Abstract
More than a decade ago, ‘plasticity’ suddenly became a ‘fashionable’ topic with overemphasized implications for regenerative medicine. The concept of ‘plasticity’ is supported by old transplantation work, at least for embryonic cells, and metaplasia is a classic example of plasticity observed in patients. Nevertheless, the publication of a series of papers showing rare conversion of a given cell type into another unrelated cell raised the possibility of using any unaffected tissue to create at will new cells to replace a different failing tissue or organ. This resulted in disingenuous interpretations and a reason not to fund anymore research on embryonic stem cells (ESc). Moreover, many papers on plasticity were difficult to reproduce and thus questioned; raising issues about plasticity as a technical artefact or a consequence of rare spontaneous cells fusion. More recently, reprogramming adult differentiated cells to a pluripotent state (iPS) became possible, and later, one type of differentiated cell could be directly reprogrammed into another (e.g. fibroblasts into neurons) without reverting to pluripotency. Although the latter results from different and more robust experimental protocols, these phenomena also exemplify ‘plasticity’. In this review, we want to place ‘plasticity’ in a historical perspective still taking into account ethical and political implications.
Keywords: cell fusion, ES and iPS cells, multipotency, nuclear reprogramming, plasticity and trans-differentiation, stem/progenitor cells
Introduction
The last years have witnessed groundbreaking results that have radically changed established concepts in stem cell biology, such as the irreversibility of the differentiated state. This was mainly due to the possibility of ‘reprogramming’ a differentiated cell into an ‘induced pluripotent stem cell’ (iPSc) by transfer of few transcription factors. iPS cells are similar to embryonic stem cells (ESc), the only truly pluripotent cells that have raised hopes for regenerative medicine and also heated ethical debates because they are derived from human embryos, a step now unnecessary with iPS. Indeed, older results obtained by cell fusion or nuclear transfer had shown that reversion to an undifferentiated state is possible, but the easy and direct approach that generates iPS cells has somehow set a milestone in the field.
More recently, direct reprogramming from one to another differentiated cell type without transit through an ‘undifferentiated, pluripotent’ state has moved further the field toward rapid and safer clinical translation, since this procedure would eliminate the risk of teratoma that both iPSc and ESc may generate in vivo.
While many excellent reviews cover these topics exhaustively, here we aim to highlight the concept of ‘plasticity’, i.e. the ability of a cell to change its fate in response to extra-cellular signals. Plasticity, that could be redefined as ‘environmental or extrinsic factor-mediated reprogramming’ at variance with the ‘transcriptional or intrinsic factor-mediated reprogramming’ mentioned above, is a complex concept often mudded by technical artefacts. We aim to discuss ‘plasticity’ in relation to the above topics and also to older concepts such as trans-determination, trans-differentiation and metaplasia. We aim to create a unifying scenario that may allow placing old and newer data under the same perspective and discuss the relative implications for clinical translation that is already taking place now.
Obviously it would be impossible and beyond our scope to review such an enormous literature. Many excellent reviews on specific topics exist, to which the reader is referred in the subsequent sections. A few keystone observations will be mentioned to create a historical frame indicating where various events fit in the recent history of the field of plasticity.
Cloning by nuclear transfer demonstrated that adult nuclei can be reprogrammed
Many classical experiments of embryology had shown that cells can change their fate when transplanted heterotopically (i.e. in an anatomical location different from that which they had been isolated from; see, e.g.: Gunhaga, 2011; Le Douarin et al, 2004). However the generation of an adult frog from a nucleus transplanted in the cytoplasm of an enucleated egg (Gurdon et al, 1958), showed for the first time that the nucleus of a somatic cell still contains all the information necessary and sufficient to make another organism, and can be re-programmed by factors that are present in the egg cytoplasm. Almost 40 years went by before the same reprogramming could be demonstrated in a mammalian nucleus that gave rise to the famous sheep ‘Dolly’ (Wilmut et al, 1997).
Cell fusion and the discovery of MyoD
On a parallel route, cell fusion experiments showed that when nuclei of two different tissues and species are artificially placed in the same cytoplasm, often one predominates and activates the genes of its own developmental program in the other nucleus (Blau et al, 1983). This suggested that some transcription factor might play a dominant role and impose its transcriptional program. Myogenic determination gene (MyoD) was the first and still most remarkable case of a single transcription factor that is able to convert a non-myogenic cell into skeletal muscle (Davis et al, 1987). Subsequent work by several laboratories showed that cells can be myogenically ‘converted’ (‘directly reprogrammed’ according to nowadays terminology) with a frequency that is proportional to the lineage relationship of the converted cells with skeletal myoblasts, i.e. high in paraxial mesoderm cells, lower in ectoderm and endoderm derived cells, almost null in amniotic cells. It was later recognized that each transcription factor works in concert with many others, and their activity also depends on the epigenetic landscape and interactions with other molecules such as microRNAs. Thus, the microenvironment inside a nucleus plays a major role in determining the frequency of conversion/reprogramming. At that time in the late 80s, many laboratories tried to find an equivalent of MyoD in other tissues. With the exception of NeuroD (Lee et al, 1995), these early attempts failed in most cases and remained largely unpublished.
