Abstract
Imaging is an integral part of the management of patients with ankylosing spondylitis and axial spondyloarthritis. Characteristic radiographic and/or magnetic resonance imaging (MRI) findings are key in the diagnosis. Radiography and MRI are also useful in monitoring the disease. Radiography is the conventional, albeit quite insensitive, gold standard method for assessment of structural damage in spine and sacroiliac joints, whereas MRI has gained a decisive role in monitoring disease activity in clinical trials and practice. MRI may also, if ongoing research demonstrates a sufficient reliability and sensitivity to change, become a new standard method for assessment of structural damage. Ultrasonography allows visualization of peripheral arthritis and enthesitis, but has no role in the assessment of axial manifestations. Computed tomography is a sensitive method for assessment of structural changes in the spine and sacroiliac joints, but its clinical utility is limited due to its use of ionizing radiation and lack of ability to assess the soft tissues. It is exciting that with continued dedicated research and the rapid technical development it is likely that even larger improvements in the use of imaging may occur in the decade to come, for the benefit of our patients.
Keywords: ankylosing spondylitis, computed tomography, imaging, magnetic resonance imaging, radiography, spondyloarthritis, ultrasonography, ultrasound
Introduction
Imaging in ankylosing spondylitis (AS) has been synonymous for decades with conventional radiography (CR). However, developments in computed tomography (CT), ultrasonography (US) and particularly magnetic resonance imaging (MRI) have dramatically increased the amount and scope of information obtainable by imaging. Imaging may be used for multiple reasons that include establishing or confirming the diagnosis, determining extent of disease in axial or peripheral joints and/or entheses, monitoring change in disease (e.g. activity and structural damage) for instance for assessment of therapeutic efficacy in trials, providing prognostic information or selecting patients for specific therapies. These entirely different contexts may favour different imaging approaches.
The present paper focuses on imaging of the spine and sacroiliac joints, i.e. the axial joints, in AS and other variants of axial spondyloarthritis (SpA). The reader is kindly referred to other review articles (e.g. Poggenborg et al. [2011]) for imaging aspects of peripheral involvement in SpA. This paper describes the virtues of CR and its current major importance in diagnosis and follow-up of AS, but also, by putting emphasis on MRI, stresses that AS management has entered a time of exciting and expanding therapeutic as well as imaging possibilities.
Conventional radiography
Technical aspects
The conventional radiograph is a two-dimensional summation image that is dependent on variable absorption of X-rays by different tissues for its inherent contrast. It has a very high spatial resolution that is rarely surpassed by other modalities but radiography only offers high contrast between a limited number of structures: bone (calcium), soft tissue and air. Fat is visible as a separate density but the distinction between soft tissue and fat is often subtle (except in mammography) and radiography cannot distinguish between the other soft tissues because cartilage, muscle, tendon, ligament, synovium and fluid all appear with the same density [Resnick, 2002]. These characteristics give the radiograph its inherent advantages and disadvantages. The conventional radiograph is relatively cheap, available worldwide and produces an image which is almost identical regardless of technical parameters or whether the image is analogue or digital.
CR shows skeletal structure very well, and because it is a summation image, it allows excellent overall assessment of skeletal trauma and alignment. The limited number of images produced facilitates rapid review and the high bone–soft tissue contrast often produces radiographic manifestations of disease in specific patterns that make the test particularly useful in daily clinical practice. The biggest disadvantage of radiography is its inherent lack of soft tissue contrast making it insensitive for the detection of soft tissue abnormalities.
Although CR uses ionizing radiation, radiography is regarded as relatively safe in older patients because of the long lag time for any negative effect to occur. However, spine and pelvis radiography doses need to be relatively high in order to penetrate the trunk and, in a younger population, MRI offers a safer and more informative alternative.
Ankylosing spondylitis
Sacroiliac joints
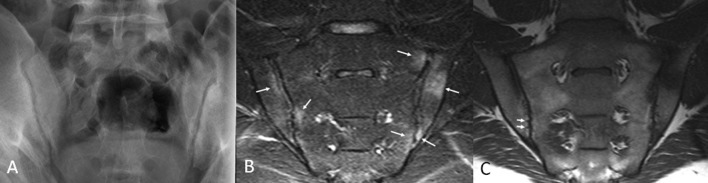
Erosion and ankylosis of the sacroiliac joints are the hallmarks of AS [Resnick, 2002; van der Linden et al. 1984]. Sacroiliitis is usually the first manifestation and is characteristically bilateral and symmetrical in AS [Berens, 1971; Resnick et al. 1977]. Early radiographic findings predominate on the iliac side of the cartilage compartment with erosion of subchondral bone causing loss of definition of the articular surfaces usually accompanied by variable degrees of adjacent osteoporosis and surrounding reactive sclerosis. Bone erosion may result in the radiographic observation of focal joint space widening (Figure 1) and, as the disease progresses, definition of the joint is completely lost with radiographic superimposition of erosion, sclerosis and new bone formation which fills in the erosions and the original cartilaginous ‘joint space’. The joint may disappear completely in late disease with ankylosis and remodelling of the bone (Figure 1). The ligamentous compartment of the sacroiliac joint is frequently affected by bony erosion and entheseal proliferation although these may be hard to see radiographically [Berens, 1971; Resnick et al. 1977].
Figure 1.

Radiographic findings in sacroiliac joints and spine in ankylosing spondylitis.
