Abstract
Salivary secretion is principally regulated by autonomic nerves. However, recent evidence from in vivo animal experiments suggests that gastrointestinal peptide hormones can also influence saliva production. The aim of the present study was to define the secretagogue activity of the gastrin-analogue pentagastrin in human salivary glands. For this purpose, parotid tissues were exposed to pentagastrin in vitro. Morphological techniques were used to evaluate modifications to serous acinar cells associated with secretion. Using a variant of the osmium maceration method, high resolution scanning electron microscopy allowed assessment of the morphology of the cytoplasmic aspect of the plasmalemma to demonstrate secretory activity. To quantify responses to pentagastrin, we recorded morphometric data on microvilli, microbuds, and protrusions. Dose-dependent morphological changes were observed, whereas protein concentration increased in the incubate. The use of selective receptor antagonists showed pentagastrin to act principally via cholecystokinin-A receptors. The morphological responses observed following exposure to pentagastrin differed from those elicited following exposure to the pan-muscarinic agonist carbachol. This study provides the first demonstration of a direct secretory action of gastrointestinal peptides on salivary glands in humans.
Keywords: CCK receptors, gastrointestinal peptides, high resolution scanning electron microscopy, human submandibular glands, osmium maceration method, pentagastrin, salivary secretion
Introduction
Salivary secretion is principally under the control of the autonomic nervous system (Ekström, 1989, 1999; Proctor & Carpenter, 2007). However, recent in vivo animal experiments of ours indicate a short-term endocrine regulation of salivary glandular activities as well. Some gastrointestinal peptide-hormones induce amylase secretion, without any accompanying overt fluid secretion, as revealed by a methacholine-induced wash-out flow of saliva and also protein synthesis, the experimental time-frame being minutes (secretion) up to 2 h (synthesis) (Çevik-Aras & Ekström, 2006a,b,c; Ekström & Çevik-Aras, 2008; Çevik-Aras et al. 2011). We have previously described ‘dynamic’ ultrastructural events acutely evoked in human salivary gland tissues, thought to reflect secretory activity in response to the in vitro administration of muscarinic and adrenergic agonists (Segawa et al. 1998a; Murakami et al. 2000; Riva et al. 2003; Testa Riva et al. 2006).
The purpose of the present study, making use of our in vitro technique (Testa Riva et al. 2006), was to investigate the secretory effect of pentagastrin, a gastrin-analogue, on serous acinar cells of the human parotid gland, as judged by morphological changes and protein output. As the results showed pentagastrin to evoke secretory activities, experiments followed to examine the involvement of cholecystokinin CCK-A or CCK-B receptors in mediating the response; in fact, not only cholecystokinin but also gastrin (pentagastrin) acts via these types of receptors (Wank, 1995). Moreover, findings were compared with those following administration of the pan-muscarinic acetylcholine receptor agonist carbachol.
Materials and methods
Samples of human parotid glands were obtained at orofacial surgery from 30 male and seven female patients aged 16–85 years. Informed consent was obtained in each case and permission was granted by the local ethical committee (ASL 8, Cagliari, Italy). All tissues came from non-irradiated patients and were normal as assessed by light microscopy. Furthermore, prior to or during surgery, patients were not treated with drugs which were likely to interfere with in vitro secretion under the present experimental set-up. Fragments of each sample were immediately cut into 1-mm3 pieces and put into pre-weighed bottles with 10 mL of oxygenated inorganic medium (NaCl 79 mm, KCl 4.9 mm, MgCl2 1.2 mm, CaCl2 1 mm, TRIS 16 mm, d-glucose 5 mm, NaHCO3 44 mm) containing pentagastrin or carbachol at 37 °C (Testa Riva et al. 2006); the bottles were then re-weighed to give the weight of the pieces of gland tissue. The final concentrations of pentagastrin were 26 μm, 65 μm, and 130 μm, and of carbachol 10 μm. After a 15-min exposure to either drug, the gland pieces were removed. In a number of experiments, the effect of pentagastrin (130 μm) was tested in the presence of the CCK-A receptor antagonist lorglumide (1.37 mm) and the CCK-B antagonist itriglumide (127 μm): in these cases the antagonists were added to the medium 5 min before pentagastrin. Specimens incubated in the same medium without the drugs served as controls. All drugs were purchased from Sigma (St. Louis, MO, USA).
