Abstract
The articular cartilage and the subchondral bone form a biocomposite that is uniquely adapted to the transfer of loads across the diarthrodial joint. During the evolution of the osteoarthritic process biomechanical and biological processes result in alterations in the composition, structure and functional properties of these tissues. Given the intimate contact between the cartilage and bone, alterations of either tissue will modulate the properties and function of the other joint component. The changes in periarticular bone tend to occur very early in the development of OA. Although chondrocytes also have the capacity to modulate their functional state in response to loading, the capacity of these cells to repair and modify their surrounding extracellular matrix is relatively limited in comparison to the adjacent subchondral bone. This differential adaptive capacity likely underlies the more rapid appearance of detectable skeletal changes in OA in comparison to the articular cartilage. The OA changes in periarticular bone include increases in subchondral cortical bone thickness, gradual decreases in subchondral trabeular bone mass, formation of marginal joint osteophytes, development of bone cysts and advancement of the zone of calcified cartilage between the articular cartilage and subchondral bone. The expansion of the zone of calcified cartilage contributes to overall thinning of the articular cartilage. The mechanisms involved in this process include the release of soluble mediators from chondrocytes in the deep zones of the articular cartilage and/or the influences of microcracks that have initiated focal remodeling in the calcified cartilage and subchondral bone in an attempt to repair the microdamage. There is the need for further studies to define the pathophysiological mechanisms involved in the interaction between subchondral bone and articular cartilage and for applying this information to the development of therapeutic interventions to improve the outcomes in patients with OA.
Keywords: osteoarthritis, bone remodeling, articular cartilage, biomechanics
Introduction
The periarticular bone changes associated with osteoarthritis (OA) can be organized into distinct patterns based on the anatomic site and the structural and composition alterations in the bone. These changes include increased subchondral cortical thickness, alterations in the architecture and volume of subchondral trabecular bone, formation of new bone at the joint margins (osteophytes), development of subchondral bone cysts, bone marrow lesions (detectible by MRI) and advancement of the tidemark associated with vascular invasion of the calcified cartilage [Goldring and Goldring, 2007; Goldring, 2009] (Figure 1). In addition, there also may be changes in the contour and shape of the subchondral cortical bone [Buckland-Wright, 2004; Bullough, 2004; Burr and Schaffler, 1997; Burstein and Gray, 2006; Messent et al. 2006; Radin and Rose, 1986].
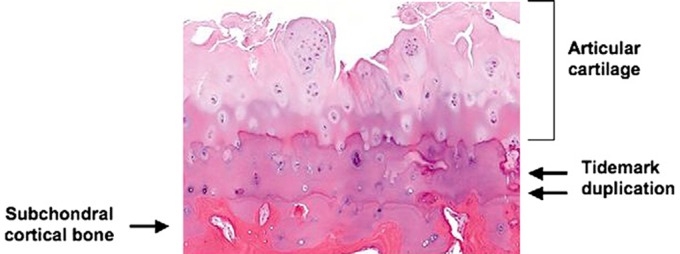
Figure 1.
Histologic features associated with advanced osteoarthritis. There is fragmentation and fissuring of the articular cartilage. Also, there is duplication of the tidemark with advancement of the calcified cartilage into the lower zones of the articular cartilage further contributing to thinning of the cartilage lining. (Courtesy of Edward DiCarlo, MD, Hospital for Special Surgery, New York, NY.)
The alterations in the periarticular bone that characterize the osteoarthritic process represent adaptations to local biomechanical and biological signals. These changes are mediated by bone cells that modify the architecture and properties of the bone through active cellular processes of modeling and remodeling [Goldring, 2009]. The cell-mediated process of adapting the bone to local mechanical influences is embodied in Wolff’s hypothesis that states that the distribution and material properties of bone are determined by the magnitude and direction of applied load [Frost, 2003]. In accordance with this principle, increases in bone volume are reflective of increased load and decreases in bone mass reflect a relative decrease in the local loading history. The mechanical effects of loading on bone remodeling not only affect bone mass but also produce alterations in the contour and shape of the subchondral bone. The term ‘bone attrition’ has been used to describe the decrease or loss of bone height and contour that accompanies the osteoarthritic changes in bone [Neogi et al. 2009; Reichenbach et al. 2011]. As discussed below, these changes affect joint congruity and may create a biomechanical environment that adversely affects the articular cartilage and contributes to joint symptoms.
In addition to the role of mechanical factors, bone remodeling also may be initiated at sites of local bone damage resulting from excessive loading. This process is characterized by the appearance of microcracks in the bone and is distinct from traumatic bone injuries associated with fractures that disrupt the gross bone architecture [Bullough, 2004; Burr, 2004; Burr and Schaffler, 1997; Frost, 2003; Martin, 2007]. The targeted bone remodeling provides a mechanism for repair of bone damage, but under certain conditions may contribute to the formation of bone cysts that represent one of the radiographic and anatomic hallmarks of OA [Bennell et al. 2011; Eriksen and Ringe, 2011; Goldring, 2009; McErlain et al. 2011]. Examination of the bone histology associated with local microdamage reveals regions of local fat necrosis and marrow fibrosis at various stages of healing [Taljanovic et al. 2008]. These regions correspond to the bone marrow lesions observed with MRI in patients with OA as described below.