(A) Radiograph of the sacroiliac joints in a 23-year-old male demonstrates established ankylosing spondylitis. Bilateral erosions cause discrete foci of loss of subchondral bone and apparent joint space widening in some areas (arrows) and ill definition of the joint margin in other areas (arrowheads). Bilateral subchondral sclerosis is most prominent in the left ilium. (B)–(D) Radiographs of the spine in a 47-year-old male with widespread ankylosis. The cervical spine (B) exhibits extensive formation of vertical syndesmophytes that have bridged the anterior vertebral corners causing ankylosis. Some facet joints are fused, best appreciated at C2/3. The lumbar spine (C; enlargement of L1–L4 in D) shows similar ankylosis. Note the thick right L1/2 bridging (arrow) and compare this to the delicate vertical syndesmophytosis on the left at L3/4. The sacroiliac joints are completely fused with barely any remnant of joint visible.
Spine
The early radiographic manifestations of AS in the spine are most often due to enthesitis at the edges of the discovertebral joints [Berens, 1971]. Focal sclerosis (the ‘shiny corner’) and erosion (the ‘Romanus lesion’) develop at the attachment of the annulus fibrosis to the anterior corner of the vertebral endplate and are characteristic features of early AS [Hermann et al. 2005]. The anterior borders of vertebrae may appear straight or ‘squared’ due to periosteal proliferation of new bone filling in the normal concavity or erosion at the anterosuperior and anteroinferior vertebral margins. This observation is much easier to make in the lumbar spine where normal vertebrae are always concave anteriorly in comparison to the thoracic and cervical spine where the normal contour is much more variable and may be square or occasionally convex.
The hallmark of spinal disease in AS is the development of characteristic bony spurs: syndesmophytes (Figure 1). These start as thin vertically oriented projections of bone that develop due to ossification within the outer fibres of the annulus fibrosus of the intervertebral disc. Syndesmophytes are radiographically visible on the anterior and lateral aspects of the spine starting from the corner of the vertebra.
Traditional literature indicates that all of the early signs of spinal involvement are most often visualized first near the thoracolumbar junction. While this may be true for radiography, MRI studies show a predilection for early involvement of the midthoracic spine. Both modalities would agree that the cervical spine is rarely affected first and spinal disease rarely occurs in the absence of significant SI joint involvement.
Progressive growth of syndesmophytes will bridge the intervertebral disc causing ankylosis (Figure 1) and extensive bone formation produces a smooth, undulating spinal contour: the ‘bamboo spine’. The syndesmophytes that characterize AS must be differentiated from other spinal and paraspinal bone formation. Degenerative bony spurring in spondylosis deformans arises several millimetres from the discovertebral junction, is typically triangular in shape and has a horizontally oriented segment of variable length at the point of origin. In diffuse idiopathic skeletal hyperostosis (DISH), bone formation in the anterior longitudinal ligament results in a flowing pattern of ossification that is usually thick and the sacroiliac joints are not involved.
Erosion of the vertebral endplate is common in later stages of AS and may be focal or diffuse. It is also seen when pseudarthrosis develops following a fracture in a previously ankylosed spine. Changes in the apophyseal joints are common and start with of ill-defined erosion and sclerosis but may be hard to see. Capsular ossification or intra-articular bony ankylosis frequently occurs in late disease. The ankylosed spine is very susceptible to fractures, which should always be suspected in case of unexplained pain exacerbations. Enthesitis at the interspinous and supraspinous ligamentous attachments is very common with bone formation causing whiskering and interspinous ankylosis.
Use in diagnosis, monitoring and prognostication
Diagnosis
While the modified New York Criteria are actually classification criteria, these criteria are the most commonly used criteria for the diagnosis of AS and are based on clinical features and radiographic sacroiliitis [van der Linden et al. 1984]. According to these criteria AS may be diagnosed if, in addition to one clinical criterion being present, grade 2 sacroiliitis (minimal sacroiliitis: loss of definition of the joint margins, minimal sclerosis, joint space narrowing and erosions) or higher occurs bilaterally, or grade 3 (moderate sacroiliitis: definite sclerosis on both sides of the joint, erosions, and loss of joint space) or grade 4 (complete bony ankylosis) occurs unilaterally [van der Linden et al. 1984]. Owing to the requirement for these radiographic structural changes, the duration of disease before diagnosis has been a median of 7–10 years [Feldtkeller et al. 2003]. The definitions of radiographic changes according to the New York Criteria are included in the Assessment of Spondyloarthritis International Society (ASAS) classification criteria for SpA [Rudwaleit et al. 2009b], the European Spondyloarthritis Study Group (ESSG) criteria for spondyloarthritis [Dougados et al. 1991] and in the modified New York Criteria [van der Linden et al. 1984].
Monitoring
Radiography of the spine is not included in the classification criteria but may be useful to follow structural disease progression in patients with spinal involvement. The bone changes seen in patients with axial SpA develop slowly and are often not present in patients with early disease, and generally only minor changes can be observed in 1–2 years. Different scoring methods, all based on assessment of lateral views, have been developed to quantify changes in the spine of patients with AS: the Stoke AS Spine Score (SASSS), Bath AS Radiology Index (BASRI) and the modified Stoke AS Spine Score (mSASSS). A comparative study of the three methods concluded that all measures were reliable but mSASSS was more sensitive to change [Wanders et al. 2004]. These spine scores are primarily used in clinical research.
