Abstract
AIMS
During the global H1N1 influenza A (swine flu) pandemic 2009–2010, swine flu vaccines were expeditiously licensed and a mass vaccination programme for high risk groups, including pregnant women, was introduced in the UK. This pilot active safety surveillance study was performed to establish the feasibility of rapidly monitoring the new swine flu vaccines in large patient numbers receiving or offered the vaccination under normal conditions of use within a short time frame.
METHODS
A cohort design with safety data capture through modern technologies was carried out in Scotland, UK during the winter swine flu vaccination programme 2009–2010 in individuals receiving or offered the swine flu vaccination. The main outcome measures were self-reported serious adverse events (SAEs) and pregnancy outcomes.
RESULTS
The cohort comprised 4066 people; 3754 vaccinated and 312 offered the vaccination but not vaccinated. There were 939 self-reported events (838 different events), 53 judged to fit SAE criteria by the investigators, with nine judged as possibly, probably or definitely vaccine related. None of the seven deaths (six in vaccinees) were judged as vaccine related. One hundred and twenty-eight women reported 130 pregnancies during the study with 117 pregnant at study start. There were reports of four miscarriages in three women and six possible congenital abnormalities in live births.
CONCLUSIONS
Overall, no significant safety issues were identified. The methodology and use of modern technologies to collect safety data from large numbers of patients was successful and could be used again in similar safety studies.
Keywords: H1N1 vaccination, post marketing, safety surveillance
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
While the new H1N1 vaccines underwent the usual rigorous safety and efficacy testing, concerns remained that there may be unexpected side effects of the vaccines.
The strategy for H1N1 vaccine pharmacovigilance in the UK consisted of two patient studies by the two vaccine manufacturers, 14 small scale studies supported by the National Institute of Health Research, monitoring by specialist neurologists and the MHRA.
There were calls to investigate the feasibility of a large-scale prospective active surveillance system for ‘near real-time’ vaccine safety monitoring that would be complementary to the other aforementioned initiatives.
WHAT THIS STUDY ADDS
This pilot study demonstrated that collecting ‘near real-time’ reporting of event data from patients who experienced side effects as well as those who reported no problems after swine flu vaccination is feasible.
The use of information technologies improves patient involvement in research as well as dramatically limiting the cost of the study.
The online methodology facilitates rapid surveillance in response to urgent safety issues.
Introduction
In April 2009, the first cases of human influenza A (H1N1) (‘swine flu’) were identified in Mexico, Canada and the US [1]. The virus spread rapidly to other parts of the world and a global flu pandemic was declared in June 2009 by the World Health Organization (WHO). The rapid development of H1N1 vaccines to prevent further morbidity and mortality became a public health priority and the first vaccines were licensed in October 2009. A mass vaccination programme for high risk groups was introduced in the UK from late October 2009 onwards. In Scotland, the vaccination programme was launched on 21 October 2009. The people initially offered vaccination included frontline health and social care workers, people over 6 months and up to 65 years in the seasonal flu vaccine clinical at-risk groups (e.g. heart, lung, kidney disease), all pregnant women, household contacts of people with compromised immune systems and people aged 65 years and over in the existing seasonal flu vaccine clinical at-risk groups. In a second phase of the programme, young children aged over 6 months and up to 5 years of age were also prioritized for vaccination between December 2009 and March 2010 [2]. Around 500 000 people in Scotland were vaccinated against H1N1 influenza between November 2009 and April 2010 (>5 million in the UK) [3].
While the new H1N1 vaccines underwent the usual rigorous safety and efficacy testing [4]–[7], concerns remained that there may be unexpected side effects of the vaccines. This was partly based on the experiences from a 1976 US national immunization programme against a swine-origin influenza A virus (H1N1 subtype A/NJ/76) in which >40 million people were vaccinated [8], [9]. The programme was stopped early, partly due to the fact that the expected pandemic failed to materialize, but also due to the appearance of an apparent excess of cases of the neurological disorder, Guillain-Barre syndrome (GBS) in vaccinated individuals. GBS usually follows a viral infection, but can also rarely follow immunization in susceptible individuals [10]. The large scale of the 2009 worldwide vaccination programme prompted many countries to improve and expand their vaccination safety monitoring procedures [11].
The strategy for H1N1 vaccine pharmacovigilance in the UK consisted of two 9000 patient studies by the two vaccine manufacturers, the monitoring of GBS via neurologists within the Health Protection Agency (HPA), 14 small scale studies supported by the National Institute of Health Research (NIHR) on behalf of the Department of Health and safety monitoring by the Medicines and Healthcare products Regulatory Agency (MHRA) including a modified yellow card system and online reporting portal. In addition, we carried out an academic active safety surveillance study to monitor for serious adverse events in the population offered H1N1 vaccination in Scotland.
The aim of this study was to investigate the feasibility of a large scale prospective active surveillance system for ‘near real-time’ vaccine safety monitoring that would be complementary to the other aforementioned initiatives. Through the use of information technologies, exposure and outcome data would be collected directly from patients in Scotland during the mass vaccination programme in an expedited manner.
