Abstract
Mycobacterial proteins interact with host macrophages and modulate their functions and cytokine gene expression profile. The protein Rv0652 is abundant in culture filtrates of Mycobacterium tuberculosis K-strain, which belongs to the Beijing family, compared with levels in the H37Rv and CDC1551 strains. Rv0652 induces strong antibody responses in patients with active tuberculosis. We investigated pro-inflammatory cytokine production induced by Rv0652 in murine macrophages and the roles of signalling pathways. In RAW264.7 cells and bone marrow-derived macrophages, recombinant Rv0652 induced predominantly tumour necrosis factor (TNF) and monocyte chemoattractant protein (MCP)-1 production, which was dependent on mitogen-activated protein kinases and nuclear factor-κB. Specific signalling pathway inhibitors revealed that the extracellular signal-regulated kinase 1/2 (ERK1/2), p38 and phosphatidylinositol 3-kinase (PI3K) pathways were essential for Rv0652-induced TNF production, whereas the ERK1/2 and PI3K pathways, but not the p38 pathway, were critical for MCP-1 production in macrophages. Rv0652-stimulated TNF and MCP-1 secretion by macrophages occurred in a Toll-like receptor 4-dependent and MyD88-dependent manner. In addition, Rv0652 significantly up-regulated the expression of the mannose receptor, CD80, CD86 and MHC class II molecules. These results suggest that Rv0652 can induce a protective immunity against M. tuberculosis through the macrophage activation.
Keywords: cytokines, Mycobacterium tuberculosis, Rv0652, macrophage, signalling
Introduction
Mycobacterium tuberculosis is the causative agent of tuberculosis (TB), a major global disease responsible for 2 million deaths annually.1 The emergence of multi-drug-resistant strains, the increased number of cases of reactive TB in immunocompromised patients such as those with AIDS, and the variable efficacy of the only currently available vaccine, bacillus Calmette–Guérin,2 have prompted the development of novel control strategies for TB. The identification and characterization of immune-modulating mycobacterial proteins are essential for understanding TB pathogenesis and for developing new TB eradication strategies.
Macrophages constitute the first line of defence against M. tuberculosis and are critical in linking innate and adaptive immunity.3 Macrophages infected with M. tuberculosis are activated to release various pro-inflammatory cytokines and chemokines, thereby promoting lymphocyte activation and recruitment, and ultimately inducing granuloma formation.4 Despite robust immune responses, M. tuberculosis survives within macrophages by modulating their antimycobacterial activity. Proteins secreted by live M. tuberculosis within macrophages elicit protective immunity or interfere with the microbicidal function of macrophages.
During M. tuberculosis infection, pro-inflammatory cytokines such as tumour necrosis factor (TNF) and interleukin-12 (IL-12) as well as chemokines are essential for anti-mycobacterial activity and granuloma formation.5 Macrophages recognize M. tuberculosis and its components via pattern-recognition receptors such as Toll-like receptors (TLRs). In response, macrophages become activated and secrete cytokines and chemokines.6 Various mycobacterial proteins and glycolipids involved in the responses through the TLR pathway have been identified. For example, a 19 000 molecular weight (MW) protein was shown to activate macrophages to secrete TNF and nitric oxide,7 and to inhibit MHC class II antigen processing via an interaction with TLR2.8 In addition, recent reports indicated that PPE18 interacts with TLR2 to activate IL-10 production in macrophages,9 and that a 38 000 MW protein acts through both TLR2 and TLR4 to induce the production of pro-inflammatory cytokines.10 Among mycobacterial PE/PPE proteins, PE_PGRS33, PE_PGRS11, and PE_PGRS17 were shown to interact with TLR2.11,12 Also glycolipids such as lipoarabinomannan, lipomannan and glycopeptidolipids interact with TLR2. 13,14 However, little is known about mycobacterial proteins that activate macrophages via TLR4.
Mycobacterial proteins recognized by a host immune system are interesting targets for the development of new vaccines and diagnostic reagents. Recently, we reported that Rv0652, the 50S ribosomal proteins L7/L12, elicits a strong serum antibody response in patients with active TB.15 This protein is one among purified protein derivative proteins that induce strong delayed-type hypersensitivity.16 A proteomics analysis revealed that this protein was abundant in culture filtrates of M. tuberculosis K-strain, and not in those of M. tuberculosis H37Rv and CDC1551.17 The K-strain, which is the most prevalent strain in Korea, belongs to the Beijing family and is considered to be highly virulent.18 Interestingly, microbial ribosomal proteins are known to be potent adjuvants.19,20 Nevertheless, there is no report of the effect of Rv0652 on macrophage activation and related signalling events.
In this study, we investigated the immunostimulatory role of Rv0652 in murine macrophages. Our data suggest that recombinant Rv0652 activates macrophages to secrete pro-inflammatory cytokines, predominantly TNF and monocyte chemoattractant protein 1 (MCP-1), through TLR4-dependent and MyD88-dependent signalling pathways. Furthermore, Rv0652 significantly up-regulated the expression of mannose receptor (MR), CD80 (B7-1), CD86 (B7-2), and MHC class II molecules, compared with expression levels in untreated cells.