Prognostication
The amount of data documenting a prognostic value of CR findings is limited. However, low-grade (grade 1) sacroiliitis has been shown to have predictive value for the development of AS [Huerta-Sil et al. 2006], and the severity of radiographic damage has been documented to be related to subsequent radiographic damage progression [Maksymowych et al. 2010a; Poddubnyy et al. 2011].
Computed tomography
Technical aspects
Developments in computing and engineering have resulted in remarkable changes in the CT technology in the last decade. CT offers fast and reliable acquisition, high resolution and multiplanar capabilities that have enhanced its use in recent years, although the soft-tissue contrast is still limited.
Although CT image acquisition is restricted to the axial plane of imaging, the ability to acquire isotropic voxels has resulted in reliable and versatile multiplanar capabilities so that many CT scans of the body are now interpreted primarily from coronal or sagittal images in the same way as MRI. The data acquisition is so fast that patient motion is rarely a problem as CT scans are now routinely acquired in few seconds. In fact CT is often faster than taking multiple radiographs. Complex requirements for patient positioning in musculoskeletal CT are now substantially less important as the majority of studies can be reconstructed in orthogonal planes. Patient tolerance is excellent and, unlike MRI, there are no absolute contraindications to CT. Spatial resolution is high, usually higher than MRI, and contrast resolution between soft tissue and bone is unsurpassed by any other modality. However, despite all of the advantages listed above, the application of CT will remain somewhat limited, because the soft-tissue contrast is inferior to MRI and US and ionizing radiation is used. Radiation dose increases with the requirements for spatial detail and the depth of the body part, which is considerable for the sacroiliac joint and spine.
Ankylosing spondylitis/axial spondyloarthritis
CT allows visualization of the same pathological processes as CR (erosion, osteoporosis / sclerosis, and new bone formation/ankylosis) with the added benefit of multiplanar imaging free from superimposition of overlying structures.
In AS, the pathological processes start in bone marrow and at sites of entheses. However, CT has poor ability to detect soft-tissue change and is usually normal until structural damage is present. CT can detect osteoporosis or osteosclerosis quite well but these changes are very nonspecific. New bone formation is also well visualized in the form of syndesmophytes, ligamentous ossification and periarticular and intra-articular ankylosis, but the use for CT in this regard is limited. The primary value of CT in AS is its ability to detect and clearly define erosion of bone at any joint or enthesis, and for documenting fractures.
Use in diagnosis, monitoring and prognostication
Diagnosis
The diagnosis of AS is primarily based on the radiographic observation of bilateral moderate or unilateral severe sacroiliitis. When good quality radiographs of the sacroiliac joints are normal or radiographic changes meet diagnostic criteria, there is no role for CT. Early detection of AS is better investigated by MRI and if clearly defined structural damage is present on CR, then there is no additional diagnostic utility for CT. However, CT of the sacroiliac joints is much easier to interpret than radiography which is notoriously subject to poor observer reliability. When the radiographic findings are unclear, CT will usually resolve this uncertainty. Since CT shows bone erosion in exquisite detail, CT may also have a role to play in the further investigation of MRI equivocal findings. It should be noted that classification criteria for AS depend on radiographic findings and more recently MRI, but not CT specifically. In the spine, CT is useful in the diagnosis of complications of late disease such as spondylodiscitis or spinal fracture when patients may be unable to tolerate MRI due to pain or spinal deformity.
Monitoring disease activity and damage
CT has no useful role in monitoring disease activity or damage. CT cannot show active inflammation and the relatively high radiation dose of CT precludes its routine use for assessment of damage progression.
Prognostication
The prognostic value of CT findings of sacroiliitis require further investigation.
Ultrasonography
US is an evolving imaging technique increasingly used by the rheumatologist in daily clinical practice. Despite the fact that contrast-enhanced Doppler US has been reported to have a high negative predictive value for the detection of sacroiliitis [Klauser et al. 2005], the role of US in assessment of sacroiliac and spine involvement in AS and other types of axial SpA is minimal.
AS and other types of axial SpA frequently involve peripheral joints and entheses. US allows assessment of peripheral involvement in SpA. The reader is referred to other reviews (e.g. Gandjbakhch et al. [2011] and Poggenborg et al. [2011]) for further information on this.
Magnetic resonance imaging
Technical aspects
MRI provides multiplanar tomographic imaging with unprecedented soft-tissue contrast and allows assessment of all of the structures involved in musculoskeletal diseases. MRI is more sensitive than clinical examination and CR for the detection of inflammation and damage in inflammatory and degenerative rheumatological disorders. Disadvantages of MRI include longer examination times and higher costs and lower availability than radiography.
MRI involves no ionizing radiation or increased risk of malignancies, and adverse effects of the contrast agents are very rare. However, contrast agent use should be avoided in case of severely impaired renal function due to risk of nephrogenic systemic fibrosis.
T1-weighted (T1w) imaging sequences are favoured for the relatively short imaging times, good anatomical detail and the ability to visualize tissues with high perfusion and permeability, including the inflamed synovium, after intravenous contrast (paramagnetic gadolinium compounds; Gd) injection. Fat and Gd-enhanced tissues have a high signal intensity on T1w images. T2-weighted fat suppressed (T2wFS) and short tau inversion recovery (STIR) images depict water with a high signal intensity. These are well-suited for detection of oedematous tissue/fluid located in areas with fatty tissue, e.g. bone marrow oedema. Bone marrow abnormalities in both sacroiliac joints and spine are detected almost equally well with the STIR and contrast-enhanced T1w FS sequences in patients with SpA, so contrast injection is generally not needed [Baraliakos et al. 2005; Madsen et al. 2010]. T1w images are mandatory for evaluation of structural (sometimes referred to as chronic) changes, such as bone erosion, new bone formation and fat infiltrations.
