Abstract
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of cells that negatively regulate the immune response during tumour progression, inflammation and infection. Only limited data are available on human MDSC because of the lack of specific markers. We have identified members of the S100 protein family – S100A8, S100A9 and S100A12 – specifically expressed in CD14+ HLA-DR−/low MDSC. S100A9 staining in combination with anti-CD14 could be used to identify MDSC in whole blood from patients with colon cancer. An increase in the population of CD14+ S100A9high MDSC was observed in the peripheral blood from colon cancer patients in comparison with healthy controls. Finally, nitric oxide synthase expression, a hallmark of MDSC, was induced in CD14+ S100A9high upon lipopolysaccharide/interferon-γ stimulation. We propose S100 proteins as useful markers for the analysis and further characterization of human MDSC.
Keywords: cancer, flow cytometry/FACS, immune response, tolerance, tumour immunology
Introduction
Myeloid-derived suppressor cells (MDSC) have been characterized as a population of cells that can negatively regulate T-cell function. They are a heterogeneous population of myeloid origin including macrophages, granulocytes and other cells, which suppress immune responses in vivo as well as in vitro. An increase in the frequency of MDSC in the peripheral blood of patients with different types of cancers has been demonstrated.1,2 Murine MDSC are characterized by co-expression of Gr-1 and CD11b, and can be further subdivided into two major groups: CD11b+ Gr-1high granulocytic MDSC (which can also be identified as CD11b+ Ly-6G+ Ly6Clow MDSC) and CD11b+ Gr-1low monocytic MDSC (which can also be identified as CD11b+ Ly-6G− Ly6Chigh MDSC). We have previously identified CD49d as another marker to distinguish these two murine cell populations from each other.3 We could demonstrate that CD11b+ CD49d+ monocytic MDSC were more potent suppressors of antigen-specific T cells in vitro than CD11b+ CD49d− granulocytic MDSC.
S100A9 has recently been reported to be essential for MDSC accumulation in tumour-bearing mice. It was also shown that S100A9 inhibits dendritic cell differentiation by up-regulation of reactive oxygen species. Finally, no increase in the frequency of MDSC was observed in S100A9 knockout mice, which also showed strong anti-tumour immune responses and rejection of implanted tumours,4 indicating the relevance of S100A9+ MDSC in tumour settings.
In contrast to murine MDSC, human MDSC are not so clearly defined because of the lack of specific markers. Human MDSC have been shown to be CD11b+, CD33+ and HLA-DR−/low. In addition, interleukin-4 receptor α, vascular endothelial growth factor receptor, CD15 and CD66b have been suggested as more specific markers for human MDSC. However, these markers can only be found on some MDSC subsets.5 It has been suggested that monocytic MDSC are CD14+2,6 and granulocytic MDSC express CD15,7,8 whereas both groups of MDSC are HLA-DR−/low and CD33+. The heterogeneous expression of these markers suggests that multiple subsets of human MDSC can exist.
We have previously shown direct ex vivo isolation of a new subset of MDSC that are significantly increased in the peripheral blood and tumours of patients with hepatocellular carcinoma. These cells express CD14, have low or no expression of HLA-DR and have high arginase activity. CD14+ HLA-DR−/low cells not only suppress the proliferation of and interferon-γ secretion by autologous T cells, but also induce CD25+ Foxp3+ regulatory T cells that are suppressive in vitro.9 Others have been able to detect CD14+ cells with suppressor activity in the peripheral blood from patients with other malignancies such as melanoma, colon cancer and head and neck cancer.8,10 We have been able to demonstrate their suppressor activity in patients with colon cancer (data not shown). Although many studies have shown the presence of human MDSC in different pathological conditions, understanding their biology in human cancer requires further characterization of these cells.