The generation of new bone at the joint margins and periarticular entheseal sites results in the formation of osteophytes that represent an additional radiologic and morphologic feature of OA. The new bone is added by a process of endochondral ossification that recapitulates the cellular mechanisms of bone growth that occur during skeletal growth and development in which new bone is formed by replacement of a cartilaginous matrix [Bullough, 2004; Scharstuhl et al. 2002, 2003; van der Kraan and van den Berg, 2007].
The periarticular bone is not a homogeneous structure and is organized into the subchondral bone plate that consists of cortical compact bone. The subchondral plate transitions into a network of cancellous trabecular bone. The subchondral bone is separated from the overlying articular cartilage by a zone of calcified cartilage. The interface between the subchondral bone and calcified cartilage is marked by the so-called tidemark, which is a region of enhanced metachromatic staining. As discussed below, during the course of OA progression the zone of calcified cartilage undergoes marked alterations in its cellular composition and structural organization with expansion and advancement into the overlying articular cartilage associated with the penetration of the calcified cartilage by vascular elements that extend from the subchondral bone and adjacent marrow space [Buckland-Wright, 2004; Bullough, 2004; Burr, 2004; Lane et al. 1977; Walsh et al. 2007; Walsh et al. 2010]. This process recapitulates the vascular invasion of the growth plate that occurs during the development and growth of long bones and results in duplication of the tidemark and advancement of the calcified cartilage into the deep zones of the articular cartilage leading to local cartilage thinning.
The properties of subchondral bone that determine its capacity to absorb, distribute and transfer load also are influenced by the organization and composition of the organic bone matrix and the mineral content [Bau et al. 2002; Burket et al. 2011; Day et al. 2001; Donnelly et al. 2010; Faibish et al. 2006; Meunier and Boivin, 1997]. In the absence of underlying genetic or acquired abnormalities of the mineralization process, the state of bone mineralization is highly dependent on the rate of bone remodeling. In the physiologic remodeling cycle, bone formation by osteoblasts is initiated by the deposition of the organic bone matrix (osteoid), which undergoes rapid mineralization. After this initial phase, the mineralization process continues, and this late phase of mineral accretion markedly affects the material properties of the bone matrix. When the rate of bone remodeling and turnover are high, the ‘late’ phase of mineral accretion is attenuated leading to a state of relative hypomineralization. This is associated with a reduction in the elastic modulus of the bone that is more easily deformed under load. In contrast, in conditions of low bone turnover, the continued deposition of mineral leads to an increase in the elastic modulus and the bone becomes resistant to deformation and more ‘brittle’. During the progression of OA, marked changes occur in the rate and extent of remodeling in the subchondral cortical plate and the underlying trabecular bone. These alterations in bone turnover affect the state of mineralization and modify the capacity of the bone to deform under load [Buckland-Wright, 2004; Day et al. 2001, 2004].
There remains controversy regarding the initial structural and composition changes in OA and whether these alterations occur first in the bone or the articular cartilage. In addressing this issue, it is important to recognize that the subchondral mineralized tissues and overlying articular cartilage form a functional biocomposite in which the individual components of this structural entity interact cooperatively and synergistically with each other to transfer and distribute load during weight bearing and locomotion. Therefore, because of their intimate structural and functional relationship any biological or biomechanical process that affects one of the components of this load bearing structure will affect the adjacent tissues.
The focus on the skeletal changes as the initial effector of the osteoarthritic process was influenced by the early studies of Radin and Rose who proposed that the pathogenesis of OA could be attributed to a primary alteration in peri-articular bone [Radin and Rose, 1986]. They speculated that the osteoarthritic process was initiated by an increase in the thickness, volume and stiffness in the subchondral bone and that these changes resulted in increased load transfer to the overlying articular cartilage that adversely affected chondrocyte function and contributed to cartilage matrix loss. Their hypothesis is supported by the numerous studies that have demonstrated that the changes in periarticular bone occur very early in the development of OA. However, the cartilage and bone both have the capacity to respond to adverse biological or biomechanical signals and, therefore, it is more likely that both tissues undergo structural and functional alterations during the initiation and evolution of OA.
The detection of bone changes in OA prior to the appearance of detectible changes in the articular cartilage can be attributed to at least two factors. The first is the inability of standard radiographs to detect changes in the composition and properties of articular cartilage. To some extent this limitation can be addressed by the application of more recent magnetic resonance imaging techniques that can detect compositional and structural changes in the articular cartilage extracellular matrix. A second and more important factor that underlies the more rapid appearance of skeletal tissue changes in OA is related to the marked differential capacity of cartilage and bone to adapt to altered mechanical loads and damage. As discussed in the preceding section, both cortical and trabecular bone have the capacity to rapidly alter their architecture and structural properties via the cell-mediated processes of modeling and remodeling. In contrast, although recent studies have shown that chondrocytes can also modulate their functional state in response to loading, the capacity of these cells to repair and modify their surrounding extracellular matrix is relatively limited in comparison to skeletal tissues [Goldring and Goldring, 2004, 2007; Hunziker, 2002; Little Goodwin et al. 2008; Maroudas et al. 1998; Plaas et al. 2007; Sandell and Aigner, 2001]. This differential adaptive capacity likely underlies the more rapid appearance of detectable skeletal changes in OA, especially after injuries that acutely alter joint mechanics.