The majority of MRI studies of the sacroiliac joint have used only one imaging plane (semicoronal, i.e. parallel with the axis of the sacral bone), usually T1W and T2wFS/STIR images. A supplementary T1w FS sequence may improve the evaluation of erosions [Madsen and Jurik, 2010a], and sequences designed for cartilage evaluation, e.g. 3D gradient echo sequences, may also be added [Puhakka et al. 2004]. To be maximally sensitive for changes in the ligamentous portion of the sacroiliac joints imaging in the semi-axial plane is required [Madsen and Jurik, 2010a]. This may therefore be recommended when MRI is used for diagnostic purposes, while it is probably not essential when used as an outcome measure in trials. While MRI for some indications (e.g. suspected disc herniation) should include axial images, MRI of the spine in SpA generally only involve sagittal images, but these should extend sufficiently lateral to include the frequently involved facet, costovertebral and costotransverse joints [Maksymowych et al. 2010b].
Ankylosing spondylitis/axial spondyloarthritis
MRI allows direct visualization of the abnormalities in peripheral and axial joints and entheses that occur in AS, psoriatic arthritis (PsA) and other forms of SpA.
MRI is, through its ability to detect inflammatory changes in bone and soft tissues, the most sensitive imaging modality for recognizing early spine and sacroiliac joint changes in AS. MRI findings indicating active disease in the sacroiliac joints (sacroiliitis) include juxta-articular bone marrow oedema and enhancement of the bone marrow and the joint space after contrast medium administration, while visible chronic changes include bone erosions, sclerosis, periarticular fatty tissue accumulation, bone spurs and ankylosis (Figure 2). Typical lesions of the spine, which indicate active disease, are spondylitis, spondylodiscitis (Figure 3) and arthritis of the facet, costovertebral and costotransverse joints (Figure 4). Structural changes, such as bone erosions, focal fat infiltration, bone spurs (syndesmophytes) and/or ankylosis (Figure 5), frequently occur. Enthesitis is also common, and may affect the interspinal and supraspinal ligaments and the interosseous ligaments in the retro-articular space of the sacroiliac joints. Some patients also have disease manifestations in peripheral joints and entheses, and these can be visualized by MRI [Hermann and Bollow, 2004; Maksymowych and Landewe, 2006]. Definitions of key pathologies in axial SpA are provided in Table 1.
Figure 2.
Early sacroiliitis on conventional radiography and MRI.
Radiograph (A) of the sacroiliac joints in a 28-year-old male reveals only subtle findings of possible erosion and minimal sclerosis. Short tau inversion recovery (STIR) MRI image (B) performed at the same time shows multiple bone marrow lesions, which appear as oedema (bright; arrows) involving the sacrum and ilium bilaterally, i.e. definite sacroiliitis was documented by MRI. The corresponding T1-weighted MRI image (C) shows some areas of diminished marrow fat signal corresponding to the intense oedema in the left upper quadrant. Some very subtle defects in the subchondral marrow in the lower quadrants, which likely represent tiny erosions (arrows), are also seen.
Figure 3.

Inflammatory and fat lesions on MRI of the spine.
MRI of the lumbar and lower thoracic spine in 27-year-old male shows multiple tiny foci of infiltration of fat in the posterior corners of vertebral bodies on the T1-weighted sequence (A; arrows). On the short tau inversion recovery (STIR) sequence (B), these discs demonstrate no evidence of degeneration of the nucleus pulposis or tear of the annulus fibrosus, which is consistent with a postinflammatory cause of the marrow fat deposition rather than trauma or degenerative disc disease. Also note the solitary focus of inflammation on STIR imaging with increased signal at the anterosuperior corner of T10 (arrowhead). The appearance is typical for a corner inflammatory lesion (CIL) associated with spondyloarthritis (i.e. a triangular shaped lesion which may or may not (as in this case) be quite as bright in the extreme corner, with adjacent normal nucleus pulposus).
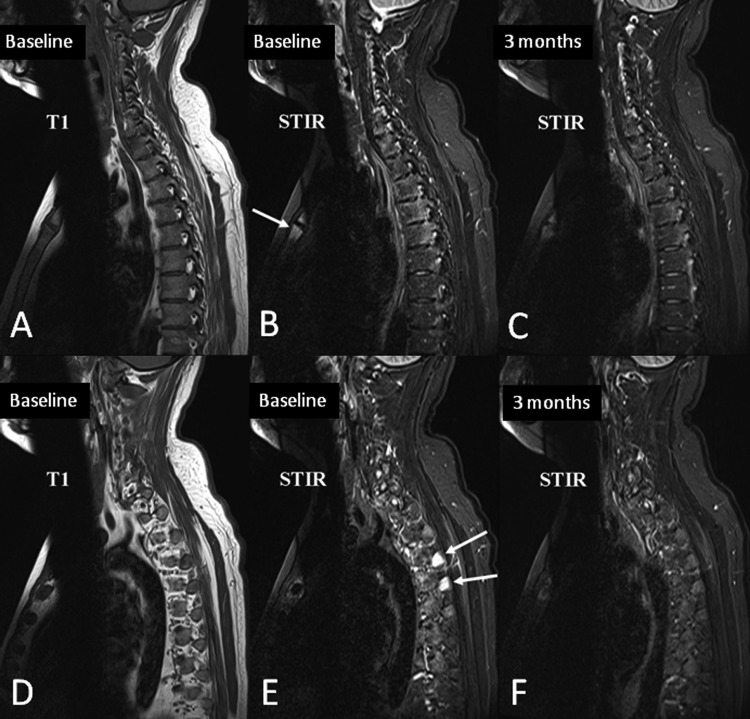
Figure 4.
