Abstract
Envelope glycoproteins of human endogenous retrovirus (HERV), such as syncytin 1 (HERV-W), are highly expressed in the placenta and some family members have immunomodulatory properties. Placental microvesicles (MV), which are shed into the maternal circulation during pregnancy, have been demonstrated to induce immune cell activation. Therefore, the aim of this study was to investigate the immunological properties of the highly expressed placental HERV-W protein, syncytin 1, and its potential involvement in placental MV modulation of immune cell activity. The MV shed from first trimester, normal term and pre-eclamptic term placentas, and from the BeWo trophoblast cell line, all contain syncytin 1. Recombinant syncytin 1 and syncytin 1-positive BeWo trophoblast MV both induced peripheral blood mononuclear cell (PBMC) activation, indicated through production of cytokines and chemokines. Reducing syncytin 1 content in BeWo MV inhibited PBMC activation. Recombinant syncytin 1 and syncytin-1-positive BeWo MV dampened PBMC responses to lipopolysaccharide challenge. Our findings suggest that syncytin 1 is shed from the placenta into the maternal circulation in association with MV, and modulates immune cell activation and the responses of immune cells to subsequent lipopolysaccharide stimulation. These studies implicate placental MV-associated HERV in fetal regulation of the maternal immune system.
Keywords: human endogenous retrovirus, immune cells, microvesicle, syncytin, trophoblast
Introduction
Approximately 8% of the human genome is comprised of sequences derived from human endogenous retroviruses (HERV),1 which have arisen as a result of retroviral infection and subsequent integration into the germ cell line. Over time, mutations have rendered most HERV non-functional and uninfective. However, some HERV have retained intact open reading frames and can be expressed as proteins, suggesting a biological function.2,3 Unlike most normal adult tissues, the placenta expresses a wide range of HERV.2,4
HERV-W, which encodes the protein, syncytin 1, is highly expressed in placenta,5 and is localized to the syncytiotrophoblast, cytotrophoblast and extravillous trophoblast.6–9 Syncytin 1 is involved in fusion of the cytotrophoblast with the syncytiotrophoblast, which is vital for normal placental development.6,9,10 As a result of their retroviral origin, placental HERV have also been hypothesized to prevent immune rejection of the feto–placental unit.11,12 This idea is supported by the identification of a putative immunosuppressive domain within the HERV-W peptide sequence, homologous to that found in other retroviruses.9 However, a more recent study has demonstrated that syncytin 1 lacks immunosuppressive function in a mouse model,3 while the envelope protein from a closely related W-family retrovirus, multiple sclerosis-associated retroviral element, has been demonstrated to have pro-inflammatory properties.13
During normal pregnancy, deported placental membrane-bound microvesicles (MV) are readily detected in the maternal circulation.14,15 We, and others have demonstrated that placental MV prepared by a number of different methods induce immune activation.16–20 The MV are shed from pre-eclamptic placentas at an increased rate,14,15,19 and they are more pro-inflammatory.16 However, despite these studies, little is known about the mechanism of immune cell activation by placental MV or the possible role of syncytin 1. Therefore, the objective of this study was to investigate the presence of syncytin 1 in placental MV and to determine whether placental syncytin 1 has any immunomodulatory function.
Materials and methods
Patient samples
First-trimester placentas were obtained following elective social terminations of pregnancy (8–12 weeks) (n = 5). Normal term placentas (n = 4) and term pre-eclamptic placentas (n = 4) were obtained following caesarean section or spontaneous vaginal delivery (37–42 weeks). Both were obtained with written informed patient consent and local ethics committee approval. Exclusion criteria were: twin pregnancy, maternal infection, diabetes, pre-existing hypertension and babies with chromosomal abnormalities. Pre-eclamptic pregnancies were defined by new hypertension arising after 20 weeks of gestation with measurements over 140/90 mmHg on two occasions and concurrent proteinuria of at least 0·3 g over 24 hr. All normal term placentas were associated with babies with a birthweight between the 10th and 90th centiles. Villous tissue was washed in sterile PBS, and dissected in 1 : 1 Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco, Grand Island, NY) containing 2 mm L-glutamine, 100 μg/ml streptomycin and 100 units/ml penicillin (GSP).
Placental microvesicle generation
Placental villous explants (2–4 mm2) were placed into 47-μm mesh Netwells (Corning, Corning, NY) in serum-free 1 : 1 DMEM/F12 with GSP (Gibco) and incubated for 24 hr in 6% oxygen, 5% CO2 at 37°. The MV were generated at 6% oxygen, because this is the estimated physiological level in normal placenta after 11 weeks.19,20 Medium containing shed material was collected from the bottom of the microplates and pooled, then transferred to sterile ultracentrifuge tubes and subjected to a three-step centrifugation at 4°: 1000 g for 10 min; 10 000 g for 10 min; and 70 000 g for 90 min. The final MV-containing pellet was resuspended in sterile PBS.