Embryonic stem cells and the discovery of pluripotency
Approximately at the same time, the discovery of ESc (Evans and Kaufman, 1981; Martin, 1981) opened previously unimaginable scenarios for regenerative medicine. Explanting mouse blastocysts in culture leads, under proper conditions, to unlimited proliferation of cells of the blastocyst inner cell mass that, strikingly, maintain the potency of generating all the cells of the body (pluripotency), including germ cells, and thus may be used to replace any lost or damaged tissue. Moreover, upon injection into the blastocele of a foster blastocyst, they colonize all tissues, including gonads and can thus produce gametes that entirely originate from ESc. Thus, mating two chimaeras can produce a normal fertile mouse, entirely derived from ES cells. Such a discovery opened the possibility to create numberless models of human diseases, and related mutant mouse strains by manipulating the genome in ESc.
Glossary
Cell fusion
Is the phenomenon by which two cells fuse their membranes so that the two nuclei end up in the same cytoplasm. It may occur naturally, e.g. in skeletal myoblasts, or experimentally by exposing cells to fusogenic agents like polyethylene glycol (PEG) or Sendai Virus.
Committed
Is used for progenitor cells that are fated to differentiate into a specific cell type. Commitment can be divided in a reversible phase (which can still be changed by external cues such as transplantation in a different anatomical site) and an irreversible phase (which can no longer be modified).
Embryonic stem cells
Are isolated and expanded in vitro from mammalian blastocyst inner cell mass (the internal part of the mammalian embryo before implantation in the uterus, destined to form all the tissues of the future organism). ES cells can be cultured indefinitely and maintain the ability to differentiate into all cell types of the body either in vitro or after injection into a blastocyst.
iPS cells
Induced pluripotent stem cells are cells (of any origin) that have been reprogrammed by expression of few (usually 3 or 4) specific transcription factors (e.g. c-Myc, Klf4, Oct4, Sox2) to an embryonic stage. The phenotype of reprogrammed cells is very similar, though not identical to that of ES cells.
Plasticity
Is the phenomenon by which a cell (usually not terminally differentiated) changes its phenotype in response to environmental signals. Trans-differentiation is the phenomenon by which an already differentiated cell changes its phenotype in response to environmental signals; metaplasia is a form of trans-differentiation that occurs in pathological conditions where usually epithelial cells adopt the phenotype of another epithelium. It is often a pre-neoplastic lesion.
Potency
Is the ability of stem/progenitor cells to differentiate into one or more types of differentiated cells. Specifically: totipotency indicates the ability to differentiate into any type of cells of the body including foetal annexes; pluripotency indicates the ability to differentiate into any type of cells of the body except foetal annexes; multipotency indicates the ability to differentiate into several type of cells of the body; unipotency indicates the ability to differentiate into only one cell type.
Regeneration
Is the process by which a tissue restores its physiological homeostatic condition. It often recapitulates many aspects of tissue histogenesis during development. When regeneration fails, tissue is progressively replaced by fat infiltration and fibrosis.
The first derivation of human ES cells (Thomson et al, 1998) made this methodology ‘immediately’ translatable to patients. Indeed, 13 years went by before FDA approved the first trial, conducted by Geron Co. in patients with spinal cord lesions. Why all these years? In our opinion, two main reasons delayed clinical translation, one scientific and one ethical that turned political.
For what concerns science, the major and still partially unsolved problem for regenerative medicine, is the need to induce differentiation in 100% of the cell population and not in 99.99% of them. In fact, even very few undifferentiated cells contaminating the ‘differentiated’ or ‘committed’ population, will continue to proliferate and give rise to a teratoma. Currently, extremely sophisticated cell separation techniques (Tang et al, 2011) and the possibility of inserting suicide genes only inducible in undifferentiated cells (Naujok et al, 2010) have reduced this risk to the point of convincing the FDA to authorize few ES cells-based human trials for spinal cord lesions and Stargardt's Macular Dystrophy (ClinicalTrials.Gov).