Preparation
Treated and control specimens were fixed in a mixture of paraformaldehyde 0.5% and glutaraldehyde 0.5%, in 0.1 m cacodylate buffer (pH 7.2) at room temperature, embedded in Epoxy resin and treated for transmission electron microscopy (TEM; Riva, 1974). Semithin sections were stained for light microscopy (LM) with Azure II/Toluidine blue and examined in a Leica DMR HC. Some of the parotid fragments were subjected to our variant of the OsO4 maceration method for high resolution scanning electron microscopy (HRSEM; Riva et al. 1993, 1999). To view the cytoplasmic aspect of the plasmalemma of parotid serous cells, we extracted all cellular organelles with a rotating agitator during rinsing and maceration. Observations were made with a JEOL 100S TEM operated at 80 kV, and a Hitachi S4000 FEG HRSEM operated at 15–20 kV.
Protein
Protein output into the incubation medium was determined by the method of Lowry et al. (1951) and expressed in terms of percentage of concentration [μg (mg of tissue)−1] with respect to controls, using bovine serum albumin as standard.
Statistics
More than 300 images of intercellular canaliculi were randomly collected by HRSEM. Data on microvilli, protrusions, and microbuds density were quantified and related to 1 μm2 of visible plasmalemma in HRSEM images of canaliculi. The measurements were performed by two independent observers. Statistical differences were determined by a non-parametric test (Mann–Whitney U-test). Data on protein output were obtained from 30 experiments in toto. Statistical differences were determined by an unpaired t-test (Welch corrected). All data are expressed as % ± SE with respect to controls. The significance level was set at P < 0.05. Image acquisitions were obtained by Quartz PCI (Quartz imaging, Vancouver, Canada). Measurement and statistical analysis was carried out with image tool v. 3.0 for Windows (University of Texas Health Science Centre in San Antonio) and with Microsoft excel 2003; Mann–Whitney U-test and the unpaired t-test (Welch corrected) were performed using graphpad instat v. 3.06 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Morphological observations
Effects of pentagastrin
LM revealed that, in comparison with control (Fig. 1 inset), lumina (including intercellular canaliculi) of serous cells were dilated as a result of exposure to pentagastrin (130 μm; Fig. 1A). There were fewer intracellular secretory granules, located principally in the apical part of the cells. TEM showed that lumina were dilated, with capital omega (Ω) exocytotic profiles (in continuity with lumen) in the luminal membrane (Fig. 1B). In such profiles, the flat lower parts of Ω represent the uninvolved luminal membrane, whereas the circular part represents secretory granules that fused during docking. After removal of cytoplasmic organelles, HRSEM revealed the cytoplasmic aspect of the luminal membrane following pentagastrin (130 μm) treatment, at variance with control (Fig. 2), with some protrusions of the size of a single granule likely corresponding to LM and TEM Ω exocytotic profiles (Fig. 3A). Moreover, among the holes, related to the bases of microvilli deprived of their cytoskeleton and observed on the cytoplasmic aspect, numerous microbuds 80–150 nm in size were found (Fig. 3A). Lower concentrations of pentagastrin evoked similar, but less prominent, morphological cellular modifications (Fig. 3B–C). No vacuoles were visible inside the cells.
Fig. 1.
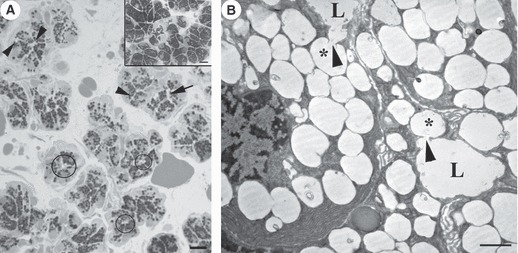
Parotid gland treated with pentagastrin 130 μm. (A) LM. Serous acini show a few dilated lumina (circles) and intercellular canaliculi (arrow) with exocytotic profiles (arrowheads). Bar: 10 μm. Inset: LM. Serous acini of control parotid glands. Bar: 10 μm. (B) TEM. Serous cells show a few Ω shape profiles (asterisks) which open (arrowheads) to the lumina (L). Bar: 1 μm.
Fig. 2.