Materials and methods
Reagents and antibodies
Enzyme-linked immunosorbent assay (ELISA) kits for the detection of TNF, MCP-1 and IL-1β were obtained from eBioscience (San Diego, CA), and ELISA kits for IL-12p70 and IL-10 were obtained from BD Biosciences (San Diego, CA). Anti-phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2), anti-phosphorylated p38, anti-phosphorylated Akt, anti-IκBα, and anti-β-actin monoclonal antibodies, and anti-p38 and anti-ERK1/2 polyclonal antibodies were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Horseradish peroxidase-conjugated anti-mouse IgG, horseradish peroxidase-conjugated anti-rabbit IgG, and specific inhibitors of ERK (U0126), p38 mitogen-activated protein kinase (MAPK; SB203580), phosphatidylinositol 3-kinase (PI3K; LY294002), and nuclear factor-κB (NF-κB; BAY 11-7082) were obtained from Calbiochem (San Diego, CA). Fluorescein isothiocyanate-conjugated monoclonal antibody against MHC class II, phycoerythrin-conjugated monoclonal antibodies against MR and CD86, and allophycocyanin-conjugated monoclonal antibody against CD80 were purchased from BD Biosciences.
Mice
Male C57BL/6 (H-2Kb and I-Ab) mice, 6–8 weeks old, were purchased from the Korean Institute of Chemistry Technology (Daejeon, Korea). C57BL/6J TLR2 knockout (KO) mice (TLR2−/−; B6.129-Tlr2tm1Kir/J), C57BL/10 TLR4 KO mice (TLR4−/−; C57BL/10ScNJ), and C57BL/6J MyD88 KO mice [MyD88−/−; B6.129P2(SJL)-MyD88tml.Defr/J] aged 6–8 weeks were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in a specific pathogen-free environment in an animal facility for at least 1 week before the experiments. The animal procedures were approved by the Institutional Animal Care and Use Committee of Chungnam National University (Permit Number: 2010-3-9), and all animal experiments were performed in accordance with Korean Food and Drug Administration guidelines.
Purification of recombinant Rv0652 protein and antigen 85 complex (Ag85)
Recombinant Rv0652 protein was prepared as described previously.15 In brief, the recombinant plasmid encoding the Rv0652 gene was transformed into Escherichia coli BL21 (DE3) for protein over-expression. The recombinant protein was purified by nickel nitrilotriacetic acid agarose (Ni-NTA) chromatography in accordance with the manufacturer’s instructions (Invitrogen, Carlsbad, CA). The purity of the prepared protein was analysed by SDS–PAGE, followed by Coomassie blue staining or immunoblotting using anti-His antibodies (Invitrogen). Purified Rv0652 protein was dialysed against PBS to remove imidazole and was then incubated overnight at 4° with polymyxin B-agarose beads (Sigma-Aldrich, St Louis, MO) to remove endotoxin contamination. Further evaluation of bacterial endotoxin was carried out using an amoebocyte lysate assay (Sigma-Aldrich). The purified protein was stored in small aliquots at −80° until used.
Native Ag85 was purified from the culture filtrate of M. tuberculosis H37Rv (ATCC 27294) by ammonium sulphate precipitation, followed by hydroxyapatite and anion exchange chromatography, as described previously.21
Cell culture and generation of bone-marrow derived macrophages
The RAW264.7 macrophage cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Lonza, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 1% HEPES, and 1%l-glutamine at 37° in an atmosphere of 5% CO2. Bone-marrow-derived macrophages (BMDM) were obtained from C57BL/6 and all KO mice. Briefly, bone marrow cells from the femur and tibia were cultured in DMEM containing 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS and 25 ng/ml recombinant mouse macrophage colony-stimulating factor (R&D Systems, Minneapolis, MN) at 37° in an atmosphere of 5% CO2. After 4 days, non-adherent cells were removed, and differentiated macrophages were incubated in antibiotic-free DMEM.
Cell cytotoxicity analysis
RAW264.7 cells were seeded at a density of 1 × 104 per well in 96-well culture plates and treated with Rv0652 (10 μg/ml). After 24 or 48 hr, cell viability was assessed using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratory, Kumamoto, Japan). Aliquots of the kit reagent were added to the culture medium, and the plate was incubated at 37° for another hour. Cell viability was determined as absorbance at 450 nm with a microplate reader.
Measurement of cytokines and chemokines by ELISA
Culture supernatants of macrophages stimulated with Rv0652 or lipopolysaccharide (LPS) were harvested at the indicated time-points and stored at −20°. Sandwich ELISAs were used to detect cytokine and chemokine levels in the culture supernatants. The assays were performed as recommended by the manufacturers (eBioscience and BD Biosciences). The cytokine concentrations were calculated using standard curves that were generated from recombinant cytokines.
LPS contamination assay in the Rv0652 preparation
Before stimulating with Rv0652, the protein preparation was heat denatured at 100° for 30 min or treated with proteinase K (1/20 concentration of protein) for 1 hr; proteinase K was inactivated by boiling. We also determined whether Rv0652-induced macrophage activation was neutralized by incubation with polymyxin B (10 μg/ml) for 30 min.