In this study, we searched for specific markers present on human MDSC by performing a differential display analysis on CD14+ HLA-DR+ monocytes and CD14+ HLA-DR−/low MDSC. S100A12 was expressed more strongly in CD14+ HLA-DR−/low MDSC than in CD14+ HLA-DR+ monocytes. Based on these results we analysed the expression of S100A8, S100A9 and S100A12 in CD14+ HLA-DR−/low MDSC in both whole blood and peripheral blood mononuclear cells (PBMC) from healthy volunteers and patients with cancer. We demonstrated that the frequency of S100A9 MDSC correlated with the frequency of CD14+ HLA-DR−/low MDSC and we found an increase in the frequency of CD14+ S100A9high MDSC in the peripheral blood from patients with cancer. Finally, we demonstrate that CD14+ S100A9high cells expressed high levels of nitric oxide synthase (NOS2), which is one of the proposed mediators of the inhibitory properties of MDSC. We therefore propose S100A9 as an additional useful marker for human MDSC.
Materials and methods
Blood samples
Blood samples were collected from patients with colon cancer and healthy controls. None of the patients were receiving chemotherapy at the time of blood collection. All patients gave written informed consent for research testing under protocols approved by the Institutional Review Board of the National Cancer Institute, National Institutes of Health. Patient information is summarized in Table 1.
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| Total number | 14 |
| Average age, years | 61·4 |
| Male/female | 9/5 |
| Average age, years (male/female) | 61·8/60·8 |
| Stage IV (male/female) | 8/5 |
Cell isolation and sorting
Human PBMC were isolated from freshly obtained blood by Ficoll density gradient centrifugation (Lonza, Walkersville, MD). Whole blood lysate was obtained by lysing whole blood with ACK Lysing Buffer (Quality Biological, Gaithersburg, MD) as the manual indicated. MDSC (CD14+ HLA-DR−/low) and control monocytes (CD14+ HLA-DR+) were sorted from PBMC using BD FACSAria II cell sorter (Becton-Dickinson, Mountain View, CA). The gating strategy is shown in Supplementary material, Fig. S1. CD4, CD8, B cells and dendritic cells were sorted by CD3+ CD4+, CD3+ CD8+, B220+ and CD11c+ (BD Biosciences, San Jose, CA) markers, respectively. The purity of the cells after sorting was > 95%. Granulocytes for the Western blotting were obtained by lysing the red blood cell pellet after the Ficoll density gradient centrifugation with ACK Lysing buffer.
Microarray analysis
The PBMC were isolated as described above. CD14+ HLA-DR−/low and CD14+ HLA-DR+ cells were isolated using CD14-MicroBeads (Miltenyi, Bergisch-Gladbach, Germany) followed by FACS sorting using a BD FACS Aria II cell sorter (Becton-Dickinson). RNA extraction was performed using NucleoSpin RNA II (Macherey-Nagel, Düren, Germany) followed by Linear T7-based amplification of the RNA. Gene expression analysis was performed using a PIQOR Immunology Microarray (Miltenyi). RNA isolation, amplification and Microarray were performed by Miltenyi-Biotec. Microarray data were deposited in the GEO database and the accession number is GSE32001.
Flow cytometry analysis
The following antibodies were used in the FACS staining: CD14-Vioblue (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), HLA-DR-allophycocyanin (BD Biosciences), S100A9-FITC (Biolegend, San Diego, CA), NOS2-phycoerythrin (Santa Cruz Biotechnology, Santa Cruz, CA). Freshly obtained PBMC or whole blood lysate were resuspended in flow cytometry buffer and surface-stained with antibodies for 15 min at 4°C. Samples for intracellular staining were additionally fixed and permeabilized using BD Cytofix/Cytoperm Fixation/Permeabilisation Kit (BD Biosciences) according to the manufacturer’s instructions. FACS acquisition was performed on LSR-II (Becton-Dickinson) and results were analysed using FlowJo software (TreeStar Inc, Ashland, OR).
Determination of mRNA expression by quantitative PCR
RNA was isolated using an RNeasy Micro Kit (Qiagen, Hilden, Germany). Complementary DNA synthesis was carried out with an iScript Kit (Bio-Rad, Munich, Germany) and quantitative PCR was performed using the following primers: S100A12: forward primer 5′-CAC ATT CCT GTG CAT TGA GG-3′, reverse primer 5′-TGC AAG CTC CTT TGT AAG CA-3′; S100A8: forward primer 5′-TGT CTC TTG TCA GCT GTC TTT CA-3′, reverse primer 5′-CCT GTA GAC GGC ATG GAA AT-3′; S100A9: forward primer 5′-GGA ATT CAA AGA GCT GGT GC-3′, reverse primer 5′-TCA GCA TGA TGA ACT CCT CG-3′; cyclophilin A: forward primer 5′-ATG CTC AAC CCC ACC GTG T-3′, reverse primer 5′-TCT GCT GTC TTT GGG ACC TTG TC-3′. Reactions were performed in triplicate using iQ SYBR Green Supermix (Bio-Rad) and normalized to endogenous cyclophilin A mRNA level using the ΔΔCt method.