Multiple different technologies have been used to detect the skeletal tissue changes in OA. Early studies utilizing isotope-labeled bone-seeking agents and radiographs [Buckland-Wright et al. 1991; Hutton et al. 1986; Macfarlane et al. 1991] demonstrated increased retention of the radiolabel at sites of enhanced bone remodeling associated with OA and provided evidence that the changes in bone turnover preceded detectable radiographic changes. Importantly these scintigraphic changes were predictive of the subsequent development of osteophytes and subchondral bone sclerosis [Dieppe et al. 1993; McCrae et al. 1992]. These studies also showed that the development of osteophytes and subchondral sclerosis preceded detectable changes in articular cartilage thickness assessed by joint space narrowing using radiographic techniques [Buckland-Wright, 2004].
A major challenge in assessing the temporal sequence of skeletal changes in OA relates to the difficulty in identifying the specific timing of disease initiation. For this reason, the study of individuals with OA associated with traumatic joint injury in which the onset of the pathologic process can be linked to the date of a specific injury have been particularly informative. For example, Buckland-Wright and co-workers [Buckland-Wright et al. 2000] employed quantitative macroradiography in a cross-sectional study of patients with anterior cruciate ligament rupture to define the sequence of periarticular bone changes. The major alterations postinjury involved thickening of the subchondral horizontal trabeculae that reached significance by 3–4 years. Osteophytes were detected in approximately 50% of the injured knees by the third year, but no changes were detected in joint space width or cortical plate thickness within these time intervals. In contrast, they observed that individuals with OA of the knee not associated with a discrete injury exhibited a different pattern of bone changes in which there was an increase in cortical plate thickness that antedated the trabecular alterations. Based on these findings they concluded that the biomechanical and adaptive influences in these two populations differed [Buckland-Wright et al. 1994, 1996].
Additional studies have provided insights into the sequential structural changes the subchondral cortical and trabecular bone in OA. Karvonen and colleagues analyzed the bone mineral density of the subchondral trabecular bone in the knee joints of patients with early OA (Kellgren’s grade 0–2) and observed reduced levels deep to the thickened cortical bone [Karvonen et al. 1998]. More recently, Messent and coworkers [Messent et al. 2005a, 2005b, 2005c] utilized a computerized method of textural image analysis (Fractal Signature Analysis) to assess the architecture of subchondral trabecular bone in the knee joints of patients with medial compartment OA. They observed that the progression of OA was associated with an increase in vertical trabeculae, which tended to be thinner and fenestrated. These changes indicate that the trabecular bone in the subcortical compartment was relatively osteoporotic and these findings are consistent with the decrease in mineral density detected in this region reported by Karvonen and colleagues [Karvonen et al. 1998]. In the studies by Messent and colleagues, they noted that the horizontal trabeculae increased throughout the course of OA and that the size varied depending on whether the patients had early or late stage OA. They speculated that the thickened cortical plate and retention of the horizontal trabeculae was associated with increased load-bearing stresses in this region, and that the development of osteoporotic changes in the trabeculae beneath this region was related to reduced transmission of load and represented a form of so-called ‘stress shielding’.
Recent studies by Chan and colleagues have provided potential mechanistic insights into the molecular signals by which mechanical factors modulate subchondral bone remodeling [Chan et al. 2011]. They examined the expression of SOST and additional components of the Wnt-β-catenin pathway in osteochondral samples from human OA tissue and from osteochondral sections from sheep and mice with surgically induced OA. The SOST gene encodes the protein sclerostin, which is a potent inhibitor of the Wnt pathway that contributes to the regulation of bone formation, and the expression of this gene has been shown to be regulated in part by mechanical factors [Robling et al. 2008]. They noted that osteocyte SOST expression was locally decreased in regions of bone sclerosis and speculated that increased mechanical loading in these regions could be responsible for the downregulation of SOST with a resultant increase in localized bone formation. Surprisingly, they also detected SOST expression in articular chondrocytes and noted that the increased expression was localized to regions of focal cartilage damage. The role of SOST in regulating chondroctye differentiation and activity is complex and the mechanisms and consequences of its increased expression by chondrocytes in regions of cartilage damage remains unclear.