BeWo microvesicle generation
BeWo cells were cultured in 1 : 1 DMEM/F12 supplemented with 10% fetal bovine serum (Gibco) and 1% GSP until 60–70% confluent and then transferred to serum-free OptiMem (Invitrogen, Grand Island, NY) containing 1% GSP for 72 hr. Medium was subjected to two sequential rounds of centrifugation (1000 g, 10 min) to remove cell debris, then ultracentrifuged (70 000 g, 180 min, 4°). The final MV-containing pellet was resuspended in sterile PBS.
Detection of syncytin 1 protein expression
Microvesicle protein content was quantified using the Pierce BCA assay (Thermo Scientific, Rockford, IL). Proteins were analysed by Western blot using a rabbit anti-human syncytin 1 surface subunit polyclonal antibody at 0·1 μg/ml (Santa Cruz Biotechnology, Santa Cruz, CA) and a secondary anti-rabbit-IgG conjugated with horseradish peroxidase at 1 : 10 000 (Dako, Carpinteria, CA), followed by enhanced chemiluminescence.
Microvesicle–macrophage interactions
Green fluorescently labelled MV were prepared by staining BeWo cells with PKH67 (Sigma, St Louis, MO), following the manufacturer’s instructions. Labelled cells were then replated and cultured, and the shed MV were isolated as before. The THP-1 monocytic cell line was differentiated for 72 hr into adherent macrophages using 10 ng/ml PMA. Fluorescently labelled MV were incubated with PMA-treated THP-1 cells for 4 hr. Cells were rinsed vigorously to remove unbound MV and fixed in ice-cold methanol before visualization with an Observer.Z1 fluorescence microscope and AxioCam MRN using axiovision 4.6 imaging software (Zeiss, Welwyn Garden City, Hertfordshire, UK).
Peripheral blood mononuclear cell isolation and activation
Venous blood from non-pregnant healthy donors was collected, and peripheral blood mononuclear cells (PBMC) were isolated using Histopaque 1077 (Sigma), and then plated in 24-well low-binding plates (HydroCell; Nunc, Rochester, NY) at 1 × 106 cells per well in 250 μl OptiMem. After resting for 2 hr at 37°, PBMC were treated with 10 μg/ml partial recombinant syncytin 1 protein (ERVWE1; NP_055405, 116 to 215 amino acids; Abnova, Walnut, CA) or 20 μg/ml sterile MV. Treatment with 10 ng/ml lipopolysaccharide (LPS) isolated from Escherichia coli (serotype 0111:B4; Sigma) served as a positive control. After 24 hr, cell-free supernatants were collected and stored at −80°. For priming experiments, PBMC were pre-treated with or without MV (20 μg/ml) or recombinant syncytin 1 (10 μg/ml) for 2 hr, followed by incubation with medium alone or LPS (10 ng/ml) for 24 hr. Supernatants were initially analysed for interleukin-1β (IL-1β) by ELISA (R&D Systems, Minneapolis, MN) as a marker for inflammation/immune activation. To explore a broader range of pro-inflammatory and anti-inflammatory cytokines and chemokines that are representative of immune cell activation and immunomodulation, levels of IL-2, IL-6, IL-8, IL-10, IL-12, IL-17, granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFNγ), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1-alpha (MIP-1α), MIP-1β, regulated on activation, normal T-cell expressed and secreted (RANTES), vascular endothelial growth factor (VEGF), tumour necrosis factor-alpha (TNF-α) and growth-related oncogene-alpha (GRO-α) were also measured using a 17-Bio-Plex assay (Bio-Rad, Hercules, CA) run on the Luminex multiplex 200 system (Upstate Biotechnology, Billerici, MA).
Knockdown of syncytin 1
Knockdown of syncytin 1 in BeWo cells was performed using two sequences from a Stealth Select RNAi™ Set (Invitrogen) designed to target HERV-W mRNA; A (5′-GGUACUGGCAUUGGCGGUAUCACAA-3′) and B (5′-CCUGUAUCUUUAACCUCCUUGUUAA-3′). Cells were suspended in Amaxa cell line solution L and mixed with 500 nm HERV-W small interfering RNA (siRNA) sequence A or B or Silencer negative control scrambled siRNA (Ambion, Grand Island, NY), then nucleofected using program X-005 (Amaxa Biosystems, Walkersville, MD). Following transfection, cells were plated and after 72 hr they were washed and changed into OptiMem. After 48 hr, MV were isolated. Twelve separate transfections were performed and were combined into four pools of three transfections each. Knockdown of HERV-W mRNA was confirmed by quantitative reverse transcription-PCR.
Quantitative real-time PCR
RNA was extracted from BeWo cells using TRIzol (Invitrogen) and quantified with the Quant-iT RiboGreen RNA kit (Invitrogen). Reverse transcription was carried out using the AffinityScript Multiple Temperature cDNA synthesis kit from Stratagene, Santa Clara, CA. Alongside each set of reactions, two control samples were included, one without reverse transcriptase and one without RNA. In addition, human reference total RNA (Stratagene) was run in quadruplicate as a calibrator for the reverse transcription reaction. The controls and calibrators were included in each subsequent PCR. The HERV-W primers have been previously reported.2 Real-time PCR was performed using the Brilliant SYBR Green qPCR master mix from Stratagene. Reactions were incubated at 95° for 10 min, followed by 40 cycles of 95° for 30 seconds, 60° for 1 min and 72° for 1 min. Each reaction was conducted in duplicate alongside the two controls and a no template control. Copy number was calculated using a standard curve prepared by serial dilution of placental cDNA and was expressed relative to the mean copy number for four internal calibrators included with each reverse transcriptase reaction. HERV-W mRNA levels were then normalized to the 18S housekeeping gene.
