Abstract
Podoplanin is a transmembrane glycoprotein indirectly linked to classic cadherins through ezrin-actin networks. Recently, the overexpression of podoplanin in high-grade malignancy brain tumors has been reported. The aim of this study was to investigate the expression of podoplanin and classic cadherins in the mouse brain. Immunohistochemistry showed that podoplanin was expressed on ependymal cells and choroid plexus epithelial cells at the ventricle side of the cell surface and at the cell–cell junctions, and on retinal pigment epithelial cells and in the pia mater; P-cadherin between choroid plexus epithelial cells and endothelial cells at the basement membrane side of cell surface, and between retinal pigment epithelial cells; VE-cadherin on the PECAM-1 positive-choroid plexus endothelial cells of the fibrovascular core; and N-cadherin on the cell surface and at the cell–cell junctions of ependymal cells, and in the pia mater. The regions expressing podoplanin, P-cadherin, and VE-cadherin did not coincide. In real-time PCR analysis, the amounts of podoplanin and P- and N-cadherin mRNA were larger in the ventricular wall with choroid plexus than in the abdominal aorta and cerebrum. In the RT-PCR analysis, the intensities of amplicon for VE-cadherin mRNA were the same for the abdominal aorta, cerebrum, and ventricular wall with the choroid plexus, suggesting that mouse ependymal cells, choroid plexus epithelial cells, and glial cells under the pia mater have the ability to express podoplanin and P- and N-cadherins. Glial cells and retinal pigment epithelial cells may create barriers by podoplanin and classic cadherins as a rate-determining step for transmission of blood components.
Keywords: choroid plexus, functional human anatomy, P-cadherin, podoplanin
Introduction
Podoplanin is a mucin-type transmembrane glycoprotein first identified in podocytes, kidney glomerular epithelial cells (Breiteneder-Geleff et al. 1997). The role of podoplanin has been studied in a rat model of nephropathy caused by puromycin aminonucleoside nephrosis with severe proteinuria. Podoplanin is thought to play a role in maintaining the shape of podocyte foot processes and glomerular permeability because of morphological alterations of cell shapes with selective losses of podoplanin in the nephrosis accompanying proteinuria (Koop et al. 2008). Podoplanin upregulates RhoA-GTPase activity, resulting in the phosphorylation of ezrin. The increase in the cell surface distribution of phosphorylated ezrin promotes the actin cytoskeleton rearrangement by the development of membrane-actin structures, thus contributing to actin filament rearrangement. Podoplanin links and stabilizes the phosphorylated ezrin mediating the anchorage of F-actin to the plasma membrane. Since an actin filament links classic cadherins through cytoplasmic catenins, podoplanin indirectly interacts with classical cadherins through ezrin-actin networks (Aberle et al. 1996; Gautreau et al. 1999; Scholl et al. 1999; Pujuguet et al. 2003; Lan et al. 2006; Lien et al. 2006; Martín-Villar et al. 2006; Wicki et al. 2006; Raica et al. 2008; Belkina et al. 2009; Harris & Tepass, 2010; Noda et al. 2010). It has been reported that podoplanin promotes the epithelial to mesenchymal transition (EMT) in cancer cells. EMT represents a phenotypic conversion by which epithelial cells lose polarity and acquire fibroblast characteristics. The podoplanin-induced EMT is accompanied by downregulation of E-cadherin and upregulation of mesenchymal N-cadherin in several cancers (Martín-Villar et al. 2006; Wicki et al. 2006). It is expected that podoplanin expression may be a close link in the regulation of classical cadherin expressions.
Classical cadherins are calcium-dependent adhesion molecules at adherens junctions and combine cytoplasmic β-catenin, which binds to α-catenin, with the actin filament networks. In cell–cell contacts, E-, P- and VE-cadherins play a key role at adherens junctions of epithelium and endothelium. Soluble E-cadherin also promotes cell survival and N-cadherin functions in the nervous system (Nose & Takeichi, 1986; Aberle et al. 1996; Halbleib & Nelson, 2006; Lien et al. 2006; Harris & Tepass, 2010; Inge et al. 2011). Recently, it has been reported that VE-cadherin is reduced in cortical microvessels with tight junctions but is present in microvessels of circumventricular organs (CVO). It was also reported that cadherin-10 is expressed in the retinal pigment epithelial cells, cortical microvessels, choroid plexuses epithelial cells, and ependymal cells lining CVO (Williams et al. 2005).
The expression of podoplanin has also been reported in tumors of the central nervous system: ependymal tumors, choroid plexus papillomas, meningiomas, astrocytic tumors, medulloblastomas, and hemangioblastomas, with variable frequency and intensity. The podoplanin expression in astrocytic tumors is associated with malignant properties as podoplanin expression is markedly higher in glioblastomas than in anaplastic astrocytomas (Mishima et al. 2006a,b; Shibahara et al. 2006; Raica et al. 2008; Ishizawa et al. 2009). Therefore, it is thought that glia cells, e.g. astrocytes and ependymal cells, usually express podoplanin; however, the distribution of podoplanin-positive cells in the normal brain has not been reported. This study set out to examine the expression of classic cadherins and podoplanin in the non-CNS tissue around ventricles and in the retina.