It is important to appreciate that changes in bone volume represent only one of the factors that determine the mechanical properties of bone and that additional influences, including the architecture and material properties of the bone, also contribute to its capacity to transmit load. The studies of Day and coworkers have been particularly informative in illustrating this concept. They constructed finite-element models from microCT scans of subchondral trabecular bone obtained from the proximal tibiae from cadaver specimens from subjects with early cartilage damage [Day et al. 2001] and used mechanical testing and finite-element analysis to determine the effective tissue modulus of the bone. They found that the volume fraction of trabecular bone was increased, consistent with the changes reported by Messent and colleagues but observed that the tissue modulus of the bone was reduced in the condyles in which there was damage in the overlying articular cartilage. They attributed the reduction in tissue modulus to a decrease in mineral density, which they speculated was related to incomplete mineralization due to an increase in the rate of bone remodeling and turnover. These observations are paradoxical and indicate that the properties of the subchondral bone in certain stages of OA progression may be associated with a decrease rather than an increase in the bone tissue modulus, i.e. stiffness, despite an increase in overall bone volume. These conclusions, if correct, have significant implications with respect to treatment strategies for targeting subchondral and periarticular bone remodeling in OA. For example, therapeutic agents such as bisphosphonates that inhibit bone resorption and reduce bone turnover would increase bone mineral content by reducing bone turnover. This would lead to an increase in subchondral bone stiffness, which in turn may alter the pattern of load transfer through the cartilage and potentially adversely affect cartilage homeostasis. The lack of efficacy of a recent trial with risedronate in reducing progression of the cartilage changes in OA highlight the complexity of the issues surrounding the influences of bone adaptation and its effects on the natural history of OA [Bingham et al. 2006; Buckland-Wright et al. 2007].
As discussed above, the presence of duplication of the tidemark and advancement of the zone of calcified cartilage into the overlying hyaline articular cartilage is an additional finding associated with OA [Bullough, 2004; Burr and Schaffler, 1997; Lane et al. 1977; Pulai et al. 2005]. Previous histologic studies of this zone demonstrated that this process was associated with the penetration of the calcified cartilage by vascular elements and formation of new calcified cartilage and bone, recapitulating morphological features of the growth plate [Bullough, 2004; Burr and Schaffler, 1997; Lane et al. 1977]. More recently, Walsh and coworkers [Ashraf et al. 2011; Suri et al. 2007; Walsh et al. 2007; Walsh et al. 2010] used immunostaining techniques to analyze the osteochondral junction in an animal model of OA and in tissue samples from patients undergoing arthroplasty for OA. They observed the presence of sensory nerve fibers expressing nerve growth factor (NGF) in the vascular channels associated with osteochondral angiogenesis and speculated that the sensory fibers in these regions could be a potential source of symptomatic pain. They also noted that the regions of vascular invasion were associated with localized bone marrow replacement by fibrovascular tissue expressing vascular endothelial factor (VEGF). VEGF expression was also detected in chondrocytes in proximity to the angiogenesis and they hypothesized that this growth factor could provide the signals for recruitment of the vascular elements. The advancement of calcified cartilage and resultant thinning of the articular cartilage would be predicted to increase the mechanical stresses in the deep zones of the cartilage matrix that could contribute to altered chondrocyte function and adversely affect cartilage matrix homeostasis.
The presence of so-called ‘bone marrow edema’ is an additional feature of OA. This term was introduced in 1988 by Wilson and colleagues who noted the presence in subchondral bone from patients with OA of localized regions of increased signal intensity using fluid sensitive magnetic resonance sequences [Wilson et al. 1988]. Analysis of the histologic changes corresponding to the anatomic site of the bone marrow lesions has revealed the presence of fat necrosis and localized marrow fibrosis associated with microfractures of the trabecular bone at various stages of healing indicating that the MRI signal was not generated by actual ‘edema’ [Leydet-Quilici et al. 2010; Taljanovic et al. 2008]. Importantly, although longitudinal studies reveal that the bone marrow lesions may come and go, their presence correlates overall with the severity of joint pain and with progression of OA cartilage and bone changes [Felson et al. 2001, 2007; Hernandez-Molina et al. 2008; Hunter et al. 2006; Lo et al. 2005; Reichenbach et al. 2008; Roemer et al. 2010; Taljanovic et al. 2008; Tanamas et al. 2010]. The correspondence of the sites of bone marrow lesions with regions of bone and cartilage damage strongly supports a primary role for a mechanical and traumatic etiology for the subchondral bone marrow changes [Bennell et al. 2011; Eriksen and Ringe, 2011]. The presence of microfractures with localized bone remodeling is consistent with activation of bone repair processes that accompany targeted bone remodeling [Burr, 2004; Burr and Schaffler, 1997; Cardoso et al. 2009; Martin, 2007]. Of interest, the bone cysts associated with OA frequently develop in the focal areas of bone damage and necrosis, supporting the concept that their pathogenesis also is related to a mechanical process [Bancroft et al. 2004; Carrino et al. 2006]. In addition to the role of mechanical loading and microdamage in the pathogenesis of bone marrow lesions, there is evidence that impaired blood flow and local ischemia may also contribute to the disturbance in subchondral bone remodeling [Findlay, 2007]. These changes in subchondral bone blood flow could also interfere with the supply of nutrients and other products to the overlying cartilage and in this way adversely affect chondrocyte function and cartilage matrix homeostasis.