Statistical analysis
Analysis of IL-1β in individual supernatants, quantified by ELISA, was performed using the Mann–Whitney U-test. Analysis of cytokines/chemokines quantified by multiplex in pooled supernatants was performed by unpaired t-test. For analysis of the effects of MV or syncytin 1 protein on the LPS response, data were normalized to the LPS response and compared with this by one-way t-test. Responses to MV from siRNA-treated cells were normalized to control MV and compared with the PBMC response to MV generated from cells treated with scrambled siRNA. Statistical significance was classified as *P < 0·05, **P < 0·01 or ***P < 0·001.
Results
Trophoblast-derived microvesicles contain syncytin 1 protein
Primary trophoblast cells and the BeWo trophoblast cell line have both been shown to express syncytin 1.6,9,21,22 Therefore, we hypothesized that syncytin 1 may be released in association with shed MV, much like other placental proteins.22–29 Western blots revealed syncytin 1 bands at 50 000 and 73 000 molecular weight in MV shed from BeWo cells and first-trimester, normal term and pre-eclamptic term placental explants (Fig. 1).
Figure 1.
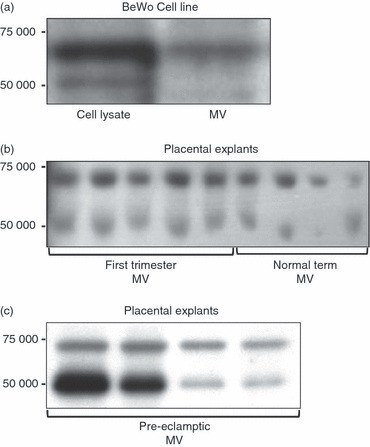
Syncytin 1 protein content in trophoblast microvesicles (MV). Western blot for syncytin 1 protein (50 000 and 73 000 molecular weight) was performed on: (a) BeWo cell lysates and BeWo-derived MV; (b) isolated MV shed from first-trimester (n = 5) and normal term (n = 4) placental explants; and (c) isolated MV shed from pre-eclamptic term placental explants (n = 4).
Trophoblast-derived microvesicles interact with macrophages
To investigate the interaction between syncytin 1-positive MV and immune cells, THP-1 monocytic cells were treated with PMA to induce differentiation into adherent macrophages, and then co-cultured with fluorescently labelled MV isolated from BeWo cells. After a 4-hr incubation, fluorescent BeWo cell-derived MV were both bound to, and internalized by, the macrophages (Fig. 2).
Figure 2.
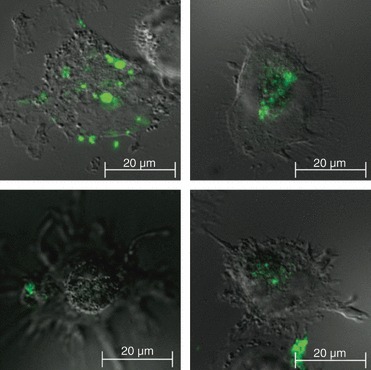
Interaction of microvesicles (MV) with macrophages. Fluoresence microscopy was used to visualize the interaction of PKH67-labelled BeWo-derived MV (green) with PMA-differentiated THP-1 cells. Images show fluorescent MV both bound to the surface of, and internalized into, the THP-1 cells. Images are representative of three independent experiments.
Syncytin 1-positive trophoblast microvesicles activate peripheral blood immune cells
We next investigated whether the interaction with syncytin 1-positive trophoblast MV or syncytin 1 protein would activate immune cells. The PBMC were maintained in suspension in low-binding plates and incubated with either syncytin 1-positive MV shed from BeWo cells or recombinant syncytin 1. Both treatments elevated the secretion of IL-1β from PBMC, although only the BeWo MV reached significance (Fig. 3a). Further investigation using multiplex analysis revealed that both BeWo MV and recombinant syncytin 1 significantly increased PBMC secretion of IL-6, IL-10, G-CSF, MCP-1, MIP-1α, MIP-1β, RANTES and GRO-α (Table 1). BeWo MV, but not the recombinant syncytin 1, also significantly increased PBMC secretion of IL-2, IL-8, IL-12 and TNF-α (Table 1).
Figure 3.