Materials and methods
Immunohistochemistry
Tissue of the brain, tongue, and heart of 7-week-old male ICR mice and the placenta from ICR mice at the 16th day of pregnancy (n = 5) (Charles River Japan Inc., Yokohama, Japan) were used. The collection of the tissue was conducted after euthanasia by intraperitoneal injection with sodium pentobarbital (10 mL kg−1, Nembutal; Abbott Laboratories, North Chicago, IL, USA). The protocol for animal use was reviewed and approved by the animal experiment committee of Fukuoka Dental College in accordance with the principles of the Helsinki Declaration. Mice were perfused through the heart with 100% methanol and tissue was fixed in 100% methanol for 10 min at −20 °C, and then immersed in 30% sucrose-phosphate-buffered saline (PBS) for 12 h at 4 °C before freezing. The frozen 10-μm sections were cut in a cryostat and fixed in 100% methanol for 5 min at −20 °C. The sections were treated with 0.1% goat serum for 30 min at 20 °C and then the sections were treated with PBS containing 0.1% goat serum and primary antibodies: 2 μg mL−1 of monoclonal hamster anti-mouse podoplanin (AngioBio Co., Del Mar, CA, USA), 4 μg mL−1 of monoclonal rat anti-mouse P-cadherin (R&D Systems Inc., Minneapolis, MN, USA), 4 μg mL−1 of polyclonal rabbit anti-mouse N-cadherin (Abcam plc., Cambridge, UK), and 1.5 μg mL−1 of polyclonal rabbit anti-mouse VE-cadherin (Abcam), for 8 h at 4 °C. Further, 2 μg mL−1 of rat monoclonal anti-mouse CD31 (PECAM-1) and rabbit polyclonal anti-mouse PECAM-1 (Abcam) were used to identify blood vessels. After the treatment with primary antibodies, the sections were washed three times in PBS for 10 min and immunostained for 0.5 h at 20 °C with 0.1 μg mL−1 of secondary antibodies: Alexa Fluor (AF) 488 or 568-conjugated goat anti-hamster, goat anti-rabbit, or goat anti-rat IgGs (Probes Invitrogen Co., Eugene, OR, USA). The immunostained sections were mounted in 50% polyvinylpyrrolidone solution and examined by fluorescence microscopy (BZ-8100; Keyence Corp., Osaka, Japan) or laser-scanning microscopy (LSM710; Carl Zeiss, Jena, Germany) with a ×63 oil planapochromatic objective lens (numerical aperture ×1.3). The Chinese hamster ovary (CHO) K1 cells (CRL-2243) from the American Type Culture Collection (Manassas, VA, USA) and the human tongue squamous cell carcinoma cell line (HSC-3) from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan) were cultured in Dulbecco’s Modified Eagle Medium (Wako Pure Chemical Industries, Osaka, Japan) including 2 mm l-glutamine (Wako) supplemented with 10% heat-inactivated fetal bovine serum (Wako) on the coverslips in six-well plates. After forming the 100% confluent monolayer, the coverslips with cells were immersed in 100% methanol for 5 min at −20 °C. After the treatment with 0.1% goat serum for 30 min at 20 °C, the cells were treated with PBS containing 0.1% goat serum and primary antibodies: 2 μg mL−1 of hamster anti-mouse podoplanin (AngioBio), 4 μg mL−1 of rat anti-mouse P-cadherin (R&D Systems), 4 μg mL−1 of rabbit anti-mouse N-cadherin (Abcam), 1.5 μg mL−1 of rabbit anti-mouse VE-cadherin (Abcam), and 2 μg mL−1 of rat and rabbit anti-mouse PECAM-1 (Abcam) for 8 h at 4 °C. After the treatment with primary antibodies the slips were washed three times in PBS for 10 min. Both cells treated with primary antibodies and cells without them were treated for 0.5 h at 20 °C with 0.1 μg mL−1 of secondary antibodies: Alexa Fluor (AF) 488 or 568-conjugated goat anti-hamster, goat anti-rabbit, or goat anti-rat IgGs (Invitrogen). The coverslips with the cells were mounted in 50% polyvinylpyrrolidone solution on slide glasses and examined by fluorescence microscopy (BZ-8100; Keyence Corp.).
Reverse transcription (RT)-PCR and real-time PCR
The abdominal aorta was cut to 5 mm length, and the cortex surface with pial and the ventricle wall with choroid plexus were peeled away from the cerebrum within a 5-mm square with an 18-gauge needle. The total RNA extraction from the tissue of the aorta, cortex surface, and ventricular wall was performed with a QIAshredder column and RNeasy kit (Qiagen, Inc., Tokyo, Japan). Contaminating genomic DNA was removed using DNAfree (Ambion, Huntingdon, UK), and the RT was performed on 30 ng of total RNA, followed by 25–30 cycles of PCR for amplification using the Ex Taq hot start version (Takara Bio Inc., Otsu, Japan) with 50 pm of primer sets (Table 1) where the specificities had been confirmed by the manufacturer (Sigma-Genosys Ltd., Cambridge, UK). The RT-PCR products were separated on 2% agarose gel (NuSieve; FMC, Rockland, ME, USA) and visualized by Syber Green (Takara). The correct size of the amplified PCR products was confirmed by gel electrophoresis and amplification of accurate targets was confirmed by sequence analysis.
Table 1.
Primer sequences.
| Gene names | Base pairs | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| Podoplanin | 332 | CACCTCAGCAACCTCAGAC | ACAGGGCAAGTTGGAAGC |
| P-cadherin | 199 | GCAGAAGTCAGCGAGAAAGGAG | GGAGGATGAAACCACCCTTCCA |
| N-cadherin | 119 | CCACAGTACCCAGTCCGATCC | ACTAAGAGGGAGTCATACGGTGG |
| VE-cadherin | 116 | AACCGCTGATCGGCACTGT | TCCCTGCTTGGTTATTCGGAAG |
| β-Actin | 411 | GTTCTACAAATGTGGCTGAGGA | ATTGGTCTCAAGTCAGTGTACAG |
To quantify podoplanin and cadherin mRNA generation, cDNA samples were analyzed by real-time quantitative PCR. A total of 1 μL of cDNA was amplified in a 25-μL volume of PowerSYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in a Stratagene mx3000p real-time PCR system (Agilent Technologies, Inc., Santa Clara, CA, USA), and the fluorescence was monitored at each cycle. Cycle parameters were 95 °C for 15 min to activate Taq followed by 35 cycles of 95 °C for 15 s, 58 °C for 1 min, and 72 °C for 1 min. For the real-time analysis, two standard curves were constructed from amplicons for both the β-actin and target genes (podoplanin, P-cadherin, N-cadherin, VE-cadherin) in three serial fourfold dilutions of cDNA from sublingual gland tissue. The β-actin or target gene cDNA levels in each sample were quantified against β-actin or the target gene standard curves by allowing the mx3000p software to determine each cDNA unit accurately. Finally, target gene cDNA units in each sample were normalized to β-actin cDNA units. Thus, the relative target gene expression units were expressed as arbitrary units, calculated according to the following formula: relative target gene expression units = target gene cDNA units/β-actin cDNA units.
Statistics
All experiments were repeated five times, and data are expressed as mean + SEM. The statistical significance of differences (P < 0.01) was determined by the Student’s t-test and one-way anova with statview 4.51 software (Abacus concepts, Calabasas, CA, USA).
Results
Control experiments for the immunostaining with antibodies for mouse podoplanin and classical cadherins
The CHO K1 and HSC-3 cells were not immunostained by anti-podoplanin and anti-cadherins, and none of the mouse tissue was immunostained with the secondary antibodies alone (data not shown). In the mouse tongue tissue, anti-podoplanin reacted with lymphatic vessels but did not cross-react with blood vessels, lingual muscle or connective tissue. Anti-VE-cadherin reacted with blood vessels and weakly with lymphatic vessels but did not cross-react with other lingual tissue. In the placenta, anti-podoplanin reacted with syncytiotrophoblasts of placenta villus and anti-P-cadherin reacted with decidua. In the heart, anti-podoplanin reacted with epicardium and anti-N-cadherin reacted with epicardium and intercalated disks but did not cross-react with cardiac muscle (Fig. 1).