Osteophytes are an additional characteristic finding in OA and their presence has served as a useful radiographic marker of OA initiation and progression. They are localized to the margins of OA joints, and numerous lines of investigation implicate local biomechanical factors in their appearance and growth. Although there remains controversy regarding their functional role, several observations suggest that they may serve to stabilize the joint rather than contributing to joint dysfunction and OA progression [Guyton and Brand, 2002; Messent et al. 2007; Perry et al. 1972; van der Kraan and van den Berg, 2007]. This conclusion is supported by the observations of Pottenger and colleagues, who noted that removal of medial and lateral osteophytes from the knee joint results in increased joint instability [Pottenger et al. 1990]. Felson and coworkers [Felson et al. 2005] provided further evidence that osteophytes may not have a direct role in OA progression but rather serve as a marker of nearby cartilage loss and joint pathology. They employed fluoroscopic techniques to examine osteophyte size and the risk for development of structural progression of knee OA. They found that the large osteophytes did not appear to affect the risk of structural progression and that the relationship between osteophytes and progression were at least in part explained by the association of the structural change with malalignment.
Studies in animal models of OA have been particularly informative in defining the sequence of events associated with osteophyte formation [van der Kraan and van den Berg, 2007]. These studies indicate that the osteophyte is initiated by proliferation of periosteal cells at the joint margin followed by differentiation of these cells into chondrocytes, which hypertrophy and through the process of endochondral ossification create an enlarging skeletal outgrowth at the joint margin. The local production of growth factors, including transforming growth factor-β and bone morphogenic protein-2, has been implicated in this process [Blaney Davidson et al. 2007; Zoricic et al. 2003]. Their role in osteophyte formation is supported by studies in which intra-articular injection of these growth factors into joints induces osteophyte formation, and inhibition of the activity or interference with their signal pathways results in inhibition of osteophyte formation [Scharstuhl et al. 2002, 2003; van Beuningen et al. 1998, 1994, 2000].
In addition to the role of mechanical factors in the pathogenesis of the structural and functional changes in cartilage and bone, there is evidence that biological processes also affect the remodeling and adaptations of these tissues. In part, these effects are mediated by products derived from other joint tissues, including for example the synovium, menisci and adipose tissues. However, there is also evidence that there is direct communication between the subchondral bone and cartilage via a process of diffusion that permits exchange of soluble products that have the capacity to modulate the activities of resident cells in these tissues [Amin et al. 2009; Findlay, 2010; Pan et al. 2009]. The vascular invasion of the calcified cartilage in OA tissues, and the advancement of the tidemark represent biological processes that may result from these types of interactions [Ashraf et al. 2011; Suri et al. 2007; Walsh et al. 2007, 2010].
In summary, OA is characterized by marked alterations in the composition, structure and functional properties of periarticular bone. These changes are mediated by the cells that remodel and model bone under physiological conditions, and an understanding of the pathophysiological processes associated with the skeletal changes in OA provides a rationale framework for developing therapeutic interventions that have the potential to modify the natural history of OA. Although results in animal models of OA have shown beneficial effects, to date, therapies that specifically modify bone cell activity and remodeling have not been shown to be of benefit in studies of humans with OA. In part, this lack of success can be attributed to the marked heterogeneity of the patient populations with OA and the complex processes associated with skeletal remodeling. In addition, further information is needed regarding the biological and biomechanical interactions between the periarticular bone and articular cartilage. Nevertheless, improved diagnostic tools for assessing bone properties and turnover and for modulating the activity and function of bone cells are becoming available, providing potential opportunity for the targeting of skeletal tissue changes in OA for therapeutic intervention.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
References
- Amin A.K., Huntley J.S., Simpson A.H., Hall A.C. (2009) Chondrocyte survival in articular cartilage: the influence of subchondral bone in a bovine model. J Bone Joint Surg Br 91(5): 691–699 [DOI] [PubMed] [Google Scholar]
- Ashraf S., Mapp P.I., Walsh D.A. (2011) Contributions of angiogenesis to inflammation, joint damage and pain in a rat model of osteoarthritis. Arthritis Rheum, in press [DOI] [PubMed] [Google Scholar]
- Bancroft L.W., Peterson J.J., Kransdorf M.J. (2004) Cysts, geodes, and erosions. Radiol Clin North Am 42: 73–87 [DOI] [PubMed] [Google Scholar]