Specific objectives were (i) to recruit cohorts of people offered H1N1 influenza A vaccination: vaccinated and unvaccinated (unexposed) cohorts (those eligible for vaccination but who did not receive it), (ii) to examine the use and safety of H1N1 influenza A vaccination during the vaccination programme with particular interest in vaccine utilization characteristics for the whole vaccinated cohort and selected clinical risk groups (pregnant women, adults with selected underlying conditions, frontline health care workers), (iii) to capture patient self-reported events, (iv) to describe cases of serious adverse events (resulting in or prolonging hospital admission, life-threatening, fatal or resulting in significant or persisting disability) and relatedness to vaccination, (v) to describe pregnancy outcomes in vaccinated and non-vaccinated women and (vi) to pilot the feasibility of using patient self-reporting via questionnaires using largely electronic data capture to streamline data entry.
Methods
The study used an observational prospective cohort design consisting of an exposed (H1N1 vaccinated) population and unexposed (non-H1N1 vaccinated) reference group. Participants were those offered the swine flu vaccination as part of the government directed strategy in Scotland. Patients were invited to participate in the study when attending swine flu vaccination centres within primary care and other HPA/NHS designated sites in Scotland. Study information was sent out to all 1015 general practices in Scotland and around one third of these practices took part by making information available to their patients. Participants were alerted to the study at the time of attendance for vaccination via leaflets and posters displayed at the vaccination site. Participants were given an information sheet about the study and directed to a bespoke study website (http://www.safetyswineflu.org–now closed) for further information. A freephone telephone enquiry line was also available to answer questions about the study. Written informed consent was collected from patients, either at the time of vaccination, or via an online form. Patients were included in the study if they provided consent to a) participate in the study and b) allow follow-up of any adverse events from healthcare professionals and their medical records if necessary.
H1N1 exposure data and outcome data were gathered directly from study participants. Participants completed baseline information about themselves on a paper or online registration form including demographics, information about risk group, e.g. healthcare worker, underlying medical conditions, vaccination site, e.g. GP surgery and date of vaccination. For non-vaccinated individuals, reasons for not having the vaccine were collected.
Participants were followed up at monthly intervals following the date of first vaccination, or if unvaccinated, following the date of registration (‘index date’). Participants were contacted via their preferred mode of contact on a monthly basis after the index date, using automated technology to generate messages by email or text (via Ja.net®) where possible, or otherwise by letter or telephone, and asked to respond to report in their own words any serious adverse events requiring emergency treatment and/or resulting in hospitalization since being offered vaccination (primary study outcome), irrespective of whether they felt the event was related to the vaccine. Other information relating to minor events was not requested. If they had no events, a simple ‘No’ response was required. If the participant did not respond, a reminder message was sent. If no response was received after the reminder, alternative contact methods were tried or a nominated proxy, where provided, was contacted. Since entry into the cohort was staggered, the observation time for each individual varied. Figure 1 shows participant flow and response rate during the study. Each patient was followed up for each month post recruitment irrespective of response (or not) to the previous month's follow-up request.
Figure 1.
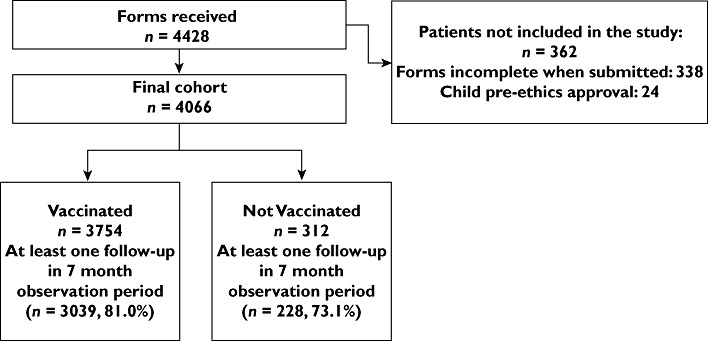
Flow diagram of participants and follow-up rates during the study
All data provided on the baseline questionnaire and at each monthly follow-up (where a response was provided) were entered onto the study database. All positive monthly follow-up reports were reviewed by two members of the research team and patient verbatim reported event data were also coded onto the database using the Drug Safety Research Unit (DSRU) Event Dictionary which is a hierarchical dictionary arranged in a system-organ classification where groups associated ‘Doctor Summary’ terms (terminology used by the prescribing physician) under ‘Lower Level’ event terms (LLT), similarly, related LLT are grouped under a broader ‘Higher Level’ event term. Serious adverse events were followed up by contacting the patient, their proxy, their general practitioner or consulting medical records for more information. Any reported pregnancies were specifically followed up to delivery date or to the end of pregnancy to ascertain the outcome of pregnancy.
The study started on 2 November 2009 and closed to new recruitment on 30 April 2010, when the 2009–2010 vaccination programme had largely ended. Follow-up of the last pregnancy outcomes was completed in January 2011.
The study was conducted in accordance with the principles of Good Clinical Practice (GCP) and received expedited favourable ethical opinion from the Fife and Forth Valley Ethics Committee and NHS R + D approvals. Initially, only adults (≥16 years) were recruited but later children were also included after additional regulatory approvals were obtained.
Data analysis included calculation of cumulative weekly participation rates (vaccinated and unvaccinated cohorts), examination of patient characteristics using descriptive statistics and univariate analysis [crude Odds Ratios (OR) with 95% confidence intervals (CI)]. Counts of events reported by participants were summarized by body system organ class. Overall event incidence densities for the 7 month observation period from the start of the vaccination programme were calculated, the numerator being the first report of event (at lower level term) and the denominator patient months of observation, censored at month of last response to follow-up. Patients for whom no response was provided to any follow-up were excluded from the denominator. Self-reported events of interest were then reviewed by two members of the clinical research team and adjudicated by study clinicians (with training in making seriousness (in accordance with the International Conference on Harmonization definitions [12]) and relatedness assessments on events) and relatedness to vaccination [the four categories used were: definite, probable, possible and not related (unlikely)][13], [14]). Confirmed serious adverse events are presented within a case series matrix.