Fig. 1.
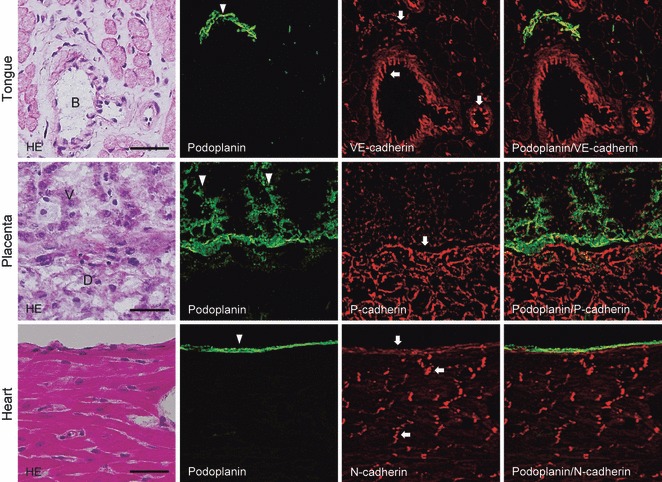
Immunostaining for podoplanin, P-, N- and VE-cadherin in mouse tissue. The immunostained sections were re-stained by hematoxylin-eosin (H-E) staining, which shows blood vessels (B) of the muscle layer of the tongue, the villus (V), and decidua (D) of the placenta, and the cardiac muscle and epicardium of the heart. The fluorescence with anti-podoplanin (arrowhead) is found on the lymphatic vessel of the tongue, on syncytiotrophoblasts of the placenta villus, and on the epicardium. The fluorescence with anti-VE-cadherin (arrows) is found on blood vessels, and weakly on the lymphatic vessel of the tongue. The lymphatic vessel immunostained with both anti-podoplanin and anti-VE-cadherin is shown in the merged image. The fluorescence with anti-P-cadherin (arrows) is found on the decidua of the placenta. The separation of the area immunostained with anti-podoplanin and anti-P-cadherin in the placenta is shown in the merged image. The fluorescence with anti-N-cadherin (arrows) is found on epicardium and intercalated disks without a cross-reaction with cardiac muscle. The epicardium immunostained with both anti-podoplanin and anti-N-cadherin is shown in the merged image. The anti-podoplanin and ant-cadherins did not show a cross-reaction with connective tissue or muscle. Bar: 50 μm.
Immunostaining for podoplanin and classic cadherins in mouse ventricles and retina
In the lateral ventricle, anti-podoplanin reacted with the surface of ependymal cells and choroid plexus epithelial cells at the region of the ventricle surrounded by corpus callosum, caudate nucleus, and fimbria hippocampus (Fig. 2). Anti-P-cadherin reacted with the inside of choroid plexus where anti-podoplanin did not react. Anti-N-cadherin reacted with ependymal cells at the region where anti-podoplanin also reacted, but not with choroid plexus epithelial cells (Fig. 2). In the third ventricle, anti-podoplanin reacted with the surface of ependymal cells and choroid plexus epithelial cells at the region of the ventricle surrounded by red nuclei (Fig. 3). Anti-P-cadherin reacted with the inside of choroid plexus where anti-podoplanin did not react. Anti-N-cadherin reacted with ependymal cells and the pia mater of the fornix at the region where anti-podoplanin reacted, but not with choroid plexus epithelial cells (Fig. 3). In the fourth ventricle, anti-podoplanin reacted with ependymal cells and choroid plexus epithelial cells at the region of the ventricle surrounded by nodulus and cerebellar lingual, and on the pia mater around the cerebellar lingual (Fig. 4). Anti-P-cadherin reacted with the inside of the choroid plexus where anti-podoplanin did not react. Anti-N-cadherin reacted with ependymal cells and the pia mater of the cerebellum at the region where anti-podoplanin reacted, but not on choroid plexus epithelial cells (Fig. 4). In the laser-scanning confocal microscopy of the immunostaining for podoplanin, and P- and VE-cadherins on the choroid plexus and ventricle epithelia, the fluorescence with anti-podoplanin was found on ependymal cells and epithelial cells at the ventricle side both on the cell surface and in the cell–cell junctions, whereas the fluorescence with anti-P-cadherin was found on the choroid plexus epithelial cells at the basement membrane side both on the cell surface and in the cell–cell junctions (Fig. 5). The fluorescence with anti-VE-cadherin was found on the choroid plexus endothelial cells of the fibrovascular core on the cell surface and in the cell–cell junctions. The regions that reacted with anti-podoplanin, anti-P-cadherin, and anti-VE-cadherin did not coincide (Fig. 5). In the laser-scanning confocal microscopy of the immunostaining for podoplanin, and P- and VE-cadherins on the choroid plexus fibrovascular core, the fluorescence with both anti-PECAM-1 and anti-VE-cadherin was found on the endothelial cells of the fibrovascular core in the choroid plexus and the fluorescence with anti-podoplanin and anti-P-cadherin was found on choroid plexus epithelial cells on the cell surface at the ventricle side and at the basement membrane side, respectively. The region reacting with anti-PECAM-1 coincided with neither podoplanin nor anti-P-cadherin (Fig. 6). In the retina, pigment epithelial cells were immunostained by anti-podoplanin on the surface and at cell–cell junctions, and with anti-P-cadherin at cell–cell junctions (Fig. 7).
Fig. 2.