Ethical issues
The ethical issue was more complex and still fuels heated and, in our opinion, outdated debates. Since human ES cells are derived from pre-implantation human embryos, they are considered ‘human beings’ by the Catholic Church, and therefore have the same moral status of a ‘person’, as defined by several constitutions such as those of US and Italy (Avila, 2001; Mazzoni, 2002). Human blastocysts are either obtained from supernumerary embryos after in vitro fertilization procedures (but in this case they would be ‘non-self’ to the patient), or from ‘ad hoc’ created blastocysts after nuclear transfer of a patient nucleus into an anucleated human oocyte. The Catholic Church condemns both procedures, and this was reflected in restrictive regulations and funding limitations in the US and several European countries. It should be noted that the political situation and the relative influence of the Catholic Church resulted in extremely different attitudes and regulations of human ESc research. Europe has yet to reach a consistent legislation, and this is reflected in unclear and ambiguous sentences that appear in EC calls related to this topic (Ralston, M, Pew Forum on Religion & Public Life: ‘Stem Cell Research Around the World’ http://pewforum.org/Science-and-Bioethics/Stem-Cell-Research-Around-the-World.aspx). While Germany, Austria, Italy, Finland, Greece, Ireland, Portugal and the Netherlands prohibit or severely restrict the use of ES, Sweden, UK and to various extent Belgium and France have regulations that allow free research on ES though all essentially ban reproductive cloning (i.e. the creation of a human being from nuclear transfer). Research on human ESc and the derivation of new cell lines from supernumerary human embryos are strictly regulated Switzerland and, it is forbidden to create embryos for the sole purpose of research. In October 2011, the European Court banned patents based on human ESc (Callaway, 2011), thus causing problems to European countries with respect to countries like China (Dennis, 2002), India, South Korea or Israel, that essentially have no regulations or patent restrictions. The situation in the US changed with the different administrations and Obama, in 2009, removed the restriction of federal funding on ES cell research imposed during Bush's presidency.
Despite this complex and problematic situation, we believe that the further consolidation of iPSc or, later in time, of direct reprogramming, will end this controversy.
Reprogramming to an embryonic-like state or directly to another mature cell
Almost 20 years after MyoD discovery, Takahashi and Yamanaka showed that transduction of embryonic mouse fibroblasts with four transcription factors now known as the Yamanaka factors (Oct4, Klf4, cMyc and Sox2), reprograms somatic cells into an embryonic-like state: ‘induced pluripotent stem cells’ or iPS cells (Takahashi & Yamanaka, 2006; Fig 1). This discovery (named by Science the discovery of the year in 2007) set an historical milestone in the field and the number of papers published yearly on the topic keeps increasing exponentially (for recent reviews see: Hanna et al, 2010; Jopling et al, 2011; Wilmut et al, 2011; Yamanaka & Blau, 2010). The possibility of deriving patient-specific ES-like cells that can be indefinitely expanded in vitro, genetically corrected if needed, and then induced to differentiate into the desired cell type, appeared as a real breakthrough over previous reprogramming approaches based on nuclear transfer; it is technically simpler and apparently bypasses the ethical controversies and their political consequences described above. For the sake of records, it should be mentioned that several recent papers have challenged the complete equivalence of iPS and ES cells (for a recent review see: Power & Rasko, 2011). Until now, the differences reported do not seem to have a major impact on the possible future clinical use of these cells, with the possible exception of immunogenicity (Zhao et al, 2011).
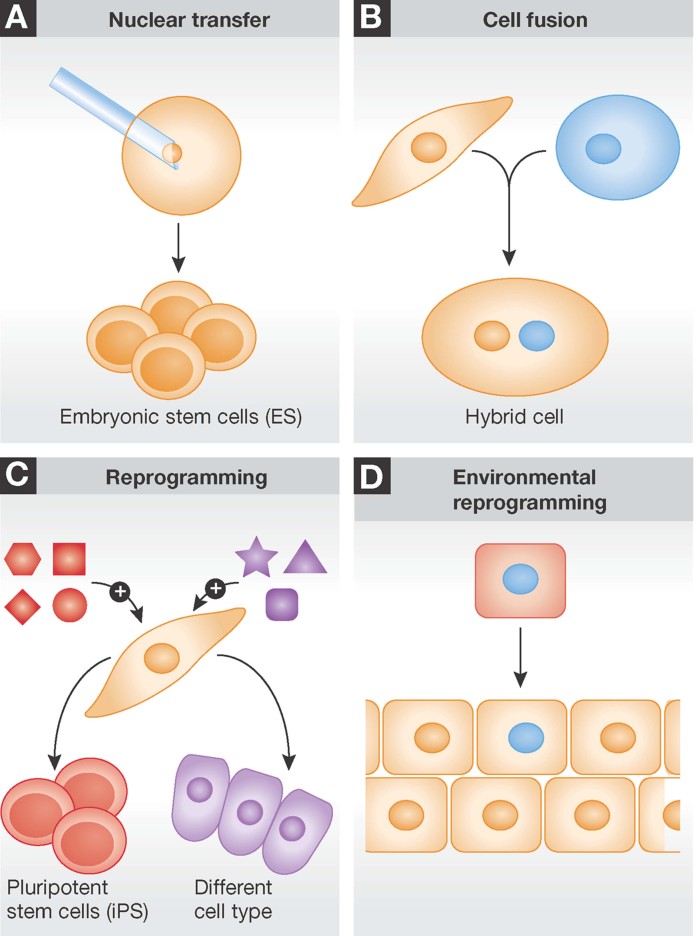
Figure 1. Cell reprogramming.