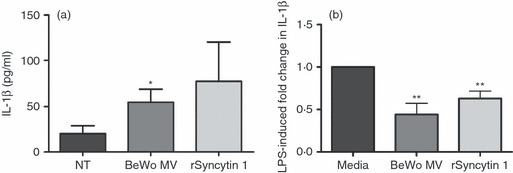
Activation and priming of peripheral blood mononuclear cells (PBMC) by syncytin 1 protein and syncytin-1-positive trophoblast-derived microvesicles (MV). The effect of syncytin-1-positive MV on baseline PBMC activation and on the ability of PBMC to respond to lipopolysaccharide (LPS) stimulation was examined. (a) PBMC were treated with: no treatment (NT), 20 μg/ml BeWo cell-derived microvesicles (BeWo MV), or 10 μg/ml recombinant syncytin 1 protein (rSyncytin 1) (n = 6). Following a 24-hr incubation, supernatants were collected and assayed for interleukin-1β (IL-1β) by ELISA. (b) PBMC were pre-treated with either: medium, 20 μg/ml BeWo MV or 10 μg/ml rSyncytin 1 for 2 hr. After this pre-treatment, all cells were then treated with or without LPS for 24 hr and the supernatants were assayed for IL-1β by ELISA. Bar chart shows fold change in IL-1β production in response to LPS stimulation relative to LPS alone with no pre-treatment (media), which was set at 1. *P < 0·05; **P < 0·01 compared with the NT/media control, as determined by Mann–Whitney U-test.
Table 1.
Fold-change in cytokine/chemokine production by peripheral blood mononuclear cells in response to BeWo microvesicles (MV) and recombinant syncytin 1 protein
| Molecule | BeWo MV | rSyncytin | ||
|---|---|---|---|---|
| IL-2 | 1·2 (± 0·11) | * | 0·9 (± 0·04) | ns |
| IL-4 | 1·1 (± 0·05) | ns | 1·1 (± 0·03) | ns |
| IL-6 | 3·3 (± 0·53) | *** | 3·1 (± 8·18) | ** |
| IL-8 | 10·4 (± 1·86) | ** | 3·5 (± 1·13) | ns |
| IL-10 | 5·1 (± 0·69) | ** | 1·9 (± 0·11) | ** |
| IL-12 | 1·4 (± 0·08) | * | 1·3 (± 0·45) | ns |
| IL-17 | 0·7 (± 0·24) | ns | 1·0 (± 0·10) | ns |
| G-CSF | 2·4 (± 0·34) | ** | 2·5 (± 0·27) | *** |
| GM-CSF | 0·9 (± 2·32) | ns | 1·0 (± 2·02) | ns |
| IFN-γ | 1·3 (± 0·31) | ns | 1·0 (± 0·32) | ns |
| MCP-1 | 1·1 (± 4·09) | * | 2·0 (± 1·55) | *** |
| MIP-1α | 6·7 (± 2·19) | *** | 3·0 (± 0·34) | *** |
| MIP-1β | 3·0 (± 16·62) | *** | 2·7 (± 46·31) | * |
| RANTES | 0·9 (± 4·29) | * | 1·6 (± 11·82) | ** |
| TNF-α | 45·0 (± 2·87) | *** | 1·0 (± 0·01) | ns |
| VEGF | 1·0 (± 0·33) | ns | 1·1 (± 0·07) | * |
| GRO-α | 2·0 (± 44·89) | * | 2·5 (± 31·24) | ** |
Peripheral blood mononuclear cells were treated with 20 μg/ml BeWo MV (n = 6) or 10 μg/ml recombinant syncytin 1 protein (rSyncytin 1; n = 4). Following 24 hr of incubation, cell-free supernatants were pooled and assayed by multiplex analysis. Numbers represent fold change of cytokine/chemokine production after treatment with MV or recombinant syncytin relative to the level produced after no treatment. Standard errors of the means are presented in parenthesis. Analysis was performed by unpaired t-test before calculation of fold-change.
P < 0·05
P < 0·01
P < 0·001. ns indicates no significant difference.
IL-2, interleukin-2; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN-γ, interferon-γ; MCP-1, monocyte chemoattractant protein 1; MIP, macrophage inhibitory protein; RANTES, regulated on activation normal T-cell expressed and secreted; TNF-α, tumour necrosis factor alpha, vascular endothelial growth factor; GRO-α, growth-related oncogene-α.
Syncytin 1-positive trophoblast microvesicles and syncytin 1 protein modulate PBMC responses to LPS
We have previously demonstrated that placental MV throughout gestation dampened PBMC responses to LPS.16 Therefore, in this current study we sought to determine the role for syncytin 1 in this process. The effect of BeWo-derived MV and syncytin 1 protein on the immune cell responses to an infectious Toll-like receptor 4 stimulus was determined by pre-treating PBMC with MV or recombinant syncytin 1, followed by challenge with low-dose LPS. Untreated PBMC up-regulated IL-1β production in response to LPS (Fig. 3b). Pre-treatment of PBMC with either syncytin-1-positive BeWo MV or recombinant syncytin 1 significantly reduced LPS-induced production of IL-1β (Fig. 3b). This was further explored using multiplex analysis of cytokine/chemokine production. In addition to IL-1β, PBMC challenged with LPS produced significantly elevated levels of IL-2, IL-6, IL-8, IL-10, G-CSF, MCP-1, MIP-1α, MIP-1β, RANTES, TNF-α and GRO-α when compared with the no treatment control (Table 2: PBMC response to LPS). As shown in Table 2, pre-treatment of PBMC with either BeWo MV (+ BeWo MV) or recombinant syncytin 1 (+ rSyncytin 1) significantly reduced the LPS-induced PBMC secretion of IL-6, MCP-1 and TNF-α. In addition, pre-treatment of PBMC with BeWo MV, but not recombinant syncytin 1, significantly down-regulated LPS-induced secretion of IL-10, G-CSF, RANTES and GRO-α. Pre-treatment of PBMC with syncytin 1 protein, but not BeWo MV, augmented the LPS-induced RANTES response by PBMC and increased VEGF secretion (Table 2).
