Abstract
It is widely accepted that during postnatal development trabecular bone adapts to the prevailing loading environment via modelling. However, very little is known about the mechanisms (whether it is predominantly modelling or remodelling) or controls (such as whether loading influences development) of fetal bone growth. In order to make inferences about these factors, we assessed the pattern of fetal trabecular development in the humerus and femur via histomorphometric parameter quantification. Growth and development (between 4 and 9 months prenatal) of trabecular architecture (i.e. thickness, number and bone volume fraction) was compared across upper and lower limb bones, proximal and distal regions, and sexes. The data presented here indicate that during prenatal development trabeculae became thicker and less numerous, whilst bone volume fraction remained constant. This partly mimics the pattern of early postnatal development (0–2 years) described by other researchers. Thickness was reported to increase whilst number reduced, but bone volume fraction decreased. This is perhaps because the balance of bone modelling (deposition vs. resorption) changes post partum. Published histological data suggest that bone deposition slows after birth, while resorption rates remain constant. Hence, fetal development may be characterized by relatively high rates of modelling and, particularly, bone deposition in comparison to postnatal. With respect to measures of thickness, number and bone volume fraction prenatal development was not bone, site, or sex specific, whilst postnatally these measures of architecture diverge. This is despite reported developmental variation in the frequency, speed and amplitude of fetal movements (which begin after 11 weeks and continue until birth), and probably therefore loading induced by muscular contractions. This may be because prenatal limb bone micro-architecture follows a generalised predetermined growth trajectory (or genetic blueprint), as appears to be the case for gross distribution of trabecular tissue.
Keywords: development, fetal, micro-computed tomography, trabecular architecture, trabecular thickness, X-ray micro-tomography
Introduction
Increasingly researchers are reporting that developmental health can impact adult disease susceptibility (Kajantie, 2008). For example, low birth weight is linked to reduced bone mass (Schlüssel et al. 2010) and increased fracture risk in adults (Cooper et al. 2006). Body mass at birth (Cooper et al. 1995) and age 1 year (Cooper et al. 1997; Dennison et al. 2005) are correlated with bone mineral content (BMC) in old age (60–75 years), even when factors thought to influence BMC are taken into account, such as exercise levels, smoking and diet. Hence, fetal development may have a significant impact on adult bone quality (Javaid & Cooper, 2002) and, therefore, senescent disease (Cooper et al. 2006). For this reason it is essential that we understand the factors that control healthy fetal bone development, which at present are unclear (Nowlan et al. 2007). A number of studies have examined postnatal development of trabecular micro-architecture but relatively few have considered the prenatal period, partly because of the lack of access to material.
Studies of postnatal development may give some insight into prenatal growth. For example, early postnatal development (0–2 years) of the femur (Glorieux et al. 2000) and tibia (Ryan & Krovitz, 2006) were characterised by an increase in trabecular thickness accompanied by a decrease in both number and volume fraction. Subsequently, between 2 and 10 (Ryan & Krovitz, 2006) or 2–25 years (Glorieux et al. 2000) thickness seems to continue to increase whilst number falls, but there is a net increase in bone volume fraction. During adolescence and early adulthood trabeculae in the capitate and navicular also appeared to get thicker and less numerous (Macho et al. 2005). However, despite the apparent developmental similarities across bones, there is evidence that postnatal development of trabecular micro-architecture could be bone region and sex specific (see Kuhn et al. 1990). Should such patterns of growth exist they might obscure general patterns of micro-architectural development if not fully understood and accounted for. In adult humans trabeculae tended to be thinner and more numerous in the navicular than in capitate (Macho et al. 2005). Perhaps this intra-specific variation reflects loading patterns, for example habitually weight-bearing lower limbs vs. habitually non-weight-bearing upper limbs. However, the adult primate humeral head has thinner, more numerous trabeculae than the femur, regardless of locomotor behaviour (Fajardo & Muller, 2001; Ryan & Walker, 2010). Furthermore, the sub-epiphyseal (methaphyseal) region of the human tibia has a greater bone volume fraction and more numerous trabeculae than subchondral (epiphyseal) bone (Ryan & Krovitz, 2006). This suggests bone development might be region specific. There is also evidence of sexual dimorphism; males tend to have thicker, less numerous trabeculae than females in both the capitate and navicular (Macho et al. 2005). Although no significant differences were found in the lumbar vertebrae (Agarwal et al. 2004). Sexually dimorphic microstructures may be related to variation in rates of bone deposition. Post-pubertal rates of osteoblastic activity and osteoid formation in the proximal femur were found to be higher in males than females (Parfitt et al. 2000). This could explain why male capitatum and naviculae had thicker trabeculae. Together these findings suggest that postnatal development of micro-architecture diverges across bones, sites and sexes, probably beginning during adolescence but possibly earlier in development.