- Nuclear transfer into an anucleated oocyte reprograms a somatic nucleus, thus generating a blastocyst from which ES cells can be derived.
- Cell fusion exposes two different nuclei to the same cytoplasm, and one nucleus may impose its transcriptional program (red) over the other (blue).
- Transfer of specific transcription factors may reprogram a somatic cell (yellow) to a pluripotent (iPS) or to another differentiated cell type (red).
- Transplantation of a genetically labeled (blue nucleus) differentiated cell (red) to a different tissue (yellow) may activate that developmental program in the transplanted cell.
Moreover, the argument that the reprogrammed nucleus is anyway ‘old’, i.e. has the same age of the patient, appears to be at least disingenuous when compared to spared embryo derived ES cells, which are ‘non-self’: a reprogrammed cell should be compared to a nuclear-transferred ES cell that has exactly the same age and ‘self-ness’. However, the efficiency of derivation of pluripotent cells from somatic cells could be affected by several factors, including genetic and epigenetic profiles that are correlated to the senescence process (Banito et al, 2009). Only time will tell whether iPS will replace ES cells, but in any case, the value of ES cells for science and medicine will remain immense, since they led to a revolution in biology, and the same iPS cells would have never been discovered without them.
Direct reprogramming
Finally, in the last 2 years, several laboratories showed that it is possible to directly reprogram an adult fibroblast to a cardiomyocyte or a neuron (and even specific neural subtypes), by forced expression of usually two or three transcription factors (Efe et al, 2011; Ieda et al, 2010; Kim et al, 2011; Szabo et al, 2010; Vierbuchen et al, 2010). In some cases a fate switch among related pancreatic epithelial cells has been induced by the forced expression of three or even a single transcription factors (Collombat et al, 2009; Zhou et al, 2008). In all cases, and examples are accumulating at a weekly pace, cells repress their own transcriptional program and activate the new one without transiting through an ES-like state. Although many issues remain to be solved, e.g. frequency of complete terminal differentiation into the desired cell type, these data appear to move the field even further toward their safe use since the tumorigenic risk, associated with ES/iPS cells, would not exist anymore. Obviously, especially in the case of certain human cells, the total number of cells that can be expanded in vitro would return as a problem due to the limited proliferation potency of human fibroblasts. Why earlier attempts (at the time of MyoD discovery) at directly reprogramming fibroblasts into other types of differentiated cells failed, and more recent, iPS cell-boosted attempts succeed, is probably due to the major advances in our understanding of the transcriptional machinery, that took place in the last 20 years.
Environmental reprogramming
Parallel to these events, and currently outdated by the development of molecular approaches, a flurry of data accumulated during decades of work showing that, following transplantation, embryonic or even adult cells, may change their fate and adopt that of the surrounding cells. Indeed, heterotopic transplantation of a group of cells, naturally fated to give rise to tissue A, into developing tissue B, is a classic assay for fate determination in embryology. If cells maintain the A phenotype, they are considered ‘determined’ or ‘committed’ to fate A, whereas if they turn into tissue B, they are considered ‘undetermined’ and ready to be instructed by signals emanating from the extra-cellular microenvironment. It is conceivable that these signalling molecules may lead to the activation of the same genes that, once transfected into adult fibroblasts in vitro, ‘reprogram’ them to the desired cell fate.
Despite the fact that the mammalian embryo is considered to be ‘regulative’, it was generally assumed, at least until Yamanaka's work, that once committed, cells could only progress towards their fixed differentiation pathway or die. Yet, numerous examples of ‘spontaneous change of fate’ exist in the old literature, but they are often linked to post-natal tissue damage and regeneration. For example, retina regeneration by pigment cells in amphibians is a classic case of trans-differentiation (or ‘spontaneous reprogramming’) that only occurs after tissue damage (Okada, 1980). On the other hand, a spontaneous trans-differentiation from smooth to skeletal muscle in the mouse oesophagus was reported in 1995 (Patapoutian et al, 1995) but later questioned based upon lineage tracing studies (Rishniw et al, 2003).