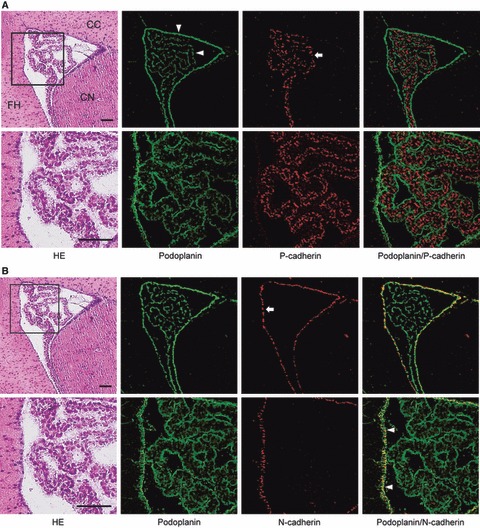
Immunostaining for podoplanin, P- and N-cadherin of the lateral ventricle. (A) Immunostaining for podoplanin and P-cadherin. The immunostained sections were re-stained by the H-E staining which shows corpus callosum (CC), caudate nucleus (CN), and fimbria hippocampus (FH). Bottom figures represent the region highlighted by the box. The fluorescence with anti-podoplanin (arrowheads) is found on ependymal cells and choroid plexus epithelia. The fluorescence with anti-P-cadherin (arrow) is found inside the choroid plexus. The anti-podoplanin-positive region did not coincide with the region with which anti-P-cadherin reacted in the merged images. Bar: 100 μm. (B) Immunostaining for podoplanin and N-cadherin on the section adjacent to (A). The fluorescence with anti-N-cadherin (arrow) is found on lateral ventricle ependymal cells at the region where anti-podoplanin reacted (arrowheads), but not found on choroid plexus epithelia in the merged images. Bar: 100 μm.
Fig. 3.
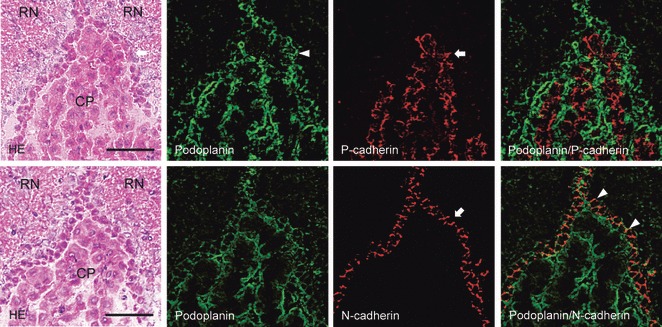
Immunostaining for podoplanin, P- and N-cadherin of the third ventricle. The double immunostained section for podoplanin and P-cadherin is adjacent to the double immunostained section for podoplanin and N-cadherin. Immunostained sections were re-stained by H-E staining, which shows red nuclei (RN), choroid plexus (CP), and ependymal cells (arrow). The fluorescence with anti-podoplanin is found on ependymal cells (arrow) and choroid plexus epithelia. The fluorescence with anti-P-cadherin (arrowhead) is found inside the choroid plexus, where anti-podoplanin did not react in the merged image. The fluorescence with anti-N-cadherin is also found on ependymal cells (arrow) where anti-podoplanin reacted (arrowheads) but was absent on choroid plexus epithelia in the merged image. Bar: 50 μm.
Fig. 4.
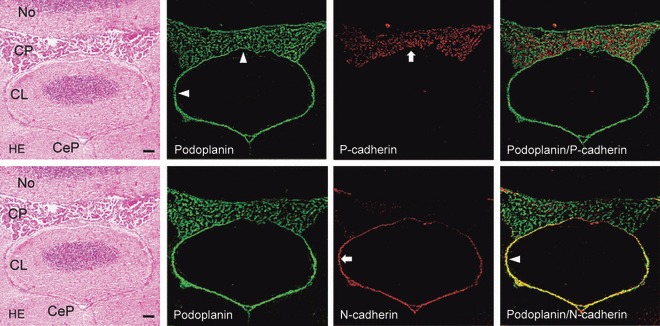
Immunostaining for podoplanin, P- and N-cadherin of the fourth ventricle. The double immunostained section for podoplanin and P-cadherin is adjacent to the double immunostained section for podoplanin and N-cadherin. The immunostained section for podoplanin and P-cadherin was re-stained by the H-E staining which shows nodulus (No), cerebellar peduncle (CeP), and cerebellar lingual (CL). The fluorescence with anti-podoplanin (arrowheads) is found on ependymal cells and choroid plexus epithelial cells, and on the pia mater around the cerebellar lingual. The fluorescence with anti-P-cadherin (arrow) is found inside the choroid plexus, where anti-podoplanin did not react in the merged image. The fluorescence with anti-N-cadherin (arrow) is also found on ependymal cells, and on the pia mater around the cerebellar lingual, where anti-podoplanin reacted (arrowheads), but not on choroid plexus epithelia. Bar: 100 μm.
Fig. 5.
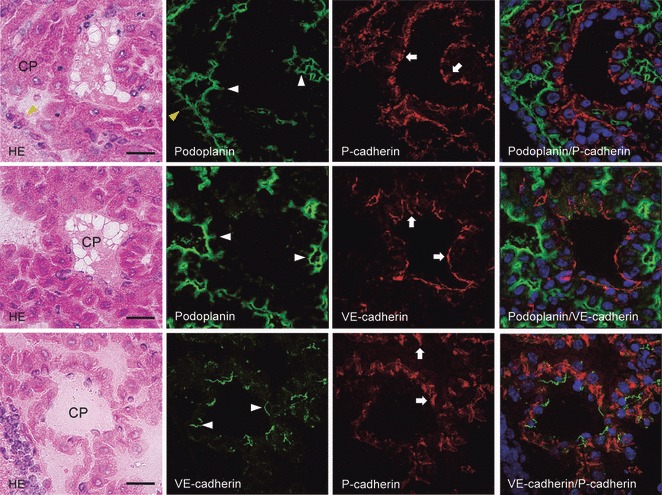
The laser-scanning confocal microscopy of immunostaining for podoplanin, P- and VE-cadherins on the choroid plexus and ventricle epithelia of the lateral ventricle. The upper, middle, and lower panels are the same sections. The immunostained sections were re-stained by the H-E staining, which shows choroid plexus (CP) and ependymal cells (arrowhead, yellow). In the immunostaining with anti-podoplanin and anti-P-cadherin, the fluorescence with anti-podoplanin is found on the choroid plexus epithelial cells (arrowheads, white) and ependymal cells (arrowhead, yellow) on the ventricle side both on the cell surface and in the cell–cell junctions, where the fluorescence with anti-P-cadherin is found on the choroid plexus epithelial cells (arrow) at the basement membrane side both on the cell surface and in the cell–cell junctions. The region reacting with anti-podoplanin did not coincide with the region reacting with anti-P-cadherin in the merged image. In the immunostaining with anti-podoplanin and anti-VE-cadherin, the fluorescence with anti-podoplanin is found on the choroid plexus epithelial cells (arrowheads) on the ventricle side both on the cell surface and in the cell–cell junctions, whereas the fluorescence with anti-VE-cadherin is found on the choroid plexus endothelial cells (arrows) both on the cell surface and in the cell–cell junctions. The region reacting with anti-podoplanin did not coincide with region reacting with anti-VE-cadherin in the merged image. In the immunostaining with anti-VE-cadherin and anti-P-cadherin, the region reacting with anti-VE-cadherin on the choroid plexus endothelial cells (arrowheads) did not coincide with the region reacting with anti-P-cadherin on the choroid plexus epithelial cells (arrows) in the merged image. Bar: 20 μm.