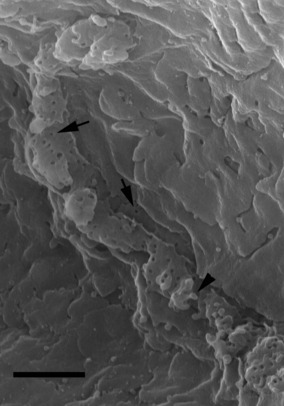
Intercellular canaliculi as seen by HRSEM, after maceration method and removal of all cellular organelles. Numerous holes (arrows) and a few microbuds (arrowhead) are evident in the cytoplasmic aspect of plasmalemma. Bar: 1 μm.
Fig. 3.
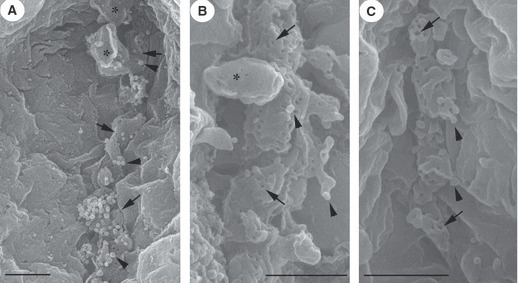
By HRSEM, intercellular canaliculi of serous acini show a few holes (arrows) and numerous microbuds (arrowheads). In the cytoplasmic aspect of the canaliculi membrane there are a few protrusions (asterisks) related to TEM Ω shape profiles. (A) Pentagastrin 130 μm stimulation; (B) pentagastrin 65 μm; (C) pentagastrin 26 μm. Bar: 1 μm.
Morphometric estimations
In response to pentagastrin, the mean density of microvilli decreased, whereas that of the microbuds and of the protrusions increased with respect to control, on the whole, dose-dependently. With 130 μm of pentagastrin, the percentage figures were (in relation to control values) −33% (P < 0.001), +280% (P < 0.001) and +145% (P < 0.001), respectively (Fig. 4A–C).
Fig. 4.
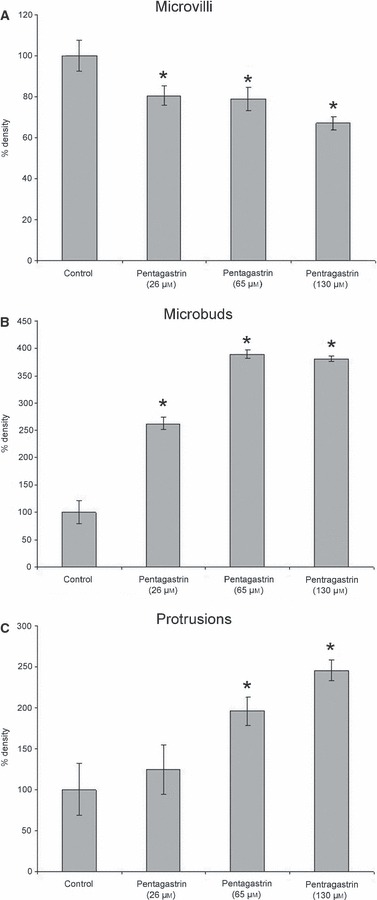
Relationship of microvilli (A), microbuds (B) and protrusion (C) density after pentagastrin (26, 65 and 130 μm) treatment and controls. All data are expressed as % with respect to control. *Statistically significant with respect to control.
Effects of cholecystokinin receptor antagonists
In the presence of the CCK-A receptor antagonist lorglumide, pentagastrin (130 μm) seemed to have no effect on morphology (Fig. 5 inset), and the morphometric assessment revealed no changes (Fig. 6A–C). In contrast, in the presence of the CCK-B receptor antagonist itriglumide (Fig. 5A–B), pentagastrin (130 μm) still exerted its expected effects on microbuds and protrusions, whereas the density of microvilli was within the range of controls (Fig. 6A–C).
Fig. 5.