To date only a few studies have considered prenatal development of trabecular micro-architecture. Fetal trabecular volume fraction increased in the vertebrae between 16 and 24 weeks (Nuzzo et al. 2003), and the proximal femur between 16 and 41 weeks (Glorieux et al. 1991; Salle et al. 2002). Trabecular thickness also increased between 16 and 41 weeks in the vertebrae (Nuzzo et al. 2003), proximal femur (Glorieux et al. 1991; Salle et al. 2002) and ilium (McColl et al. 2006). Intraspecific comparison of bones across these studies was difficult because the regions of interest were not homologous and the histomorphometric methods varied. However, Nuzzo et al. (2003) and McColl et al. (2006) compared regions of interest within fetal vertebrae and ilia, respectively. The central region of vertebrae was found to possess thicker, less numerous trabeculae than the external region. This is probably because the external elements were younger in comparison to the central region, which had already undergone sculpting, i.e. some trabeculae had become thicker whilst others were resorbed. In contrast, trabeculae within the iliac crest and acetabulum exhibited comparable thickness between the ages of 16 and 41 weeks (McColl et al. 2006). Ossification of the ilium proceeds from a centre anterior to the sciatic notch and progresses to the epiphyses. Perhaps the tissue at the crest and acetabulum were of comparable age. To date only one study has considered sexual dimorphism of fetal trabeculae, and found that between 4 and 9 months males and females possess identical morphology (McColl et al. 2006).
The limited data available indicate that the divergence in trabecular structure between bones, regions or sexes that occurs postnatally does not begin before birth. The aim of this study was to document fetal development of trabecular architecture more comprehensively and to determine whether the divergent growth patterns observed postnatally begin to appear prenatally. The results are interpreted with regard to the mechanisms of, and factors influencing, bone growth and development.
Materials and Methods
Sample
A sample of 38 fetal human skeletons of known sex, aged between 4 and 9 months (at monthly intervals), was analysed (see Table 1 for breakdown of sample sizes). The collection originated from a Liverpool workhouse at the turn of the 19th–20th century and was held in the Department of Human Anatomy and Cell Biology, Liverpool (McColl et al. 2006). The specimens were stillbirths and the age was originally ascertained to the nearest month according to the mother’s testimony. Specimens had been de-fleshed by bicuspid beetles and stored without further treatment, wrapped in newspaper. Specimens with evidence of pathological alteration to morphology (e.g. club foot) were excluded from the study.
Table 1.
Breakdown of age categories, sexes and sample size.
| Age (months) | Males (n) | Females (n) | Total (n) |
|---|---|---|---|
| 4 | 3 | 4 | 7 |
| 5 | 4 | 3 | 7 |
| 6 | 3 | 3 | 6 |
| 7 | 3 | 3 | 6 |
| 8 | 3 | 3 | 6 |
| 9 | 3 | 3 | 6 |
Micro-computed tomography (CT)
The morphology of fetal trabecular architecture was visualised and measured using micro-CT data sets. Digital histomorphometric measurements are non-destructive. Furthermore, the measurements are not subject to errors associated with traditional histological techniques, such as damage from sawing or shrinkage during preparation (see Lane & Ralis, 1983; Uchiyama et al. 1997; Ferguson et al. 1999). The limb bones were scanned at the University of Liverpool (following Macho et al. 2005, Macho et al. 2011; McColl et al. 2006) using an ACTIS 420/600 system (BIR, IL). The system was equipped with a tungsten X-ray target, a 250-mm-thick columnar caesium iodide scintillator, and a Toshiba AI5877 JP dual-field image intensifier. The scan parameters were as follows: voltage 40–60 kV; current 140–200 mA; effective monochromatic X-ray energy 20–30 kV; focal spot size 50 μm; and contrast resolution was approximately 0.5%. The original voxel size was 60 × 60 × 100 μm for smaller specimens and 120 × 120 × 100 μm for larger specimens. To avoid measurement errors and bias due to the differences in resolution, the in-plane voxel size of the smaller 60 μm data was increased by a factor of two to create a set of scans each with identical voxel sizes.