Bone marrow oedema in the transverse processes, costovertebral joints and manubriosternal joint.
MRI of the cervical and upper thoracic spine of a 29-year-old male patient scans were performed before (A–B and D–E) and 3 months after (C and F) initiation of anti-TNF therapy. Sagittal slices lateral to the spinal canal are shown.
(A)–(C) Sagittal slice through the pedicle and the lateral parts of the vertebrae, shows moderate bone oedema on the baseline STIR image (B) in the posterolateral aspects of all of the thoracic vertebral bodies, most pronounced at T4 and T5. The distribution is typical for inflammation on the vertebral side of the costovertebral joints. Also note inflammation in the manubriosternal joint anteriorly (arrow). Most foci of inflammation are still faintly visible after anti-TNF therapy (C), but are clearly less intense.
(D)–(F) Far lateral slice through the transverse processes and ribs. Intense bone marrow oedema is seen in the transverse processes of the upper thoracic spine (E; arrows), which resolves completely with treatment. This pattern of bone marrow oedema in the transverse processes, costovertebral joints and manubriosternal joint are pathognomonic of spondyloarthritis.
Figure 5.
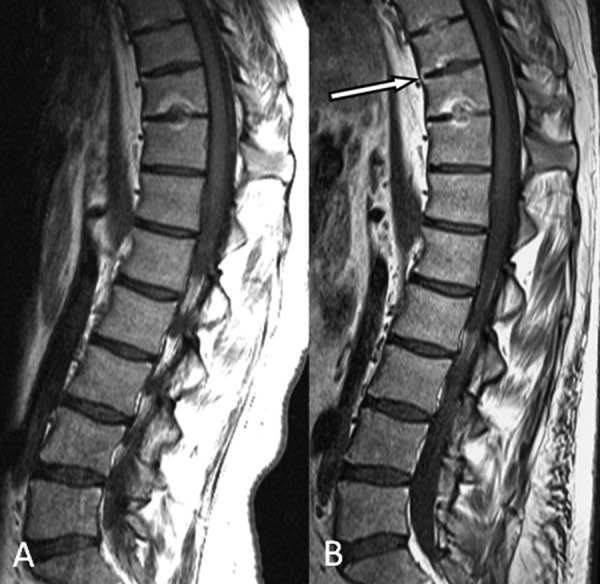
Progression of structural damage in the spine in ankylosing spondylitis, visualized by MRI.
T1-weighted MRIs of the thoracolumbar spine with a 3-year interval in a baseline (A) 23-year-old male with established ankylosing spondylitis. The follow-up MRI (B) demonstrates new anterior ankylosis at T9/10 with bridging anterior syndesmophytosis containing marrow fat signal. Note also several other findings indicating the progression of structural damage: the intervertebral disc at T10/11 is completely fused on the second scan; a new endplate defect has appeared at the inferior endplate of T9 with new fat infiltration; and the signal within the T8/9 disc has increased suggesting progression of disc ossification.
Table 1.
Magnetic resonance imaging definitions of inflammatory and structural lesions in axial spondyloarthritis (by Canada-Denmark MRI Working Group and MORPHO group [Lambert et al. 2009; Østergaard et al. 2009; Weber et al. 2010a, 2010b]).
| A. Inflammatory lesions |
| Bone marrow oedema: Increase in bone marrow signal* on STIR images. |
| B. Structural lesions |
| Bone erosion: Full-thickness loss of dark appearance of the cortical bone and change in normal bright appearance of adjacent bone marrow on T1-weighted images** |
| Fat infiltration: Focal increased signal in bone marrow on T1-weighted images**. |
| Bone spur: Bright signal on T1-weighted images extending from the vertebral endplate towards the adjacent vertebra (spine) |
| Ankylosis: Bright signal on T1-weighted images extending across the sacroiliac joints or extending from one vertebra being continuous with the adjacent vertebra (spine) |
Reference point for bone marrow signal on STIR images: sacroiliac joints, the centre of the sacrum at the same craniocaudal level; spine, the centre of the vertebra, if normal; if not normal, the signal in the centre of the closest available normal vertebra.
Reference point for bone marrow signal on T1-weighted images: sacral bone, the centre of the sacrum at the same craniocaudal level; iliac bone, normal iliac marrow at the same craniocaudal level; spine, the centre of the vertebra, if normal; if not normal, the signal in the centre of the closest available normal vertebra.
