Abstract
Nardilysin (NRDc), a metalloendopeptidase of the M16 family, promotes ectodomain shedding of the precursor forms of various growth factors and cytokines by enhancing the protease activities of ADAM proteins. Here, we show the growth-promoting role of NRDc in gastric cancer cells. Analyses of clinical samples demonstrated that NRDc protein expression was frequently elevated both in the serum and cancer epithelium of gastric cancer patients. After NRDc knockdown, tumour cell growth was suppressed both in vitro and in xenograft experiments. In gastric cancer cells, NRDc promotes shedding of pro-tumour necrosis factor-alpha (pro-TNF-α), which stimulates expression of NF-κB-regulated multiple cytokines such as interleukin (IL)-6. In turn, IL-6 activates STAT3, leading to transcriptional upregulation of downstream growth-related genes. Gene silencing of ADAM17 or ADAM10, representative ADAM proteases, phenocopied the changes in cytokine expression and cell growth induced by NRDc knockdown. Our results demonstrate that gastric cancer cell growth is maintained by autonomous TNF-α–NF-κB and IL-6–STAT3 signalling, and that NRDc and ADAM proteases turn on these signalling cascades by stimulating ectodomain shedding of TNF-α.
Keywords: ADAM proteases, gastric cancer, IL-6, nardilysin, NF-κB
INTRODUCTION
Gastric cancer is a common epithelial malignancy. Although our understanding of this disease has improved during the past decades, prognosis of patients with advanced gastric cancer remains poor. The 5-year survival rate of patients with localized disease is 61.4%, whereas, that of patients with distant metastases is only 3.6% (http://seer.cancer.gov/csr/1975_2008/). Further elucidation of the molecular basis of gastric cancer development may lead to the identification of novel therapeutic targets that are expected to improve patient outcomes.
Biological behaviours of cancer cells are regulated by the tumour microenvironment, and multiple growth factors and cytokines secreted by cancer and stromal cells participate in creating this microenvironment. Various membrane-anchored proteins are shed from the cell surface by proteolytic cleavage, a process referred to as ectodomain shedding (Hooper et al, 1997). Matrix metalloproteinases (MMPs) and “a disintegrin and metalloproteinase” (ADAM) family of proteins are representative proteases that mediate ectodomain shedding, and this process regulates the biological activities of diverse proteins, including growth factors, cytokines, receptors and cell adhesion molecules in a context-dependent manner (Blobel, 2005; Seals & Courtneidge, 2003). Therefore, dysregulation of ectodomain shedding seems to be involved in the pathophysiology of various diseases such as inflammation and cancer (Murphy, 2008). For instance, recent reports demonstrated that several genetically modified mice with attenuated ADAM17 expression showed increased susceptibility to colitis induced by dextran sulphate sodium (DSS), presumably due to decreased shedding of EGFR ligands such as TGF-α or amphiregulin (Brandl et al, 2010; Chalaris et al, 2010).
Nardilysin (N-arginine dibasic convertase, NRDc) is a zinc peptidase of the M16 family that selectively cleaves dibasic sites (Pierotti et al, 1994). NRDc localizes diffusely in the cytoplasm and is secreted to the cell surface by an undetermined mechanism, although it lacks a conventional signal peptide sequence (Hospital et al, 2000). We identified NRDc as a specific binding partner of the heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF; Nishi et al, 2001). In addition, NRDc has been found to enhance shedding of HB-EGF and tumour necrosis factor-α (TNF-α) through activation of ADAM proteases such as ADAM17, also known as TNF-α-converting enzyme (TACE; Hiraoka et al, 2008; Nishi et al, 2006). NRDc binds to the extracellular domain of ADAM17 and directly enhances the catalytic activity of ADAM17, but the metalloendopeptidase activity of NRDc was not required for this enhancement (Hiraoka et al, 2007, 2008; Nishi et al, 2006). Our recent study demonstrated that NRDc regulates axonal maturation and myelination by enhancing ectodomain shedding and activation of neuregulin 1 (Ohno et al, 2009). These findings indicated that NRDc regulates various signalling pathways by promoting ectodomain shedding, but its role in cancer biology is unknown.
In this report, we investigated the role of NRDc in gastric cancer development and identified NRDc as a novel cancer-promoting gene. We found that NRDc expression is frequently increased in the serum and cancer epithelium of gastric cancer patients. Furthermore, our data demonstrated that NRDc enhances ectodomain shedding of TNF-α, which activates IKK–NF-κB and interleukin (IL)-6–STAT3 signalling pathways, thereby, leading to maintenance of gastric cancer cell growth.