Digital histomorphometric analysis
Rather than applying a global value, the half-maximum height (HMH) method was used to threshold the micro-CT scans, i.e. define bone/non-bone boundaries. The HMH method involves thresholding each trabecule individually to minimize problems associated with beam hardening and partial volume averaging effects, as well as locate bone–non-bone boundaries within voxels (Macho et al. 2005).
Measures of trabecular thickness (Tb.Th), number (Tb.N) and a proxy for bone volume fraction (BV/TV) were collected from two transects parallel to the long axis of the bone, proximal and distal, in the fetal humerus and femur (Fig. 1). Transects were sampled from a longitudinal (∼coronal) micro-CT slice (Fig. 1) in a plane defined by three external cortical landmarks: medial apex of the proximal metaphysic; medial and lateral apices of the distal metaphysis. Transects were placed at 10% and 90% of the distance between the proximal and distal landmarks, respectively. Measurements were collected by plotting a profile of CT numbers along a transect, and then calculating the distance between the HMH points (edges) of each trabecule. The HMH points were located on the transect halfway up and down the grey value profile for a single trabecule. Thickness was defined as the distance between the two HMH points (for a full description, see Macho et al. 2005; McColl et al. 2006; Spoor et al. 1993). Number was calculated as the number of elements per millimetre along a transect, and volume fraction as the total width of bone in relation to the length of a transect.
Fig. 1.
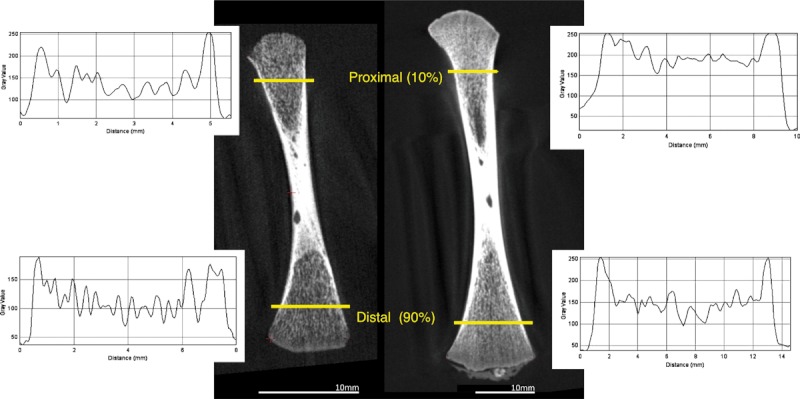
Threshold and quantification of trabecular structure using the HMH method. Profile plots were used to measure peaks (trabeculae) and troughs (non-bone) in grey values along two transects. Frequency and width of peaks corresponded to the number and thickness of trabeculae, respectively. The total width of the elements was used to calculate a measure of bone volume fraction.
Statistical analysis
Development of trabecular architecture in the humeri and femora was compared across six age categories (4, 5, 6, 7, 8 and 9 months prenatal) using one-way anova with Tukey’s post-hoc. At each stage of development morphology was also compared between regions within bones (i.e. proximal and distal transects) and sexes. Statistical tests were carried out using PAST version 2.08 (Hammer et al. 2001).
Results
Both the humeri and femora exhibited an increase in Tb.Th and decrease in Tb.N during fetal development, whilst BV/TV remained constant (Fig. 2). Post-hoc analysis revealed that this pattern of change in Tb.Th and Tb.N was significant. Older age groups, 5 to 9 months, had significantly thicker and less numerous trabeculae than 4-month-old limb bones (Table 2). Only one comparison, humeral trabecular thickness in the 4- and 6-months-old categories, was not statistically significant (Table 2). Conversely, measures of BV/TV were comparable and did not differ significantly at all across the 4-months and older age categories (Table 2).
Fig. 2.
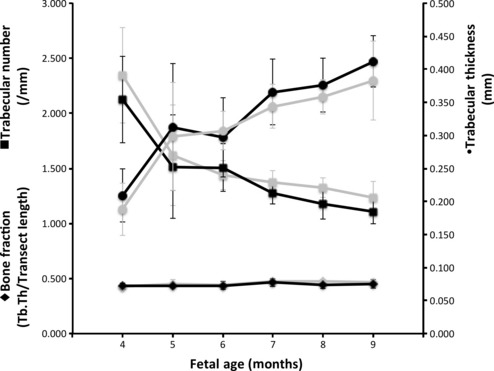
Humeri and femora share comparable patterns of trabecular micro-architectural growth and development. Tb.Th, trabecular thickness.