Use in diagnosis, monitoring and prognostication
Diagnosis
The introduction of MRI has resulted in a major improvement in the evaluation and management of patients with SpA. Diagnosis was previously dependent on the presence of bilateral moderate or unilateral severe radiographic sacroiliitis, as part of the modified New York criteria for AS [van der Linden et al. 1984]. This frequently delayed the diagnosis by 7–10 years [Feldtkeller et al. 2003]. Now, through the recent ASAS (ASsessment of SpondyloArthitis) classification criteria for axial SpA, MRI forms an integral part, as patients with active sacroiliitis on MRI plus one clinical feature (e.g. psoriasis, enthesitis or uveitis (see Rudwaleit et al. [2009b] for a complete list), should be classified as axial SpA [Rudwaleit et al. 2009b]. A consensus-based definition of the requirements to constitute active sacroiliitis, i.e. fulfil the MRI criterion of the ASAS criteria (‘a positive MRI’) has been defined: bone marrow oedema, located in ≥2 sites and/or in ≥2 slices [Rudwaleit et al. 2009a].
Recent data demonstrate that incorporating structural damage lesions (erosions) into the criteria, would improve the diagnostic utility of MRI [Weber et al. 2010a, 2010b]. However, ASAS in January 2011 decided to await further data before considering revision of the definition of a positive MRI in the axial SpA criteria.
Monitoring disease activity and damage
MRI can provide objective evidence of currently active inflammation in patients with SpA (Figure 4) [Hermann and Bollow, 2004; Maksymowych and Landewe, 2006]. Until the introduction of MRI, disease activity assessment was restricted to patient-reported outcomes, such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI), because disease activity could not be assessed in a sensitive manner by biochemical (mainly C-reactive protein [CRP]) or physical evaluation.
Several systems for assessment of disease activity in the sacroiliac joints and in the spine have been proposed (see a recent review by Østergaard et al. [2010] for details). Reproducible and responsive methods are available [Lukas et al. 2007]. The sensitivity to change and discriminatory ability of the three most used spine scoring systems (The Ankylosing Spondylitis spine Magnetic Resonance Imaging-activity [ASspiMRI-a] score, the Berlin modification of the ASspiMRI-a score and the Spondyloarthritis Research Consortium of Canada [SPARCC] scoring system) [Braun et al. 2003; Haibel et al. 2006; Maksymowych et al. 2005] have been demonstrated in clinical trials, and they have been tested against each other by the ASAS/OMERACT MRI in AS group [Lukas et al. 2007]. All methods were feasible, reliable, sensitive to change and discriminative. The SPARCC method had the highest sensitivity to change, as judged by Guyatt’s effect size, and the highest reliability as judged by the inter-reader intraclass correlation coefficient (ICC) [Lukas et al. 2007].
MRI is much less established for assessment of structural changes (often referred to as chronic changes) than inflammatory changes. Since MRI undoubtedly provides otherwise inaccessible information on inflammatory activity, just ‘equality’ of MRI with radiography concerning structural damage assessment is a step forward, because radiography, and the ensuing need for two examinations and exposure to ionizing radiation, could then be avoided. Scoring methods assess erosions, sclerosis, fat deposition and/or bone bridges separately or as global score [Braun et al. 2003; Madsen and Jurik, 2010b; Østergaard et al. 2009]. The validation of the methods for damage assessment is limited and their value is not yet clarified.
Prognostication
Several published spine studies have documented an association between the presence of bone marrow oedema at the anterior corners of the vertebrae on MRI and subsequent development of syndesmophytes on radiography after 2 years of follow up. Presence as opposed to absence of MRI anterior inflammation provides relative risks of 3–5 for a new anterior radiographic syndesmophyte at that level [Baraliakos et al. 2008; Maksymowych et al. 2009; Pedersen et al. 2011]. In two studies, the association was even more pronounced in those vertebral corners in which the inflammation had resolved following institution of anti-TNF therapy, possibly explained by tumour necrosis factor (TNF) in an active inflammatory lesion restricting new bone formation, whereas reduction of TNF by applying a TNF-antagonist allows tissue repair to manifest as new bone formation [Maksymowych et al. 2009; Pedersen et al. 2011]. A very recent study has demonstrated that fat infiltrations in vertebral corners increase the risk of subsequent radiographic syndesmophyte formation [Chiowchanwisawakit et al. 2011], bearing very interesting implications for fat infiltration (which can be easily recognized and reliably scored by MRI [Chiowchanwisawakit et al. 2009, 2011]), as a potentially valuable surrogate marker for new bone formation in AS. This, however, needs to be investigated in further longitudinal studies.
One study suggests that in early inflammatory back pain, severe sacroiliac MRI bone marrow oedema together with HLA-B27 positivity is a strong predictor of future AS, whereas mild or no sacroiliitis, irrespective of HLA-B27 status, was a predictor of not developing AS [Bennett et al. 2008]. Data on the value of MRI for predicting therapeutic response in SpA are very limited. A high spine MRI inflammation score and short disease duration have been reported as statistically significant predictors of clinical response (BASDAI improvement >50%) to anti-TNF therapy [Rudwaleit et al. 2008]. Further and larger studies are needed to clarify the role of MRI in the prediction of disease course and therapeutic response.
Conclusion
Imaging is an integral part of the management of patients with AS and axial SpA (Table 2). Characteristic radiographic and/or MRI findings are key in the diagnosis, and these modalities are also useful in monitoring the disease. CR is the conventional, albeit quite insensitive, gold standard method for assessment of structural damage in spine and sacroiliac joints, whereas MRI has developed a decisive role in monitoring disease activity in clinical trials and practice. MRI may also, if ongoing research demonstrates a sufficient reliability and sensitivity to change, become a new standard method for assessment of structural damage. It is exciting that with continued dedicated research and the rapid technical development it is likely that even larger improvements in the use of imaging may occur in the decade to come, for the benefit of our patients.