Fig. 6.
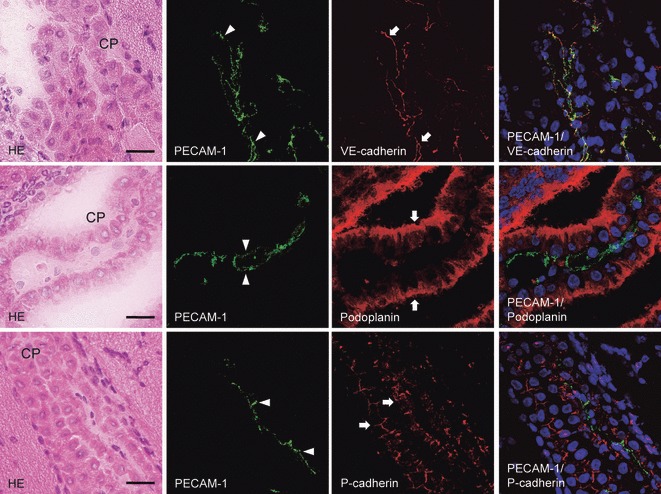
The laser-scanning confocal microscopy of immunostaining for podoplanin, P- and VE-cadherins on the choroid plexus fibrovascular core of the lateral ventricle. The upper, middle, and lower panels are the same sections. The immunostained sections were re-stained by the H-E staining, which shows the choroid plexus (CP). In the immunostaining with anti-PECAM-1 and anti-VE-cadherin, the fluorescence with anti-VE-cadherin is present on the choroid plexus endothelial cells (arrows) of the fibrovascular core as well as the region reacted with anti-PECAM-1 (arrowheads). The region reacting with anti-PECAM-1 coincided with the region reacting with anti-VE-cadherin in the merged image. In the immunostaining with anti-PECAM-1 and anti-podoplanin, there is fluorescence with anti-PECAM-1 on the choroid plexus endothelial cells (arrowheads) of the fibrovascular core, whereas fluorescence with anti-podoplanin occurs on the choroid plexus epithelial cells (arrows) on the ventricle side both on the cell surface and in the cell–cell junctions. The region reacting with anti-PECAM-1 did not coincide with the region reacting with anti-podoplanin in the merged image. In the immunostaining with anti-PECAM-1 and anti-P-cadherin, the fluorescence with anti-P-cadherin is on the choroid plexus epithelial cells (arrows) at the basement membrane side both on the cell surface and in the cell–cell junctions, whereas the fluorescence with anti-PECAM-1 is on the choroid plexus endothelial cells (arrowheads) of the fibrovascular core. The region reacting with anti-PECAM-1 did not coincide with the region reacting with anti-P-cadherin in the merged image. Bar: 20 μm.
Fig. 7.
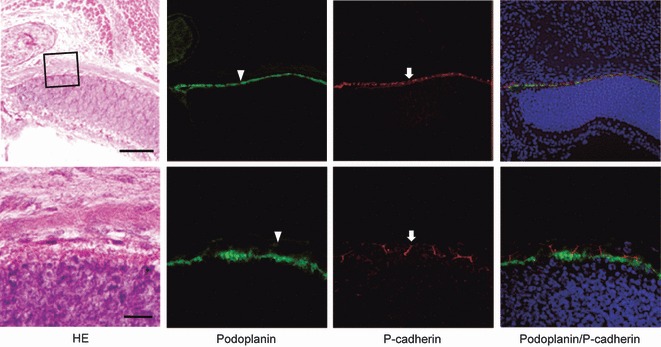
Immunostaining for podoplanin and P-cadherin of the retina. Upper sections were examined by a fluorescence microscopy (bar: 100 μm) and lower sections by laser-scanning confocal microscopy (bar: 20 μm). The fluorescence with anti-podoplanin is found on the surface of retinal pigment epithelial cells (arrowheads), whereas the fluorescence with anti-P-cadherin is localized on retinal pigment epithelial cells at cell–cell junctions (arrows).
The expression of podoplanin and classic cadherin mRNAs in the choroid plexus
Total RNA extraction from the abdominal aorta, the surface of the cerebrum with the pia mater, and the ventricular wall with choroid plexus were examined (Fig. 8). In the real-time PCR analysis, the podoplanin, and P- and N-cadherin mRNA amounts were significantly larger in ventricular walls with the choroid plexus than in the abdominal aorta and cerebrum. The VE-cadherin mRNA amount is smaller in ventricular walls with choroid plexus than in the abdominal aorta and cerebrum. Podoplanin and N-cadherin mRNA amounts were significantly larger in the surface of cerebrum with the pia mater than in the abdominal aorta. In the RT-PCR analysis, the intensities of the amplicon for β-actin and VE-cadherin mRNAs were similar for abdominal aorta, cerebrum, and ventricular walls with choroid plexus. The intensities of amplicon for podoplanin, and P- and N-cadherin mRNA were increasingly stronger, in order ventricular wall, cerebrum, and abdominal aorta.
Fig. 8.
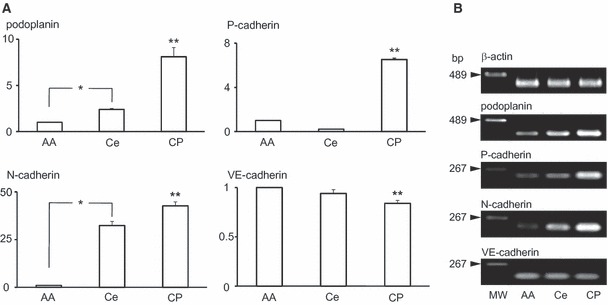
Gene analysis for the expression of podoplanin, P-, N-, and VE-cadherin mRNAs in the choroid plexus. Total RNA extraction from tissue of the abdominal aorta (AA), the surface of cerebrum with the pia mater (Ce), and the ventricular wall with choroid plexus (CP) were examined. (A) Real-time PCR analysis. Relative mRNA amounts are expressed in arbitrary units. Target gene cDNA units in each sample were normalized to β-actin cDNA units. Podoplanin, P- and N-cadherin mRNA amounts were significantly larger in the ventricular wall with choroid plexus than in cerebrum tissue or in abdominal aorta as a negative control. The VE-cadherin mRNA amount was significantly smaller in the ventricular wall with choroid plexus than in cerebrum tissue and in abdominal aorta as a positive control. The amounts of podoplanin and N-cadherin mRNA were significantly larger in the surface of cerebrum with the pia mater than in abdominal aorta. *Statistically significant difference by Student’s t-test and one-way anova** (P < 0.01). (B) RT-PCR analysis. Intensities of amplicon for β-actin mRNA and VE-cadherin were similar in abdominal aorta, cerebrum, and ventricular wall with choroid plexus. Intensities of amplicon for podoplanin, P- and N-cadherin mRNA increased in strength in the order ventricular wall with choroid plexus, cerebrum, and abdominal aorta.