Table 2.
Trabecular architecture of the humerus and femur is sculpted during fetal development, i.e. trabecular thickness (Tb.Th) increases whilst number (Tb.N) decreases and volume fraction (BV/TV) remains constant. Age categories compared vs. 4 months using one-way anova with Tukey’s post-hoc. Grey text indicates non-significant difference in architecture.
| One way-anova | Tukey’s post-hoc 4 months vs. n months P = | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bone | Variable | F | P | 4 months | 5 months | 6 months | 7 months | 8 months | 9 months |
| Humerus | Tb.Th | 9.7900 | < 0.001 | Increase | 0.0409 | 0.1147 | 0.0007 | 0.0004 | 0.0001 |
| Tb. N | 10.7900 | < 0.001 | Decrease | 0.0092 | 0.0081 | 0.0003 | 0.0002 | 0.0001 | |
| BV/TV | 0.9972 | 0.4352 | Comparable | 0.9998 | 0.9998 | 0.4559 | 0.9963 | 0.7730 | |
| Femur | Tb.Th | 12.0300 | < 0.001 | Increase | 0.0096 | 0.0033 | 0.0002 | 0.0002 | 0.0001 |
| Tb.N | 13.4200 | < 0.001 | Decrease | 0.0013 | 0.0002 | 0.0001 | 0.0001 | 0.0001 | |
| BV/TV | 3.1570 | 0.0199 | Comparable | 0.6703 | 0.9853 | 0.7780 | 0.0564 | 0.1689 | |
Trabecular micro-architecture (thickness, number and bone volume fraction) did not differ significantly between the humeri and femora in any age category (Table 3). Trabecular microstructure of proximal and distal transects was also comparable in both the humeri and femora in all six age categories (Table 3). Similarly, within each of the defined age groups there were no significant differences between males and females in either the humerus or femur (Table 3).
Table 3.
Development of trabecular architectural variables is comparable across regions, sexes and bones, i.e. age categories have the same structure assessed using one-way anova with Tukey’s post-hoc. Abbreviations denote: Tb.Th, trabecular thickness; Tb.N, trabecular number; BV/TV, bone volume fraction. Grey text indicates non-significant difference in architecture.
| One way-anova Humerus vs. Femur | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bone | Variable | F | P | 4 months | 5 months | 6 months | 7 months | 8 months | 9 months |
| Humerus and femur | Tb.Th | 9.9260 | < 0.001 | 0.9999 | 1.0000 | 1.0000 | 0.9999 | 1.0000 | 0.9982 |
| Tb.N | 11.2400 | < 0.001 | 0.9641 | 1.0000 | 1.0000 | 1.0000 | 0.9989 | 0.9997 | |
| BV/TV | 1.8940 | 0.0566 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.6767 | 1.0000 | |
| One way-anova proximal vs. distal | |||||||||
| Humerus | Tb.Th | 5.7810 | < 0.001 | 1.0000 | 0.9903 | 0.9995 | 0.9999 | 0.9994 | 0.9995 |
| Tb.N | 8.6510 | < 0.001 | 0.9999 | 1.0000 | 0.9966 | 1.0000 | 0.9277 | 1.0000 | |
| BV/TV | 1.2910 | 0.2502 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.1971 | 1.0000 | |
| Femur | Tb.Th | 8.3080 | < 0.001 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.9651 |
| Tb.N | 8.3030 | < 0.001 | 1.0000 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| BV/TV | 2.1640 | 0.0277 | 0.9931 | 0.9901 | 1.0000 | 0.9581 | 0.9425 | 0.9988 | |
| One way-anova male vs. female | |||||||||
| Humerus | Tb.Th | 5.4980 | < 0.001 | 1.0000 | 0.9987 | 0.9983 | 1.0000 | 1.0000 | 1.0000 |
| Tb.N | 9.0170 | < 0.001 | 0.7049 | 0.9986 | 0.9993 | 1.0000 | 1.0000 | 1.0000 | |
| BF | 0.8425 | 0.5991 | 0.9747 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.9884 | |
| Femur | Tb.Th | 3.4350 | < 0.001 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Tb.N | 9.2940 | < 0.001 | 0.5335 | 0.9963 | 1.0000 | 0.9999 | 0.9998 | 1.0000 | |
| BV/TV | 2.6110 | 0.0080 | 0.7027 | 0.9947 | 1.0000 | 0.9802 | 1.0000 | 0.6357 | |
Discussion
Trabecular micro-architectural development in the fetal humerus and femur, between 4 and 9 months, was analysed using non-destructive digital histomorphometric measures of trabecular thickness, number and bone volume fraction. Fetal remains from both past and contemporary populations are rare. To find and gain access to such a wealth of specimens that were available for this study was very difficult. Furthermore, transport of the specimens was not permitted and on-site CT facilities had to be used, as opposed to better resolution systems. It is not possible to obtain scans of present day populations because it is unethical to ask parents for access to fetal remains. Thus, the data presented in this study are both unique and valuable. However, before the usefulness of the data can be examined in a wider context it is important to assess validity of the sample and measurements.
