Abstract
Objectives:
All biologic agents approved for the treatment of rheumatoid arthritis (RA) have been tested versus methotrexate (MTX) for efficacy on damage progression in several randomized clinical trials (RCTs), but direct head-to-head comparisons have never been conducted. The purpose of this investigation is to analyse data coming from main RA RCTs and to perform an indirect comparison.
Methods:
A systematic review of literature from 1988 to 2011 was conducted. Only randomized, double-blind, controlled, comparative trials, with evaluation of radiographic progression were included. The radiographic score was standardized and mean difference in the percentage of the annual radiographic progression rate was used as the effect measure. Heterogeneity between studies was estimated by I2 test. For each trial, the effect was plotted according to its standard error in a funnel plot.
Results:
Of 44 potentially relevant trials, 12 RCTs were included in the study. In order to optimize RCTs comparison, studies were stratified in early and late RA group. Main population characteristics were similar in both early and late RA groups, whereas the standardized baseline radiographic score value significantly differs among trials in both early (range 2.7–21.9) and late (range 23.46–75) RA groups. The standardized annual estimated progression is similar across the late RA group. Strong evidence of heterogeneity (I2 = 97%, p = 0.00001) but no asymmetry of the funnel plot was observed in the early RA group. Total mean difference was −16.28 (95% confidence interval [CI] −24.42 to −8.14). For the late RA group a random model was used (I2 = 99%, p = 0.00001) and a total mean difference of −39.25 (95% CI −53.77 to −24.73) was found.
Conclusions:
All biologic agents provide a favourable effect on disease progression both in early and late RA. The significant heterogeneity among various RCTs did not allow an effective comparison of the performance of biologic agents in each study.
Keywords: Rheumatoid arthritis, biologic therapy, radiographic progression, metaanalysis
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory disease of the joints. The definition of RA usually describes a symmetrical, persistent and destructive polyarthritis often associated with positive results for rheumatoid factor and/or anticyclic citrullinated peptide antibodies (ACPAs).
RA is a very heterogeneous disease, the outcome of which is difficult to predict. Some RA patients do not develop any erosion even after long-term disease, but the vast majority will have bone erosions and cartilage breakdown resulting in joint destruction, functional impairment and increased mortality. The outcome of the disease has improved considerably in recent years with the availability of very effective therapies. In addition, the recognition that early and intensive treatment strategies result in better outcomes has been highlighted by recent guidelines focused on the management of early arthritis [Smolen et al. 2010]. New disease-modifying antirheumatic drugs (DMARDs) and DMARD combinations have shown their ability to slow disease progression [Landewé et al. 2002; Korpela et al. 2004]. Furthermore, biological therapies have demonstrated rapid and sustained disease control, which is associated with impressive prevention of joint destruction [Goekoop-Ruiterman et al. 2005]. Several biological agents have now been approved by regulatory authorities in many countries for the treatment of patients with RA. These biologics target various immune cells or cytokines that play a key role in local and systemic inflammation. Some biologics target tumour necrosis factor (TNF)-alpha in the joint lining, bone and other tissues; while others target T cells, B cells and interleukin (IL). Anti-TNF biologics include both soluble receptors that serve as decoy receptors competing with TNF receptors (etanercept) and monoclonal antibodies targeting the TNF receptors (infliximab, adalimumab, golimumab and certolizumab pegol). Rituximab is a monoclonal antibody against CD20, which is found primarily on B cells. Abatacept is a fusion protein against CTLA-4, inhibiting costimulation of T cells. Owing to the diverse mechanisms of action of these biologics, it is conceivable to expect a different impact on RA damage progression. All of these agents have been tested versus methotrexate (MTX) for efficacy on disease progression prevention in several randomized clinical trials (RCTs).
Direct head-to-head comparisons of these agents would be useful to inform decision making in the context of clinical management of patients or in drug formulary development, but these comparisons have not been conducted yet.
The purpose of this investigation is to analyse data on RA progression coming from main RCTs one by one and to perform an indirect comparison.
Methods
Literature search and study selection
A systematic review of literature was conducted using MEDLINE, EMBASE and the Cochrane Library (from 1988 to September 2011), conformed to standard reporting guidelines [Irwig et al. 1994].
Clinical study reports, published systematic reviews and health technology assessments (1988–2011), Internet sites for the US Food and Drug Administration, ClinicalTrials.gov and ClinicalStudyResults.org, and abstracts presented at the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) congresses (2004–11) were screened.
MEDLINE was searched using the medical subject headings (MeSH) terms ‘rheumatoid arthritis’ and ‘randomized controlled trial’ (resulting in 866 references) combined with each of the eight included drugs: adalimumab, etanercept, infliximab, certolizumab, golimumab, tocilizumab, abatacept and rituximab (resulting in 20,238 references).
Search limits were set up in MEDLINE to limit the studies to the date ranges indicated above, English language and humans. A similar search of EMBASE and the Cochrane Library did not disclose further studies. The last search was conducted on 30 September 2011.