Discussion
The expression of podoplanin and classic cadherins in mouse tissues
Podoplanin is a mucin-type 38-kDa transmembrane glycoprotein first identified in kidney glomerular epithelial cells (Breiteneder-Geleff et al. 1997; Koop et al. 2008). Podoplanin is homologous to the T1α-2 gene which encodes the type I alveolar cell specific antigen and to the oncofetal antigen M2A recognized by the D2-40 antibody (Wetterwald et al. 1996; Williams et al. 1996; Ramirez et al. 2003; Schacht et al. 2003; Chu et al. 2005; Ordóñez, 2005; Sonne et al. 2006; Yu et al. 2007; Marsee et al. 2009). The role of podoplanin has been studied in the puromycin-induced nephropathy rat. It has been thought that podoplanin plays a role in maintaining the shape of podocyte foot processes and glomerular permeability because podocyte foot processes have extensive flattening with selective loss of podoplanin to < 30% in the kidneys of nephropathy model rats (Koop et al. 2008). Podoplanin is also well known as a lymphatic endothelial cell marker (Schacht et al. 2003; Iwasawa et al. 2008; Noda et al. 2010). Podoplanin-deficient mice have defects in lymphatic formation but not in blood vessels. Further, there have been reports of the podoplanin expression in osteocytes and osteoblasts (Wetterwald et al. 1996; Hantusch et al. 2007), mesothelial cells (Chu et al. 2005; Ordóñez, 2005), epidermal basal layer cells (Schacht et al. 2003), placenta villus (Wang et al. 2011), follicular dendritic cells (Yu et al. 2007; Marsee et al. 2009), and immature cells such as fetal germ cells and Sertoli cells (Mishima et al. 2006a,b; Sonne et al. 2006). We have recently shown that tooth germ epithelium and salivary gland myoepithelium express podoplanin (Hata et al. 2008, 2010; Sawa et al. 2008; Imaizumi et al. 2010; Noda et al. 2010). In the study reported here, CHO K1 cells from Chinese hamster ovary and HSC-3 cells from human tongue squamous cell carcinoma were not immunostained by anti-mouse podoplanin, and the anti-podoplanin reacted to lymphatic vessels and epicardium without cross-reacting with blood vessels, muscle cells or connective tissue cells in the mouse tissue, indicating the reaction of anti-mouse podoplanin (Fig. 1). Especially in the placenta, anti-podoplanin reacted only with syncytiotrophoblasts. Podoplanin may function in the placental barrier in syncytiotrophoblasts, restricting the villus to the decidua in the endometrium.
Classic cadherins are involved in cell–cell adhesion at adherens junctions. Further, P-cadherin that has been identified in the mouse placenta acts as an adhesion molecule and has unknown roles in the decidua of the placenta and in mammary glands (Nose & Takeichi, 1986; Shimoyama et al. 1989; Hatta et al. 1991; Aberle et al. 1996; Radice et al. 1997; Angst et al. 2001; Soler et al. 2002; Gama et al. 2004; Halbleib & Nelson, 2006; Hantusch et al. 2007; Shapiro & Weis, 2009); N-cadherin functions in the nervous system and the expression is clearly observed in the intercalated disks of cardiac muscle (Angst et al. 2001; Ferreira-Cornwell et al. 2002; Halbleib & Nelson, 2006; Shapiro & Weis, 2009). In vascular systems, VE-cadherin regulates the vascular permeability at adherens junctions (Angst et al. 2001; Vincent et al. 2004; Dejana et al. 2008; Shapiro & Weis, 2009). In this study, CHO K1 and HSC-3 cells were not immunostained by anti-mouse cadherins, and the anti-VE-cadherin reacted with blood and lymphatic endothelial cells, anti-P-cadherin with placenta decidua, and anti-N-cadherin with epicardium and intercalated disks, but there were no cross-reactions with muscle or connective tissue in the mouse tissue, suggesting that the reactions of anti-cadherins can reliably be expected to occur (Fig. 1).
Distribution of podoplanin and classical cadherin-expressing cells in mouse ventricles
There have been no detailed reports on podoplanin expression in the normal brain. The study here showed that on the lateral, third, and fourth ventricles, the surfaces of ependymal cells, choroid plexus epithelia, and the pia mater reacted with anti-podoplanin (Figs 2–4), suggesting that ependymal cells, choroid plexus epithelial cells, and glial cells under the pia mater have the ability to express podoplanin. Investigation for the distribution of classic cadherin-expressing cells at the ventricles showed that anti-P-cadherin reacted with the inside of choroid plexus epithelial cells, and that anti-N-cadherin reacted with ependymal cells and the pia mater (Figs 2–4), indicating that choroid plexus epithelial cells express P-cadherin, and that ependymal cells and glial cells under the pia mater express N-cadherin.
The choroid plexus consists of epithelial cells and a fibrovascular core formed by both fibroblasts originating in the pia mater and endothelial cells of the fenestrated choroidal capillaries which produce cerebrospinal fluid (CSF). Blood components immediately permeate into extravascular space under epithelial cells surrounding the fibrovascular core because of the absence of a tight junction at the cell–cell junctions between choroid plexus endothelial cells. Blood-CSF barriers are formed by the tight junctions at cell–cell junctions of choroid plexus epithelial cells and limit the permeability of blood components into CSF. The CSF reflects brain interstitial fluid because ependymal cells connect via the gap junctions and desmosomes, which have a limiting transmission that is weaker than at a tight junction (Zamora & Thiesson, 1980; Williams et al. 2005). In the laser-scanning confocal microscopy, both podoplanin and P-cadherin were expressed on choroid plexus epithelial cells on the cell surface and in the cell–cell junctions, but the regions did not coincide; podoplanin was found at the ventricular side and P-cadherin at the basement membrane side (Fig. 5). Further, the expression of VE-cadherin was observed in the choroid plexus but the VE-cadherin-positive region did not coincide with the podoplanin- or P-cadherin-positive regions, suggesting that choroid plexus epithelial cells do not express VE-cadherin. Taken together, this may suggest that podoplanin and P-cadherin play a role as a rate-determining step in the permeability of CSF on choroid plexus epithelial cells at the ventricle side and at the vascular side, respectively.