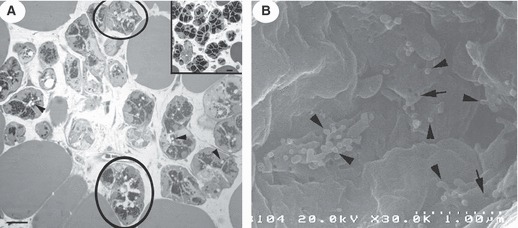
Parotid gland. (A) Treatment with itriglumide and with lorglumide (inset) before pentagastrin stimulation, as seen by LM. After inhibition of CCK-B receptors only, lumina and intercellular canaliculi are dilated (circles) and a few exocytotic profiles (arrowheads) are evident. Bar: 10 μm. (B) After inhibition with itriglumide, as shown by HRSEM, intercellular canaliculi show a few holes (arrows) and some microbuds (arrowheads). Protrusions were often observed. Bar: 1 μm.
Fig. 6.
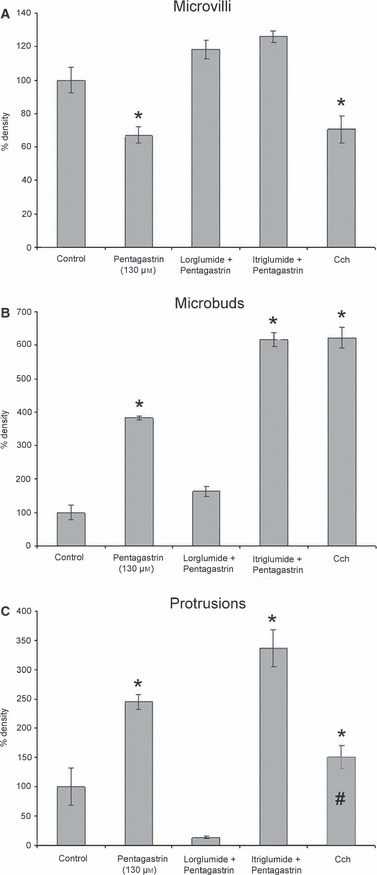
Relationship of microvilli (A), microbuds (B) and protrusion (C) density after pentagastrin (130 μm) and carbachol (Cch) treatment, and after inhibition of CCK-A and -B receptors, with respect to control. All data are expressed as % with respect to control. *Statistically significant with respect to control. #Protrusion data, after Cch treatment, should be related to intracellular vacuoles (see text).
Effects of carbachol
In response to the muscarinic agonist, lumen and canaliculi were dilated with numerous Ω profiles of exocytosis and large vacuoles in the cytoplasm; further on, large protrusions were observed by HRSEM. The density of microvilli was decreased (−30%, P < 0.001), whereas that of microbuds was increased (+520%, P < 0.0001) (Fig. 6A–B). Protrusion density was calculated, but not considered (Fig. 6C), because some granules fused together to form intracellular vacuoles which were removed, together with all the cytoplasmic organelles, by our HRSEM technique.
Protein output
Pentagastrin (130 μm) increased protein concentration (expressed per mg of tissue) in the medium by +300% as compared with that of the controls (P < 0.0001) (Fig. 7). The percentages were lower in the presence of the CCK-A receptor antagonist lorglumide (−200%, P < 0.01) and in the presence of the CCK-B receptor antagonist itriglumide (−124%, P > 0.05) with respect to that of pentagastrin (Fig. 7).
Fig. 7.
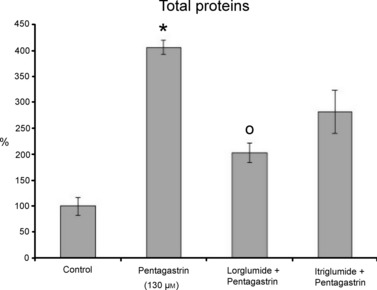
Percentage of total protein per mg of tissue, as detected in the media after treatment with pentagastrin (130 μm), and after inhibition with lorglumide and itriglumide. All data are expressed as % with respect to control. *Statistically significant with respect to control. ○ Statistically significant with respect to pentagastrin.
Discussion
The in vitro approach allowed us to study the morphological and functional responses of the human parotid gland by using operational specimens, avoiding patient exposure to the systemic administration of drugs. The results suggest a potential role for gastrin in the regulation of parotid secretion in humans. At LM level, the serous acinar cells lost secretory granules, and, at ultrastructural level, the number of microvilli decreased, whereas that of microbuds and protrusions increased. Moreover, the protein output from the gland pieces in the medium was elevated. The morphometric assessment was focused on the cytoplasmic aspect of the canaliculi plasmalemma in that a great part of the secretion occurs through these membranes (Sakai, 1984; Murakami et al. 2000).