Western blot analysis
Lysates from FACS sorted CD14+ HLA-DR−/low MDSC and CD14+ HLA-DR+ monocytes were denatured at 95° for 5 min and subjected to SDS–PAGE. The gel was blotted onto nitrocellulose membrane followed by incubation with anti-S100A12 antibody (Abcam, Cambridge, UK) or a control anti-glyceraldehyde 3-phosphate dehydrogenase antibody (Sigma, St Louis, MO). Binding of the antibodies was visualized using horseradish peroxidase-conjugated rabbit anti-mouse IgG (Abcam). Western blot imaging and quantitative analysis were performed using FluorChem HD2 Multiplex Fluorescent Imaging System (Cell Biosciences Inc., Santa Clara, CA).
Statistical analysis
All the statistical analyses were based on two-tailed Student’s t-test. All P-values < 0·05 were considered to be significant.
Results
S100A8/A9/A12 is differentially expressed in CD14+ HLA-DR−/low MDSC and CD14+ HLA-DR+ monocytes
Differential gene expression analysis was performed to identify genes expressed in CD14+ HLA-DR−/low MDSC but not in CD14+ HLA-DR+ monocytes. Using PIQOR Immunology Microarrays (Miltenyi), we found that S100A12 was 40-fold more strongly expressed in MDSC than in monocytes (GEO database accession no. GSE32001). Real time PCR was performed on FACS-sorted MDSC (CD14+ HLA-DR−/low) and monocytes (CD14+ HLA-DR+) from peripheral blood to confirm these results. Higher S100A12 expression was seen in MDSC than in monocytes (Fig. 1a). S100 is a family of proteins including 21 calcium-binding proteins.11 Among them, S100A8, S100A9 and S100A12 are closely related. We focused on these three proteins because monoclonal antibodies for FACS and Western blotting were available for them. First, we analysed the expression of S100A8 and S100A9 genes in the PBMC of healthy donors. Both S100A8 and S100A9 were about 10-fold to 15-fold more expressed in MDSC than in monocytes (Fig. 1a). CD4+ and CD8+ T cells, as well as B cells and dendritic cells, were used as controls and no relevant expression of S100A8, S100A9 or S100A12 was found in these cells. The higher expression of S100 in MDSC was further confirmed by Western blot analysis in which S100A12 expression was seen in MDSC from several healthy donors and in patients with colon cancer but not in monocytes (Fig. 1b–e).
Figure 1.
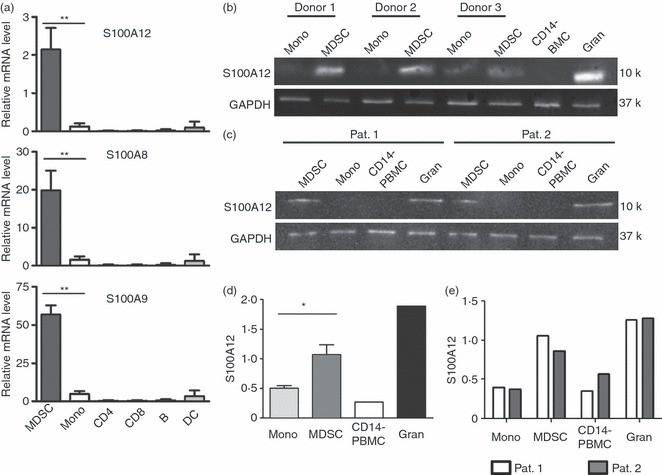
Myeloid-derived suppressor cells (MDSC) express high amounts of S100A8, S100A9 and S100A12. (a) CD14+ HLA-DR−/low MDSC, CD14+ HLA-DR+ monocytes (mono) and control cell populations (CD4+ T cells, CD8+ T cells, B cells and dendritic cells) were sorted from peripheral blood mononuclear cells (PBMC) of healthy donors. Expression level of S100A8, S100A9 and S100A12 are examined by quantitative real-time PCR. Expression is set relative to cyclophilin A mRNA. Cumulative results of four independent experiments are shown (**P < 0·001). (b, c) S100A12 expression was tested by Western blot analysis on 105 sorted MDSC and monocytes (Mono) of healthy donors (b) or colon cancer patients (c). CD14− PBMC were used as negative and granulocytes (Gran) as positive controls, respectively. (d, e) Quantitative bioluminescence analysis for S100A12 expression. S100A12 protein concentration is shown as a ratio of S100A12 to glyceraldehyde 3-phosphate dehydrogenase. The S100 expression in cells derived from healthy donors (n = 3) (d) and colon cancer patients (n = 2) (e) is shown (*P < 0·05).