Table 2.
Fold-change in cytokine/chemokine production by immune cells in response to lipopolysaccharide (LPS) alone, and after priming with BeWo microvesicles (MV) or recombinant syncytin 1 protein
| Molecule | PBMC response to LPS | Change in response to LPS + BeWo MV | Change in response to LPS + rSyncytin 1 | |||
|---|---|---|---|---|---|---|
| IL-2 | 0·82 (± 0·02) | ** | 1·3 (± 0·11) | ns | 1·2 (± 0·22) | ns |
| IL-4 | 1·12 (± 0·03) | ns | 0·9 (± 0·02) | ns | 0·9 (± 0·01) | ns |
| IL-6 | 19·08 (± 68·19) | ** | 0·8 (± 4·76) | * | 0·8 (± 11·84) | * |
| IL-8 | 20·30 (± 150·10) | ** | 1·1 (± 2·30) | ns | 1·3 (± 3·27) | ns |
| IL-10 | 22·35 (± 1·38) | *** | 0·6 (± 1·63) | * | 1·1 (± 0·53) | ns |
| IL-12 | 0·95 (± 0·03) | ns | 0·9 (± 0·27) | ns | 1·3 (± 0·09) | ns |
| IL-17 | 0·90 (± 0·10) | ns | 0·6 (± 0·05) | ns | 0·7 (± 0·33) | ns |
| G-CSF | 23·93 (± 5·72) | ** | 0·7 (± 3·72) | * | 1·1 (± 0·73) | ns |
| GM-CSF | 0·75 (± 1·75) | ns | 1·1 (± 1·33) | ns | 1·2 (± 1·95) | ns |
| IFN-γ | 1·43 (± 0·16) | ns | 1·2 (± 0·30) | ns | 1·2 (± 0·61) | ns |
| MCP-1 | 1·12 (± 2·30) | * | 0·8 (± 4·14) | * | 0·9 (± 2·33) | * |
| MIP-1α | 19·65 (± 30·12) | ** | 0·8 (± 11·88) | ns | 1·0 (± 5·22) | ns |
| MIP-1β | 4·03 (± 4·72) | *** | 0·9 (± 50·45) | ns | 1·8 (± 13·75) | ns |
| RANTES | 1·41 (± 12·04) | ** | 0·7 (± 1·12) | ** | 1·4 (± 14·62) | * |
| TNF-α | 316·59 (± 33·35) | *** | 0·8 (± 43·42) | * | 0·2 (± 8·03) | ** |
| VEGF | 1·03 (± 0·14) | ns | 0·9 (± 0·10) | ns | 1·2 (± 0·14) | * |
| GRO-α | 3·79 (± 100·68) | ** | 0·8 (± 0·76) | ** | 1·0 (± 87·22) | ns |
Peripheral blood mononuclear cells were pre-treated with 20 μg/ml BeWo MV (n = 6) or 10 μg/ml recombinant syncytin 1 protein (rSyncytin 1; n = 4) for 2 hr followed by LPS for 24 hr. Supernatants were collected, pooled and assayed by multiplex. The peripheral blood mononuclear cells response to LPS alone was determined by comparison with no treatment and is represented in the table as fold change relative to no treatment. Change in the LPS response in the presence of BeWo MV and syncytin 1 was determined by comparison with LPS treatment alone and is represented in the table as fold change relative to the LPS response. Standard errors of the means are presented in parenthesis. Analysis was performed by one-way t test before calculation of fold-change.
P < 0·05
P < 0·01
P < 0·001. ns indicates no significant difference.
IL-2, interleukin-2; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN-γ, interferon-γ; MCP-1, monocyte chemoattractant protein 1; MIP, macrophage inhibitory protein; RANTES, regulated on activation normal T-cell expressed and secreted; TNF-α, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor; GRO-α, growth-related oncogene-α.
Activation of PBMC by trophoblast-derived MV is dependent on the expression of syncytin 1
To determine whether syncytin 1 plays a direct role in mediating the activation of immune cells by MV, siRNA-knockdown was performed in BeWo cells. Using two specific siRNA sequences, HERV-W mRNA levels were significantly reduced by ∼ 65% compared with cells treated with non-targeting siRNA (Fig. 4a). The PBMC were then treated with MV generated from either untransfected BeWo cells (wild-type MV); BeWo cells transfected with non-targeting siRNA (non-targeting siRNA MV); or BeWo cells transfected with HERV-W-specific siRNA sequences (syncytin siRNA A/B MV). As shown in Fig. 4(b), IL-1β production by PBMC was significantly elevated following treatment with MV shed from both wild-type and non-targeting siRNA control cells. Although MV shed from BeWo cells transfected with HERV-W siRNA also induced an increase in IL-1β, this was significantly less (∼ fourfold) than IL-1β secretion induced by MV from the control cells (Fig. 4b). As shown in Table 3, syncytin 1 knockdown significantly reduced the non-targeting siRNA MV induced up-regulation of IL-6, IL-10, G-CSF, MIP-1α, MIP-1β, TNF-α and GRO-α.