Results
Recruitment
Four thousand and sixty-six patients gave valid consent and entered the study. Figure 2 shows the pattern of recruitment of participants to the study cohort between November 2009 and April 2010, according to vaccination status. Fifty percent of the cohort was recruited by the fourth week of the study. Where place of vaccine was reported in vaccinated participants (n = 3624), vaccination took place most commonly in GP surgeries (83.1%, n = 3012) followed by the workplace (including health and social care workers vaccinated in hospitals) (10.2%, n = 371) and other settings (6.9%, n = 251).
Figure 2.
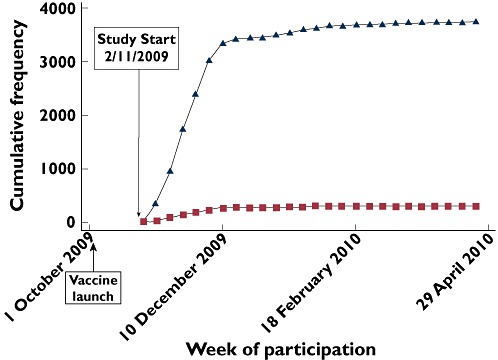
Recruitment of participants to the study cohort shown by date and vaccination status. Had H1N1 vaccine ( ); declined H1N1 vaccine (
); declined H1N1 vaccine ( )
)
Baseline characteristics
The mean (±SD) age of all participants was 53.6 ± 17.0 years; 4028 participants were aged ≥16 years (mean 53.2 ± 17.5 years and 38 participants were children aged <16 years [median 4.8 years (IQR 2.3–8.1)]. Data on the adult (≥16 years) and children (<16 years) groups are presented separately.
Adult participants (aged ≥16 years)
The baseline characteristics of the adult participants are presented in Table 1. More than half of participants were female (57.2%). Male participants were significantly older than females [males (n = 1660) mean age 57.9 ± 15.9 years; females (n = 2284) mean age 50.4 ± 17.2 years; (P < 0.001)]; 3708 (92.2%) participants were vaccinated. The mean time from start of study (2 November 2009) to vaccine uptake was 26.5 ± 16.3 days. The proportion of participants who did not receive the H1N1 vaccine was small (7.8%, n = 312).
Table 1.
Baseline characteristics of participants aged ≥ 16 years, (significant OR in bold)
| Had H1N1 vaccine | Yes (n = 3716) | No (n = 312) | Total (n = 4028) | P | OR (95% CI) |
|---|---|---|---|---|---|
| Gender (Female) | 2157 (58.7%) | 148 (47.9%) | 2305 (57.9) | P < 0.0001 | 1.55 (1.22, 1.97) |
| 46 | |||||
| Age at consent (years): | |||||
| Mean (SD) | 53.6 (16.9) | 54.4 (17.9) | 53.6 (17.0) | P = 0.8016 | |
| Missing | 32 | ||||
| Caregiver | 247 (7.3) | 18 (6.6) | 265 (7.3) | P = 0.654 | 1.12 (0.68, 1.95) |
| Missing | 384 | ||||
| Healthcare professional | 614 (17.4) | 27 (9.4) | 641 (16.8) | P = 0.001 | 2.03 (1.35, 3.17) |
| Missing | 208 | ||||
| Past history vaccination in prior 3 months** | |||||
| Seasonal flu vaccine | 1998 (53.8) | 134 (43.0) | 2132 (53.0) | P < 0.0001 | 1.55 (1.22,1.97) |
| Travel vaccine | 42 (1.1) | 1 (0.3) | 43 (1.1) | P = 0.181 | 3.56(0.60, 144.12) |
| Other vaccine | 128 (3.4) | 6 (1.9) | 134 (3.3) | P = 0.150 | 1.82 (0.80,5.09) |
| Any one or more vaccinations in prior 3 months | 2068 (55.6) | 137 (43.9) | 2205 (54.8) | P < 0.0001 | 1.60 (1.26, 2.04) |
| Type of concurrent vaccination on same day, if vaccinated**: | |||||
| Seasonal flu | 469 (12.6%) | NA | 469 (12.6%) | NA | NA |
| Other | 17 (0.5%) | 16 (0.5%) | |||
| Medical conditions** | |||||
| Asthma | 886 (23.8) | 83 (26.6) | 969 (24.1) | P = 0.272 | 0.86 (0.66, 1.14) |
| Diabetes | 630 (17.0) | 61 (19.6) | 691 (17.2) | P = 0.242 | 0.84 (0.62, 1.14) |
| Heart disease | 565 (15.2) | 54 (17.3) | 619 (15.4) | P = 0.321 | 0.86 (0.63, 1.19) |
| Kidney disease | 55 (1.5) | 10 (3.2) | 65 (1.6) | P = 0.020 | 0.45 (0.23, 1.00) |
| Liver disease | 42 (1.1) | 6 (1.9) | 48 (1.2) | P = 0.215 | 0.58 (0.24, 1.70) |
| Blood disorder | 77 (2.1) | 6 (1.9) | 83 (2.1) | P = 0.859 | 1.08 (0.47, 3.06) |
| Immunosuppression | 217 (5.9) | 10 (3.2) | 227 (5.6) | P = 0.053 | 1.87 (0.98, 4.00) |
| Other condition | 458 (12.3) | 22 (7.1) | 480 (11.9) | P = 0.006 | 1.85 (1.18, 3.04) |
| One or more reported medical condition of interest† | 2457 (66.1) | 207 (66.4) | 2664 (66.1) | P = 0.930 | 0.99 (0.77, 1.27) |
| Pregnant at consent (n, % of females) | 104 (7.5) | 13 (12.5) | 117 (10.7) | P = 0.065 | 0.57 (0.30, 1.14) |
| Missing (female any age) | 810 | ||||
| Other regular medication | 2966 (81.5%) | 247 (83.2%) | 3213 (81.6) | P = 0.525 | 0.89 (0.64, 1.23) |
| Missing | 91 | ||||
| Had flu since April 2009 | 537 (14.7) | 59 (19.5) | 597 (14.9) | P = 0.024 | 0.71 (0.53, 0.98) |
| Missing | 69 |
Percent given of prognostic variable where response provided.