To determinate whether choroid plexus endothelial cells express podoplanin and P-cadherin, double immunostaining for the endothelial cell marker CD31/PECAM-1 and VE-cadherin, podoplanin, or P-cadherin was performed (Fig. 6) (Hata et al. 2008). It is known that VE-cadherin is also a marker for vascular endothelial cells (Vincent et al. 2004; Dejana et al. 2008). Since the PECAM-1-positive region coincided with the VE-cadherin-positive region in the fibrovascular core of the choroid plexus, it is thought that choroid plexus endothelial cells were successfully identified by PECAM-1, and that choroid plexus endothelial cells express VE-cadherin. In the double immunostaining for PECAM-1 and podoplanin or P-cadherin, the PECAM-1-positive region did not coincide with the regions reacted with anti-podoplanin or anti-P-cadherin, suggesting that choroid plexus endothelial cells do not express podoplanin or P-cadherin.
In the retina, anti-podoplanin reacted with pigment epithelial cells on the cell surface while anti-P-cadherin was only found on pigment epithelial cells at the cell–cell junctions (Fig. 7). Earlier, Williams et al. (2005) examined cadherin expression in brain tissue and reported that the general vascular endothelial cadherin, VE-cadherin, was reduced or absent in tight cortical microvessels. T reciprocal to VE-cadherin, the type II classical cadherin, cadherin-10, was expressed in retinal pigment epithelial cells in the blood-retinal barrier as brain barriers, in microvessel endothelial cells of the cerebral cortex as the blood–brain barrier, in choroid plexus epithelial cells as the blood–CSF barrier, and in ependymal cells as the CSF–brain barrier, but not in leaky microvessels such as the choroid plexus where the blood–brain barrier is absent (Williams et al. 2005). Therefore it seems that the expression pattern of P-cadherin in the brain is similar to that of cadherin-10, although cadherin expression in the blood–brain barrier was not clearly established. The P-cadherin may play a role similar to cadherin-10 in the blood-retinal barrier, and in brain barriers.
Total RNA extraction from the surface of cerebrum with the pia mater and the ventricular wall with choroid plexus contained more podoplanin mRNA compared with the abdominal aorta as a negative control (Fig. 8). Total RNA in the ventricular wall with choroid plexus contained more P-cadherin and N-cadherin mRNAs compared with the surface of cerebrum with the pia mater and in the abdominal aorta as a negative control. Further, total RNA in the cerebrum surface contained significantly more N-cadherin mRNA compared with the abdominal aorta (Fig. 8). Total RNA in ventricular walls with choroid plexus contained less VE-cadherin mRNA compared with the surface of cerebrum with the pia mater and in the abdominal aorta as a positive control (Fig. 8). These results suggest that the glial cells of the ventricular wall including the choroid plexus have the ability to express podoplanin as well as P- and N-cadherins to a greater extent compared with the glial cells in the cerebrum. Podoplanin and P-cadherin may play a role in a blood–CSF barrier on choroid plexus epithelial cells on the ventricle side and on the basement membrane side, respectively (Fig. 9). Podoplanin is also able to interact with P- and N-cadherins through the actin filament networks (Aberle et al. 1996; Pujuguet et al. 2003; Lien et al. 2006; Martín-Villar et al. 2006; Wicki et al. 2006; Raica et al. 2008). Podoplanin and N-cadherin may function in coordination on the ependymal cells and glial cells with the pia mater as a CSF-brain barrier (Fig. 9).
Fig. 9.
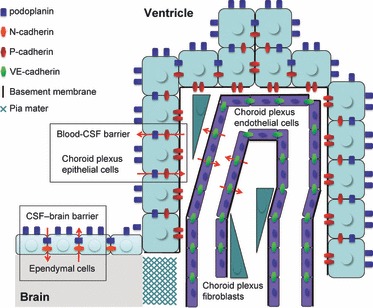
A schematic outline of the expression for podoplanin, P-, N-, and VE-cadherin in the choroid plexus. Podoplanin is expressed on the cell surface and at the cell–cell junctions of ependymal cells and choroid plexus epithelial cells. P-cadherin is expressed on choroid plexus epithelial cells at the vascular side between epithelial cells and endothelial cells or fibroblasts. VE-cadherin is expressed on choroid plexus endothelial cells at the cell–cell junctions. N-cadherin is expressed on the pia mater and ependymal cells at the cell surface and cell–cell junctions. Podoplanin and P-cadherin participate in the blood-CSF barrier, and podoplanin and N-cadherin play roles in the CSF-brain barrier.
In conclusion, the results would seem to suggest that podoplanin, and P- and N-cadherins act as a rate-determining step in the blood and CSF component transmission on choroid plexus epithelial cells and ependymal cells as participants in the blood-CSF and CSF-brain barriers.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (B) 2390345 (principal investigator: Sawa Y., corresponding author) and Exploratory Research 23659884 (principal investigator: Sawa) from Japan Society for the Promotion of Science.