The Ω profiles, seen by TEM in the luminal membrane, correspond to the exocytosis of single secretory granules by the major regulated pathway. The protrusions, seen by HRSEM in the inner side of the plasmalemma of secretory canaliculi, are related to Ω profiles and to granular secretion. In fact, HRSEM protrusions are often similar in size to a single granule. The reduction in the number of microvilli (Figs 4A and 6A) reflects the re-absorption of the microvilli cytoskeleton as a consequence of the cytoskeletal actin terminal web re-organization, permitting granules to be translocated to the luminal membrane (Segawa et al. 1998b; Testa Riva et al. 2006). Thus, the decrease of microvilli could be related to the major regulated secretory pathway, that is, due to stimulated exocytosis of granules (Castle & Castle, 1996, 1998).
Microbuds are located among the bases of microvilli (seen as holes by HRSEM after removal of organelles) and, often, on the cytoplasmic surface of protrusions. Some of the HRSEM-visualized microbuds could be explained by membrane recycling processes, i.e. endocytosis, and related to coated pits (which are limited regions of luminal plasmalemma covered by clathrin) visible by TEM (Testa Riva et al. 2006). Moreover, the increase in the number of microbuds (Figs 4B and 6B) should be related to microexocytotic events. In fact, small vesicles budding from the Golgi network, from immature secretory granules, and/or from different intracellular membrane complexes, are the product of different kinds of secretion mechanisms such as constitutive and constitutive-like secretion (Castle & Castle, 1996, 1998; Castle, 1998). A quantification of the size of microbuds reveals no sub-populations (data not shown); this fact, and the incompatibility of our osmium maceration technique with immunostaining methods, prevented us from distinguishing endocytotic from microexocytotic microbuds. Microbuds docking on the luminal membrane, even if they may provide fusion sites for granule exocytosis (Castle et al. 2002), should not be related to re-organization of the microvilli cytoskeleton and, directly, to their decrease. In fact, we noted that, after inhibition of CCK-B receptors with itriglumide, microbuds density increased with respect to controls but microvilli density remained similar to that of controls.
Compared with the morphological response to pentagastrin, carbachol caused a profound granular exocytosis and, in addition, the formation of intracellular vacuoles (Segawa et al. 1998a). The decrease in number of microvilli was about the same (Fig. 6A), and the number of microbuds was higher after carbachol than after pentagastrin treatment (Fig. 6B). However, the protrusions (Fig. 6C) could not be evaluated because, after carbachol stimulation, granules dock together, forming intracellular vacuoles. In contrast to carbachol, the response to pentagastrin was, qualitatively, similar to that of the β-adrenergic receptor agonist isoprenaline (Segawa et al. 1998a). From in vivo animal experiments, isoprenaline is known to evoke a sparse secretion of fluid and an extensive secretion of protein (Murakami et al. 2000).
In the light of previous in vivo studies on rat parotid glands, cholecystokinin receptors of type A were probably engaged in responses seen here in human parotid gland (Çevik-Aras & Ekström, 2006a,b). This is confirmed in human by the results obtained after inhibition of CCK-A and -B receptors (Fig. 6A–C), as the use of selective receptor antagonists showed pentagastrin to act principally via cholecystokinin-A receptors. However, with respect to protein synthesis, both types of cholecystokinin receptors are involved in mediating response (Çevik-Aras & Ekström, 2006a,b).
Admittedly, it may be argued that the gland tissue presently under study was exposed to a concentration of pentagastrin non-comparable to the circulating levels of gastrin produced in response to a meal. Although the present experimental in vitro set-up did not mimic physiological conditions, it provided reproducible data indicating a possible involvement of natural occurring gastrin as well as of cholecystokinin in salivary gland protein secretion, as the latter hormone also exerts its action via cholecystokinin receptors. In the previous in vivo studies on rats, the doses of pentagastrin were undoubtedly supraphysiological (Çevik-Aras & Ekström, 2006a,b,c). However, under natural conditions, in response to a meal, the gland response does not rest only on the action of gastrin. Hormones including gastrin, cholecystokinin and also melatonin (of intestinal origin; Bubenik, 2002) most likely act in concert with parasympathetic acetylcholine and sympathetic noradrenaline (and possible circulating adrenaline), as well as with a number of non-adrenergic, non-cholinergic transmitters (Ekström et al. 1998). Few studies dealing with acute hormonal effects on salivary glandular activities in humans are on record. Interestingly, there are two brief notes that report increases in amylase concentration in resting flow of saliva from human parotid glands upon infusions of gastrin, secretin and cholecystokinin (Mulcahy et al. 1972; Malfertheiner et al. 1980). It may be wondered whether gastrinaemia under conditions such as gastrinoma (Zollinger–Ellison syndrome) and treatments with proton-pump inhibitors or histamine 2-receptor antagonists influence the secretory activity and output of proteins in human salivary glands.