Figure 4.
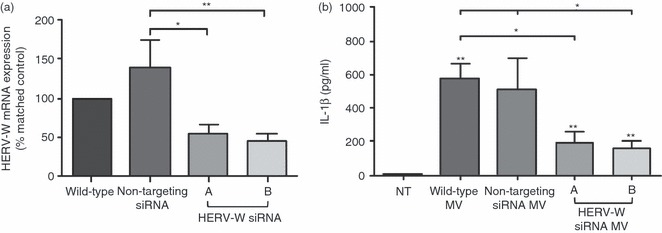
Microvesicle (MV) -induced peripheral blood mononuclear cell (PBMC) activation is dependent upon the presence of syncytin 1. (a) BeWo cells, either non-transfected (wild-type) or transfected with a non-targeting small interfering (si) RNA (500 nm) or two different HERV-W-specific siRNA sequences (a, b; 500 nm) were cultured for 72 hr and then analysed for HERV-W mRNA expression. HERV-W mRNA expression normalized to 18S mRNA was expressed relative to the wild-type control cells for each experiment and analysed by Mann–Whitney U-test (*P < 0·05, **P < 0·01 versus cells treated with non-targeting siRNA) (n = 5). (b) PBMC were incubated with either: no treatment (NT); or 20 μg/ml isolated MV derived from: wild-type BeWo cells (Wild-type MV); BeWo cells transfected with non-targeting siRNA (non-targeting MV); or BeWo cells transfected with HERV-W specific siRNA sequences a or b (HERV-W siRNA MV). After 24 hr, supernatants were collected and interleukin-1β (IL-1β) was measured by ELISA and analysed by Mann–Whitney U-test. *P < 0·05, **P < 0·01 compared with NT control unless otherwise specified (n = 6).
Table 3.
Fold-change in cytokine/chemokine production by immune cells in response to syncytin small interfering RNA microvesicles (syncytin siRNA MV)
| Molecule | Fold change relative to non-targeting siRNA MV | |
|---|---|---|
| IL-2 | 0·9 (± 0·04) | ns |
| IL-4 | 1·0 (± 0·04) | ns |
| IL-6 | 0·5 (± 0·05) | * |
| IL-8 | 1·5 (± 0·38) | ns |
| IL-10 | 0·8 (± 0·04) | * |
| IL-12 | 0·8 (± 0·02) | ns |
| IL-17 | 0·7 (± 0·05) | ns |
| G-CSF | 0·6 (± 0·05) | * |
| GM-CSF | 0·9 (± 0·04) | ns |
| IFN-γ | 0·9 (± 0·08) | ns |
| MCP-1 | 0·9 (± 0·04) | ns |
| MIP-1α | 0·4 (± 0·01) | ** |
| MIP-1β | 0·8 (± 0·01) | * |
| RANTES | 1·4 (± 0·04) | * |
| TNF-α | 0·5 (± 0·01) | * |
| VEGF | 1·0 (± 0·03) | ns |
| GRO-α | 0·9 (± 0·01) | * |
Peripheral blood mononuclear cells were treated with 20 μg/ml isolated MV derived from BeWo cells transfected with non-targeting siRNA, or BeWo cells transfected with human endogenous retrovirus W (HERV-W) -specific siRNA. Following a 24-hr incubation, cell-free supernatants were pooled and assayed by multiplex analysis. Numbers represent fold change of cytokine/chemokine production after treatment with syncytin siRNA MV relative to the level produced after treatment with non-targeting siRNA MV. Standard errors of the means are presented in parenthesis. Analysis was performed by unpaired t-test before calculation of fold-change.
P < 0·05
P < 0·01. ns indicates no significant difference.
IL-2, interleukin-2; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN-γ, interferon-γ; MCP-1, monocyte chemoattractant protein 1; MIP, macrophage inhibitory protein; RANTES, regulated on activation normal T-cell expressed and secreted; TNF-α, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor; GRO-α, growth-related oncogene-α.
Discussion
Human endogenous retroviral proteins such as syncytin 1 are highly expressed in the placenta, and are potential candidates for immunomodulators in pregnancy.11,12 Normal pregnancy is associated with the presence of circulating placental MV.30 It has been demonstrated that these MV have immunomodulatory activities,17–19,31 a finding supported by our own recent studies.16 In this study we have shown, for the first time, that trophoblast/placental-derived MV contain syncytin 1, and that MV-mediated immune cell activation is, at least partly, dependent on the presence of syncytin 1. Furthermore, we demonstrated that both syncytin-1-positive MV and recombinant syncytin 1 modulate immune cell responses to LPS. These studies implicate syncytin 1 in MV-mediated immune cell activation and immunomodulation in pregnancy.