Determination of MAPK phosphorylation
The BMDM stimulated with Rv0652 for the times indicated were detached, collected by centrifugation, and lysed in lysis buffer (10 mm Tris–HCl, pH 7·4, 5 mm EDTA, 150 mm NaCl, 1% Triton X-100, 1 mm PMSF, and protease inhibitor cocktail). Protein concentrations were determined with the Bradford assay, and 30 μg of protein were separated by SDS–PAGE, followed by electrotransfer to a nitrocellulose membrane (Hybond-ECL; GE Healthcare, Little Chalfont, UK). The blots were probed with an appropriate primary antibody (anti-phospho-ERK, anti-ERK, anti-phospho-p38, anti-p38, anti-phospho-Akt, anti-IκBα, or anti-β-actin) at the optimized concentration, followed by horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were detected by enhanced chemiluminescence (ECL; GE Healthcare) and exposure to film.
To determine the effects of pharmacological inhibitors on Rv0652-induced TNF and MCP-1 production, BMDM were incubated with an inhibitor of ERK (U0126, 5–20 μm), p38 (SB203580, 5–20 μm), PI3K (LY294002, 5–20 μm), or NF-κB (BAY 11-7082, 5–20 μm), reconstituted in sterile cell culture-grade DMSO (Sigma-Aldrich), for 1 hr before treatment with Rv0652 (5 μg/ml). As a control, 0·1% (volume/volume) DMSO was added to some samples. After incubation for 24 hr, the TNF-α and MCP-1 levels were measured by ELISA.
Flow cytometric analysis of surface molecules in macrophages
RAW264.7 macrophages were stimulated with Rv0652 (5 μg/ml), LPS (100 ng/ml), or Ag85 (5 μg/ml). After incubation for 24 hr, the cells were harvested, washed with PBS, and suspended in FACS wash buffer (2% FBS and 0·1% sodium azide in PBS). The cells were pre-incubated with 0·5% BSA in PBS for 30 min and washed with PBS. The cells were then stained with fluorescein isothiocyanate-conjugated anti-MHC class II antibody or phycoerythrin-conjugated anti-MR, anti-CD86, or anti-CD80 antibody for 45 min at 4°. After the cells were washed three times with PBS and resuspended in 500 μl PBS, flow cytometric analysis was performed (FACSCanto, BD Biosciences). The data were analysed using Cell Quest software (Becton Dickinson, San Jose, CA).
Statistical analysis
The data are presented as means ± standard deviation (SD) from at least three independent experiments. Statistical analyses were performed using an unpaired Student’s t-test with Bonferroni adjustment. Significance was indicated by a P-value of < 0·05.
Results
Purification of recombinant Rv0652 protein
Rv0652 was expressed as a His-tagged protein in E. coli and purified by Ni-NTA affinity chromatography. The purified protein appeared as a major band at approximately 23 000 MW, although faint contaminating bands were also observed (Fig. 1). The 23 000-MW band reacted with anti-His antibody on an immunoblot (data not shown). Its purity was > 97% based on SDS–PAGE.
Figure 1.

SDS–PAGE analysis of purified Rv0652 protein. The protein was expressed in Escherichia coli and purified by Ni-NTA affinity chromatography. The gel was stained with Coomassie blue.
Rv0652 protein induces the secretion of pro-inflammatory cytokines and MCP-1
To assess whether Rv0652 can stimulate a macrophage response, its ability to induce cytokine production in macrophages was determined. Cytotoxicity of Rv0652 to RAW264.7 cells was tested using a Cell Counting Kit-8. As shown in Fig. 2, no cytotoxic effect of Rv0652 at the tested concentration was observed after 48 hr. The levels of TNF, IL-1β, IL-12p70, IL-10 and MCP-1 in the culture supernatant of BMDM treated with Rv0652 at 5 μg/ml were determined. TNF, MCP-1, IL-12p70 and IL-10 were detected 24 hr after stimulation (Fig. 3). TNF and MCP-1 predominated, with Rv0652-stimulated TNF and MCP-1 levels increasing in a time-dependent and dose-dependent manner (Fig. 4). Production of TNF was noted after 3 hr, and MCP-1 was detected after 18 hr. Both were produced maximally at an Rv0652 concentration of 5 μg/ml.
Figure 2.
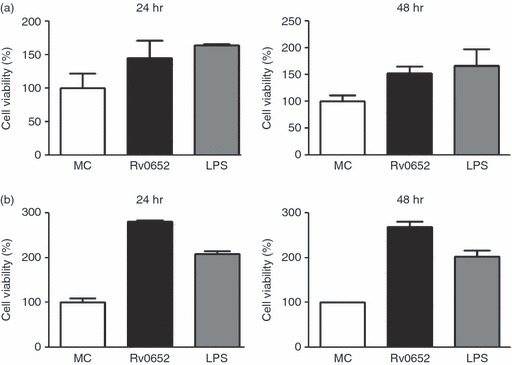
Rv0652 is not cytotoxic to RAW264.7 cells (a) and bone-marrow-derived macrophages (BMDM) (b). RAW264.7 cells and BMDM were treated with Rv0652 (10 μg/ml) or lipopolysaccharide (LPS; 100 ng/ml) for 24 or 48 hr. Cell viability was measured using a CCK-8 assay. Data are presented as means ± SD of three experiments. MC, medium control.