The field was changed in 1998, by a paper showing that the bone marrow of normal adult mice contains cells that can participate in skeletal muscle regeneration and give rise to new muscle fibre nuclei (Ferrari et al, 1998; Fig 2). Bone marrow-derived muscle cells were very few (less than 1%) and were easily detected in the host muscle despite their low frequency, because they expressed a muscle-specific nuclear LacZ. Unpredicted by the authors, this paper opened a Pandora's box, whose ethical and political consequences far exceeded the relevance of the data reported. Within a few years, the literature was flooded with papers, often in high profile journals (e.g. Bjornson et al, 1999; Krause et al, 2001; Lagasse et al, 2000; Orlic et al, 2001), showing that many cells of adult tissues, when transplanted in a different regenerating tissue, may give rise to one or more cell types that are typical of that recipient environment. Often, conclusions were based on double fluorescence, where one cell would show a tracer of its origin and an antigen typical of the tissue where it had been transplanted. Nevertheless, these data suggested that it would have been possible to isolate patient's own cells from an unaffected tissue, expand and, if needed, genetically correct them for transplantation into the affected tissue or organ. It was obvious that the frequency of these events was almost invariably very low, far below the threshold of any possible clinical efficacy. In addition, in some cases it was clearly demonstrated that bone marrow-derived Purkinije neurons, cardiomyocytes and hepatocytes were the result of cell fusion rather than reprogramming (Alvarez-Dolado et al, 2003; Balsam et al, 2004; Wang et al, 2003). Yet, the idea of using this form of environmental ‘reprogramming’ for clinical future aims circulated and was rapidly adopted by ethicists and politicians to reach the conclusion that ESc work was not only morally condemnable, but also useless.
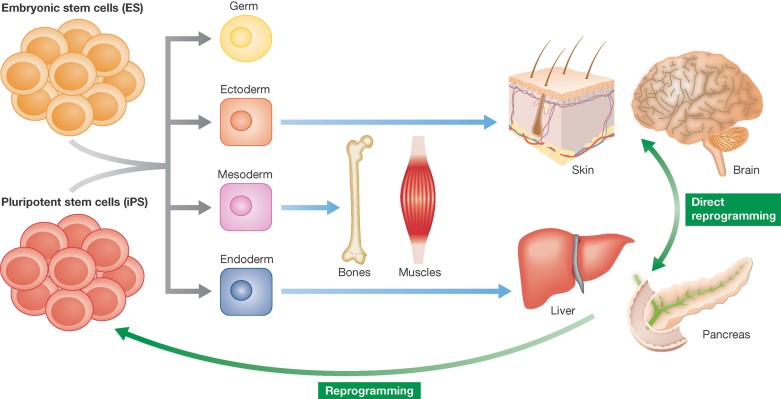
Figure 2. Changing the model of cell determination and differentiation.
Grey arrows indicate the ability of ES and iPS cells to give rise to germ and somatic layers, that proceed (light blue arrows) towards their various differentiated tissues. Green arrows represent the possibility of reprogramming differentiated cells to a pluripotent state or directly to a different differentiated cell type, independently from the germ layer of origin.
As mentioned above, the Bush Administration prohibited any NIH funded project to work with human ES cells derived after year 2001. Also, several European countries made the life of people working with ESc difficult, and the papers mentioned above unwillingly put a potent political weapon in their hands (see, e.g.: Marwick, 2001). It is ironic that one of the senior authors of the original article was actively engaged with the Italian Radical Party to defend the possibility of working with human ES in Italy, by promoting a referendum to abolish a law (no. 40) of February 2004 that practically, though not formally, prohibited work on human ES cells in Italy, and also introduced a number of illogical and unscientific restrictions that fortunately were later abolished because they were found to be unconstitutional.