Study quality assessment
Two investigators independently reviewed the titles and abstracts from the initial literature applying the predefined inclusion criteria in a hierarchical manner.
First, only double-blind RCTs that compared adalimumab, etanercept, infliximab, certolizumab, golimumab, tocilizumab, abatacept and rituximab with any other agent, including placebo or alternative doses of the agent, in adult patients with RA, were included. Second, only trials that were published in a peer-reviewed medical journal, available as a complete study report (for studies that had completed enrolment), or abstracts with primary endpoints that had been presented at ACR or EULAR congresses were included. Third, only RCTs with evaluation of radiographic progression as a primary or secondary endpoint were considered. Last, only trials with at least 24 weeks of follow up and at least 50 patients were included. Studies with a nonrandomized trial design (e.g. open-label studies, observational studies, case reports, noncomparative studies, systematic reviews or health technology assessments), preclinical (animal) or phase I studies, or studies designed to evaluate patients with conditions other than RA (e.g. Crohn’s disease, ulcerative colitis, juvenile RA, ankylosing spondylitis or psoriatic arthritis) were excluded. Studies that pooled patients from different disease cohorts were also excluded. All publications identified as potentially relevant by at least one reviewer were retrieved. The reviewers discussed publications that were considered to be potentially relevant and came to a consensus on inclusion based on the inclusion criteria.
Data extraction
One reviewer examined all publications for duplication of study populations. After removing duplicates, the study characteristics, including study design, baseline demographics, patient enrolment dates, clinical characteristics and relevant clinical outcomes, were recorded for all studies. Particularly, data on baseline and mean change from baseline of radiographic score (irrespective of scoring system) were collected.
Publications were also identified as studies having either methotrexate-experienced or methotrexate-naïve populations.
Data statistical analysis
In RCTs on biologic agents, the radiographic progression is assessed by using at least three different scoring methods: Sharp score [Sharp et al. 1971] and its modifications by van der Heijde [van der Heijde, 2000] and Genant [Genant, 1983]. All of these three total methods involve separate scores for erosions and joint space narrowing assessed in different sites and are scored as a continuous quantitative scale of more than 200 units (398, 292 and 448 for Sharp, van der Heijde–Sharp and Genant–Sharp, respectively).
The analysed outcome was the difference in the percentage of the annual radiographic progression rate (PARPR) between two randomized groups. The radiographic score (irrespective of scoring system) was standardized by calculating the score as the percentage of the maximum score according to the formula: score percentage = (score/maximum possible score) × 100. The score change percentage was calculated as follows: end study score × 100/maximum possible score – baseline study score × 100/maximum possible score.
Since the primary outcome consisted of continuous data, the mean difference was used as the effect measure. In articles where the median instead the mean was used to describe the radiographic score, this median value was used as substitute for the mean value. If standard deviation was not given, it could be calculated from a 95% confidence interval (CI), a standard error of the mean, a p-value and a T table, or from data estimated from a figure [Elbourne et al. 2002]
Heterogeneity between studies was estimated by I2 test. In cases of homogeneity, a fixed-effects model was used, and in cases of heterogeneity, a random-effects model was used [Normand, 1999].
For each trial, the effect was plotted according to its standard error in a funnel plot. The possibility of publication bias was assessed by evaluating the funnel plot for asymmetry, which can result from nonpublication of small trials that yielded negative results, from differences in trial quality or from true study heterogeneity.
Results
Study selection
The first identified paper was from 2000, and the last was from 2011. The trial flow (Figure 1) was as follows: from the MEDLINE MeSH-term search of 187 MEDLINE references, 44 potentially relevant trials were selected. Of these 44 trials, 24 were excluded because radiographic outcome was not estimated. The 20 that estimated radiographic outcome were evaluated in detail. One of those was excluded because it was not specially designed to evaluate radiographic progression in biologic- versus nonbiologic-treated patients, two were excluded because radiographic data were insufficient to calculate a radiographic progression rate and five were excluded because they were extension studies of otherwise included studies.
Figure 1.
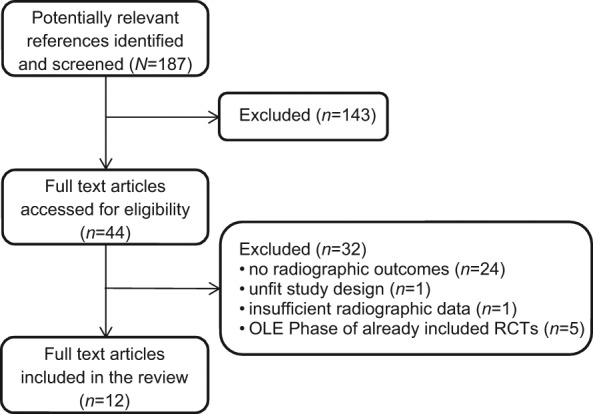
Flow chart of the study selection process.