Figure 3.
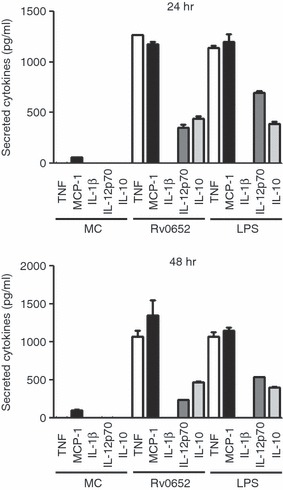
Rv0652-induced cytokine and chemokine production in bone-marrow-derived macrophages (BMDM). BMDM were stimulated with Rv0652 (5 μg/ml) or lipopolysaccharide (LPS; 100 ng/ml) for 24 or 48 hr. Levels of tumour necrosis factor (TNF), interleukin-1β (IL-1β), IL-12p70, IL-10 and monocyte chemoattractant protein 1 (MCP-1) in culture medium were measured by ELISA. Data are presented as means ± SD of three experiments. MC, medium control.
Figure 4.
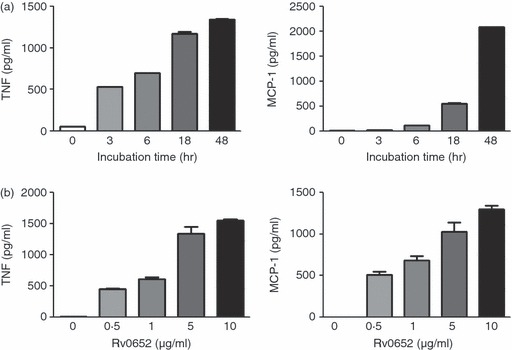
Rv0652-stimulated production of tumour necrosis factor (TNF) and monocyte chemoattractant protein 1 (MCP-1) in a time-dependent and dose-dependent manner in RAW264.7 cells. (a) RAW264.7 cells were stimulated with Rv0652 (5 μg/ml) for the times indicated. (b) RAW264.7 cells were stimulated with Rv0652 at the concentrations indicated for 18 hr. Levels of TNF and MCP-1 in culture medium were measured by ELISA. Data are presented as means ± SD of three experiments.
The pattern of cytokine production stimulated by Rv0652 was similar to that stimulated by LPS, which was used as a positive control (Fig. 3). We therefore tested whether Rv0652-induced macrophage activation was attributable to LPS contamination. Proteinase K digestion or heat denaturation of the Rv0652 preparation abrogated MCP-1 and TNF production (Fig. 5). Furthermore, pre-incubation of BMDM with polymyxin B for 30 min did not suppress Rv0652-induced MCP-1 or TNF production, but did reduce the stimulatory effects of LPS. These results verify that macrophage activation was induced by intact Rv0652 and was not the result of LPS contamination. In fact, the endotoxin content of the purified recombinant Rv0652 was extremely low (< 0·05 EU/ml).
Figure 5.
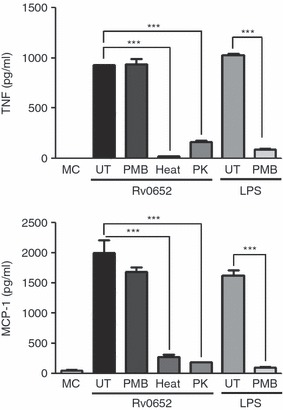
Rv0652-induced tumour necrosis factor (TNF) and monocyte chemoattractant protein 1 (MCP-1) production is not the result of lipopolysaccharide (LPS) contamination. Bone-marrow-derived macrophages (BMDM) were stimulated with Rv0652 (5 μg/ml), heat-treated Rv0652, or proteinase K (PK)-treated Rv0652. The BMDM were also incubated with polymyxin B (PMB, 10 μg/ml) for 30 min before treatment with Rv0652 (5 μg/ml) or LPS (100 ng/ml). Culture supernatants were harvested after 24 hr, and the levels of TNF and MCP-1 were measured by ELISA. Data are presented as means ± SD of three experiments. ***P < 0·001 versus untreated (UT) control. MC, medium control.
Rv0652 protein leads to MAPK phosphorylation
The MAPK are critical for cellular responses to many external stimuli and are important signal molecules for production of pro-inflammatory cytokines in M. tuberculosis-infected macrophages.6,22 We examined the activation of MAPK in response to Rv0652. Phosphorylation profiles of ERK1/2, p38 and Akt in BMDM were analysed at various time-points (15–240 min) after stimulation with Rv0652. At 15 min after stimulation, ERK1/2, p38 MAPK and Akt were strongly phosphorylated (Fig. 6a). In addition, the expression of IκBα was rapidly lost at 15 min and then reappeared. These results suggest that Rv0652-induced macrophage activation is mediated by signalling pathways involving MAPKs and NF-κB.
Figure 6.