Where only positive response provided, percent given of adult cohort (thus missing assumed ‘No’)’. †excluding pregnancy.
Factors affecting uptake of vaccination
Simple exploratory univariate analysis of data stratified by H1N1 vaccination status was conducted to look for associations between patient baseline characteristics and uptake of H1N1 vaccination (Table 1). Those participants who had the vaccine were significantly more likely to be female (OR 1.6, 95% CI 1.2, 2.0), health professionals (OR 2.0, 95% CI 1.4, 3.2), to have received one or more vaccines in the prior 3 months (OR 1.6, 95% CI 1.2, 2.0) (mainly seasonal flu vaccination) and to have medical conditions other than those specified within the vaccination programme target groups (OR 1.9, 95% CI 1.2, 3.0). Sixteen patients reported that they had previously been medically diagnosed with swine flu.
Non-vaccinated cohort: Reasons for not having H1N1 vaccine
Of the 312 patients who decided not to have the H1N1 vaccination, 142 reasons were provided (Table 2). Of these, the most frequent reason cited for non-vaccination was ‘considering having it later’ (13.5%).
Table 2.
Patients aged ≥16 years: Reasons given for not having vaccination in non-vaccinated cohort (n = 312)
| Reasons for not having H1N1 vaccination | n (% of non-vaccinated subgroup) |
|---|---|
| Consider having it later | 42 (13.5%) |
| Other reason | 35 (11.2%) |
| Worried about side effects | 25 (8.1%) |
| Think they are at low risk | 13 (4.2%) |
| Advised not to | 10 (3.2%) |
| Would rather have flu | 9 (2.9%) |
| Needlephobic | 8 (2.6%) |
| Total reasons given | 142 |
Patients could provide more than one reason.
Child and adolescent participants (aged < 16 years)
All 38 children who participated in the study received the H1N1 vaccination (Table 3). Consent/assent was given by the parent or guardian (and also by the child if old enough to understand the study). Numbers of participants were small as further ethical and R & D approvals were required prior to including children in the study and by the time these were obtained, the vaccination of children had already been largely completed in many parts of Scotland. The majority of this group of participants were female (56.7%, n = 21). The median age was 4.8 years (IQR 2.3–8.1), with participants aged <4 years of age being the most represented, the youngest child being 6 months of age. The most frequent medical condition reported was asthma (26.3%, n = 10).
Table 3.
Patients aged < 16 years: characteristics on consent (all had H1N1 vaccine)
| Characteristics | n (%); n = 38 |
|---|---|
| Gender (Female) | 21 (56.7) |
| Missing | 1 |
| Age at consent (years): | |
| Up to but not including 5 | 23 (60.5) |
| Between 5 and 9 (inclusive) | 9 (23.7) |
| Between 10 and 15 (inclusive) | 6 (15.8) |
| Median (IQR) | 4.8 (2.3, 8.1) |
| Place where vaccinated | |
| GP surgery | 37 (97.3) |
| Parent's workplace | 1 (2.6) |
| Other | 0 (0) |
| Past history of vaccination in prior 3 months* | |
| Seasonal flu vaccine | 5 (13.2) |
| Travel vaccine | 1 (2.6) |
| Other vaccine | 3 (7.9) |
| Any one or more vaccinations in prior 3 months | 9 (23.7) |
| Concurrent vaccination given same day*: Seasonal flu | 6 (15.8) |
| Medical conditions* | |
| Asthma | 10 (26.3) |
| Other condition | 3 (7.9) |
| One or more reported medical conditions of interest | 13 (32.4) |
| Other regular medication | 12 (32.4) |
| Missing | 1 |
Missing assumed ‘No’.
Self-reported events
There were 939 self-reported events (838 different events once duplicates were removed, 827 in the 7 month observation period. The remaining 11 events occurred in the eighth and ninth month after index date and were all related to pregnancies. Overall follow-up response rates to at least one follow-up request over the 7 month observation period for the H1N1 vaccinated and unvaccinated cohorts were 81.0% and 73.1%, respectively, which were significantly different (Chi2 d.f.1, P < 0.001). The most commonly reported clinical events (other than pregnancies and births which were prompted outcomes, hospital referrals and tests) in H1N1 vaccinated subjects were chest infection (n = 53; 2.9 cases per 1000 patient months' follow-up), coryza (n = 24, 1.3 cases per 1000 patient months' follow-up) and malaise (n = 21, 1.2 cases per 1000 patient months' follow-up). Pregnancy and congenital abnormalities data are presented separately later. The most frequently self-reported events are presented in Table 4. There were 38 self-reported incident events reported by unvaccinated subjects, the most frequent of which were also chest infection (n = 5, 4.0 cases per 1000 patient months' follow-up) and coryza (n = 2, 1.6 cases per 1000 patient months' follow-up). There were no events reported for the children (aged < 16 years). All self-reported events were reviewed by the investigators and where necessary further information was obtained from the patient, their proxy, healthcare professionals or from medical records.