The fetal specimens were collected from a workhouse at the turn of the 19th–20th century. As such, the individuals were probably from the lowest socio-economic classes and may therefore have been undernourished. Nutrition is known to affect bone growth and development (Specker, 2004). Furthermore, stillbirths are an indicator of prenatal developmental problems; but the specimens analysed did not have any apparent pathology. A comparison of age-related change in femoral and humeral length between the Victorian and a modern population (Johnsen et al. 2006) sampled in vivo reveals that samples were not dissimilar (Fig. 3). However, the Victorian population grew more slowly and were about 15% shorter at term. The high standard deviation of limb lengths, and overlap between developmental stages, indicates that some of the ages may be inaccurate. The degree of accuracy is not known. Nevertheless, mean humeral and femoral lengths, as well as the overall pattern of development are broadly similar in the Victorian and present day cohorts. Hence, the growth curves for trabecular micro-architecture might be considered representative of healthier and modern individuals.
Fig. 3.
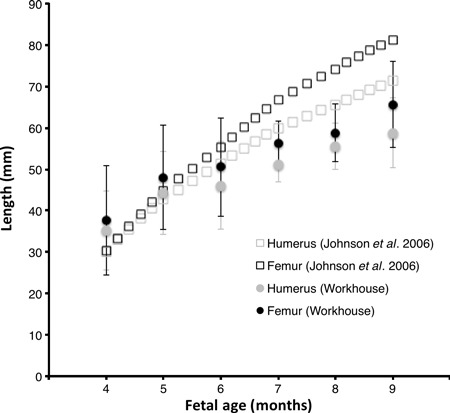
Growth of femoral and humeral length is similar in modern and Victorian populations, but the workhouse sample is approximately 15% shorter at term.
Voxel size (120 × 120 × 100 μm) was large in relation to the size of the objects being measured, given that fetal trabeculae were reported to range from 45 to 240 μm in thickness (Salle et al. 2002; Nuzzo et al. 2003; McColl et al. 2006). However, the smallest trabeculae measured were 50 μm in size. This is because the HMH method adopted here can pinpoint material boundaries within voxels. In general however the thickness data (Fig. 2) indicate that most trabeculae were above 140 μm in width. Accordingly, the interpretations that follow may only apply to the upper half of the size range of trabeculae. Furthermore, the thickest ‘trabeculae’ are about 450 μm. This suggests that some of the trabeculae were measured longitudinally (length) as opposed to perpendicularly (width), a problem of the 2D method adopted here. It is recommended that should material become available again, higher resolution scans are collected (e.g. voxel size 10–20 μm) and that trabecular architecture be measured in 3D.
Fetal development might be characterised by relatively high rates of modelling
Postnatally trabecular development is widely considered to proceed mainly via modelling. Overall, development of fetal micro-architecture in both the humerus and femur is characterised by an increase in trabecular thickness, which is matched by a decrease in number. Therefore, bone volume fraction remains constant (Fig. 2; Table 2). While some trabeculae get thicker, others are lost. This is consistent with a pattern of fetal growth and development via modelling (Salle et al. 2002) as opposed to remodelling (Glorieux et al. 1991). Although remodelling does have the capacity to alter morphology, it usually affects bone on a local, rather than global scale. Thus, modelling is the more likely mechanism. This pattern of change in morphology can be described as sculpting (see Abel & Macho, 2011) and continues postnatally. During postnatal development this pattern of change is thought to be associated with the thickening of trabeculae aligned along the principal direction of loading, and removal of elements perpendicular to the load. Accordingly, future studies of fetal development might find that anisotropy decreases during fetal development.