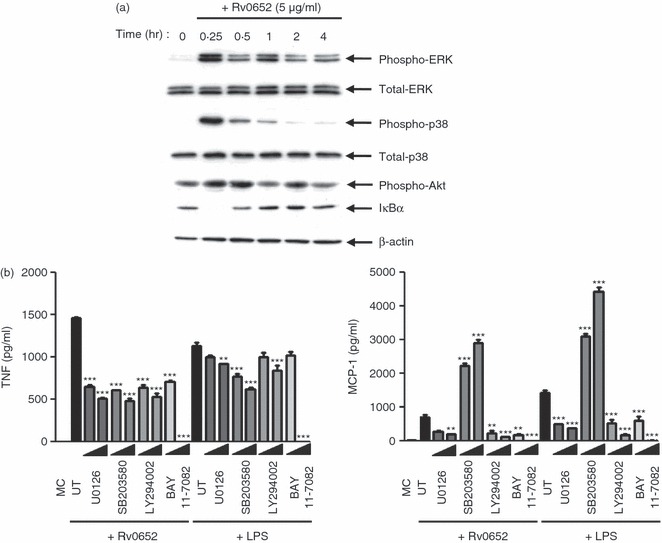
Rv0652 induces phosphorylation of mitogen-activated protein kinases (MAPK). (a) Bone-marrow-derived macrophages (BMDM) stimulated with Rv0652 for the times indicated were lysed, and the proteins in the total cell lysate samples were separated by SDS–PAGE, followed by immunoblot analysis using antibodies against phospho-extracellular signal-regulated kinase 1/2 (ERK1/2), ERK1/2, phospho-p38, p38, phospho-Akt, IκBα, and β-actin. This image is representative of three experiments showing similar results. (b) Effect of MAPKs, p38, phosphatidylinositol 3-kinase (PI3K) and nuclear factor-κB (NF-κB) inhibitors on lipopolysaccharide (LPS) -induced and Rv0652-induced tumour necrosis factor (TNF) and monocyte chemoattractant protein 1 (MCP-1) production. BMDM were treated with inhibitors of ERK (U0126, 5–20 μm), p38 (SB203580, 5–20 μm), PI3K (LY294002, 5–20 μm), or NF-κB (BAY 11-7082, 5–20 μm) for 1 hr before treatment with Rv0652 (5 μg/ml). After 24 hr, the levels of TNF and MCP-1 were measured by ELISA. Data are presented as means ± SD of three experiments. **P < 0·01 and ***P < 0·001 versus untreated controls. MC, medium control; UT, untreated control.
To examine the role of MAPKs in Rv0652-induced MCP-1 and TNF production, BMDM were pre-treated with a specific inhibitor of ERK1/2 (U0126), p38 (SB203580), PI3K (LY294002), or NF-κB (BAY 11-7082) for 1 hr, and cytokine levels were assayed at 24 hr. As shown in Fig. 6(b), TNF production in Rv0652-treated BMDM was significantly inhibited by the ERK1/2, PI3K and p38 MAPK inhibitors in a dose-dependent manner. The LPS-induced TNF production was significantly inhibited by the p38 inhibitor, and moderately inhibited by a high concentration of the ERK1/2 and PI3K inhibitors. In contrast, Rv0652-stimulated and LPS-stimulated MCP-1 production by BMDM was significantly depressed by the ERK1/2 and PI3K inhibitors, and the p38 inhibitor SB203580 significantly increased Rv0652-stimulated and LPS-stimulated MCP-1 production. The production of TNF and MCP-1 was completely abrogated by the NF-κB inhibitor. The observed inhibition was not caused by DMSO, because DMSO alone did not exhibit any inhibitory effects (data not shown). These results suggest that the ERK1/2, p38 and PI3K pathways are critical for Rv0652-induced TNF production, whereas ERK1/2 and PI3K are required for MCP-1 production.
Rv0652-mediated induction of TNF and MCP-1 is dependent on signalling via TLR4 and MyD88
Various mycobacterial proteins activate macrophages through TLR2 and/or TLR4-dependent signals.6 To determine whether Rv0652-induced macrophage activation is TLR-dependent, we prepared BMDM from C57BL/6J wild-type (WT) and TLR2−/− mice, and C57BL/10 WT and TLR4−/− mice, treated the BMDM with Rv0652 (5 μg/ml), Pam3 (100 ng/ml) or LPS (100 ng/ml) for 24 hr, and then measured the TNF and MCP-1 levels in the culture supernatants, by ELISA. As shown in Fig. 7(a), TNF and MCP-1 were produced in BMDM from TLR2−/− and WT mice after stimulation with Rv0652. As expected, the response to Pam3, TLR2 agonist, was significantly depressed in TLR2−/− mice. Interestingly, the response to Rv0652 was significantly depressed in TLR4−/− mice, which was similar to the response to LPS. These findings suggest a specific role for TLR4 in Rv0652-induced macrophage activation.
Figure 7.