Table 4.
Most frequently self-reported clinical incident events in 7 month study observation period, ranked by total count, by vaccine exposure cohort
| Follow-up period (month after vaccinated or offered vaccination) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Incident events | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total | Number per 1000 patient months of follow-up |
| Received H1N1 vaccine | |||||||||
| Chest infection | 10 | 14 | 14 | 10 | 5 | 0 | 0 | 53 | 2.91 |
| Coryza | 5 | 8 | 6 | 1 | 3 | 1 | 0 | 24 | 1.32 |
| Malaise | 12 | 4 | 4 | 1 | 0 | 0 | 0 | 21 | 1.15 |
| Cough | 5 | 7 | 2 | 1 | 4 | 0 | 0 | 19 | 1.04 |
| Allergy | 9 | 1 | 1 | 1 | 0 | 0 | 0 | 12 | 0.66 |
| Flu like symptoms | 2 | 3 | 2 | 1 | 3 | 1 | 0 | 12 | 0.66 |
| Pyrexia | 7 | 4 | 1 | 0 | 0 | 0 | 0 | 12 | 0.66 |
| Dyspnoea | 1 | 4 | 2 | 1 | 2 | 1 | 0 | 11 | 0.60 |
| Pharyngitis | 4 | 2 | 3 | 0 | 2 | 0 | 0 | 11 | 0.60 |
| Pain in limb | 3 | 1 | 4 | 1 | 1 | 0 | 0 | 10 | 0.55 |
| Headache | 1 | 0 | 5 | 1 | 2 | 0 | 0 | 9 | 0.49 |
| Myalgia | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 8 | 0.44 |
| Rash | 2 | 1 | 3 | 1 | 1 | 0 | 0 | 8 | 0.44 |
| Diarrhoea | 3 | 1 | 1 | 2 | 0 | 0 | 0 | 7 | 0.38 |
| Chest pain | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 7 | 0.38 |
| Sinusitis | 1 | 0 | 0 | 2 | 2 | 2 | 0 | 7 | 0.38 |
| Viral infection | 0 | 2 | 0 | 3 | 1 | 0 | 0 | 6 | 0.33 |
| Influenza | 3 | 2 | 0 | 1 | 0 | 0 | 0 | 6 | 0.33 |
| Nausea | 3 | 1 | 0 | 2 | 0 | 0 | 0 | 6 | 0.33 |
| Pain | 4 | 0 | 2 | 0 | 0 | 0 | 0 | 6 | 0.33 |
| Did not receive H1N1 vaccine | |||||||||
| Chest infection | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 5 | 3.98 |
| Coryza | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 1.59 |
| Angina | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.80 |
| Asthma worse | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.80 |
| Carcinoma stomach | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.80 |
| Cardiac failure | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.80 |
| Cardiovascular system unspecified* | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.80 |
| Constipation | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.80 |
| Dyspnoea | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.80 |
| Fracture | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.80 |
Patient verbatim term given as ‘heart problems’.
Serious adverse events
After review by study investigators, 53 of the 838 self-reported events (6.4%) met the protocol definition for serious adverse event (SAE) i.e. resulting in or prolonging hospital admission, life-threatening, fatal or resulting in significant or persisting disability (NB, pregnancy events were counted and reported separately, see later). Of the 53 SAEs, nine were assessed as possibly, probably or definitely related to vaccination (Table 5). One event was judged as probably related to vaccination (neurological symptoms within 1 day of vaccination), and one as definitely related to vaccination (local skin reaction developing into possible cellulitis at vaccination site starting 6 days after vaccination). There were seven deaths (one of which was in the non-vaccinated cohort), none of which were judged to be related to vaccination (Table 6). These deaths were due to underlying conditions (e.g. cancer), exacerbations of pre-existing chronic conditions and in one case, consequences of a fall, and the causes of death would not be unexpected in the age groups in which the deaths occurred. In some cases, the underlying condition leading to death may have been the reason why the patient was offered vaccination. Of note, those patients offered vaccination are by definition in higher risk groups than the general population so one might expect higher event rates in the vaccinated population.
Table 5.