Study characteristics
In order to optimize study characteristics matching and improve the RCTs comparison, we stratified the 12 RCTs in two different groups: 5 studies [Breedveld et al. 2005; Genovese et al. 2002; St Clair et al. 2004; Emery et al. 2010, 2011]) for the early RA group (patients who were methotrexate naïve and/or with disease duration <3 years) and 7 studies [Lipsky et al. 2000; Klareskog et al. 2004; Keystone et al. 2004, 2008; Kremer et al. 2006, 2011; Emery et al. 2011]) for late RA group (patients who were methotrexate insufficient responders and/or with disease duration ≥3 years).
In the early RA group the main patient characteristics were similar across the five selected trials: most patients were women (68–84.3%), with a mean age at baseline between 44.7 and 52.1 years, a mean disease duration between 0.59 and 1 year (with the exception of GO-BEFORE trial, 2.9–3.5 years) and rheumatoid factor positivity in 71.4–88% of patients (Table 1). Disease activity (DAS28, swollen and tender joint count, CRP) and disability (HAQ-DI) measures were significantly lower in the ADJUST trial (because of different patient selection criteria), whereas they were similar across the remaining four studies.
Table 1.
Baseline characteristics of population in early RA group studies.
| ERA [Genovese et al. 2002] | PREMIER [Breedveld et al. 2005] | ASPIRE [St Clair et al. 2004] | GO-BEFORE [Emery et al. 2011] | ADJUST [Emery et al. 2010] | |
|---|---|---|---|---|---|
| Biologic agent | Etanercept | Adalimumab | Infliximab | Golimumab | Abatacept |
| Demographics | |||||
| Mean age (years) | 49–51 | 51.9–52.1 | 50–51 | 48.6–50.9 | 44.7–44.8 |
| Mean RA duration (years) | 1 | 0.7–0.8 | 0.8–0.9 | 2.9–3.5 | 0.59–0.73 |
| Female (%) | 74.5 | 75 | 68–75 | 84.3 | 71.4 |
| RF positive (%) | 88 | N.I. | 72 | N.I. | 71.4–85.7 |
| Disease activity measures | |||||
| Mean CRP (mg/dl) | 3.5 | 4 | 2.6-3 | 2.5 | 1.07–1.12 |
| Mean DAS28 | N.I. | 6.35 | 6.7 | 6.3 | 3.6 |
| Mean TJC (68 joint) | 30–31 | 30.7–32.3 | 32–34 | 27.3–29.2 | N.I. |
| Mean SJC (66 joint) | 24 | 21.1–22.1 | 21–22 | 14.9–16 | N.I. |
| Mean HAQ | N.I. | 1.5–1.6 | 1.5 | 1.5 | 0.8 |
| Treatments | |||||
| Previous DMARDs (%) | 43 | 32.5 | 32 | 51.1 | 0 |
| Previous MTX (%) | 0 | 0 | 5.8 | 0 | 0 |
| Radiographics | |||||
| Scoring method | Sharp | vdH–Sharp | vdH–Sharp | vdH–Sharp | Genant–Sharp |
| Baseline score | 2.4–12.9 | 18.1–21.9 | 11.3–11.6 | 18.7–19.7 | 3.3–4 |
| Annual estimated progression | 2.4–12.9 | 25.8–27.3 | 12.5–14.5 | 5.3–6.7 | 4.5–6.7 |
RA, rheumatoid arthritis; RF, rheumatoid factor; CRP, C-reactive protein; DAS28, Disease Activity Score 28; TJC, tender joints count; SJC, swollen joints count; HAQ, Health Assessment Questionnaire; DMARD, disease-modifying antirheumatic drug; MTX, methotrexate, N.I., not indicated; vdH, van der Heijde.
The standardized baseline radiographic score value significantly differs in various trials, ranging between 2.7 (ERA trial) and 21.9 (PREMIER trial). Similarly, based on the standardized annual estimated progression, it is possible to discriminate between population of very slow progressors (2.7/year, ERA trial) and population of very rapid progressors (27.35/year, PREMIER trial).
The main population characteristics of the 7 RCTs in the late RA group are reported in Table 2: most patients were women (74.6–85.4%), with a mean age at baseline ranging from 49 to 56.1 years, a mean duration of the disease between 5.6 and 11 years and rheumatoid factor positivity in 73–85.5% of patients. Disease activity measures did not significantly differ across the studies, nor did HAQ-DI score.
Table 2.