Herein, we report that syncytin 1 is expressed in MV shed from first-trimester, term normal and pre-eclamptic term placental tissue, as well as from the BeWo trophoblast cell line. The presence of syncytin 1 in MV suggests active deportation of this placenta-specific protein into the maternal circulation during pregnancy. We, and others, have demonstrated the presence of syncytin 1 in the syncytiotrophoblast microvillous membrane,6,9,32,33 from where MV are shed. As a model, we show that BeWo cell-derived MV, positive for syncytin 1, are bound and taken up by monocyte-derived macrophages, an observation also made by others using trophoblast-derived MV or exosomes,19,34 although this is not necessarily a prerequisite for immune cell activation.34
Following incubation with either the BeWo trophoblast cell-derived, syncytin-1-positive MV, or a recombinant syncytin 1 fragment comprising 100 amino acids from the extracellularly facing SU subunit, PBMC secretion of an array of cytokines and chemokines was up-regulated, which, in general is consistent with a pro-inflammatory profile. This observation conflicts with speculations that syncytin 1 is immunosuppressive and tolerogenic, based on the presence of a putative immunosuppressive peptide.9 However, this sequence is located in the transmembrane subunit, which was not present in the recombinant protein. More importantly, an animal model of transplant rejection identifies syncytin 1 (uniquely among HERV) as lacking immunosuppressive activity;3 and studies of the highly related multiple sclerosis-associated retroviral element have identified the corresponding protein as having potent pro-inflammatory properties.13,35
We have recently demonstrated that first-trimester placental explant-derived MV induce PBMC activation, as shown by the up-regulation of IL-1β and a number of other cytokines.16 In this study, we have shown that these placental-derived MV16 express syncytin 1, and that both syncytin-1-positive BeWo MV and recombinant syncytin 1 have similar pro-inflammatory properties, so we developed an siRNA-knockdown model in which the shed MV lacked syncytin 1. For this we used the BeWo cell line, as such experiments are difficult to perform in primary trophoblast cells or explant cultures. The MV in which syncytin 1 was knocked down exhibited a markedly reduced pro-inflammatory activity compared with MV expressing syncytin, suggesting that syncytin 1 is, at least in part, responsible for the immune cell activation induced by placental MV.
We have also recently demonstrated that placental explant-derived MV modulate PBMC responses to LPS, with both first-trimester and term placental-derived MV dampening LPS-induced PBMC IL-1β production.16 Here, we also demonstrate the same effect with both BeWo cell-derived MV and recombinant syncytin 1. This suggests the induction of a state of endotoxin tolerance.36 When further cytokines and chemokines were evaluated, we found that IL-6, MCP-1 and TNF-α production in response to LPS were also reduced in the presence of either the MV or syncytin 1. This pattern for MCP-1 and TNF-α has been demonstrated before with placental MV from normal pregnancies.16 However, there were some differences between the MV-mediated and syncytin-mediated regulation of PBMC responses to LPS. BeWo-MV, but not syncytin 1, reduced the LPS-induced secretion of PBMC IL-10, G-SCF and GRO-α, whereas syncytin 1 enhanced the production of RANTES and VEGF. This might be explained by differences in the native and recombinant partial synctin 1 protein. For example, as mentioned earlier, the recombinant syncytin 1 used in these studies is a partial protein. Alternatively, these differences may be attributed to other immunomodulatory proteins within the trophoblast MV.23–29,34 For example, trophoblast-derived exosomes can also induce IL-1β production by macrophages through their expression of fibronectin.34 Whether the MV from our studies express fibronectin, however, is currently unknown.
This study is the first demonstration that a placental human endogenous retroviral protein may be pro-inflammatory and strengthens evidence that the immunomodulatory function of HERV may be ‘uncoupled’ from their fusogenic function.3 The shedding of placental HERV into maternal circulation via MV may represent a means of interaction between placental HERV and the mother that facilitates an immune dialogue at a systemic level. The role of syncytin 1 in the aberrant immune responses associated with pregnancy pathologies, such as pre-eclampsia warrants further investigation, particularly in light of our finding that MV from pre-eclamptic placentas are intrinsically more pro-inflammatory.16
Acknowledgments
The authors would like to thank Rebecca Jones and Sylvia Lui for help and support. We thank staff at St Marys Hospital, Manchester for help in obtaining placentas. This work was supported in part by the McKern Scholar Award for Perinatal Research (VMA); the Medical Research Council; and Tommy’s, the baby charity.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 2.de Parseval N, Lazar V, Casella JF, Benit L, Heidmann T. Survey of human genes of retroviral origin: identification and transcriptome of the genes with coding capacity for complete envelope proteins. J Virol. 2003;77:10414–22. doi: 10.1128/JVI.77.19.10414-10422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangeney M, Renard M, Schlecht-Louf G, et al. Placental syncytins: genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci U S A. 2007;104:20534–9. doi: 10.1073/pnas.0707873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muir A, Lever A, Moffett A. Expression and functions of human endogenous retroviruses in the placenta: an update. Placenta. 2004;25(Suppl. A):S16–25. doi: 10.1016/j.placenta.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Blond J-L, Beseme F, Duret L, Bouton O, Bedin F, Perron H, Mandrand B, Mallet F. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J Virol. 1999;73:1175–85. doi: 10.1128/jvi.73.2.1175-1185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frendo JL, Olivier D, Cheynet V, et al. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol. 2003;23:3566–74. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holder BS, Abrahams VM, Tower CL, Jones C, Aplin JD. Syncytin 1 in the human placenta. Placenta. 2012 doi: 10.1016/j.placenta.2012.02.012. ; In Press. [DOI] [PubMed] [Google Scholar]