Serious adverse events judged to be possibly, probably or definitely related to vaccination (i.e. serious adverse reactions)
| Patient gender/age (years) | Follow-up period event reported | Received H1N1 vaccine | Time from vaccination to onset of symptoms (days) | Patient self report of symptoms | Duration of symptoms (days) | Patient sought medical attention | Diagnosis | Treatment received | Symptoms Resolved | Ongoing Treatment | Possible alternative causes | Relatedness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/46 | 2 | Y | 7 | Flu like symptoms 5 days. Admitted to hospital, shortness of breath and productive cough, flushed and sweating | 5 | Y (Hospital doctor) | Lower respiratory tract infection | Antibiotics | Y | N | Viral infection | Possibly |
| F/62 | 2 | Y | 53 | Facial droop | Ongoing at follow-up | Y (GP) | Bell's palsy | Steroids | N | N | NS | Possibly |
| F/59 | 2 | Y | 12 | Epileptic seizure, post-seizure paralysis in right arm and leg for 2 days. Had past history of epilepsy | 5 | Y (Hospital doctor) | Epileptic seizure | Medication NS | Y | Y | NS | Possibly |
| F/71 | 1 | Y | 14 | Severe headaches, admitted to hospital with suspected meningitis. No meningitis found. Diagnosis- chronic migraines | NS | Y (Hospital doctor) | Chronic migraines | NS | NS | NS | NS | Possibly |
| M/39 | 1 | Y | 1 | Pins and needles and numbness in hands, feet, legs and arms. Pins and needles and numbness left side of head, tongue, nose and lips, muscle weakness, poor co-ordination, lack of sleep, slurred speech, dizzy, light headed spells, migraines, extreme tiredness. | NS | Y (Hospital doctor) | Seen by neurologist- suggested nerve fibre damage | NS | NS | NS | NS | Probably |
| F/38 | 1 | Y | 2 | Swelling at injection site, painful and hard. Felt unwell, flu like symptoms. Had three seizures. Admitted to hospital, no history of previous seizures. Had CT scan – normal. Patient had allergy to eggs. Suffered muscular damage during seizures – causing severe back pain. | Ongoing at follow-up | Y (Hospital doctor) | Epileptic seizures | NS | NS | NS | Allergy to eggs | Possibly |
| F/48 | 2 | Y | 32 | Chronic fatigue, dizzy turns | Ongoing at follow-up | Y (GP) | ? Anaemia/ problem with thyroxine concentrations | Medication NS | N | Y | Unknown | Possibly |
| M/57 | 2 | Y | 2 | Pulmonary embolism | 37 days | Y (Hospital doctor) | Pulmonary embolus | Medication NS | Y | Y | Had hernia repair prior to immunisation. | Possibly |
| F/56 | 1 | Y | 6 | Skin inflamed and red, area spreading, then developed white itchy spots in same area | 18 days | Y (Hospital Dr) | Local reaction to vaccine | Antibiotics | Y | N | Vaccine | Definitely |
NS = not specified
Table 6.
Deaths
| Age (years) | Sex | Had H1N1 Vaccine | H1N1 Vaccination date (month/year) | Cause of death | Date of death (month/year) | SAE | Relatedness assessment |
|---|---|---|---|---|---|---|---|
| 77 | F | Yes | 11/2009 | Lung cancer | 08/2010 | Yes | Unrelated |
| 75 | F | Yes | 11/2009 | Heart failure, post-operative complications after fractured femur | 08/2010 | Yes | Unrelated |
| 65 | F | Yes | 12/2009 | Myocardial infarction | 01/2010 | Yes | Unrelated |
| 61 | F | Yes | 11/2009 | Lung infection (longstanding COPD) | 03/2010 | Yes | Unrelated |
| 60 | F | Yes | 12/2009 | Metastatic cancer | 03/2010 | Yes | Unrelated |
| 75 | F | Yes | 12/2009 | Lung cancer | 02/2010 | Yes | Unrelated |
| 70 | M | No | N/A | Sepsis (had valvular heart disease, gastric adenocarcinoma, COPD and cardiovascular disease) | 11/2010 | Yes | Unrelated |
Pregnancies
One hundred and twenty-eight women reported pregnancy either at the time of entry to the study or later during the study period. Two women reported two pregnancies each (total of 130 pregnancies). All women reporting pregnancies were followed up where possible. The response rate was 75.8% (97/128). Two women self-reported events unrelated to pregnancy (respiratory infection with sleep disturbance and abdominal pain with gastroenteritis in month 1 and 2, respectively). These were not judged to be serious. The outcomes of the pregnancies (n = 130) are given in Table 7, stratified by vaccination status. Trimester of pregnancy at vaccination could not be established for two women. Of the remaining 126 women, 13 were vaccinated before their last menstrual period, 100 received the vaccination during their pregnancy and 13 were not vaccinated.
Table 7.
Outcomes of 130 confirmed pregnancies in 128 women*
| Delivery type | ||||||
|---|---|---|---|---|---|---|
| Normal birth (Possible congenital abnormalities) | Caesarean section | Forceps delivery | No reply | Spontaneous miscarriage | Total | |
| Exposed to H1N1 vaccine | ||||||
| Injection before last menstrual period | 5 (Hypospadias [n = 1]) | 3 | 0 | 3 | 2 | 13 (9.3%) |
| Injection given in 1st trimester | 9 (Skin tag on finger [n = 1]) | 2 | 2 | 4 | 2 | 19 (14.7%) |
| Injection given in 2nd trimester | 27 (Down's syndrome [n = 1]); (Hydrocephalus [n = 1]); (Umbilical hernia [n = 1]); (Cleft palate [n = 1]) | 12 | 4 | 10 | 0 | 53 (45.0%) |
| Injection given in 3rd trimester | 18 | 4 | 0 | 8 | 0 | 30 (24.0%) |
| Exposure uncertain | 0 | 0 | 0 | 2 | 0 | 2 (7.0%) |
| Not exposed to H1N1 vaccine | ||||||
| Not vaccinated | 7 | 3 | 1 | 2 | 0 | 13 (10.0%) |
| Total | 66 (6) | 24 | 7 | 29 | 4 | |
| Live births: | 97 | 33 | 130 | |||
Two women were reported to have two pregnancies; one woman reported miscarriages in both pregnancies, the other woman reported one miscarriage, then upon follow-up reported a second early pregnancy, but no further details were available on the outcome of the second pregnancy.