Like prenatal, early postnatal development (0–2 years) is also characterised by an increase in thickness and decrease in number (Glorieux et al. 2000; Ryan & Krovitz, 2006). In this context our findings suggest that there is some continuity in the pattern of micro-structural development before and after birth. However, it appears as though the balance of bone modelling (deposition vs. resorption) changes post partum, resulting in a net loss of bone tissue, as evidenced by a decrease in bone volume fraction (Glorieux et al. 2000; Ryan & Krovitz, 2006). Published data suggest that bone deposition slows after birth, while resorption rates remain constant. Parfitt et al. (2000) reported that in the proximal femur measures of deposition (such as osteoblastic activity and amount of osteoid) decreased after 1.5 years, whilst measures of resorption remained constant. Thus, the apparent change from constant (before birth) to reducing volume fraction (after birth) might be mediated by a decrease in the rate of bone formation, as opposed to an increase in resorption. Hence, fetal development may be characterized by relatively high rates of modelling, and particularly bone deposition, in comparison to postnatal.
Fetal development is not selectively affected by intrauterine movement
Bone-, site- and sex-specific growth of postnatal trabecular architecture is (at least partly) an adaptive response to different loading environments. So, it is reasonable to expect that in utero development might also exhibit an adaptive response to loading. During prenatal development the fetus is shielded from gravitational loads in utero. However, the fetal skeleton is subjected to forces imparted by muscular contractions. From about 11 weeks (∼3 months) onwards muscles contract in a sporadic and uncoordinated manner, for example kicking and punching (Delaere et al. 1992; Delaere & Dhem, 1999; Scheuer & Black, 2000). At about 5 months, limb movements become more complex, with flexing of the limb joints, and by 6 months the fetus begins to develop a regular schedule of movement (Vaughan, 1996). During normal development the frequency, speed and amplitude of these movements varies across body parts, for example the head, rump, upper and lower limbs, etc. (Adolf & Berger, 2005; de Vries & Fong, 2006), and perhaps sexes (Almli et al. 2001; but see also de Vries et al. 1987; Robles de Medina et al. 2003). Therefore, it is reasonable to assume that the loads imparted by movements might also vary across the upper and lower limbs or sexes. Yet, the development of fetal trabecular micro-architecture was comparable across the femur and humerus, the proximal and distal aspects as well as males and females (Fig. 2; Table 3). Given the apparent lack of bone-, region- or sex-specific growth, our findings suggest that the fetal movement does not selectively affect micro-architectural development.
This interpretation is consistent with the findings of previous researchers, who have reported that fetal trabecular development may be attributable to non-weight-bearing anatomical interactions, or even to a predetermined genetic blueprint. Cunningham & Black (2009) found that gross distribution of trabecular tissue (i.e. macro-structure) in the human non-habitually weight-bearing neonatal ilium is comparable to habitually weight-bearing adults. Similarly, Skedros et al. (2007) noted that adult-type main trabecular orientations (arches) in the ovine calcaneus develop in utero. Thus, familial history might be an important feature of fetal and possibly downstream adult bone health (and could be used to identify individuals at greater risk of fragility fracture), whilst loading regime and exercise might be more important after early postnatal development when habitual bipedal loading begins.
Conclusions
Despite the limitations of the 2D and relatively large-voxel CT study presented here, the findings can contribute to our understanding of fetal growth and development. Fetal development of trabecular tissue appears to be characterised by high rates of modelling, particularly bone deposition, with respect to postnatal development (0–2 years). However, bone-, site- and sex-specific patterns of trabecular growth, which are observed postnatally, do not seem to occur prenatally. This is despite variation in the frequency of muscular contractions. This finding could suggest that intrauterine kicking and punching does not selectively affect micro-architectural growth and development. This is consistent with previous researchers who have reported that fetal trabecular development may follow a predetermined path (e.g. genetic blueprint).
Acknowledgments
The authors would like to thank Dr Lauren Howard and the Micro-CT Laboratory at the Natural History Museum (London, UK) for assistance with data processing and access to computing facilities; also, Dr. Kate Robson Brown; Prof. Justin Cobb and Dr Sandra Shefelbine for advice and comments on the manuscript.