- 8.Malassiné A, Handschuh K, Tsatsaris V, Gerbaud P, Cheynet V, Oriol G, Mallet F, Evain-Brion D. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta. 2005;26:556–62. doi: 10.1016/j.placenta.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Mi S, Lee X, Li X, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–9. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 10.Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochim Biophys Acta. 2003;1638:63–71. doi: 10.1016/s0925-4439(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 11.Noorali S, Rotar IC, Lewis C, Pestaner JP, Pace DG, Sison A, Bagasra O. Role of HERV-W syncytin-1 in placentation and maintenance of human pregnancy. Appl Immunohistochem Mol Morphol. 2009;17:319–28. doi: 10.1097/PAI.0b013e31819640f9. [DOI] [PubMed] [Google Scholar]
- 12.Villarreal LLP, Villareal LLP. On viruses, sex, and motherhood. J Virol. 1997;71:859–65. doi: 10.1128/jvi.71.2.859-865.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol. 2006;176:7636–44. doi: 10.4049/jimmunol.176.12.7636. [DOI] [PubMed] [Google Scholar]
- 14.Knight MM, Redman CCW, Linton EEA, Sargent IIL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–40. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 15.Goswami DD, Tannetta DDS, Magee LLA, Fuchisawa AA, Redman CCWG, Sargent IIL, von Dadelszen PP. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Holder BS, Tower CL, Jones C, Aplin JD, Abrahams VM. Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.097014. ; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Messerli M, May K, Hansson SR, Schneider H, Holzgreve W, Hahn S, Rusterholz C. Feto-maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes. Placenta. 2010;31:106–12. doi: 10.1016/j.placenta.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Germain SJ, Sacks GP, Soorana SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–56. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 19.Patel J, Landers K, Mortimer RH, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010;31:951–7. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Robinson NJ, Wareing M, Hudson NK, Blankley RT, Baker PN, Aplin JD, Crocker IP. Oxygen and the liberation of placental factors responsible for vascular compromise. Lab Invest. 2008;88:293–305. doi: 10.1038/labinvest.3700746. [DOI] [PubMed] [Google Scholar]
- 21.Kudo Y, Boyd CA. Changes in expression and function of syncytin and its receptor, amino acid transport system B(0) (ASCT2), in human placental choriocarcinoma BeWo cells during syncytialization. Placenta. 2002;23:536–41. doi: 10.1053/plac.2002.0839. [DOI] [PubMed] [Google Scholar]
- 22.Vargas A, Moreau J, Landry S, LeBellego F, Toufaily C, Rassart E, Lafond J, Barbeau B. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J Mol Biol. 2009;392:301–18. doi: 10.1016/j.jmb.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 24.Atay S, Gercel-Taylor C, Kesimer M, Taylor DD. Morphologic and proteomic characterization of exosomes released by cultured extravillous trophoblast cells. Exp Cell Res. 2011;317:1192–202. doi: 10.1016/j.yexcr.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Frängsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva-Nilsson L. Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod. 2005;11:35–41. doi: 10.1093/molehr/gah129. [DOI] [PubMed] [Google Scholar]
- 26.Guller S, Tang Z, Ma YY, Di Santo S, Sager R, Schneider H. Protein composition of microparticles shed from human placenta during placental perfusion: potential role in angiogenesis and fibrinolysis in preeclampsia. Placenta. 2011;32:63–9. doi: 10.1016/j.placenta.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lok CA, Boing AN, Sargent IL, Sooranna SR, van der Post JA, Nieuwland R, Sturk A. Circulating platelet-derived and placenta-derived microparticles expose Flt-1 in preeclampsia. Reprod Sci. 2008;15:1002–10. doi: 10.1177/1933719108324133. [DOI] [PubMed] [Google Scholar]
- 28.Mincheva-Nilsson L, Nagaeva O, Chen T, Stendahl U, Antsiferova J, Mogren I, Hernestal J, Baranov V. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J Immunol. 2006;176:3585–92. doi: 10.4049/jimmunol.176.6.3585. [DOI] [PubMed] [Google Scholar]
- 29.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–33. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 30.Redman CWG, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29:73–7. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–54. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Muir A, Lever AM, Moffett A. Human endogenous retrovirus-W envelope (syncytin) is expressed in both villous and extravillous trophoblast populations. J Gen Virol. 2006;87:2067–71. doi: 10.1099/vir.0.81412-0. [DOI] [PubMed] [Google Scholar]
- 33.Blond JL, Lavillette D, Cheynet V, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–9. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory Il-1β production by macrophages. Am J Reprod Immunol. 2011;66:259–69. doi: 10.1111/j.1600-0897.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 35.Antony JM, van Marle G, Opii W, et al. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7:1088–95. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- 36.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:476–87. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]