- Bau B., Gebhard P.M., Haag J., Knorr T., Bartnik E., Aigner T. (2002) Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum 46: 2648–2657 [DOI] [PubMed] [Google Scholar]
- Bennell K.L., Creaby M.W., Wrigley T.V., Bowles K.A., Hinman R.S., Cicuttini F., et al. (2011) Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann Rheum Dis 69: 1151–1154 [DOI] [PubMed] [Google Scholar]
- Bingham C.O., III, Buckland-Wright J.C., Garnero P., Cohen S.B., Dougados M., Adami S., et al. (2006) Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum 54: 3494–3507 [DOI] [PubMed] [Google Scholar]
- Blaney Davidson E.N., van der Kraan P.M., van den Berg W.B. (2007) TGF-beta and osteoarthritis. Osteoarthritis Cartilage 15: 597–604 [DOI] [PubMed] [Google Scholar]
- Buckland-Wright C. (2004) Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage 12(Suppl. A): S10–S19 [DOI] [PubMed] [Google Scholar]
- Buckland-Wright J.C., Lynch J.A., Dave B. (2000) Early radiographic features in patients with anterior cruciate ligament rupture. Ann Rheum Dis 59: 641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland-Wright J.C., Lynch J.A., Macfarlane D.G. (1996) Fractal signature analysis measures cancellous bone organisation in macroradiographs of patients with knee osteoarthritis. Ann Rheum Dis 55: 749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland-Wright J.C., Macfarlane D.G., Fogelman I., Emery P., Lynch J.A. (1991) Techetium 99m methylene diphosphonate bone scanning in osteoarthritic hands. Eur J Nucl Med 18: 12–16 [DOI] [PubMed] [Google Scholar]
- Buckland-Wright J.C., Macfarlane D.G., Jasani M.K., Lynch J.A. (1994) Quantitative microfocal radiographic assessment of osteoarthritis of the knee from weight bearing tunnel and semiflexed standing views. J Rheumatol 21: 1734–1741 [PubMed] [Google Scholar]
- Buckland-Wright J.C., Messent E.A., Bingham C.O., III, Ward R.J., Tonkin C. (2007) A 2 yr longitudinal radiographic study examining the effect of a bisphosphonate (risedronate) upon subchondral bone loss in osteoarthritic knee patients. Rheumatology (Oxford) 46: 257–264 [DOI] [PubMed] [Google Scholar]
- Bullough P.G. (2004) The role of joint architecture in the etiology of arthritis. Osteoarthritis Cartilage 12(Suppl. A): S2–S9 [DOI] [PubMed] [Google Scholar]
- Burket J., Gourion-Arsiquaud S., Havill L.M., Baker S.P., Boskey A.L., van der Meulen M.C. (2011) Microstructure and nanomechanical properties in osteons relate to tissue and animal age. J Biomech 44: 277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D.B. (2004) Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage 12(Suppl. A): S20–S30 [DOI] [PubMed] [Google Scholar]
- Burr D.B., Schaffler M.B. (1997) The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Microsc Res Tech 37: 343–357 [DOI] [PubMed] [Google Scholar]
- Burstein D., Gray M.L. (2006) Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis? Osteoarthritis Cartilage 14: 1087–1090 [DOI] [PubMed] [Google Scholar]
- Cardoso L., Herman B.C., Verborgt O., Laudier D., Majeska R.J., Schaffler M.B. (2009) Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res 24: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrino J.A., Blum J., Parellada J.A., Schweitzer M.E., Morrison W.B. (2006) MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage 14: 1081–1085 [DOI] [PubMed] [Google Scholar]
- Chan B.Y., Fuller E.S., Russell A.K., Smith S.M., Smith M.M., Jackson M.T., et al. (2011) Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage 19: 874–885 [DOI] [PubMed] [Google Scholar]
- Day J.S., Ding M., van der Linden J.C., Hvid I., Sumner D.R., Weinans H. (2001) A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res 19: 914–918 [DOI] [PubMed] [Google Scholar]
- Day J.S., Van Der Linden J.C., Bank R.A., Ding M., Hvid I., Sumner D.R., et al. (2004) Adaptation of subchondral bone in osteoarthritis. Biorheology 41: 359–368 [PubMed] [Google Scholar]
- Dieppe P., Cushnaghan J., Young P., Kirwan J. (1993) Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis 52: 557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly E., Chen D.X., Boskey A.L., Baker S.P., van der Meulen M.C. (2010) Contribution of mineral to bone structural behavior and tissue mechanical properties. Calcif Tissue Int 87: 450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen E.F., Ringe J.D. (2011) Bone marrow lesions: a universal bone response to injury? Rheumatol Int, in press [DOI] [PubMed] [Google Scholar]
- Faibish D., Ott S.M., Boskey A.L. (2006) Mineral changes in osteoporosis: a review. Clin Orthop Relat Res 443: 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D.T., Chaisson C.E., Hill C.L., Totterman S.M., Gale M.E., Skinner K.M., et al. (2001) The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 134: 541–549 [DOI] [PubMed] [Google Scholar]
- Felson D.T., Gale D.R., Elon Gale M., Niu J., Hunter D.J., Goggins J., et al. (2005) Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxford) 44: 100–104 [DOI] [PubMed] [Google Scholar]
- Felson D.T., Niu J., Guermazi A., Roemer F., Aliabadi P., Clancy M., et al. (2007) Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 56: 2986–2992 [DOI] [PubMed] [Google Scholar]
- Findlay D.M. (2007) Vascular pathology and osteoarthritis. Rheumatology (Oxford) 46: 1763–1768 [DOI] [PubMed] [Google Scholar]