References
- Abel RL, Macho GA. Ontogenetic changes in the internal and external morphology of the ilium in modern humans. J Anat. 2011;218:324–335. doi: 10.1111/j.1469-7580.2011.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolf KE, Berger SE. Physical and motor development. In: MH Bornstein, ME Lamb., editors. Developmental Science: An Advanced Textbook. 5th edn. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 223–281. [Google Scholar]
- Agarwal SC, Dumitriu M, Tomlinson GA, et al. Medieval trabecular bone architecture: the influence of age, sex and lifestyle. Am J Phys Anthropol. 2004;124:33–44. doi: 10.1002/ajpa.10335. [DOI] [PubMed] [Google Scholar]
- Almli CR, Ball RH, Wheeler ME. Human fetal and neonatal movement patterns: gender differences and fetal-to-neonatal continuity. Dev Psychobiol. 2001;38:252–273. doi: 10.1002/dev.1019. [DOI] [PubMed] [Google Scholar]
- Cooper C, Cawley MID, Bhalla A, et al. Childhood growth, physical activity and peak bone mass in women. J Bone Miner Res. 1995;10:940–947. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- Cooper C, Fall C, Egger P, et al. Growth in infancy and bone mass in later life. Ann Rheum Dis. 1997;56:17–21. doi: 10.1136/ard.56.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Westlake S, Harvey N, et al. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17:337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- Cunningham CA, Black SM. Anticipating bipedalism: trabecular organization in the newborn ilium. J Anat. 2009;214:817–829. doi: 10.1111/j.1469-7580.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaere O, Dhem A. Prenatal development of the human pelvis and acetabulum. Acta Orthop Belg. 1999;65:255–260. [PubMed] [Google Scholar]
- Delaere O, Kok V, Nyssen-Behets C, et al. Ossification of the human fetal ilium. Acta Anat. 1992;143:330–334. doi: 10.1159/000147271. [DOI] [PubMed] [Google Scholar]
- Dennison EM, Syddall HE, Sayer AA, et al. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57:582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- Fajardo RJ, Muller R. Three-dimensional analysis of non-human primate trabecular architecture using microcomputed tomography. Am J Phys Anthropol. 2001;115:327–336. doi: 10.1002/ajpa.1089. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ, Bryant JT, Ito K. Three-dimensional computational reconstruction of mixed anatomical tissues following histological preparation. Med Eng Phys. 1999;21:111–117. doi: 10.1016/s1350-4533(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Glorieux FH, Salle BL, Travers R, et al. Dynamic histomorphometric evaluation of human fetal bone formation. Bone. 1991;12:377–381. doi: 10.1016/8756-3282(91)90025-e. [DOI] [PubMed] [Google Scholar]
- Glorieux FH, Travers R, Taylor A, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000;26:103–109. doi: 10.1016/s8756-3282(99)00257-4. [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electronica. 2001;4:9. [Google Scholar]
- Javaid MK, Cooper C. Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab. 2002;16:349–367. doi: 10.1053/beem.2002.0199. [DOI] [PubMed] [Google Scholar]
- Johnsen SL, Wilsgaard T, Rasmussen S, et al. Longitudinal reference charts for growth of the fetal head, abdomen and femur. Eur J Obstet Gynecol Reprod Biol. 2006;127:286–297. doi: 10.1016/j.ejogrb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kajantie E. Early-life events. Effects on aging. Hormones. 2008;7:101–113. doi: 10.1007/BF03401501. [DOI] [PubMed] [Google Scholar]
- Kuhn JL, Goldstein SA, Feldkamp LA, et al. Evaluation of a microcomputed tomography system to study trabecular bone structure. J Orthop Res. 1990;8:833–842. doi: 10.1002/jor.1100080608. [DOI] [PubMed] [Google Scholar]
- Lane J, Ralis ZA. Changes in dimensions of large cancellous bone specimens during histological preparation as measured on slabs from human femoral heads. Calcif Tissue Int. 1983;35:1–4. doi: 10.1007/BF02404997. [DOI] [PubMed] [Google Scholar]