Table 2.
Practical use of imaging of axial joints in AS and axial SpA.
| A. Use in clinical practice: |
| • to establish a diagnosis of AS / SpA: CR / CR and MRI |
| • to monitor disease activity: MRI |
| • to monitor structural joint damage: CR, MRI, (CT*) |
| B. Use in research |
| • to assess structural progression: CR, (MRI**) |
| • to assess anti-inflammatory effectiveness: MRI |
| • for pretrial selection of the patients most likely to progress (‘enrichment’): CR, MRI |
CT allows this but cannot be used due to radiation exposure.
Promising, but more data needed.
AS, ankylosing spondylitis; CR, conventional radiography; SpA, axial spondyloarthritis; CT, computed tomography; MRI, magnetic resonance.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Mikkel Østergaard, Department of Rheumatology, Copenhagen University Hospital at Glostrup, Nordre Ringvej 57, DK-2600 Glostrup, Denmark.
Robert G.W. Lambert, Department of Radiology and Diagnostic Imaging, University of Alberta, Edmonton, AB, Canada
References
- Baraliakos X., Hermann K.G., Landewe R., Listing J., Golder W., Brandt J., et al. (2005) Assessment of acute spinal inflammation in patients with ankylosing spondylitis by magnetic resonance imaging: a comparison between contrast enhanced T1 and short tau inversion recovery (STIR) sequences. Ann Rheum Dis 64: 1141–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraliakos X., Listing J., Rudwaleit M., Sieper J., Braun J. (2008) The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther 10: R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A.N., McGonagle D., O’Connor P., Hensor E.M., Sivera F., Coates L.C., et al. (2008) Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 58: 3413–3418 [DOI] [PubMed] [Google Scholar]
- Berens D.L. (1971) Roentgen features of ankylosing spondylitis. Clin Orthop Relat Res 74: 20–33 [PubMed] [Google Scholar]
- Braun J., Baraliakos X., Golder W., Brandt J., Rudwaleit M., Listing J., et al. (2003) Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 48: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Chiowchanwisawakit P., Lambert R.G., Conner-Spady B., Maksymowych W.P. (2011) Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum 63: 2215–2225 [DOI] [PubMed] [Google Scholar]
- Chiowchanwisawakit P., Østergaard M., Pedersen S.J., Lambert R.G.W., Conner-Spady B., Maksymowych W.P. (2009) Validation of definitions for structural lesions detected by MRI in the spine of patients with spondyloarthritis. J Rheumatol 36(Suppl. 84): 39–47 [Google Scholar]
- Dougados M., van der Linden S., Juhlin R., Huitfeldt B., Amor B., Calin A., et al. (1991) The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 34: 1218–1227 [DOI] [PubMed] [Google Scholar]
- Feldtkeller E., Khan M.A., van der Heijde D., van der Linden S., Braun J. (2003) Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 23: 61–66 [DOI] [PubMed] [Google Scholar]
- Gandjbakhch F., Terslev L., Joshua F., Wakefield R.J., Naredo E., d’Agostino M.A., et al. (2011) Ultrasound in the evaluation of enthesitis: status and perspectives. Arthritis Res Ther 13: R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibel H., Rudwaleit M., Brandt H.C., Grozdanovic Z., Listing J., Kupper H., et al. (2006) Adalimumab reduces spinal symptoms in active ankylosing spondylitis: clinical and magnetic resonance imaging results of a fifty-two-week open-label trial. Arthritis Rheum 54: 678–681 [DOI] [PubMed] [Google Scholar]
- Hermann K.G., Althoff C.E., Schneider U., Zuhlsdorf S., Lembcke A., Hamm B., et al. (2005) Spinal changes in patients with spondyloarthritis: comparison of MR imaging and radiographic appearances. Radiographics 25: 559–569 [DOI] [PubMed] [Google Scholar]
- Hermann K.G., Bollow M. (2004) Magnetic resonance imaging of the axial skeleton in rheumatoid disease. Best Pract Res Clin Rheumatol 18: 881–907 [DOI] [PubMed] [Google Scholar]
- Huerta-Sil G., Casasola-Vargas J.C., Londono J.D., Rivas-Ruiz R., Chavez J., Pacheco-Tena C., et al. (2006) Low grade radiographic sacroiliitis as prognostic factor in patients with undifferentiated spondyloarthritis fulfilling diagnostic criteria for ankylosing spondylitis throughout follow up. Ann Rheum Dis 65: 642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser A., Halpern E.J., Frauscher F., Gvozdic D., Duftner C., Springer P., et al. (2005) Inflammatory low back pain: high negative predictive value of contrast-enhanced color Doppler ultrasound in the detection of inflamed sacroiliac joints. Arthritis Rheum 53: 440–444 [DOI] [PubMed] [Google Scholar]
- Lambert R.G.W., Pedersen S.J., Maksymowych W.P., Chiowchanwisawakit P., Østergaard M. (2009) Active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis - Definitions, assessment system and reference image set. J Rheumatol 36(Suppl. 84): 3–17 [Google Scholar]
- Lukas C., Braun J., van der Heijde D., Hermann K.G., Rudwaleit M., Østergaard M., et al. (2007) Scoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: a multireader experiment. J Rheumatol 34: 862–870 [PubMed] [Google Scholar]