Live births were reported for 97 women for whom trimester stage upon vaccination was known. There were six reported potentially congenital abnormalities (one report each of hypospadias, Down's syndrome, hydrocephalus, umbilical hernia, cleft palate and skin tag on finger) in six live offspring of six vaccinated women. No potentially congenital abnormalities or other adverse outcomes were reported in the 13 live offspring of the 13 unvaccinated women. There were no reported stillbirths, but four spontaneous miscarriages reported by three (vaccinated) women during the study period. There was no further information available on the outcome of the remaining 29 pregnancies.
Discussion
The results of this study indicate that a large-scale prospective active surveillance system for ‘near real-time’ vaccine safety monitoring that would be complementary to other initiatives is feasible. Through the use of information technologies, the study was relatively novel in that patients could enrol for the study and securely enter their data online, which improves patient involvement in research as well as dramatically limiting the cost of the study. The online methodology also allowed the study to be set up very quickly in response to the urgent nature of the introduction of the vaccination programme at a time when the pandemic proportions could not yet be predicted. The study was done without any specific external funding. The predominant material costs were the printing of patient information sheets and advertising posters that were sent to GP surgeries.
One advantage of our study design was that it was prospective in nature and it encouraged ‘near real-time’ reporting of event data from patients who experienced no problems after swine flu vaccination, as well as from those experiencing side effects, unlike many other reporting systems that only collect data on affected individuals. Since outcome data were collected at monthly intervals after consent for participation was given, bias introduced by over-reporting from patients who may have participated because they experienced an event was likely to be minimal. We also recruited a group of non-vaccinated individuals who had been offered vaccination but had decided not to have it, as a comparator group, although this group eventually only comprised less than 10% of the total study population. Overall, we recruited approximately 1% of the vaccinated population in Scotland to this study. There was good representation of patients from GP practices throughout Scotland, so we have no reason to believe that the study cohort is not representative of the general Scottish population for whom vaccination was indicated. However, we did not collect the characteristics of individuals offered vaccination who did not participate in the study. Another advantage of our study was that we were able to recruit and follow up a cohort of pregnant women, one of the target groups for vaccination, and also ascertain the outcomes of births in these women as well as those who became pregnant shortly after receiving the vaccine.
Limitations of the study included an incomplete response rate to follow-up requests, some difficulties contacting patients where contact details had been incorrectly completed at time of registration, e.g. incorrect e-mail addresses and no other contact details provided, and the fact that only around one third of general practices in Scotland were able to make our study information available to their patients attending for vaccination (mainly due to timing and workload concerns) [15]. We were also not able to distinguish between patients receiving the two available types of vaccination in the UK [Celvapan® (Baxter) and Pandemrix® (GSK)], although it is known that Pandemrix® was much more widely used than Celvapan®during the vaccination programme. We only asked participants to report what they considered to be serious (requiring urgent medical attention) reactions to the vaccine, to allow us to concentrate on capturing more important adverse reactions. Thus minor local side effects and minor systemic reactions are by design likely to be under-reported and recorded, with the additional possibility of differential reporting between exposed and unexposed groups. Data on children were limited because additional approvals to extend the study to include children aged 16 years or less were only obtained towards the end of the study period (February 2010) and any forms received from children who tried to register earlier in the study had to be discarded until the appropriate approvals were in place.
The results of this study support those of most other studies on the safety of swine flu vaccination [16], [17] and the results of the MHRA yellow card system surveillance programme suggesting that overall, H1N1 vaccination is safe [3]. Ascertaining accurate background rates of events in any population is difficult [11], [18], [19] and we acknowledge that this study is unable to inform on excess risk attributable to use of the vaccine, in addition to those risks likely as a result of complex morbidities seen in target vaccination groups, particularly since the number of unexposed participants was low. There were no confirmed cases of GBS in this study, which was one of the main concerns regarding similar influenza vaccinations in the past, although one patient developed widespread neurological symptoms thought to be a form of generalized nerve damage within hours of vaccination. We also had no reported cases of narcolepsy. Narcolepsy was identified as a possible risk of H1N1 vaccination (Pandemrix®) in Scandinavian countries and the possible link is currently undergoing further investigation although at present no causal relationship has been established [20]. The six deaths that occurred in vaccinated patients in this study were due to other factors and were judged to be unrelated to vaccination.
There were four miscarriages within the population of 128 women reporting 130 pregnancies in this study (incidence of 3.1%). Miscarriage is recognized to occur in up to 15% of confirmed pregnancies [21], although often remains unrecognized or unreported. The incidence observed in our study was well within the expected incidence in the population. Of the six reported possible congenital abnormalities in neonates born to the pregnant women taking part in the study (all of whom received the H1N1 vaccine), some of these were minor (e.g. umbilical hernia and skin tag on finger) and occur commonly, but all are reported for completeness. There was no pattern of any one type of congenital abnormality that occurred in more than one neonate.
With more time for preparation, more broad inclusion criteria to include special populations such as children earlier, more publicity and funding for recruitment advertising, and if rolled out to the entire UK population (approximately 55 million) rather than limited to Scotland (approximately 5.5 million) we could use this study methodology again to recruit much larger numbers of patients with high quality data at a reasonable research cost.