The reaction of the ESc scientific community was prompt and vibrant; it started by heavily criticizing most of these papers, by implying that ‘plasticity’ was the consequence of immune staining or tissue culture artefacts, and explaining that the ones that were confirmed by other independent laboratories were the result of spontaneous cell fusion, where, as described above, one nucleus would impose its transcriptional program to the other. This culminated in three papers published at the same time and practically burying the field of environmental reprogramming and plasticity (Terada et al, 2002; Wagers et al, 2002; Ying et al, 2002). The fact that spontaneous cell fusion is the natural mechanism through which skeletal muscle forms in vertebrates (Mintz & Baker, 1967) was not considered at that time, even though it did explain, at least in part, the result of bone marrow giving rise to skeletal muscle (Ferrari et al, 1998), and later and unexpectedly, to Purkinjie cells (Weimann et al, 2003). Nevertheless, years went by and ‘plasticity’ was considered a concept not supported by solid experimental evidence. However, in recent years, several papers, scientifically unquestionable and in high profile journals, showed that cells can be environmentally reprogrammed to a complete and mature fate. For example, the group of G. Smith, showed that both embryonic and adult neural stem cells can be reprogrammed by the mammary gland microenvironment where they give rise to chimaeric glands and progressively lose their neuronal identity (Booth et al, 2008). Even more strikingly, clonally expanded epithelial cells of both embryonic and adult rodent thymus were reprogrammed to multipotent hair follicle stem cells in vivo, as they were found able to give rise to all skin lineages, i.e. hair follicle, epidermis and sebaceous glands, upon serial transplantation. Moreover, reprogrammed thymic cells re-isolated from the hair follicle, were able, to a variable extent, to revert to their primitive fate once transplanted back into the thymus (Bonfanti et al, 2010). In all these examples the morphogenetic signalling that drives cell fate switch and broaden potency of differentiated or ‘committed’ cells remains to be elucidated. However, the complexity at the basis of such events should stimulate instead of discouraging deeper investigation into the molecular mechanisms of environmental reprogramming. This may have relevance not only for cell therapy but also for cancer. Indeed, there is increasing evidence that specific microenvironments such as skeletal muscle, mammary gland or neural crest can inhibit tumorigenic fate and reprogram cancer cells to a ‘normal’ phenotype (Booth et al, 2011; Bussard et al, 2010; Kasemeier-Kulesa et al, 2008; Parlakian et al, 2010). Other examples keep accumulating, such as those showing extra-cellular signals enhancing transcription factor-mediated reprogramming to iPS (Lluis et al, 2008) or to another differentiated cell type (Aviv et al, 2009). The recent work of Blanpain and colleagues describes the existence of distinct unipotent stem cells that maintain different lineages of the mammary gland (Van Keymeulen et al, 2011). Authors' conclusions are in contrast to previous evidence of multipotent epithelial stem cells in the mammary gland and are explained by distinguishing how cell potency is defined during physiological tissue homeostasis versus injury or transplantation models that may broaden their differentiation capacity. Also, pathological conditions such as atherosclerosis induce smooth muscle cells to differentiate into osteochondrogenic precursors and chondrocytes (Speer et al, 2009). Importantly, epithelial metaplasias are conditions in which an epithelium adopts the phenotype of another epithelium, for instance when tracheal stem cells undergo squamous metaplasia in response to microenvironmental stress or, in the pancreas, when exocrine acinar cells become endocrine islet cells. As emphasized by Tosh and Slack (2002), understanding the molecular basis beyond the tissue-type switching that occurs in metaplasias is important, as it can improve our ability to reprogram stem cells for therapeutic purposes. This may be complex as recent evidence suggests that Barret's metaplasia (a transition from esophageal to intestinal epithelium) can be mimicked in p63 null mice and may depend upon competitive survival of embryonic cells in the adult tissue rather than from genetic lesions of adult cells (Wang et al, 2011). Finally, it was also shown that physical cues, such as substrate stiffness may, by themselves, direct mesenchymal stem cell fate towards one or another differentiation pathway (Engler et al, 2006).
What is the difference between ‘direct, i.e. intrinsic factor-mediated’ and ‘environmental, i.e. extrinsic factor-mediated’ reprogramming'?
‘Plasticity’, defined as the ability of a cell to change its phenotype in response to extra-cellular signals, has now acquired a broad and ill-defined general meaning. It does not literally correspond to ‘trans-differentiation’, because the latter only refers to already differentiated cells that directly switch to another differentiation program without regressing to an ES-like state. Plasticity instead refers also to a still undifferentiated but ‘committed’ cell, either embryonic or adult, that during its pathway towards the expected terminal differentiation can be diverted towards another type of terminal differentiation. At first sight, it would appear that approaches such as cell fusion, exposure to oocyte extract, and environmental cell reprogramming may be outdated by the most direct transfer of defined transcription factors.