The fact that morphological responses following pentagastrin were more reminiscent of responses following isoprenaline than following carbachol may suggest that the effects observed here depended on intracellular mobilization of cAMP and, probably, as in the rat, also the engagement of intracellularly mobilized nitric oxide (Çevik-Aras & Ekström, 2006b,c) rather than calcium. In pancreatic acinar cells, activation of CCK receptors increases cAMP and promotes granular exocytosis and amylase secretion (Marino et al. 1993; Wank, 1995; Williams, 2001).
In conclusion, the regulation of the digestive tract secretion is usually divided into separate phases depending on the location of the stimulus. The present findings in the human parotid gland support the opinion, based on recent animal experiments, that salivary gland secretion and its composition is under the control not only of a cephalic phase but also of gastric (gastrin) and intestinal (cholecystokinin and melatonin) phases.
Acknowledgments
We are grateful to Ms Silvana Bernardini for cutting specimens for TEM. Mr Alessandro Cadau helped with photography. Prof. J. Ekström acknowledges support from the University of Cagliari for periods as Visiting Professor. Supported by a Grant from Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 2008).
Author contributions
Authors certify that there is no conflict of interest in this manuscript. In this manuscript, concepts have been provided by J. Ekström, A. Riva, F. Loy and R. Isola, experiments and TEM observations were performed by P. Solinas, M. Isola, R. Isola, and M. Diana, and HRSEM analysis by F. Loy and G. Conti. Protein analysis was carried out by M. Diana, data interpretation by F. Loy, A. Riva and J. Ekström. Critical revisions were made by M.S. Lantini, M. Cossu, A. Riva and J. Ekström.
References
- Bubenik GA. Gastrointestinal melatonin, localization, function and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- Castle JD. Protein secretion by rat parotid acinar cells. Pathways and regulation. Ann N Y Acad Sci. 1998;842:115–124. doi: 10.1111/j.1749-6632.1998.tb09639.x. [DOI] [PubMed] [Google Scholar]
- Castle JD, Castle AM. Two regulated secretory pathways for newly synthesized parotid salivary proteins are distinguished by doses of secretagogues. J Cell Sci. 1996;109:2591–2599. doi: 10.1242/jcs.109.10.2591. [DOI] [PubMed] [Google Scholar]
- Castle JD, Castle AM. Intracellular transport and secretion of salivary proteins. Crit Rev Oral Biol Med. 1998;9:4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- Castle AM, Huang AY, Castle JD. The minor regulated pathway, a rapid component of salivary secretion, may provide docking/fusion sites for granule exocytosis at the apical surface of acinar cells. J Cell Sci. 2002;115:2963–2973. doi: 10.1242/jcs.115.14.2963. [DOI] [PubMed] [Google Scholar]
- Çevik-Aras H, Ekström J. Cholecystokinin- and gastrin-induced protein and amylase secretion from the parotid gland of the anaesthetized rat. Regul Pept. 2006a;134:89–96. doi: 10.1016/j.regpep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Çevik-Aras H, Ekström J. Pentagastrin-induced protein synthesis in the parotid gland of the anaesthetized rat, and its dependence on CCK-A and -B receptors and nitric oxide generation. Exp Physiol. 2006b;91:673–679. doi: 10.1113/expphysiol.2006.033639. [DOI] [PubMed] [Google Scholar]
- Çevik-Aras H, Ekström J. Pentagastrin-induced nitric oxide-dependent protein secretion from the parotid gland of the anaesthetized rat. Exp Physiol. 2006c;91:977–982. doi: 10.1113/expphysiol.2006.034710. [DOI] [PubMed] [Google Scholar]
- Çevik-Aras H, Godoy T, Ekström J. Melatonin-induced protein synthesis in the rat parotid gland. J Physiol Pharmacol. 2011;62:95–99. [PubMed] [Google Scholar]