In conclusion, no significant safety issues were identified in patients exposed to H1N1 influenza A vaccination in this study. The use of web-based technology in the study was successful in reducing costs and allowing the collection of high quality data directly from patients. This method for near ‘real-time’ monitoring, with minimal additional workload for healthcare staff, should be considered as an additional pharmacovigilance tool for other safety studies.
Acknowledgments
We would like to thank the patients and health professionals who participated in this study and acknowledge the contributions of the following people to early discussions about this study: Professor Sir Lewis D Ritchie OBE Centre of Academic Primary Care (University of Aberdeen), Professor Helen Smith, Head of Division of Public Health and Primary Care, Chair of Primary Care, Director of Student Support (University of Brighton), Professor Richard Martin, Professor of Clinical Epidemiology, Department of Social Medicine (University of Bristol), Professor Munir Pirmohamed, NHS Chair of Pharmacogenetics, Deputy Director, MRC Centre for Drug Safety Science, Department of Pharmacology (University of Liverpool), Professor Jonathan Van-Tam, Professor of Health Protection and Professor Tony Avery, Professor in Primary Care, Institute of Clinical Research (University of Nottingham), Professor C.P. Farrington (Open University), Dr Claire Doe, Clinical Research Fellow, Dr Victoria Cornelius, Statistician and Mr Shayne Freemantle (DSRU) and Dr Daniel Rutherford, Clinical Trials Physician and General Practitioner (University of Dundee).
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.The Scottish Government. Swine flu pandemic [online] 2011. Available at http://www.scotland.gov.uk/Topics/Health/health/flu/swineflu (last accessed 13 April 2011). The Scottish Government 2011 March 1.
- 3.MHRA. Final Public Summary. UK suspected adverse reaction analysis. Swine Flu (H1N1) Vaccines – Celvapan and Pandemrix. [online] 2010. Available at http://www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con078911.pdf (last accessed 10 May 2011)
- 4.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 5.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 6.Vajo Z, Tamas F, Sinka L, Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009-10 influenza season: a multicentre, randomised controlled trial. Lancet. 2010;375:49–55. doi: 10.1016/S0140-6736(09)62039-0. [DOI] [PubMed] [Google Scholar]
- 7.Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, Li RC, Xia SL, Zhao YL, Li FJ, Yan SH, Yin WD, An K, Feng DJ, Cui XL, Qi FC, Ju CJ, Zhang YH, Guo ZJ, Chen PY, Chen Z, Yan KM, Wang Y. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 8.Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12:29–33. doi: 10.3201/eid1201.051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans D, Cauchemez S, Hayden FG. ‘Prepandemic’ immunization for novel influenza viruses, ‘swine flu’ vaccine, Guillain-Barre syndrome, and the detection of rare severe adverse events. J Infect Dis. 2009;200:321–8. doi: 10.1086/603560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barre syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10:643–51. doi: 10.1016/S1473-3099(10)70140-7. [DOI] [PubMed] [Google Scholar]
- 11.Destefano F, Tokars J. H1N1 vaccine safety monitoring: beyond background rates. Lancet. 2010;375:1146–7. doi: 10.1016/S0140-6736(09)61917-6. [DOI] [PubMed] [Google Scholar]
- 12.ICH Harmonised Tripartite Guideline. Post-approval safety data management: definitions and standards for expedited reporting E2D. 2003.
- 13.Shakir S. Causality and correlation in pharmacovigilance. In: Talbot J, Waller P, editors. Stephens' Detection of New Adverse Drug Reactions. 5th. Chichester: John Wiley & Sons Ltd; 2004. pp. 329–43. [Google Scholar]
- 14.Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf. 1994;10:93–102. doi: 10.2165/00002018-199410020-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie I, Dryburgh M, Rutherford D, MacDonald TM, Shakir SAW, Layton D. Abstract no: 733. Participation of general practices in a swine flu vaccination study. 26th International Conference on Pharmacoepidemiology & Therapuetic Risk management (ISPE). 19-22ndth August, 2010 – Brighton, UK. Pharmacoepidemiol Drug Saf. 2010;19:s328. [Google Scholar]
- 16.Harmark L, van HF, Hak E, van GK. Monitoring the safety of influenza A (H1N1) vaccine using web-based intensive monitoring. Vaccine. 2011;29:1941–7. doi: 10.1016/j.vaccine.2010.12.123. [DOI] [PubMed] [Google Scholar]
- 17.Folkenberg M, Callreus T, Svanstrom H, Valentiner-Branth P, Hviid A. Spontaneous reporting of adverse events following immunisation against pandemic influenza in Denmark November 2009-March 2010. Vaccine. 2011;29:1180–4. doi: 10.1016/j.vaccine.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Black S, Eskola J, Siegrist CA, Halsey N, Macdonald N, Law B, Miller E, Andrews N, Stowe J, Salmon D, Vannice K, Izurieta HS, Akhtar A, Gold M, Oselka G, Zuber P, Pfeifer D, Vellozzi C. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374:2115–22. doi: 10.1016/S0140-6736(09)61877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan P, Seabroke S, Davies C. H1N1 vaccine safety: real-time surveillance in the UK. Lancet. 2010;376:417–8. doi: 10.1016/S0140-6736(10)61221-4. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency. Press release. European Medicines Agency reviews further data on narcolepsy and possible association with Pandemrix Doc Ref: EMEA/CHMP/130422/2011. [online] 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2011/02/WC500102213.pdf (last accessed 12 December 2011). The European Medicines Agency (EMEA) 2011 February 18.
- 21.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]