- Madsen K.B., Egund N., Jurik A.G. (2010) Grading of inflammatory disease activity in the sacroiliac joints with magnetic resonance imaging: comparison between short-tau inversion recovery and gadolinium contrast-enhanced sequences. J Rheumatol 37: 393–400 [DOI] [PubMed] [Google Scholar]
- Madsen K.B., Jurik A.G. (2010a) Magnetic resonance imaging grading system for active and chronic spondylarthritis changes in the sacroiliac joint. Arthritis Care Res (Hoboken) 62: 11–18 [DOI] [PubMed] [Google Scholar]
- Madsen K.B., Jurik A.G. (2010b) MRI grading method for active and chronic spinal changes in spondyloarthritis. Clin Radiol 65: 6–14 [DOI] [PubMed] [Google Scholar]
- Maksymowych W., van der Heijde D., Landewe R., Pangan A., Brown S., Lavie F. (2010a) Predictors of radiographic progression in adalimumab-treated patients with ankylosing spondylitis (abstract). Arthritis Rheum 62(Suppl. 10): S815 [Google Scholar]
- Maksymowych W.P., Chiowchanwisawakit P., Clare T., Pedersen S.J., Østergaard M., Lambert R.G. (2009) Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 60: 93–102 [DOI] [PubMed] [Google Scholar]
- Maksymowych W.P., Crowther S.M., Dhillon S.S., Conner-Spady B., Lambert R.G. (2010b) Systematic assessment of inflammation by magnetic resonance imaging in the posterior elements of the spine in ankylosing spondylitis. Arthritis Care Res (Hoboken) 62: 4–10 [DOI] [PubMed] [Google Scholar]
- Maksymowych W.P., Inman R.D., Salonen D., Dhillon S.S., Krishnananthan R., Stone M., et al. (2005) Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 53: 502–509 [DOI] [PubMed] [Google Scholar]
- Maksymowych W.P., Landewe R. (2006) Imaging in ankylosing spondylitis. Best Pract Res Clin Rheumatol 20: 507–519 [DOI] [PubMed] [Google Scholar]
- Østergaard M., Maksymowych W.P., Pedersen S.J., Chiowchanwisawakit P., Lambert R.G.W. (2009) Structural lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis - Definitions, assessment system and reference image set. J Rheumatol 36(Suppl. 84): 18–34 [Google Scholar]
- Østergaard M., Poggenborg R.P., Axelsen M.B., Pedersen S.J. (2010) Magnetic resonance imaging in spondyloarthritis—how to quantify findings and measure response. Best Pract Res Clin Rheumatol 24: 637–657 [DOI] [PubMed] [Google Scholar]
- Pedersen S.J., Chiowchanwisawakit P., Lambert R.G.W., Østergaard M., Maksymowych W.P. (2011) Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation. J Rheumatol, in press [DOI] [PubMed] [Google Scholar]
- Poddubnyy D., Haibel H., Listing J., Marker-Hermann E., Zeidler H., Braun J., et al. (2011) Baseline radiographic damage, elevated acute phase reactants and cigarette smoking status predict radiographic progression in the spine in early axial spondyloarthritis. Arthritis Rheum, in press [DOI] [PubMed] [Google Scholar]
- Poggenborg R.P., Terslev L., Pedersen S.J., Østergaard M. (2011) Recent advances in imaging in psoriatic arthritis. Ther Adv Musculoskel Dis 3: 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhakka K.B., Melsen F., Jurik A.G., Boel L.W., Vesterby A., Egund N. (2004) MR imaging of the normal sacroiliac joint with correlation to histology. Skeletal Radiol 33: 15–28 [DOI] [PubMed] [Google Scholar]
- Resnick D. (2002) Diagnosis of bone and joint disorders. Philadelphia, PA: W.B. Saunders [Google Scholar]
- Resnick D., Niwayama G., Goergen T.G. (1977) Comparison of radiographic abnormalities of the sacroiliac joint in degenerative disease and ankylosing spondylitis. AJR Am J Roentgenol 128: 189–196 [DOI] [PubMed] [Google Scholar]
- Rudwaleit M., Jurik A.G., Hermann K.G., Landewe R., van der Heijde D., Baraliakos X., et al. (2009a) Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 68: 1520–1527 [DOI] [PubMed] [Google Scholar]
- Rudwaleit M., Schwarzlose S., Hilgert E.S., Listing J., Braun J., Sieper J. (2008) MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis 67: 1276–1281 [DOI] [PubMed] [Google Scholar]
- Rudwaleit M., van der Heijde D., Landewe R., Listing J., Akkoc N., Brandt J., et al. (2009b) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68: 777–783 [DOI] [PubMed] [Google Scholar]
- van der Linden S., Valkenburg H.A., Cats A. (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368 [DOI] [PubMed] [Google Scholar]
- Wanders A.J., Landewe R.B., Spoorenberg A., Dougados M., van der Linden S., Mielants H., et al. (2004) What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum 50: 2622–2632 [DOI] [PubMed] [Google Scholar]
- Weber U., Lambert R.G., Østergaard M., Hodler J., Pedersen S.J., Maksymowych W.P. (2010a) The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum 62: 3048–3058 [DOI] [PubMed] [Google Scholar]
- Weber U., Lambert R.G., Pedersen S.J., Hodler J., Østergaard M., Maksymowych W.P. (2010b) Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res (Hoboken) 62: 1763–1771 [DOI] [PubMed] [Google Scholar]