Baseline characteristics of population in late RA group studies
| ATTRACT [Lipsky et al. 2000] | TEMPO [Klareskog et al. 2004] | RAPID 1 [Keystone et al. 2008] | GO-FORWARD [Emery et al. 2011] | AIM [Kremer et al. 2006] | KEYSTONE [Keystone et al. 2004] | LITHE [Kremer et al. 2011] | |
|---|---|---|---|---|---|---|---|
| Biologic agent | Infliximab | Etanercept | Certolizumab | Golimumab | Abatacept | Adalimumab | Tocilizumab |
| Demographics | |||||||
| Mean age (years) | 51–54 | 52.5–53 | 51.5–52.2 | 52 | 49–49.4 | 56.1 | 51.3–53.4 |
| Mean RA duration (years) | 10–11 | 6.3–6.8 | 5.6–6.1 | 4.5–6.5 | 7.9–8.4 | 10.9–11 | 9–9.3 |
| Female (%) | 80.5 | 76.5 | 83.9 | 81.5 | 85.4 | 74.6 | 82.5 |
| RF positive (%) | 80.5 | 73 | 75.8 | 83.3 | 82.1 | 85.5 | 82.5 |
| Disease activity measures | |||||||
| Mean CRP (mg/dl) | 3.9–4 | 2.5–3.2 | 1.3–1.4 | 0.8–1 | 2.7–3.1 | 1.8 | 2.2 |
| Mean DAS28 | N.I. | 6.87 | 6.84 | 6.11 | 6.85 | N.I. | 6.55 |
| Mean TJC (68 joint) | 31–32 | 33.1–35 | 30.1–30.4 | 21–26 | 30.3–31.6 | 27.3–28.1 | 27.9–29.3 |
| Mean SJC (66 joint) | 21–22 | 22.1–23 | 20.5–21.9 | 12–13 | 20.1–21.3 | 19–19.3 | 16.6-17.3 |
| Mean HAQ | 1.7–1.8 | 1.7–1.8 | 1.6 | 1.25–1.375 | 1.8 | 1.45–1.48 | 1.5 |
| Treatments | |||||||
| Number of previous DMARDs | N.I. | 2.3 (no MTX) | 1.2 | N.I. | N.I. | 2.4 | 1.6 |
| Mean MTX dose (mg/week) | 16 | 16.9–17.2 | 12.2–12.5 | 15 | 16.5 | N.I. | 15.2 |
| Radiographic | |||||||
| Scoring method | vdH–Sharp | vdH–Sharp | vdH–Sharp | vdH–Sharp | Genant–Sharp | Sharp | Genant–Sharp |
| Baseline score | 79–82 | 21.8–26.8 | 36.24 | 35.85 | 44.7 | 66.4–72.1 | 28.5-28.8 |
| Annual estimated progression | 7.07 | 8.4–11 | 5.88 | 5.37 | 5.45 | 6.09–6.55 | 3.13 |
RA, rheumatoid arthritis; RF, rheumatoid factor; CRP, C-reactive protein; DAS28, Disease Activity Score 28; TJC, tender joints count; SJC, swollen joints count; HAQ, Health Assessment Questionnaire; DMARD, disease-modifying antirheumatic drug; MTX, methotrexate, N.I., not indicated; vdH, van der Heijde.
Once again, the standardized baseline radiographic score value significantly differs in various trials, ranging between 23.46 (TEMPO trial) and 75 (ATTRACT study). However, the standardized annual estimated progression, ranging between 2.40 and 7.07, is more similar across these 7 trials than that in the early RA group.
Meta-analysis of the all RCTs and risk of bias across studies
Analysis consisted of meta-analysis of all 12 main RCTs. The results are shown in Table 3 and Figure 2.
Table 3.
Study summaries for the different effects of biologic and placebo on standardized annual estimated disease progression in five early RA main RCTs.
| RCTs | Favours biologic + MTX |
Favours MTX |
Mean Difference |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Sample size | Mean | SD | Sample size | (Random, 95% CI) | |
| ERA | 6.9 | 10.1 | 177 | 16.1 | 9.86 | 169 | −9.20 [−11.30 to −7.10] |
| ADJUST | 0.24 | 7.43 | 28 | 26.18 | 9.23 | 28 | −25.94 [−30.33 to −21.55] |
| GO-BEFORE | 6.07 | 14.97 | 159 | 11.23 | 13.06 | 160 | −5.16 [−8.24 to −2.08] |
| ASPIRE | 3.03 | 15.96 | 359 | 28.03 | 26.42 | 282 | −25.00 [−28.50 to −21.50] |
| PREMIER | 4.89 | 29.55 | 268 | 21.46 | 29.55 | 257 | −16.57 [−21.63 to −11.51] |
| Total 95% CI | −16.28 [−24.42 to −8.14] | ||||||
SD, standard deviation; MTX, methotrexate; CI, confidence interval.
Figure 2.
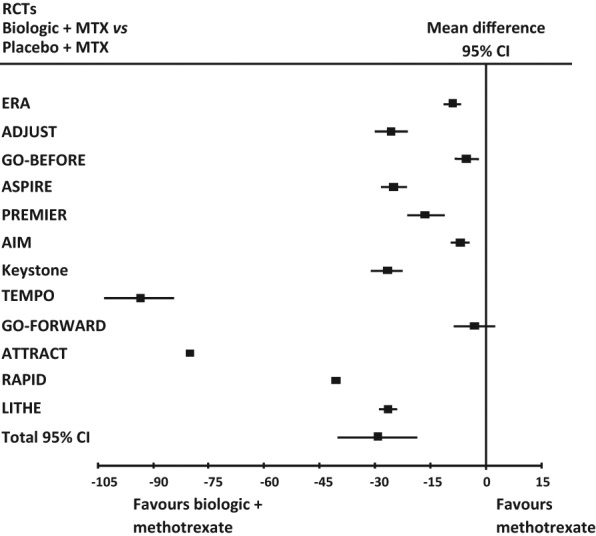
Forest plot of the findings of the meta-analysis of studies of biologic agents plus methotrexate (MTX) versus methotrexate alone in all RA main RCTs. 95% CI = 95% confidence interval.