- Macho GA, Abel RL, Schutkowski H. Age changes in bone microstructure: do they occur uniformly? Int J Osteoarchaeol. 2005;15:421–430. [Google Scholar]
- Macho GA, Spears IR, Leakey MG, et al. An exploratory study on the combined effects of external and internal morphology on load dissipation in primate Capitates: Its potential for an understanding of the positional and locomotor repertoire of Early Hominins. Folia Primatol. 2011;81:292–304. doi: 10.1159/000322631. [DOI] [PubMed] [Google Scholar]
- McColl DJ, Abel RL, Spears IR, et al. An automated method to measure trabecular thickness from microcomputed tomographic scans and its application. Anat Rec Part A. 2006;288:982–988. doi: 10.1002/ar.a.20371. [DOI] [PubMed] [Google Scholar]
- Nowlan NC, Murphy P, Prendergast PJ. Mechanobiology of embryonic limb development. Ann NY Acad Sci. 2007;1101:389–411. doi: 10.1196/annals.1389.003. [DOI] [PubMed] [Google Scholar]
- Nuzzo S, Meneghini C, Braillon P, et al. Microarchitectural and physical changes during fetal growth in human vertebral bone. J Bone Miner Res. 2003;18:760–768. doi: 10.1359/jbmr.2003.18.4.760. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Travers R, Rauch F, et al. Structural and cellular changes during bone growth in healthy children. Bone. 2000;27:487–494. doi: 10.1016/s8756-3282(00)00353-7. [DOI] [PubMed] [Google Scholar]
- Robles de Medina PG, Visser GH, Huizink AC, et al. Fetal behaviour does not differ between boys and girls. Early Hum Dev. 2003;73:17–26. doi: 10.1016/s0378-3782(03)00047-1. [DOI] [PubMed] [Google Scholar]
- Ryan TM, Krovitz GE. Trabecular bone ontogeny in the human proximal femur. J Hum Evol. 2006;51:591–602. doi: 10.1016/j.jhevol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ryan TM, Walker A. Trabecular bone structure in the humeral and femoral heads of anthropoid primates. Anat Rec. 2010;293:719–729. doi: 10.1002/ar.21139. [DOI] [PubMed] [Google Scholar]
- Salle BL, Rauch F, Travers R, et al. Human fetal bone development: histomorphometric evaluation of the proximal femoral metaphysis. Bone. 2002;30:823–828. doi: 10.1016/s8756-3282(02)00724-x. [DOI] [PubMed] [Google Scholar]
- Scheuer L, Black S. Developmental Juvenile Osteology. London: Academic Press; 2000. [Google Scholar]
- Schlüssel MM, dos Santos Vaz J, Kac G. Birth weight and adult bone mass: a systematic literature review. Osteoporos Int. 2010;21:1981–1991. doi: 10.1007/s00198-010-1236-z. [DOI] [PubMed] [Google Scholar]
- Skedros JG, Sorenson SM, Hunt KJ, et al. Ontogenetic structural and material variations in ovine calcanei: a model for interpreting bone adaptation. Anat Rec. 2007;290:284–300. doi: 10.1002/ar.20423. [DOI] [PubMed] [Google Scholar]
- Specker B. Nutrition influences bone development from infancy through toddler years. J Nutr. 2004;134:691S–695S. doi: 10.1093/jn/134.3.691S. [DOI] [PubMed] [Google Scholar]
- Spoor CF, Zonneveld FW, Macho GA. Linear measurements of cortical bone and dental enamel by computed tomography: applications and problems. Am J Phys Anthropol. 1993;91:469–484. doi: 10.1002/ajpa.1330910405. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Tanizawa T, Muramatsu H, et al. A morphometric comparison of trabecular structure of human ilium between microcomputed tomography and conventional histomorphometry. Calcif Tissue Int. 1997;61:493–498. doi: 10.1007/s002239900373. [DOI] [PubMed] [Google Scholar]
- Vaughan C. How Life Begins: The Science of Life in the Womb. New York: Times Books (Random House); 1996. [Google Scholar]
- de Vries JI, Fong BF. Normal fetal motility: an overview. Ultrasound Obst Gyn. 2006;27:701–711. doi: 10.1002/uog.2740. [DOI] [PubMed] [Google Scholar]
- de Vries JI, Visser GHA, Mulder EJ, et al. Diurnal and other variations in fetal movement and heart rate patterns at 20–22 weeks. Early Hum Dev. 1987;15:333–348. doi: 10.1016/0378-3782(87)90029-6. [DOI] [PubMed] [Google Scholar]