However, in our opinion, reprogramming by extrinsic factors maintains an important role in stem cell biology for three main reasons. First, the mammalian body is composed of thousands of different cell types and we currently have the recipe to convert ‘fibroblasts’ or direct ES/iPSc towards a specific terminally differentiated cell (e.g. a dopaminergic neuron: Caiazzo et al, 2011) only in a handful of cases. The ‘environmental’ approaches may still be invaluable to identify extra-cellular signals and downstream transcription factors that are required to obtain a functional beta or alpha cell of pancreatic islets or a cone or a cell of the heart conduction system. Second, evidence is accumulating that fusion may occur in vivo in many tissues, resulting in cell reprogramming and thus contributing to regeneration (Sanges et al, 2011). Third and more importantly, environmental reprogramming may mimic in vitro, or following experimental transplantation, natural processes that occur in vivo, though likely at low frequency, and may be needed to finely tune the amount of progenitor cells that are distributed among neighbouring developing tissues. In this regard, we showed in the past that, upon transplantation, human pericytes from skeletal muscle are recruited to a skeletal muscle fate rather than following their default pathway, i.e. the formation of the smooth muscle layers surrounding the endothelium of blood vessels (Dellavalle et al, 2007). We now have evidence that pericytes spontaneously change their fate, contributing to up to 7% of developing skeletal muscle fibres and 20% of their associated satellite cells during unperturbed post-natal development of the mouse (Dellavalle et al, 2011). This supports the hypothesis that pericytes represent a resident progenitor of post-natal tissues endowed with the potency to generate the differentiated cell types of that specific tissue (Bianco et al, 2008). The implications of this concept for regenerative medicine can be already appreciated as a phase I/II clinical trial, based upon transplantation of mesoangioblasts (the in vitro counterpart of skeletal muscle pericytes) from HLA-identical donors, is ongoing at San Raffaele Hospital in Milan. It is important that the cells to be transplanted possess, as a natural developmental option, the ability to give rise to the desired tissue, i.e. skeletal muscle in our case, at a frequency that might be clinically relevant.
Although what we discussed here raises novel and extraordinary possibilities for efficiently repairing tissues and organs using the patients' own cells, we should not underestimate the importance of carefully designing both controls and protocols. In fact, cell transplantation clinical trials have been conducted in the past where skeletal muscle cells, once transplanted into the infarcted heart, were able to survive, differentiate and spontaneously contract but did not integrate electrically within the surrounding myocardium. As a consequence severe arrhythmias developed, in some cases with a fatal outcome (Menasche, 2011). Thus, even if short-term safety and efficacy can be assessed in pre-clinical models, the biological features of ‘the human cell’ as a medicinal product can only be definitively assessed in patients, with unavoidable associated clinical risks. In the case mentioned above, no arrhythmias had developed in rodents or large animal models. In addition, careful analysis of chromosome stability, growth factor dependence and maintenance of full differentiation potency is particularly important for cells that have to be expanded in culture prior to transplantation. The capacity of cells to adapt from an in vitro to an in vivo microenvironment where they need to perform like host resident cells will be understood only disclosing the basis of intrinsic and extrinsic cell plasticity. Indeed, complete functional integration of transplanted cells may become the major hurdle for those tissues where complex intercellular interactions and communication are required for optimal function, reiterating the importance of in depth knowledge of the host microenvironment. Of notice, this consideration applies to any kind of ‘reprogramming’ also if achieved by transfer of transcription factors or cell fusion.
Conclusion
Plasticity is not an artefact. It is likely a compensatory mechanism by which developing or regenerating tissues adjust their cell number. It is rare but important. It may occur by cell fusion or environmental reprogramming that are mimicked in the laboratory by transfer of nuclei or transcription factors. The years to come will likely provide answers to these intriguing issues that are crucial for the future of regenerative medicine.
Pending issues
Efficient reprogramming: Efficiency is the challenge for the future, both for iPSc and ‘direct, i.e. intrinsic factor-mediated’ or ‘environmental, i.e. extrinsic factor-mediated’ reprogramming. This implies that all the cells will need to be reprogrammed to the desired phenotype, within a short period of time.
Safety issues: Safety is a consequence of efficiency. If all cells are reprogrammed, then undifferentiated, potentially tumorigenic cells will no longer be present in the cell population. Safety related to insertional mutagenesis, is rather related to the risk that vectors integrate in dangerous regions of the genome and is not discussed in this review.
Age and rejuvenation: Another crucial issue for the future clinical translation of these different reprogramming strategies is the age of the organism from which cells are reprogrammed. If cells are ‘old’ and cannot be rejuvenated during reprogramming (i.e. by telomere elongation) then also the ‘reprogrammed’ cells will remain as old as the patient. In addition, progression of many diseases leads to tissue alterations, such as fibrosis, that will hamper transplantation of any cell, including reprogrammed ones.
Acknowledgments
We apologize with colleagues whose work was not quoted for space limits. We thank Kevin Goudy and Alessandro W. Amici for critical reading of the manuscript and helpful suggestions. PB is supported by an EMBO fellowship and an EFSD/JDRF grant. Work in the authors' laboratories is supported by grants from the European Community (Optistem, Angioscaff, Eurosystem), ERC, Telethon and Duchenne Parent Project.