Strong evidence of heterogeneity (I2 = 99%, p < 0.00001) was observed. A funnel plot of these 12 studies showed asymmetry of 2 studies (TEMPO trial and ATTRACT trial). When these were excluded, there was still wide heterogeneity (I2 = 100%, p < 0.00001), but not asymmetry of the funnel plot. When these two studies were not considered the total mean difference was −18.69 (95% CI −29.94 to −7.44).
To deeply evaluate the heterogeneity of these main RCTs we stratified the RCTs in two different groups: early and late RA studies.
Meta-analysis of the two groups
Group of early RA, comparing biologic agents plus MTX versus MTX alone
Analysis consisted of meta-analysis of five main RCTs in early RA patients. Strong evidence of heterogeneity (I2 = 97%, p = 0.00001) but no asymmetry of the funnel plot was observed in the analysis of all studies in this group and then a random model was applied. Total mean difference was −16.28 (95% CI −24.42 to −8.14). The results are shown in Table 4 and Figure 3.
Table 4.
Study summaries for the different effect of biologic and placebo on standardized annual estimated disease progression in seven late RA main RCTs.
| RCTs | Favours biologic + MTX |
Favours MTX |
Mean Difference |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Sample size | Mean | SD | Sample size | (Random, 95% CI) | |
| AIM | 6.62 | 9.28 | 391 | 13.49 | 15.84 | 195 | −6.87 [−9.28 to −4.46] |
| Keystone | 11.41 | 18.51 | 207 | 38.52 | 25.68 | 200 | −27.11 [−31.47 to −22.75] |
| TEMPO | −15.3 | 18.64 | 218 | 79.32 | 67.81 | 212 | −94.62 [−104.08 to −85.16] |
| GO-FORWARD | 17.32 | 20.97 | 89 | 20.48 | 20.2 | 133 | −3.16 [−8.71 to 2.39] |
| ATTRACT | 18.39 | 22.57 | 71 | 99.01 | 38.74 | 64 | −80.62 [−91.47 to −69.77] |
| RAPID I | 6.8 | 2.47 | 393 | 47.62 | 4.95 | 199 | −40.82 [−41.55 to −40.09] |
| LITHE | 9.27 | 16.95 | 390 | 36.1 | 16.95 | 390 | −26.83 [−29.21 to −24.45] |
| Total 95% CI | −39.25 [−53.77 to −24.73] | ||||||
SD, standard deviation; MTX, methotrexate; CI, confidence interval.
Figure 3.
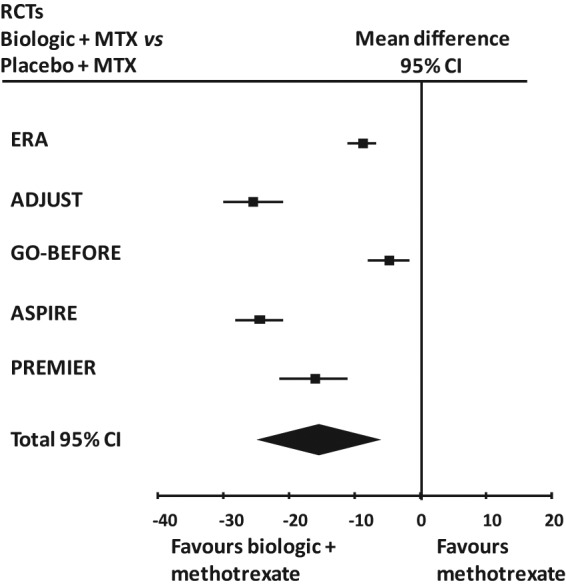
Forest plot of the findings of the meta-analysis of studies of biologic agents plus methotrexate (MTX) versus methotrexate alone in early RA main RCTs. 95% CI = 95% confidence interval.
Group of late RA, comparing biologic agents plus MTX versus MTX alone
Analysis consisted of meta-analysis of seven main RCTs on late RA patients. A random model was used (I2 = 99%, p = 0.00001) and a total mean difference of −39.25 (95% CI −53.77 to −24.73) was found. The results are shown in Table 5 and Figure 4.
Table 5.