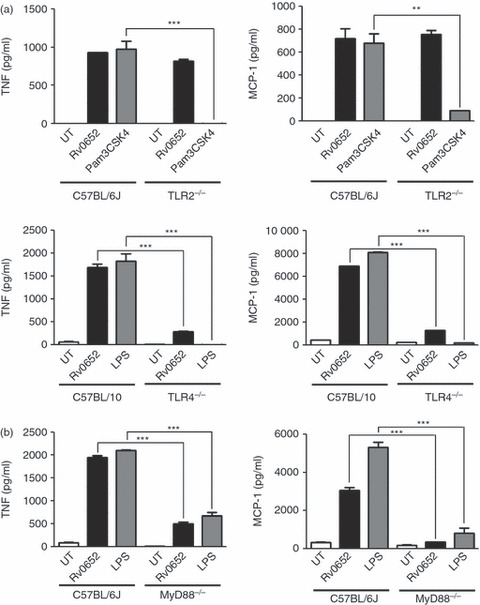
Rv0652 stimulates tumour necrosis factor (TNF) and monocyte chemoattractant protein 1 (MCP-1) release in a Toll-like receptor 4 (TLR4) -dependent and MyD88-dependent manner. (a) Bone-marrow-derived macrophages (BMDM) from C57BL/6J wild-type (WT), TLR2−/−, C57BL/10 WT, and TLR4−/− mice were stimulated with Rv0652 (5 μg/ml), Pam3 (100 ng/ml), or lipopolysaccharide (LPS; 100 ng/ml) for 24 hr. The levels of TNF and MCP-1 in the culture supernatants were measured by ELISA. (b) BMDM from C57BL/6J WT and MyD88−/− mice were stimulated with Rv0652 (5 μg/ml) or LPS (100 ng/ml) for 24 hr. The levels of TNF and MCP-1 in the culture supernatants were measured by ELISA. Data are presented as means ± SD of three determinations. ***P < 0·001 versus BMDM from WT mice.
We also tested the MyD88-dependence of the response to Rv0652 in BMDM prepared from C57BL/6J WT and MyD88−/− mice. As shown in Fig. 7(b), Rv0652-induced and LPS-induced production of TNF and MCP-1 were significantly depressed in BMDM from MyD88−/− mice.
Rv0652 induces expression of surface molecules related to macrophage function
Finally, we examined whether Rv0652 had a modulatory role in the expression of macrophage surface molecules such as MR, CD80, CD86 and MHC class II, which are important for the function of macrophages as antigen-presenting cells and phagocytes. RAW264.7 cells were treated with Rv0652 (5 μg/ml) or LPS (100 ng/ml), and after 24 hr, expression was examined by two-colour or three-colour flow cytometry. Native Ag85 (5 μg/ml) served as a mycobacterial control protein. Ag85, the major protein secreted by M. tuberculosis, has fibronectin-binding activity and is strongly immunoreactive.21,23 Rv0652 and LPS significantly up-regulated the expression of MR, CD80 and MHC class II molecules compared with levels in untreated cells (Fig. 8), and Ag85 induced significant expression of MHC class II molecules. However, the expression of CD86 was increased significantly only by stimulation with Rv0652. Rv0652 more effectively induced the expression of these surface molecules than did LPS. Hence, in addition to stimulating the production of pro-inflammatory cytokines, Rv0652 can influence the function of macrophages as antigen-presenting cells.
Figure 8.
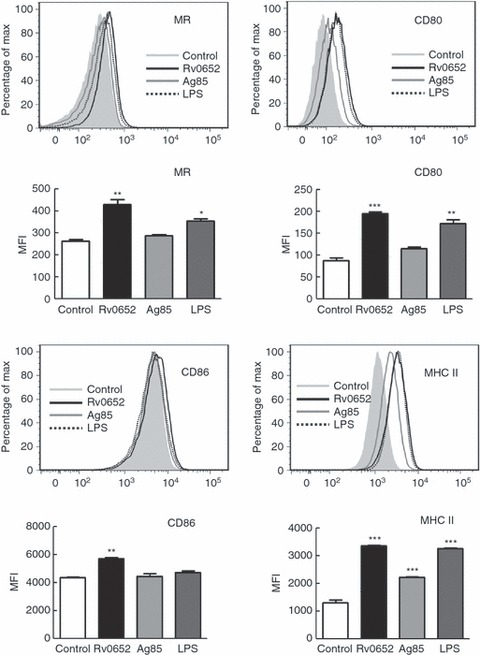
Rv0652 induces the expression of macrophage surface molecules on RAW264.7 macrophages. RAW264.7 cells were cultured for 24 hr in the presence of Rv0652 (5 μg/ml), Ag85 (5 μg/ml), or LPS (100 ng/ml), and the expression of the surface markers MR, CD80, CD86 and MHC class II was analysed by flow cytometry. Data are representative of three experiments showing similar results. Mean fluorescence intensities (MFI) calculated from histograms are presented as means ± SD of three independent experiments. *P < 0·05 or **P < 0·01 or ***P < 0·001 versus untreated cells.
Discussion
The present investigation aimed to determine the regulatory mechanism of Rv0652 in macrophage activation. Macrophages contribute directly to protective immunity against M. tuberculosis through the production of pro-inflammatory cytokines and chemokines. Mycobacterial proteins interact with host macrophages and modulate their functions and cytokine gene expression profile. Rv0652 activates macrophages to release predominantly TNF and MCP-1 through TLR4 signalling. Chemokines recruit immune cells by chemotaxis and activate leucocytes during inflammatory disease. In addition, MCP-1 substantially contributes to host antimycobacterial inflammatory responses.24 TNF is essential for maintaining granulomas and controlling TB; mice with a deficiency in TNF or its receptors are more susceptible to mycobacterial infection.25,26 On the other hand, intracellular survival of M. tuberculosis and its products stimulate continuous production of the inflammatory mediators including TNF, chemokines, nitric oxide and reactive oxygen intermediates from macrophages, and eventually induce tissue damage. Hence, our data suggest that Rv0652 can induce a protective immunity and also may be involved in TB pathogenesis through inducing TNF and MCP-1 production by macrophages.