- Findlay D.M. (2010) If good things come from above, do bad things come from below? Arthritis Res Ther 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost H.M. (2003) Perspective: genetic and hormonal roles in bone disorders: insights of an updated bone physiology. J Musculoskelet Neuronal Interact 3: 118–135 [PubMed] [Google Scholar]
- Goldring M.B., Goldring S.R. (2007) Osteoarthritis. J Cell Physiol 213: 626–634 [DOI] [PubMed] [Google Scholar]
- Goldring S.R. (2009) Role of bone in osteoarthritis pathogenesis. Med Clin North Am 93: 25–35, xv [DOI] [PubMed] [Google Scholar]
- Goldring S.R., Goldring M.B. (2004) The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop (427 Suppl.): S27–S36 [DOI] [PubMed] [Google Scholar]
- Guyton G.P., Brand R.A. (2002) Apparent spontaneous joint restoration in hip osteoarthritis. Clin Orthop Relat Res 404: 302–307 [DOI] [PubMed] [Google Scholar]
- Hernandez-Molina G., Guermazi A., Niu J., Gale D., Goggins J., Amin S., et al. (2008) Central bone marrow lesions in symptomatic knee osteoarthritis and their relationship to anterior cruciate ligament tears and cartilage loss. Arthritis Rheum 58: 130–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D.J., Zhang Y., Niu J., Goggins J., Amin S., LaValley M.P., et al. (2006) Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum 54: 1529–1535 [DOI] [PubMed] [Google Scholar]
- Hunziker E.B. (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10: 432–463 [DOI] [PubMed] [Google Scholar]
- Hutton C.W., Higgs E.R., Jackson P.C., Watt I., Dieppe P.A. (1986) 99mTc HMDP bone scanning in generalised nodal osteoarthritis. I. Comparison of the standard radiograph and four hour bone scan image of the hand. Ann Rheum Dis 45: 617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen R.L., Miller P.R., Nelson D.A., Granda J.L., Fernandez-Madrid F. (1998) Periarticular osteoporosis in osteoarthritis of the knee. J Rheumatol 25: 2187–2194 [PubMed] [Google Scholar]
- Lane L.B., Villacin A., Bullough P.G. (1977) The vascularity and remodelling of subchondrial bone and calcified cartilage in adult human femoral and humeral heads. An age- and stress-related phenomenon. J Bone Joint Surg Br 59: 272–278 [DOI] [PubMed] [Google Scholar]
- Leydet-Quilici H., Le Corroller T., Bouvier C., Giorgi R., Argenson J.N., Champsaur P., et al. (2010) Advanced hip osteoarthritis: magnetic resonance imaging aspects and histopathology correlations. Osteoarthritis Cartilage 18: 1429–1435 [DOI] [PubMed] [Google Scholar]
- Little Goodwin J., Farley M.L., Swaim B., Goldring S.R., Goldring M.B., Bierbaum B.E., et al. (2008) Dual proline labeling protocol for individual “baseline” and “response” biosynthesis measurements in human articular cartilage. Osteoarthritis Cartilage, in press [DOI] [PubMed] [Google Scholar]
- Lo G.H., Hunter D.J., Zhang Y., McLennan C.E., Lavalley M.P., Kiel D.P., et al. (2005) Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum 52: 2814–2821 [DOI] [PubMed] [Google Scholar]
- Macfarlane D.G., Buckland-Wright J.C., Emery P., Fogelman I., Clark B., Lynch J. (1991) Comparison of clinical, radionuclide, and radiographic features of osteoarthritis of the hands. Ann Rheum Dis 50: 623–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Bayliss M.T., Uchitel-Kaushansky N., Schneiderman R., Gilav E. (1998) Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys 350: 61–71 [DOI] [PubMed] [Google Scholar]
- Martin R.B. (2007) Targeted bone remodeling involves BMU steering as well as activation. Bone 40: 1574–1580 [DOI] [PubMed] [Google Scholar]
- McCrae F., Shouls J., Dieppe P., Watt I. (1992) Scintigraphic assessment of osteoarthritis of the knee joint. Ann Rheum Dis 51: 938–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McErlain D.D., Milner J.S., Ivanov T.G., Jencikova-Celerin L., Pollmann S.I., Holdsworth D.W. (2011) Subchondral cysts create increased intra-osseous stress in early knee OA: A finite element analysis using simulated lesions. Bone 48: 639–646 [DOI] [PubMed] [Google Scholar]
- Messent E.A., Buckland-Wright J.C., Blake G.M. (2005a) Fractal analysis of trabecular bone in knee osteoarthritis (OA) is a more sensitive marker of disease status than bone mineral density (BMD). Calcif Tissue Int 76: 419–425 [DOI] [PubMed] [Google Scholar]
- Messent E.A., Ward R.J., Tonkin C.J., Buckland-Wright C. (2005b) Cancellous bone differences between knees with early, definite and advanced joint space loss; a comparative quantitative macroradiographic study. Osteoarthritis Cartilage 13: 39–47 [DOI] [PubMed] [Google Scholar]
- Messent E.A., Ward R.J., Tonkin C.J., Buckland-Wright C. (2005c) Tibial cancellous bone changes in patients with knee osteoarthritis. A short-term longitudinal study using Fractal Signature Analysis. Osteoarthritis Cartilage 13: 463–470 [DOI] [PubMed] [Google Scholar]
- Messent E.A., Ward R.J., Tonkin C.J., Buckland-Wright C. (2006) Differences in trabecular structure between knees with and without osteoarthritis quantified by macro and standard radiography, respectively. Osteoarthritis Cartilage 14: 1302–1305 [DOI] [PubMed] [Google Scholar]
- Messent E.A., Ward R.J., Tonkin C.J., Buckland-Wright C. (2007) Osteophytes, juxta-articular radiolucencies and cancellous bone changes in the proximal tibia of patients with knee osteoarthritis. Osteoarthritis Cartilage 15: 179–186 [DOI] [PubMed] [Google Scholar]