Study summaries for the different effect of biologic and placebo on standardized annual estimated disease progression in seven late RA main RCTs.
| RCTs | Favours biologic + MTX |
Favours MTX |
Mean Difference |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Sample size | Mean | SD | Sample size | (Random, 95% CI) | |
| AIM | 6.62 | 9.28 | 391 | 13.49 | 15.84 | 195 | −6.87 [−9.28 to −4.46] |
| Keystone | 11.41 | 18.51 | 207 | 38.52 | 25.68 | 200 | −27.11 [−31.47 to −22.75] |
| TEMPO | -15.3 | 18.64 | 218 | 79.32 | 67.81 | 212 | −94.62 [−104.08 to −85.16] |
| GO-FORWARD | 17.32 | 20.97 | 89 | 20.48 | 20.2 | 133 | −3.16 [−8.71 to 2.39] |
| ATTRACT | 18.39 | 22.57 | 71 | 99.01 | 38.74 | 64 | −80.62 [−91.47 to −69.77] |
| RAPID I | 6.8 | 2.47 | 393 | 47.62 | 4.95 | 199 | −40.82 [−41.55 to −40.09] |
| LITHE | 9.27 | 16.95 | 390 | 36.1 | 16.95 | 390 | −26.83 [−29.21 to −24.45] |
| Total 95% CI | −39.25 [−53.77 to −24.73] | ||||||
SD, standard deviation; MTX, methotrexate; CI, confidence interval.
Figure 4.
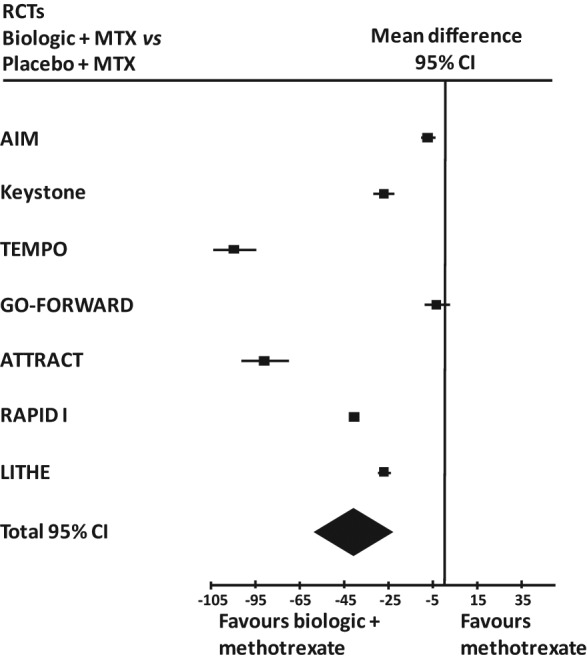
Forest plot of the findings of the meta-analysis of studies of biologic agents plus methotrexate (MTX) versus methotrexate alone in late RA main RCTs. 95% CI = 95% confidence interval.
A funnel plot showed a slight asymmetry which can be avoided by eliminating the two above studies (TEMPO trial and ATTRACT trial). In this last case the total mean difference was −21.03 (95% CI −36.79 to −5.28) keeping a very wide heterogeneity (I2 = 100%, p < 0.00001).
Supplementary analysis on possible influence of the scoring method and the time between radiographic assessment were not investigated. In fact, in all 12 RCTs the outcome was expressed as the difference in the percentage of the annual radiographic progression rate between two randomized groups and radiographic progression was assessed at 52 weeks.
Discussion
The multiplicity of biologic agents currently available provides a strong rationale for comparing the efficacy of these agents on damage progression in order to help rheumatologists to make reasonable therapeutic choices in the management of RA. In the absence of direct comparisons between different biologicals, the aim of this study was to indirectly compare the effects at 12 months in slowing radiographic progression in the contest of RCTs.
The first issue in comparing data coming from different studies concerns the scoring method used. Three methods have been accepted by regulatory agencies as valid and have been used successfully in RCTs to gain regulatory approval of structure-modifying biological therapies: the Sharp score and its two modifications by van der Heijde and Genant. These three measures differ primarily in the scales used to grade erosions, but there are also minor differences in their joint space narrowing scales and the locations scored in the hands and wrists. The relative performance of the Genant–Sharp and van der Heijde–Sharp methods has been compared directly in the same cohort of patients in a study by Peterfy and colleagues [Peterfy et al. 2011] who demonstrated relatively similar performances for scoring erosion and joint space narrowing in the hands, wrists and feet. Anyway, the comparison requires a score standardization because, given the previously mentioned scale differences, direct comparisons of scores generated by different methods are not meaningful. At least three different methods for standardizing radiographic scores have been reported in the literature. The first is the calculation of the standardized mean difference, based on the number of standard deviations [Sharp et al. 2004]. The second alternative method is to produce conversion reference tables by using regression equations [Lassere et al. 2001]. The third, given that different methods strongly correlate each other, is the simple calculation of standardized score as a percentage of the maximum possible score achievable by that method [Lassere, 2000]. The last may be very useful in the case of data coming uniquely from published papers, reported as mean plus standard deviation or confidence interval of baseline score or change from baseline score. For this reason, we chose this method for the comparison approach in this study.