CD14+ S100A9high cells correspond to CD14+ HLA-DR−/low MDSC
Next, we analysed S100A9 and HLA-DR expression in CD14+ cells in PBMC or whole blood of healthy controls. CD14+ S100A9high and CD14+ S100A9low cells from whole blood and PBMC were analysed for HLA-DR expression. As shown in Fig. 2(a), S100A9 expression was higher in CD14+ HLA-DR−/low MDSC than in CD14+ HLA-DR+ monocytes. Correspondingly, CD14+ S100A9high cells expressed less HLA-DR than CD14+ S100A9low cells (Fig. 2b). Mean fluorescence intensity (MFI) of S100A9 or HLA-DR was also analysed. Both PBMC and whole blood lysate showed higher S100A9 expression in CD14+ HLA-DR−/low MDSC (MFI 573·6 ± 152·5 in whole blood and 1723·6 ± 317·1 in PBMC; P < 0·05) than in CD14+ HLA-DR+ monocytes (MFI 172·8 ± 28·9 in whole blood and 1142·0 ± 201·4 in PBMC; Fig. 2c). This difference was statistically significant when cells were analysed from whole blood. Next, we also compared HLA-DR expression on CD14+ S100A9low and CD14+ S100A9high cells from whole blood. HLA-DR MFI was lower on CD14+ S100A9high than on CD14+ S100A9low cells (MFI 187·5 ± 15·8 versus 594·7 ± 101·9; P < 0·001). Similar results were seen when HLA-DR expression was tested on CD14+ S100A9high or CD14+ S100A9low PBMC (203·0 ± 29·1 versus 423·1 ± 72·7; P < 0·05; Fig. 2d).
Figure 2.
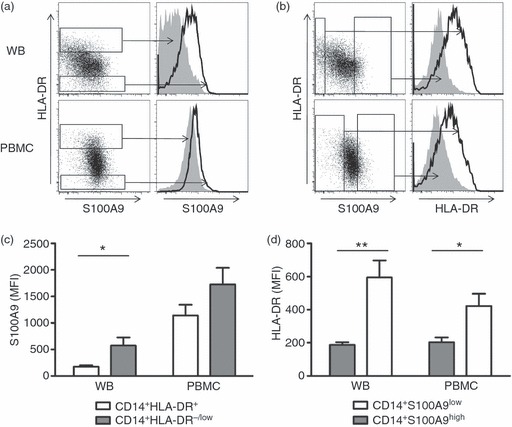
Combined S100A9, CD14 and HLA-DR analysis on peripheral blood mononuclear cells (PBMC) and whole blood cells from healthy donors. Freshly isolated PBMC or whole blood lysate (WB) from healthy donors were stained with CD14, HLA-DR and S100A9. Gates were set on the upper/lower 10% of S100A9/HLA-DR cells. (a, c) S100A9 expression compared between CD14+ HLA-DR−/low myeloid-derived suppressor cells (MDSC) and CD14+ HLA-DR+ monocytes. Representative FACS data (a) and average mean fluorescence intensity (MFI) analysis from nine healthy controls (c) are shown. *P < 0·05. (b, d) HLA-DR expression is compared between CD14+ S100A9high and CD14+ S100A9low cells. Representative FACS data (a) and average MFI analysis (c) are shown. *P < 0·05, **P < 0·001.