Macrophage activation in response to mycobacteria and their antigens initiates MAPK signalling pathways, resulting in inflammatory mediator production.6 In the present study, TNF-α production by macrophages stimulated with Rv0652 required the ERK1/2, p38 and PI3K pathways, whereas MCP-1 production required ERK1/2 and PI3K. In contrast to TNF, MCP-1 production was significantly increased by pre-incubation with a p38 inhibitor, possibly as a result of cross-talk between the MAPK pathways. Previous studies indicated that TNF production induced upon M. tuberculosis infection was dependent on both p38 and ERK1/2; on the other hand, MCP-1 production was significantly up-regulated by a p38 inhibitor.22 In addition, we demonstrated that PI3K activation was critical for TNF and MCP-1 production by monocytes in response to Rv0652. These data are consistent with another study in which PI3K was necessary for induction of MCP-1 production in M. tuberculosis-stimulated human monocytes.27 The other report also demonstrates that mycobacteria trigger other intracellular signalling cascades such as the PI3K pathway.28,29 These data demonstrate that signal pathways triggered by Rv0652 in TNF and MCP-1 production are similar to those by M. tuberculosis.
Toll-like receptors, particularly TLR2 and TLR4, recognize M. tuberculosis and initiate macrophage responses that influence both innate and adaptive immunity.30 Many mycobacterial components such as lipoarabinomannan, 19 000-MW lipoprotein, and 38 000-MW glycolipoprotein (PhoS1) also trigger secretion of pro-inflammatory cytokines in a TLR2- and/or TLR4-dependent manner.6 Although TLR2 appears to be the major mediator of M. tuberculosis-induced cellular activation, TLR4-activating components that appear to be heat labile and cell-associated have been described.30 Most mycobacterial TLR2 agonists are lipoproteins or glycolipids, which suggests that acylation is important for interaction with TLR2.31 Recombinant mycobacterial heat-shock protein 70 expressed in E. coli signals through both TLR4 and TLR2, whereas heat-shock protein 65 signals exclusively through TLR4 to activate the innate immune system.32 Moreover, native 38 000-MW protein purified from M. tuberculosis culture filtrate acts through both TLR2 and TLR4 to induce macrophage activation.10 The TLR4 generates signals in both MyD88-dependent and MyD88-independent manners. To date, little is known about mycobacterial proteins and glycolipids that signal exclusively through TLR4.
The present study demonstrated that Rv0652 activates macrophages to secrete TNF and MCP-1 in a TLR4-dependent and MyD88-dependent manner. The similarity between the cytokine patterns induced by Rv0652 and LPS (Fig. 3) and the signalling of RV0652 chiefly through TLR4 suggested the possibility of LPS contamination in the protein preparation. However, several lines of evidence showed that signalling via TLR4 and activation of macrophages were attributable to Rv0652, and not to LPS contamination: no LPS was detected in purified Rv0652, the stimulatory effects of Rv0652 were abolished by boiling the preparation or treating it with proteinase K, and polymyxin B treatment did not suppress the effect of Rv0652.
Macrophages participate in anti-mycobacterial immunity through the production of pro-inflammatory cytokines or chemokines as well as by directly killing M. tuberculosis within phagosomes. In addition, macrophages interact with T cells, which is a critical process for the activation of both T cells and macrophages.3 In the present study, we showed Rv0652-stimulated increases in the expression of several macrophage surface molecules: CD80 and CD86, which act as co-stimulators of T-cell activation; MR, which participates in phagocytosis, and MHC class II molecules, which are involved in antigen presentation, were increased by Rv0652. The effects of Rv0652 on the expression of these surface molecules were more pronounced than those of LPS or immunodominant Ag85. The 19 000-MW protein activates macrophages to secrete TNF and nitric oxide, but inhibits MHC II antigen processing by macrophages.7,8 Rv2626c induces pro-inflammatory cytokines and up-regulates various co-stimulatory molecules such as CD80, CD86 and CD4.33 In light of these previous findings, our data indicate that Rv0652 enhances the ability of macrophages to effectively induce activation of T cells.
The interactions between M. tuberculosis and host macrophages and the resultant host immune response are complex processes that appear to involve diverse host and bacterial molecules. Understanding the immunoregulatory mechanisms of the mycobacterial components involved in the pathogenesis of TB is critical for the development of effective vaccines and more efficacious treatments for TB. Rv0652 may play an immune-stimulatory role during M. tuberculosis infection and may be a candidate for the development of an immune-adjuvant.
Acknowledgments
This work was supported by the Mid-career Researcher Program through a National Research Foundation grant funded by the Ministry of Education, Science and Technology (2010-0025985).
Disclosures
Authors declare that there is no conflict of interest.