- Meunier P.J., Boivin G. (1997) Bone mineral density reflects bone mass but also the degree of mineralization of bone: therapeutic implications. Bone 21: 373–377 [DOI] [PubMed] [Google Scholar]
- Neogi T., Felson D., Niu J., Lynch J., Nevitt M., Guermazi A., et al. (2009) Cartilage loss occurs in the same subregions as subchondral bone attrition: a within-knee subregion-matched approach from the Multicenter Osteoarthritis Study. Arthritis Rheum 61: 1539–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Zhou X., Li W., Novotny J.E., Doty S.B., Wang L. (2009) In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res 27: 1347–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G.H., Smith M.J., Whiteside C.G. (1972) Spontaneous recovery of the joint space in degenerative hip disease. Ann Rheum Dis 31: 440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas A., Osborn B., Yoshihara Y., Bai Y., Bloom T., Nelson F., et al. (2007) Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage 15: 719–734 [DOI] [PubMed] [Google Scholar]
- Pottenger L.A., Phillips F.M., Draganich L.F. (1990) The effect of marginal osteophytes on reduction of varus-valgus instability in osteoarthritic knees. Arthritis Rheum 33: 853–858 [DOI] [PubMed] [Google Scholar]
- Pulai J.I., Chen H., Im H.J., Kumar S., Hanning C., Hegde P.S., et al. (2005) NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol 174: 5781–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin E.L., Rose R.M. (1986) Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res 213: 34–40 [PubMed] [Google Scholar]
- Reichenbach S., Dieppe P.A., Nuesch E., Williams S., Villiger P.M., Juni P. (2011) Association of bone attrition with knee pain, stiffness and disability: a cross-sectional study. Ann Rheum Dis 70: 293–298 [DOI] [PubMed] [Google Scholar]
- Reichenbach S., Guermazi A., Niu J., Neogi T., Hunter D.J., Roemer F.W., et al. (2008) Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling A.G., Niziolek P.J., Baldridge L.A., Condon K.W., Allen M.R., Alam I., et al. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of SOST/sclerostin. J Biol Chem 283: 5866–5875 [DOI] [PubMed] [Google Scholar]
- Roemer F.W., Neogi T., Nevitt M.C., Felson D.T., Zhu Y., Zhang Y., et al. (2010) Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: the MOST study. Osteoarthritis Cartilage 18: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L.J., Aigner T. (2001) Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 3: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharstuhl A., Glansbeek H.L., van Beuningen H.M., Vitters E.L., van der Kraan P.M., van den Berg W.B. (2002) Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol 169: 507–514 [DOI] [PubMed] [Google Scholar]
- Scharstuhl A., Vitters E.L., van der Kraan P.M., van den Berg W.B. (2003) Reduction of osteophyte formation and synovial thickening by adenoviral overexpression of transforming growth factor beta/bone morphogenetic protein inhibitors during experimental osteoarthritis. Arthritis Rheum 48: 3442–3451 [DOI] [PubMed] [Google Scholar]
- Suri S., Gill S.E., Massena de, Camin S., Wilson D., McWilliams D.F., Walsh D.A. (2007) Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis 66: 1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taljanovic M.S., Graham A.R., Benjamin J.B., Gmitro A.F., Krupinski E.A., Schwartz S.A., et al. (2008) Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol 37: 423–431 [DOI] [PubMed] [Google Scholar]
- Tanamas S.K., Wluka A.E., Pelletier J.P., Pelletier J.M., Abram F., Berry P.A., et al. (2010) Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford) 49: 2413–2419 [DOI] [PubMed] [Google Scholar]
- van Beuningen H.M., Glansbeek H.L., van der Kraan P.M., van den Berg W.B. (1998) Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage 6: 306–317 [DOI] [PubMed] [Google Scholar]
- van Beuningen H.M., Glansbeek H.L., van der Kraan P.M., van den Berg W.B. (2000) Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage 8: 25–33 [DOI] [PubMed] [Google Scholar]
- van Beuningen H.M., van der Kraan P.M., Arntz O.J., van den Berg W.B. (1994) Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest 71: 279–290 [PubMed] [Google Scholar]
- van der Kraan P.M., van den Berg W.B. (2007) Osteophytes: relevance and biology. Osteoarthritis Cartilage 15: 237–244 [DOI] [PubMed] [Google Scholar]
- Walsh D.A., Bonnet C.S., Turner E.L., Wilson D., Situ M., McWilliams D.F. (2007) Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage 15: 743–751 [DOI] [PubMed] [Google Scholar]
- Walsh D.A., McWilliams D.F., Turley M.J., Dixon M.R., Franses R.E., Mapp P.I., et al. (2010) Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 49: 1852–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.J., Murphy W.A., Hardy D.C., Totty W.G. (1988) Transient osteoporosis: transient bone marrow edema? Radiology 167: 757–760 [DOI] [PubMed] [Google Scholar]
- Zoricic S., Maric I., Bobinac D., Vukicevic S. (2003) Expression of bone morphogenetic proteins and cartilage-derived morphogenetic proteins during osteophyte formation in humans. J Anat 202: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]