Another main issue is the different rate of damage progression in various RCTs, because each RCT enrolled a unique patient population that differed significantly across trials in terms of demographics and baseline disease characteristics. Given these differences in protocol populations, it is not appropriate to directly compare changes in total composite scores across studies. However, it is possible to compare the data using an estimated annual progression of radiographic damage, based on previous progression and assuming that patients remained on their previous treatment regimen or remained untreated. The estimated annual progression can be used as a benchmark and it is possible to measure in each RCT the effect of treatments and placebo on damage progression as the difference in the PARPR.
The first finding of the present study is the favourable effect of biologic agents used in combination with methotrexate versus methotrexate monotherapy in both early (95% CI −24.42 to −8.14) and late RA group (95% CI −53.77 to −24.73). This is an expected result since in all 12 considered RCTs a statistically significant difference between treatment and placebo groups was found.
The impact of treatments reported as mean difference in the PARPR surprisingly appears to be deeper in the late (−39.25) than in the early (−16.28) RA group. Radiographic progression in RA can follow a linear or a sigmoid curve, but is more rapid during the first 2 years of the disease, with most of the damage occurring within 5 years [Courvoisier et al. 2008]. However, early RA showed a better responsiveness to all disease-modified therapies, biological or synthetic (especially MTX, used as comparator in the placebo group in all 12 considered RCTs). Since population in late RA studies was enrolled based on irresponsiveness to MTX, it is reasonable to expect a significantly more important effect of MTX on damage progression in the placebo groups in the early than late RA RCTs. For these reasons, the mean difference in PARPR between MTX and biologic agents is higher in late than in early RA studies because of the deeper impact of MTX (and not biologic therapy) on early than late RA damage progression. Moreover, estimated progression rates are limited by errors in dates of disease onset and are less valid in patients with short disease durations (<1 year) because of the amplification effect of dividing by fractions.
The third analysed item was the comparison between one biologic agent and another. Given the data in the forest plot analysis, the effect of various biologic agents seems to be different in both early and late RA groups. In particular, in the early RA group the performance of abatacept in the ADJUST trial and infliximab in the ASPIRE study seems to be better than that of etanercept in ERA trial and golimumab in GO-BEFORE trial. Similarly, in the late RA group etanercept in the TEMPO trial and infliximab in the ATTRACT study seem to be more effective in slowing damage progression than all five of the other agents considered. However, this comparison is impaired by an important limitation. In order to minimize the impact of different inclusion and exclusion criteria, baseline sample characteristics were analysed and two different groups of studies were identified according to mean disease duration and responsiveness to methotrexate. Using these criteria, matching in both groups appears to be acceptable with the only exception of ADJUST trial, whose population of undifferentiated arthritis significantly differs from those of other early RA trials. Nevertheless, statistical analysis showed a significant evidence of heterogeneity (I2 was 97% and 99% in early and late RA group, respectively) and it is impossible to state the real weight of several confounders related to inclusion and exclusion criteria of each study. In conclusion, due to various biases, it was deemed inappropriate to rank the individual biologic agents. Consequently, we do not believe that the necessary evidence exists to judge which individual treatment is to be preferred.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Ennio Giulio Favalli, Division of Rheumatology, University of Milan, Via G. Pini, 9 20122 Milan, Italy.
Francesca Pregnolato, Experimental Laboratory of Immunological and Rheumatologic Researches, IRCCS Istituto Auxologico Italiano, Milan, Italy.
Martina Biggioggero, Division of Rheumatology, Department of Internal Medicine, University of Milan, Istituto Ortopedico G. Pini, Milan, Italy.
Pier Luigi Meroni, Division of Rheumatology, Department of Internal Medicine, University of Milan, Istituto Ortopedico G. Pini, Milan, Italy.