As MDSC are increased in patients with different types of cancer, we next tested PBMC and whole blood from patients with colon cancer. Peripheral blood from 14 randomly selected patients with colon cancer (Table 1) was analysed. Similarly, CD14+ HLA-DR−/low MDSC showed higher S100A9 expression than CD14+ HLA-DR+ monocytes both in whole blood lysate (335·0 ± 39·8 versus 209·7 ± 22·8; P < 0·05) and PBMC (3435·5 ± 952·0 versus 2113·7 ± 617·5; Fig. 3a). The CD14+ S100A9high cells showed lower HLA-DR expression than CD14+ S100A9low cells (238·2 ± 23·3 versus 430·3 ± 70·2 for whole blood and 153·2 ± 26·8 versus 311·6 ± 61·9 for PBMC; P < 0·05 for both; Fig. 3b).
Figure 3.
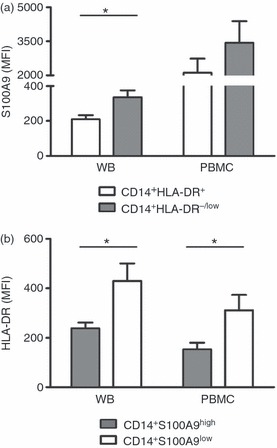
Combined S100A9, CD14 and HLA-DR analysis on peripheral blood mononuclear cells (PBMC) and whole blood cells from patients with colon cancer. Fresh isolated PBMC or whole blood lysate from 10 colon cancer patients are stained with CD14, HLA-DR and S100A9. Gates are set on the upper/lower 10% of S100A9/HLA-DR cells. (a) S100A9 expression is compared between CD14+ HLA-DR−/low MDSC (grey bar) and CD14+ HLA-DR+ monocytes (white bar). *P < 0·05. (b) HLA-DR expression is compared between CD14+ S100A9high (grey bar) and CD14+ S100A9low (white bar) cells. *P < 0·05.
Next, we analysed whether the frequency of CD14+ S100A9high cells in the peripheral blood of healthy donors and cancer patients correlates with the frequency of CD14+ HLA-DR−/low MDSC. We have previously shown that CD14+ HLA-DR−/low cells are significantly increased in the peripheral blood and tumours of patients with cancer.9 As shown in Fig. 4, the frequency of CD14+ S100A9high cells correlated with that of CD14+ HLA-DR−/low cells in both healthy donors and cancer patients. Similar to the increase in CD14+ HLA-DR−/low cells, there was also a significant increase in CD14+ S100A9high cells in the peripheral blood of cancer patients as compared with healthy donors.
Figure 4.
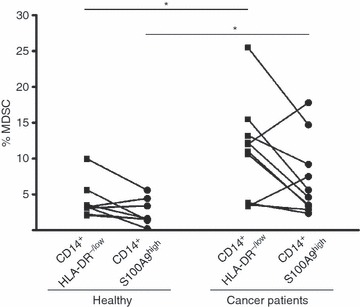
Frequency of CD14+ S100A9high cells corresponds to the myeloid-derived suppressor cell (MDSC) frequency defined by CD14+ HLA-DR−/low cells in whole blood lysate. Frequencies of CD14+ S100A9high cells and CD14+ HLA-DR−/low MDSC were analysed and calculated in whole blood lysate from healthy donors and colon cancer patients. Each pair of dots indicates the frequency of CD14+ S100A9high cells and CD14+ HLA-DR−/low MDSC from the same blood donor. The granulocytes were excluded based on forward and side scatter analysis. *P < 0·05.
NOS2 expression in CD14+ S100A9high cells
As S100A9 is an intracellular protein, it was not possible to sort these cells to test their suppressor function. However, it has been shown that MDSC suppress T-cell function by Arginase-1 and NOS2-dependent mechanisms. We therefore tested CD14+ S100A9high cells for expression of NOS2 in cancer patients. Whole blood lysate was stimulated with lipopolysaccharide and interferon-γ before expression of NOS2 was analysed. Upon lipopolysaccharide and interferon-γ stimulation, a significant induction of NOS2 was observed both in CD14+ HLA-DR−/low as well as in CD14+ S100A9high cells (Fig. 5a,b). The MFI of NOS2 was increased in both CD14+ S100A9high and CD14+ S100A9low cells (1003·7 ± 236·3 versus 209·7 ± 12·8; P < 0·05) and CD14+ HLA-DR−/low MDSC versus CD14+ HLA-DR+ monocytes (630·0 ± 50·0 versus 222·0 ± 25·0; P < 0·05; Fig. 5c,d).