The authors declare that they have no conflict of interest.
References
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Avila D. The present standing of the human embryo in U.S. law. Natl Cathol Bioeth Q. 2001;1:203–226. doi: 10.5840/ncbq20011254. [DOI] [PubMed] [Google Scholar]
- Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem. 2009;284:33509–33520. doi: 10.1074/jbc.M109.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y. Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature. 2010;466:978–982. doi: 10.1038/nature09269. [DOI] [PubMed] [Google Scholar]
- Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci USA. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Boulanger CA, Anderson LH, Smith GH. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene. 2011;30:679–689. doi: 10.1038/onc.2010.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussard KM, Boulanger CA, Booth BW, Bruno RD, Smith GH. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010;70:6336–6343. doi: 10.1158/0008-5472.CAN-10-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Callaway E. European ban on stem-cell patents has a silver lining. Nature. 2011;478:441. doi: 10.1038/478441a. [DOI] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Dennis C. Stem cells rise in the East. Nature. 2002;419:334–336. doi: 10.1038/419334a. [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-DeAngelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Gunhaga L. The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos Trans R Soc Lond B Biol Sci. 2011;366:1193–1203. doi: 10.1098/rstb.2010.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–65. doi: 10.1038/182064a0. [DOI] [PubMed] [Google Scholar]
- Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Teddy JM, Postovit LM, Seftor EA, Seftor RE, Hendrix MJ, Kulesa PM. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev Dyn. 2008;237:2657–2666. doi: 10.1002/dvdy.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung CY, Dawlaty MM, Tsai LH, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9:413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Lluis F, Pedone E, Pepe S, Cosma MP. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick C. Embryonic stem cell debate brings politics, ethics to the bench. J Natl Cancer Inst. 2001;93:1192–1193. doi: 10.1093/jnci/93.16.1192. [DOI] [PubMed] [Google Scholar]
- Mazzoni CM. The rights of the embryo and the foetus in private law: the Italian experience. Rev Derecho Genoma Hum. 2002;17:83–97. [PubMed] [Google Scholar]
- Menasche P. Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol. 2011;50:258–265. doi: 10.1016/j.yjmcc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Mintz B, Baker WW. Normal mammalian muscle differentiation and gene control of isocitrate dehydrogenase synthesis. Proc Natl Acad Sci USA. 1967;58:592–598. doi: 10.1073/pnas.58.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujok O, Kaldrack J, Taivankhuu T, Jorns A, Lenzen S. Selective removal of undifferentiated embryonic stem cells from differentiation cultures through HSV1 thymidine kinase and ganciclovir treatment. Stem Cell Rev. 2010;6:450–461. doi: 10.1007/s12015-010-9148-z. [DOI] [PubMed] [Google Scholar]
- Okada TS. Cellular metaplasia or transdifferentiation as a model for retinal cell differentiation. Curr Top Dev Biol. 1980;16:349–380. [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Parlakian A, Gomaa I, Solly S, Arandel L, Mahale A, Born G, Marazzi G, Sassoon D. Skeletal muscle phenotypically converts and selectively inhibits metastatic cells in mice. PLoS One. 2010;5:e9299. doi: 10.1371/journal.pone.0009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Wold BJ, Wagner RA. Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science. 1995;270:1818–1821. doi: 10.1126/science.270.5243.1818. [DOI] [PubMed] [Google Scholar]
- Power C, Rasko JE. Will cell reprogramming resolve the embryonic stem cell controversy? A narrative review. Ann Intern Med. 2011;155:114–121. doi: 10.7326/0003-4819-155-2-201107190-00007. [DOI] [PubMed] [Google Scholar]
- Rishniw M, Xin HB, Deng KY, Kotlikoff MI. Skeletal myogenesis in the mouse esophagus does not occur through transdifferentiation. Genesis. 2003;36:81–82. doi: 10.1002/gene.10198. [DOI] [PubMed] [Google Scholar]
- Sanges D, Lluis F, Cosma MP. Cell-fusion-mediated reprogramming: pluripotency or transdifferentiation? Implications for regenerative medicine. Adv Exp Med Biol. 2011;713:137–159. doi: 10.1007/978-94-007-0763-4_9. [DOI] [PubMed] [Google Scholar]
- Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, et al. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959–966. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Sullivan G, Chambers I. The evolving biology of cell reprogramming. Philos Trans R Soc Lond B Biol Sci. 2011;366:2183–2197. doi: 10.1098/rstb.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]