References
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- Belkina NV, Liu Y, Hao JJ, et al. LOK is a major ERM kinase in resting lymphocytes and regulates cytoskeletal rearrangement through ERM phosphorylation. Proc Natl Acad Sci U S A. 2009;106:4707–4712. doi: 10.1073/pnas.0805963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Matsui K, Soleiman A, et al. Podoplanin, novel43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- Chu AY, Litzky LA, Pasha TL, et al. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005;18:105–110. doi: 10.1038/modpathol.3800259. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Ferreira-Cornwell MC, Luo Y, Narula N, et al. Remodeling the intercalated disc leads to cardiomyopathy in mice misexpressing cadherins in the heart. J Cell Sci. 2002;115:1623–1634. doi: 10.1242/jcs.115.8.1623. [DOI] [PubMed] [Google Scholar]
- Gama A, Paredes J, Albergaria A, et al. P-cadherin expression in canine mammary tissues. J Comp Pathol. 2004;130:13–20. doi: 10.1016/s0021-9975(03)00064-1. [DOI] [PubMed] [Google Scholar]
- Gautreau A, Poullet P, Louvard D, et al. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Hantusch B, Kalt R, Krieger S, et al. Sp1/Sp3 and DNA-methylation contribute to basal transcriptional activation of human podoplanin in MG63 versus Saos-2 osteoblastic cells. BMC Mol Biol. 2007;8:20. doi: 10.1186/1471-2199-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Hata M, Ueki T, Sato A, et al. Expression of podoplanin in the mouse salivary glands. Arch Oral Biol. 2008;53:835–841. doi: 10.1016/j.archoralbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Hata M, Amano I, Tsuruga E, et al. Immunoelectron microscopic study of podoplanin localization in mouse salivary gland myoepithelium. Acta Histochem Cytochem. 2010;43:77–82. doi: 10.1267/ahc.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta M, Miyatani S, Copeland NG, et al. Genomic organization and chromosomal mapping of the mouse P-cadherin gene. Nucleic Acids Res. 1991;19:4437–4441. doi: 10.1093/nar/19.16.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y, Amano I, Tsuruga E, et al. Immunohistochemical examination for the distribution of podoplanin-expressing cells in developing mouse molar tooth germs. Acta Histochem Cytochem. 2010;43:115–121. doi: 10.1267/ahc.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inge LJ, Barwe SP, D’Ambrosio J, et al. Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Exp Cell Res. 2011;317:838–848. doi: 10.1016/j.yexcr.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Komori T, Shimada S, et al. Podoplanin is a potential marker for the diagnosis of ependymoma: a comparative study with epithelial membrane antigen (EMA) Clin Neuropathol. 2009;28:373–378. [PubMed] [Google Scholar]
- Iwasawa K, Kameyama T, Ishikawa H, et al. Induction of ICAM-1 and VCAM-1 on the mouse lingual lymphatic endothelium with TNF-α. Acta Histochem Cytochem. 2008;41:115–120. doi: 10.1267/ahc.08017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop K, Eikmans M, Wehland M, et al. Selective loss of podoplanin protein expression accompanies proteinuria and precedes alterations in podocyte morphology in a spontaneous proteinuric rat model. Am J Pathol. 2008;173:315–326. doi: 10.2353/ajpath.2008.080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M, Kojima T, Murata M, et al. Phosphorylation of ezrin enhances microvillus length via a p38 MAP-kinase pathway in an immortalized mouse hepatic cell line. Exp Cell Res. 2006;312:111–120. doi: 10.1016/j.yexcr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Vasioukhin V. Cadherin-catenin proteins in vertebrate development. Curr Opin Cell Biol. 2006;18:499–506. doi: 10.1016/j.ceb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Marsee DK, Pinkus GS, Hornick JL. Podoplanin (D2-40) is a highly effective marker of follicular dendritic cells. Appl Immunohistochem Mol Morphol. 2009;17:102–107. doi: 10.1097/PAI.0b013e318183a8e2. [DOI] [PubMed] [Google Scholar]
- Martín-Villar E, Megías D, Castel S, et al. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci. 2006;119:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- Mishima K, Kato Y, Kaneko MK, et al. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006a;111:483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- Mishima K, Kato Y, Kaneko MK, et al. Podoplanin expression in primary central nervous system germ cell tumors: a useful histological marker for the diagnosis of germinoma. Acta Neuropathol. 2006b;111:563–568. doi: 10.1007/s00401-006-0033-4. [DOI] [PubMed] [Google Scholar]
- Noda Y, Amano I, Hata M, et al. Immunohistochemical examination of the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta Histochem Cytochem. 2010;43:61–68. doi: 10.1267/ahc.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;10:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordóñez NG. D2-40 and podoplanin are highly specific and sensitive immuno-histochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372–380. doi: 10.1016/j.humpath.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Pujuguet P, Del Maestro L, Gautreau A, et al. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181–2191. doi: 10.1091/mbc.E02-07-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice GL, Ferreira-Cornwell MC, Robinson SD, et al. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025–1032. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008;28:2997–3006. [PubMed] [Google Scholar]
- Ramirez MI, Millien G, Hinds A, et al. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolous formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Sawa Y, Iwasawa K, Ishikawa H. Expression of podoplanin in the mouse tooth germ and apical bud cells. Acta Histochem Cytochem. 2008;41:121–126. doi: 10.1267/ahc.08019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, et al. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FG, Gamallo C, Vilar S, et al. Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J Cell Sci. 1999;112:4601–4613. doi: 10.1242/jcs.112.24.4601. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;11:a003–a053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara J, Kashima T, Kikuchi Y, et al. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Arch. 2006;448:493–499. doi: 10.1007/s00428-005-0133-x. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Yoshida T, Terada M, et al. Molecular cloning of a human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol. 1989;109:1787–1794. doi: 10.1083/jcb.109.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler AP, Russo J, Russo IH, et al. Soluble fragment of P-cadherin adhesion protein found in human milk. J Cell Biochem. 2002;85:180–184. [PubMed] [Google Scholar]
- Sonne SB, Herlihy AS, Hoei-Hansen CE, et al. Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2, podoplanin) in human developing testis, testicular carcinoma in situ and germ-cell tumours. Virchows Arch. 2006;449:200–206. doi: 10.1007/s00428-006-0223-4. [DOI] [PubMed] [Google Scholar]
- Vincent PA, Xiao K, Buckley KM, et al. VE-cadherin: adhesion at arm’s length. Am J Physiol Cell Physiol. 2004;286:C987–C997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sun J, Gu Y, et al. D2-40/podoplanin expression in the human placenta. Placenta. 2011;32:27–32. doi: 10.1016/j.placenta.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterwald A, Hoffstetter W, Cecchini MG, et al. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- Wicki A, Lehembre F, Wick N, et al. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Williams MC, Cao Y, Hinds A, et al. T1alpha protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus and ciliary epithelia of adult rats. Am J Respir Cell Mol Biol. 1996;14:577–585. doi: 10.1165/ajrcmb.14.6.8652186. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Lowrie MB, Bennett JP, et al. Cadherin-10 is a novel blood-brain barrier adhesion molecule in human and mouse. Brain Res. 2005;1058:62–72. doi: 10.1016/j.brainres.2005.07.078. [DOI] [PubMed] [Google Scholar]
- Yu H, Gibson JA, Pinkus GS, et al. Podoplanin (D2-40) is a novel marker for follicular dendritic cell tumors. Am J Clin Pathol. 2007;128:776–782. doi: 10.1309/7P8U659JBJCV6EEU. [DOI] [PubMed] [Google Scholar]
- Zamora AJ, Thiesson D. Tight junctions in the ependyma of the spinal cord of the urodele Pleurodeles waltlii. Anat Embryol. 1980;160:263–274. doi: 10.1007/BF00305107. [DOI] [PubMed] [Google Scholar]