Figure 5.
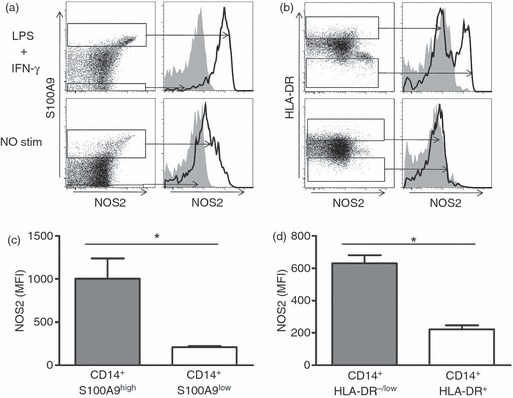
CD14+ S100A9high cells express higher amount of nitric oxide synthase (NOS2). (a, b) Representative FACS data are shown to compare NOS2 expression between CD14+ S100A9high and CD14+ S100A9low cells (a), or CD14+ HLA-DR−/low myeloid-derived suppressor cells (MDSC) and CD14+ HLA-DR+ monocytes (b). Lower panel represents NOS2 expression in unstimulated cells, and upper panel represents analysis 2 hr after lipopolysaccharide (LPS) and interferon-γ (IFN-γ) stimulation. Gates were set on the upper/lower 10% of S100A9/HLA-DR cells. (c, d) Average NOS2 mean fluorescence intensity (MFI) is analysed and compared between CD14+ S100A9high and CD14+ S100A9low cells (c), as well as between CD14+ HLA-DR−/low MDSC and CD14+ HLA-DR+ monocytes (d). *P < 0·05. (n = 3).
Discussion
Numerous studies have shown the existence of counter-regulatory immune mechanisms in patients with cancer. One of the recently identified mechanisms involves the recruitment of the heterogeneous population of MDSC. These cells have been widely studied in different mouse and human cancer models.12 We have previously reported the accumulation of CD14+ HLA-DR−/low MDSC in patients with hepatocellular carcinoma. These cells suppressed T cells and natural killer cells directly and could also suppress T-cell responses indirectly by inducing regulatory T cells.9,13,14 However, their heterogeneous nature and lack of a specific marker that clearly defines these cells limits the full understanding of the biology of MDSC.
Murine MDSC have been divided into two major groups: CD11b+ Gr-1high granulocytic MDSC (also CD11b+ Ly-6G+ Ly6Clow MDSC) and CD11b+ Gr-1low monocytic MDSC (which can also be identified as CD11b+ Ly-6GLy6Chigh MDSC).15,16 We have previously identified CD49d as another marker on murine MDSC, which distinguishes these two cell populations from each other. We have also shown that monocytic CD11b+ CD49d+ MDSC were more potent suppressors of antigen-specific T cells in vitro than CD11b+ CD49d− granulocytic MDSC and suppressed T-cell responses through a nitric oxide-mediated mechanism.3
Limited data are available on the biology of MDSC in human diseases and their interpretation is complicated by the different markers that have been used to analyse human MDSC subtypes in various clinical settings.17 Most studies concur with the observation that MDSC express CD11b and CD33 but lack the expression of markers of mature myeloid cells such as CD40, CD80, CD83 and HLA-DR. Both CD14+ HLA-DR−/low and CD14− CD15+ HLA-DR−/low MDSC have been described5 and molecules such as interleukin-4 receptor-α and vascular endothelial growth factor receptor have been used as additional markers.18 However, these markers cannot be used to distinguish HLA-DR−/low MDSC from HLA-DR+ monocytes.