- Ekström J. Autonomic control of salivary secretion. Proc Finn Dent Soc. 1989;85:323–331. [PubMed] [Google Scholar]
- Ekström J. Role of nonadrenergic, noncholinergic autonomic transmitters in salivary glandular activities in vivo. In: Garrett JR, Ekström J, Anderson LC, editors. Neural Mechanisms of Salivary Gland Secretion. Basel: Karger; 1999. Front Oral Biol 11, 94–130. [Google Scholar]
- Ekström J, Asztély A, Tobin G. Parasympathetic non-adrenergic, non-cholinergic mechanisms in salivary glands and their role in reflex secretion. Eur J Morphol. 1998;36:208–212. [PubMed] [Google Scholar]
- Ekström J, Çevik-Aras H. Parasympathetic non-adrenergic, non-cholinergic transmission in rat parotid glands: effects of cholecystokinin-A and -B receptor antagonists on the secretory response. Regul Pept. 2008;146:278–284. doi: 10.1016/j.regpep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Malfertheiner P, Fussgánger R, Minne H, et al. Influence of gastrointestinal hormones on human salivary secretion. Horm Metab Res. 1980;12:485. doi: 10.1055/s-2007-999181. [DOI] [PubMed] [Google Scholar]
- Marino CR, Leach SD, Schaefer JF, et al. Characterization of cAMP-dependent protein kinase activation by CCK in rat pancreas. FEBS Lett. 1993;316:48–52. doi: 10.1016/0014-5793(93)81734-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy H, Fitzgerald O, McGeeney KF. Secretin and pancreozymin effect on salivary amylase concentration in man. Gut. 1972;13:850. [PubMed] [Google Scholar]
- Murakami M, Yoshimura K, Segawa A, et al. Relationship between amylase and fluid secretion in the isolated perfused whole parotid gland of the rat. Eur J Morphol. 2000;38:243–247. doi: 10.1076/0924-3860(200010)38:4;1-o;ft243. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Riva A. A simple and rapid staining method for enhancing the contrast of tissues previously treated with uranyl-acetate. J Microsc. 1974;19:105–108. [Google Scholar]
- Riva A, Congiu T, Faa G. The application of the OsO4 maceration method to the study of human bioptic material. A procedure avoiding freeze-fracture. Microsc Res Tech. 1993;26:526–527. doi: 10.1002/jemt.1070260610. [DOI] [PubMed] [Google Scholar]
- Riva A, Faa G, Loffredo F, et al. An improved OsO4 maceration method for the visualization of internal structures and surfaces by high resolution scanning electron microscopy. Scanning Microsc. 1999;13:111–122. [Google Scholar]
- Riva A, Puxeddu R, Loy F, et al. Serous and mucous cells of human submandibular salivary gland stimulated in vitro by isoproterenol, carbachol and clozapine: an LM, TEM, and HRSEM study. Eur J Morphol. 2003;41:83–87. doi: 10.1080/09243860412331282174. [DOI] [PubMed] [Google Scholar]
- Sakai T. Intercellular canaliculi of salivary glands serve as a device for secretion of electrolytes and fluids. Biomed Res. 1984;5:433–440. [Google Scholar]
- Segawa A, Loffredo F, Puxeddu R, et al. Exocytosis in human salivary glands visualized by high-resolution scanning electron microscopy. Cell Tissue Res. 1998a;291:325–336. doi: 10.1007/s004410051002. [DOI] [PubMed] [Google Scholar]
- Segawa A, Riva A, Loffredo F, et al. Cytoskeletal regulation of human salivary secretion studied by high resolution electron microscopy and confocal laser microscopy. Eur J Morphol. 1998b;36:41–45. [PubMed] [Google Scholar]
- Testa Riva F, Puxeddu R, Loy F, et al. Cytomorphological study on human submandibular gland following treatment with secretagogue drugs. Cell Tissue Res. 2006;324:347–352. doi: 10.1007/s00441-005-0149-1. [DOI] [PubMed] [Google Scholar]
- Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:628–646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- Williams JA. Intracellular signalling mechanism activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77–97. doi: 10.1146/annurev.physiol.63.1.77. [DOI] [PubMed] [Google Scholar]