References
- Breedveld F.C., Weisman M.H., Kavanaugh A.F., Cohen S.B., Pavelka K., Vollenhoven R.V., et al. for the G.T.PREMIER Investigators (2005) The PREMIER study: A multicenter, randomised, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 54: 26–37 [DOI] [PubMed] [Google Scholar]
- Courvoisier N., Dougados M., Cantagrel A., Goupille P., Meyer O., Sibilia J., et al. (2008) Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther 10(5): R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne D.R., Altman D.G., Higgins J.P.T., Curtin F., Worthington H.V., Vail A. (2002) Meta-analyses involving cross-over trials: methodological issues. Intern J Epidemiol 31: 140–149 [DOI] [PubMed] [Google Scholar]
- Emery P., Durez P., Dougados M., Legerton C.W., Becker J.-C., Vratsanos G., et al. (2010) Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann Rheum Dis 69: 510–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Fleischmann R., van der Heijde D., Keystone E.C., Genovese M.C., Conaghan P.G., et al. (2011) The effects of golimumab on radiographic progression in rheumatoid arthritis: results of randomised controlled studies of golimumab before methotrexate therapy and golimumab after methotrexate therapy. Arthritis Rheum 63: 1200–1210 [DOI] [PubMed] [Google Scholar]
- Genant H.K. (1983) Methods of assessing radiographic change in rheumatoid arthritis. Am J Med 75(6A): 35–47 [DOI] [PubMed] [Google Scholar]
- Genovese M.C., Bathon J.M., Martin R.W., Fleischmann R.M., Tesser J.R., Schiff M.H., et al. (2002) Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum 46: 1443–1450 [DOI] [PubMed] [Google Scholar]
- Goekoop-Ruiterman Y.P.M., de Vries-Bouwstra J.K., Allaart C.F., van Zeben D., Kerstens P.J.S.M., Hazes J.M.W., et al. (2005) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomised, controlled trial. Arthritis Rheum 52: 3381–3390 [DOI] [PubMed] [Google Scholar]
- Irwig L., Tosteson A.N., Gatsonis C., Lau J., Colditz G., Chalmers T.C., et al. (1994) Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med 120: 667–676 [DOI] [PubMed] [Google Scholar]
- Keystone E., Heijde D.V.D., Mason D., Jr, Landewé R., Vollenhoven R.V., Combe B., et al. (2008) Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: Findings of a fifty-two-week, phase III, multicenter, randomised, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 58: 3319–3329 [DOI] [PubMed] [Google Scholar]
- Keystone E.C., Kavanaugh A.F., Sharp J.T., Tannenbaum H., Hua Y., Teoh L.S., et al. (2004) Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: A randomised, placebo-controlled, 52-week trial. Arthritis Rheum 50: 1400–1411 [DOI] [PubMed] [Google Scholar]
- Klareskog L., van der Heijde D., de Jager J.P., Gough A., Kalden J., Malaise M., et al. , for the M.TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators (2004) Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363: 675–681 [DOI] [PubMed] [Google Scholar]
- Korpela M., Laasonen L., Hannonen P., Kautiainen H., Leirisalo-Repo M., Hakala M., et al. for the T.FIN-RACo Trial Group (2004) Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: Five-year experience from the FIN-RACo study. Arthritis Rheum 50: 2072–2081 [DOI] [PubMed] [Google Scholar]
- Kremer J.M., Genant H.K., Moreland L.W., Russell A.S., Emery P., Abud-Mendoza C., et al. (2006) Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomised trial. Ann Intern Med 144: 865–876 [DOI] [PubMed] [Google Scholar]
- Kremer J.M., Russell A.S., Emery P., Abud-Mendoza C., Szechinski J., Westhovens R., et al. (2011) Long-term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3-year results from the AIM trial. Ann Rheum Dis 70: 1826–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landewé R., Boers M., Verhoeven A.C., Westhovens R., van de, Laar M.A.F.J., Markusse H.M., et al. (2002) COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 46: 347–356 [DOI] [PubMed] [Google Scholar]
- Lassere M. (2000) Pooled metaanalysis of radiographic progression: comparison of Sharp and Larsen methods. J Rheumatol 27: 269–275; discussion 276 [PubMed] [Google Scholar]
- Lassere M.N., van der Heijde D., Johnson K., Bruynesteyn K., Molenaar E., Boonen A., et al. (2001) Robustness and generalizability of smallest detectable difference in radiological progression. J Rheumatol 28: 911–913 [PubMed] [Google Scholar]
- Lipsky P.E., van der Heijde D.M., Clair E.W.S., Furst D.E., Breedveld F.C., Kalden J.R., et al. for the Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group (2000) Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med 343: 1594–1602 [DOI] [PubMed] [Google Scholar]
- Normand S.L. (1999) Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med 18: 321–359 [DOI] [PubMed] [Google Scholar]
- Peterfy C.G., Wu C., Szechinski J., DiCarlo J.C., Lu Y., Genovese M., et al. (2011) Comparison of the Genant-modified Sharp and van der Heijde-modified Sharp scoring methods for radiographic assessment in rheumatoid arthritis. Intern J Clin Rheumatol 6: 15–24 [Google Scholar]
- Sharp J.T., Lidsky M.D., Collins L.C., Moreland J. (1971) Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum 14: 706–720 [DOI] [PubMed] [Google Scholar]
- Sharp J.T., Wolfe F., Lassere M., Boers M., van der Heijde D., Larsen A., et al. (2004) Variability of precision in scoring radiographic abnormalities in rheumatoid arthritis by experienced readers. J Rheumatol 31: 1062–1072 [PubMed] [Google Scholar]
- Smolen J.S., Landewe R., Breedveld F.C., Dougados M., Emery P., Gaujoux-Viala C., et al. (2010) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 69: 964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair E.W., van der Heijde D.S.E.M.F.M., Smolen J.S., Maini R.N., Bathon J.M., Emery P., et al. for the Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset Study Group (2004) Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: A randomised, controlled trial. Arthritis Rheum 50: 3432–3443 [DOI] [PubMed] [Google Scholar]
- van der Heijde D. (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27: 261–263 [PubMed] [Google Scholar]