References
- 1.World Health Organization. Report. Global Tuberculosis Control-Surveillance, Planning, Financing. Geneva, Switzerland: WHO; 2008. WHO/HTM/TB/2008.393. [Google Scholar]
- 2.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–68. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–91. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 6.Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signaling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–98. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 7.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 8.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–8. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 9.Nair S, Ramaswamy PA, Ghosh S, et al. The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J Immunol. 2009;183:6269–81. doi: 10.4049/jimmunol.0901367. [DOI] [PubMed] [Google Scholar]
- 10.Jung SB, Yang CS, Lee JS, et al. The mycobacterial 38-kilodalton glycoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74:2686–96. doi: 10.1128/IAI.74.5.2686-2696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu S, Pathak SK, Banerjee A, et al. Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated toll-like receptor 2-dependent release of tumor necrosis factor-α. J Biol Chem. 2007;282:1039–50. doi: 10.1074/jbc.M604379200. [DOI] [PubMed] [Google Scholar]
- 12.Bansal K, Elluru SR, Narayana Y, Chaturvedi R, Patil SA, Kaveri SV, Bayry J, Balaji KN. PE_PGERS antigens of Mycobacterium tuberculosis induce maturation and activation of human dendritic cells. J Immunol. 2010;184:3495–504. doi: 10.4049/jimmunol.0903299. [DOI] [PubMed] [Google Scholar]
- 13.Quesniaux V, Nicolle DM, Torres D, et al. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172:4425–34. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- 14.Sweet L, Schorey JS. Glycopeptidolipids from Mycobacterium avium promote macrophage activation in a TLR2- and MyD88-dependent manner. J Leukoc Biol. 2006;80:415–23. doi: 10.1189/jlb.1205702. [DOI] [PubMed] [Google Scholar]
- 15.Kwon YM, Jung KH, Choi GE, et al. Identification and diagnostic utility of serologic reactive antigens from Mycobacterium tuberculosis sonic extracts. J Bacteriol Virol. 2009;39:329–36. [Google Scholar]
- 16.Kitaura H, Kinomoto M, Yamada T. Ribosomal protein L7 included in tuberculin purified protein derivative (PPD) is a major heat-resistant protein inducing strong delayed-type hypersensitivity. Scand J Immunol. 1999;50:580–7. doi: 10.1046/j.1365-3083.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 17.Bahk YY, Kim SA, Kim JS, Euh HJ, Bai GH, Cho SN, Kim YS. Antigens secreted from Mycobacterium tuberculosis identification by proteomic approach and test for diagnostic marker. Proteomics. 2004;4:3299–307. doi: 10.1002/pmic.200400980. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Bai GH, Lee H, Kim HJ, Lew WJ, Park YK, Kim Y. Transmission of Mycobacterium tuberculosis among high school students in Korea. Int J Tuberc Lung Dis. 2001;5:824–30. [PubMed] [Google Scholar]
- 19.Gregory RL. Microbial ribosomal vaccines. Rev Infect Dis. 1986;8:208–17. doi: 10.1093/clinids/8.2.208. [DOI] [PubMed] [Google Scholar]
- 20.Pregliasco F, Terracciano L, Marcassa S, Zava D, Anselmi G. Rationale for the clinical use of a ribosome-components immune modulator. Allergy Asthma Proc. 2009;30(Suppl 1):S5–12. doi: 10.2500/aap.2009.30.3249. [DOI] [PubMed] [Google Scholar]
- 21.Lim JH, Park JK, Jo EK, Song CH, Min D, Song YJ, Kim HJ. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect Immun. 1999;67:6187–90. doi: 10.1128/iai.67.11.6187-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song CH, Lee JS, Lee SU, Lim K, Kim HJ, Park JK, Paik TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-a, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003;23:194–201. doi: 10.1023/a:1023309928879. [DOI] [PubMed] [Google Scholar]
- 23.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–61. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majumder N, Bhattacharjee S, Bhattacharyya Majumdar S, Dey R, Guha P, Pal NK, Majumdar S. Restoration of impaired free radical generation and inflammatory cytokines by MCP-1 in mycobacterial pathogenesis. Scand J Immunol. 2008;67:329–39. doi: 10.1111/j.1365-3083.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 25.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–11. [PubMed] [Google Scholar]
- 26.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 27.Fietta AM, Morosini M, Meloni F, Bianco AM, Pozzi E. Pharmacological analysis of signal transduction pathways required for Mycobacterium tuberculosis-induced IL-8 and MCP-1 production in human peripheral monocytes. Cytokines. 2002;19:242–9. [PubMed] [Google Scholar]
- 28.Maiti D, Bhattacharyya A, Basu J. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2001;276:329–33. doi: 10.1074/jbc.M002650200. [DOI] [PubMed] [Google Scholar]
- 29.Yang CS, Song CH, Lee JS, et al. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol. 2006;8:1158–71. doi: 10.1111/j.1462-5822.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 30.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 31.Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8:296–307. doi: 10.1038/nrmicro2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 2005;280:20961–7. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- 33.Bashir N, Kounsar F, Mukhopadhyay S, Hasnain SE. Mycobacterium tuberculosis conserved hypothetical protein rRv2626c modulates macrophage effector function. Immunology. 2010;130:34–45. doi: 10.1111/j.1365-2567.2009.03196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