Differential expression analysis of CD14+ HLA-DR−/low MDSC and CD14+ HLA-DR+ monocytes revealed S100A8, S100A9 and S100A12 as new markers in MDSC. The over-expression of S100 in MDSC was confirmed by real-time PCR as well as by Western blot and FACS analysis. Members of the S100 family of calcium-binding proteins play essential roles in epithelial tissues and participate in a wide range of cellular processes, including transcription, proliferation and differentiation.19,20 S100A8, S100A9 and S100A12 are specifically linked to innate immune functions by their expression in cells of myeloid origin.21 S100A8 and S100A9 are found in granulocytes, monocytes and the early differentiation stages of macrophages. Their expression can also be induced in keratinocytes and epithelial cells under inflammatory conditions. In contrast, S100A12 is restricted more to granulocytes.22 S100 proteins are related to pro-inflammatory mechanisms and a significant over-expression can be found at sites of inflammation.23 These proteins have been shown to exert their pro-inflammatory activity through receptor for advanced glycation end products (RAGE).24 Interestingly, Gebhardt et al. have demonstrated that RAGE-deficient mice are resistant to DMBA (7,12-dimethyl-benz[a]anthracene)/TPA (12-O-Tetradecanoylphorbol-13-Acetate)-induced skin carcinogenesis and exhibit a severe defect in sustaining inflammation during the promotion phase, indicating a pivotal role for S100/RAGE in promoting inflammation-induced carcinogenesis.25 S100A8 and S100A9 are reportedly up-regulated in many cancers, including colon cancer,26 and have been implicated in the regulation of tumour cell proliferation and metastasis. Murine MDSC have been shown to secrete S100A8/A9 proteins and blocking of the binding of S100A8/A9 to MDSC reduces MDSC levels in blood.27
Multiple suppressor functions still remain as the major hallmark of MDSC. NOS2 and arginase-1 are both strongly expressed in MDSC and have been shown to be responsible for immune suppression. Because S100 is an intracellular protein we could not use this marker for direct isolation of cells followed by functional analysis. Instead, we were able to demonstrate that CD14+ S100A9high but not CD14+ S100A9low cells expressed NOS2 in response to lipopolysaccharide and interferon-γ stimulation, suggesting that S100A9 can specifically identify MDSC and distinguish them from CD14+ HLA-DR+ monocytes.
It should be noted that S100 family members are all intracellular proteins, which makes it impossible to use this marker to isolate MDSC and use them in functional studies. Therefore, we were able to provide indirect data indicating that CD14+ S100A9high cells are MDSC. In addition, our data clearly demonstrated a better discrimination between MDSC and non-MDSC when MDSC in whole blood were analysed for S100A9 expression. Therefore, we would suggest using this marker when whole blood is available for the analysis of MDSC.
In summary, we describe S100A9, as a new marker in MDSC that can be used to identify CD14+ MDSC. S100A9 can be used instead of or in combination with HLA-DR staining. The latter has never been a precise marker for the identification of MDSC. Future studies are needed to examine the role of S100A8, S100A9 and S100A12 in other human MDSC subtypes with the aim of further characterization of these cells. This will help further our understanding of their mechanism of action and help to target them for immunotherapeutic approaches.
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the National institutes of Health, National Cancer Institute, Center for Cancer Research. This work was supported by a grant to MPM from the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer. We would like to thank the Experimental Transplantation and Immunology Branch cell sorting facility for technical assistance with cell sorting.
Disclosures
None of the authors have any financial conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. PBMC were isolated by Ficoll density gradient and stained for CD14 and HLA-DR expression.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 2.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–210. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 4.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–806. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusmartsev S, Su Z, Heiser A, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandruzzato S, Solito S, Falisi E, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 9.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+ CD25+ Foxp3+ T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravasi T, Hsu K, Goyette J, et al. Probing the S100 protein family through genomic and functional analysis. Genomics. 2004;84:10–22. doi: 10.1016/j.ygeno.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–6541. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Ishigaki H, Ishida H, Itoh Y, Noda Y, Ogasawara K. Analysis of splenic Gr-1int immature myeloid cells in tumor-bearing mice. Microbiol Immunol. 2008;52:47–53. doi: 10.1111/j.1348-0421.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 16.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 17.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 20.Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–d1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 21.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 22.Vogl T, Propper C, Hartmann M, Strey A, Strupat K, van den Bos C, Sorg C, Roth J. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 23.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50:3762–3771. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 25.Gebhardt C, Riehl A, Durchdewald M, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


