Evolution: Effects of the Origin and Evolution of Life on Planet Earth. Margulis L, Olendzenski L, editors. Cambridge, MA: MIT Press; 1992. pp. 275–292. [Google Scholar]
- 4.White M J D. Animal Cytology and Evolution. 3rd Ed. Cambridge, U.K.: Cambridge Univ. Press; 1973. [Google Scholar]
- 5.Egozcue J. Experientia. 1971;27:969–971. doi: 10.1007/BF02135779. [DOI] [PubMed] [Google Scholar]
- 6.Imai H T, Crozier R H. Am Nat. 1980;116:537–569. doi: 10.1086/283646. [DOI] [PubMed] [Google Scholar]
- 7.Martin R D. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton: Princeton Univ. Press; 1990. [Google Scholar]
- 8.Giusto J P, Margulis L. BioSystems. 1981;13:267–302. doi: 10.1016/0303-2647(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 9.Stanyon R. BioSystems. 1983;16:57–63. doi: 10.1016/0303-2647(83)90025-4. [DOI] [PubMed] [Google Scholar]
- 10.Kolnicki R L. Symbiosis. 1999;26:123–141. [Google Scholar]
- 11.Finelli P, Stanyon R, Plesker R, Ferguson-Smith M A, O'Brien P C, Wienberg J. Mamm Genome. 1999;10:713–718. doi: 10.1007/s003359901077. [DOI] [PubMed] [Google Scholar]
- 12.Kolnicki R L. Proc Natl Acad Sci USA. 2000;97:9493–9497. doi: 10.1073/pnas.97.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mather K, Stone L H A. J Genet. 1933;28:1–24. [Google Scholar]
- 14.Schubert I, Oud J L. Cell. 1997;88:515–520. doi: 10.1016/s0092-8674(00)81891-7. [DOI] [PubMed] [Google Scholar]
- 15.Ohno S, Becak W, Becak M L. Chromosoma. 1964;15:14–30. doi: 10.1007/BF00326912. [DOI] [PubMed] [Google Scholar]
- 16.Robertson W R B. J Morphol. 1916;27:129–331. [Google Scholar]
- 17.Matthey R. Experientia. 1945;1:50–56. , 78–86. [Google Scholar]
- 18.Matthey R. Les Chromosomes des Vertébrés. Lausanne, Switzerland: F. Rouge; 1949. [Google Scholar]
- 19.Liming S, Yingying Y, Xingsheng D. Cytogenet Cell Genet. 1980;26:22–27. doi: 10.1159/000131417. [DOI] [PubMed] [Google Scholar]
- 20.Dobzhansky T. Genetics and the Evolutionary Process. New York: Columbia Univ. Press; 1970. [Google Scholar]
- 21.Lande R. Evolution (Lawrence, KS) 1979;33:234–251. doi: 10.1111/j.1558-5646.1979.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 22.Futuyma D J, Mayer G C. Syst Zool. 1980;29:254–271. [Google Scholar]
- 23.Barton N H, Hewitt G M. In: Evolution and Speciation: Essays in Honor of M. J. D. White. Atchley W R, Woodruff D S, editors. Cambridge, U.K.: Cambridge Univ. Press; 1981. pp. 109–145. [Google Scholar]
- 24.Paterson H E H. Evolution and the Recognition Concept of Species. Baltimore: Johns Hopkins Univ. Press; 1993. [Google Scholar]