RESULTS
NRDc expression is elevated in the serum and parenchyma of cancer tissue in gastric cancer patients
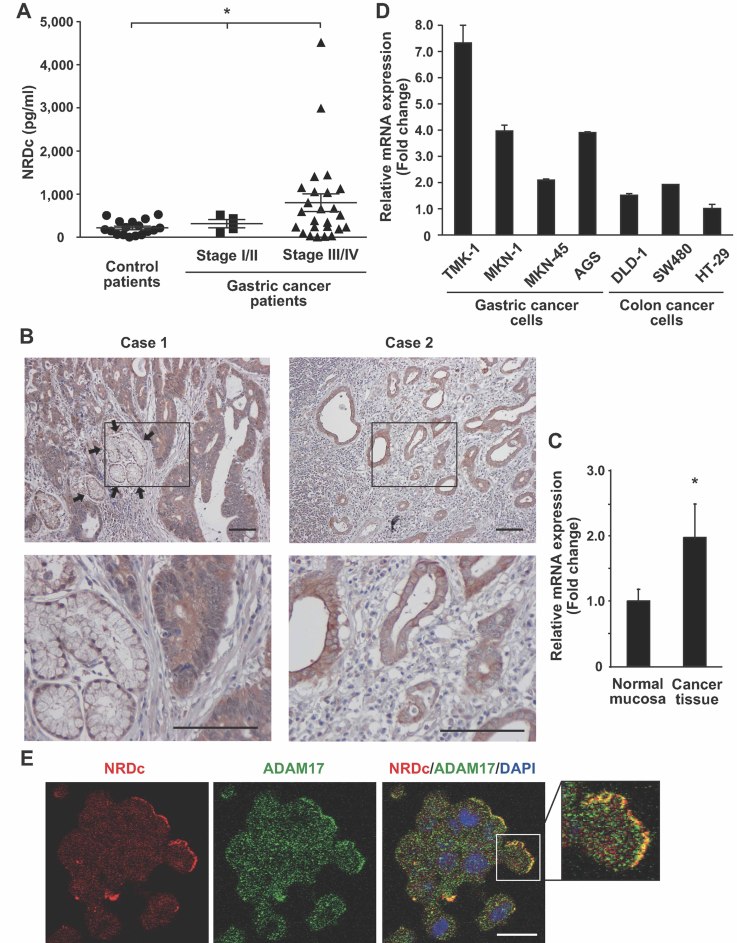
Because NRDc modulates a diverse range of signalling pathways, we postulated that it might be involved in the development of human cancers such as gastric cancer. To test this hypothesis, we first investigated NRDc protein concentration in the serum of gastric cancer patients by enzyme-linked immunosorbent assay (ELISA), which was established in our laboratory. As shown in Fig 1A, serum NRDc concentration was higher in far-advanced (stage III or IV) gastric cancer patients (mean ± SEM, 802 ± 204 pg/ml) than in patients at a relatively early clinical stage (stage I or II; 316 ± 82.3 pg/ml) or in control non-cancerous persons (218 ± 40.1 pg/ml). Next, NRDc protein expression in gastric cancer tissues was examined by immunohistochemistry using surgically resected specimens. In all cases examined (n = 22, including stage I gastric cancer), NRDc was diffusely expressed in the cytoplasm as well as at the cell surface of cancer epithelium, in contrast to the adjacent non-cancerous gastric foveolar epithelium where NRDc was expressed predominantly at the plasma membrane but hardly in the cytoplasm (Fig 1B and Fig S1 of Supporting Information). Quantitative polymerase chain reaction (after reverse transcription) (qRT-PCR) experiments also demonstrated that NRDc mRNA expression was higher in gastric cancer tissue than in adjacent non-cancerous mucosa (Fig 1C). Furthermore, NRDc mRNA level was compared between human gastric cancer and colon cancer cell lines by qRT-PCR; NRDc tended to be expressed more strongly in gastric cancer cell lines (Fig 1D). These results indicate that NRDc is highly expressed in cancer epithelium and that serum NRDc concentration correlates with tumour burden.
Figure 1. Elevated NRDc expression in the serum and cancer epithelium of gastric cancer patients.
- Serum NRDc concentration in the control (n = 17) or gastric cancer patients (stage I/II, n = 4; stage III/IV, n = 25) quantified by ELISA is plotted. *p < 0.05 by the non-parametric Kruskal–Wallis test.
- NRDc expression in advanced gastric cancer tissues was examined by immunohistochemistry using surgically resected specimens. Microphotographs from two representative cases are shown. Arrows indicate the non-cancerous gastric foveolar epithelium. Lower panels are magnified images of the boxed areas in the upper panels. Scale bars, 100 µm.
- Total RNA was extracted separately from resected gastric cancer tissue and adjacent non-cancerous mucosa (n = 28), and NRDc mRNA was quantified by RT-PCR. *p < 0.05 by paired Student's t-test.
- NRDc mRNA expression in four gastric cancer cell lines (TMK-1, MKN-1, MKN-45 and AGS) and three colon cancer cell lines (DLD-1, SW480 and HT-29) was quantified by RT-PCR. Data are shown as mean ± SD of duplicate assays.
- AGS cells were treated with 100 nM PMA at 37°C for 1 h, followed by staining with anti-NRDc and ADAM17 antibodies. Scale bar, 20 µm.
A known molecular function of NRDc is to promote ectodomain shedding by enhancing the protease activities of ADAM proteins such as ADAM17 (Nishi et al, 2006). In AGS cells, immunocytochemical analysis demonstrated that NRDc and ADAM17 colocalized at the plasma membrane but not in the cytoplasm, while both molecules were immunostained throughout the cytoplasm (Fig 1E). Taken together with the previous finding that NRDc binds to the extracellular domain of ADAM17 (Nishi et al, 2006), this result suggests that NRDc, which lacks a conventional signal peptide, is exported by a non-classical secretory pathway, and binds to ADAM proteases at the cell surface outside the plasma membrane.
Knockdown of NRDc expression attenuates gastric cancer cell growth in vitro
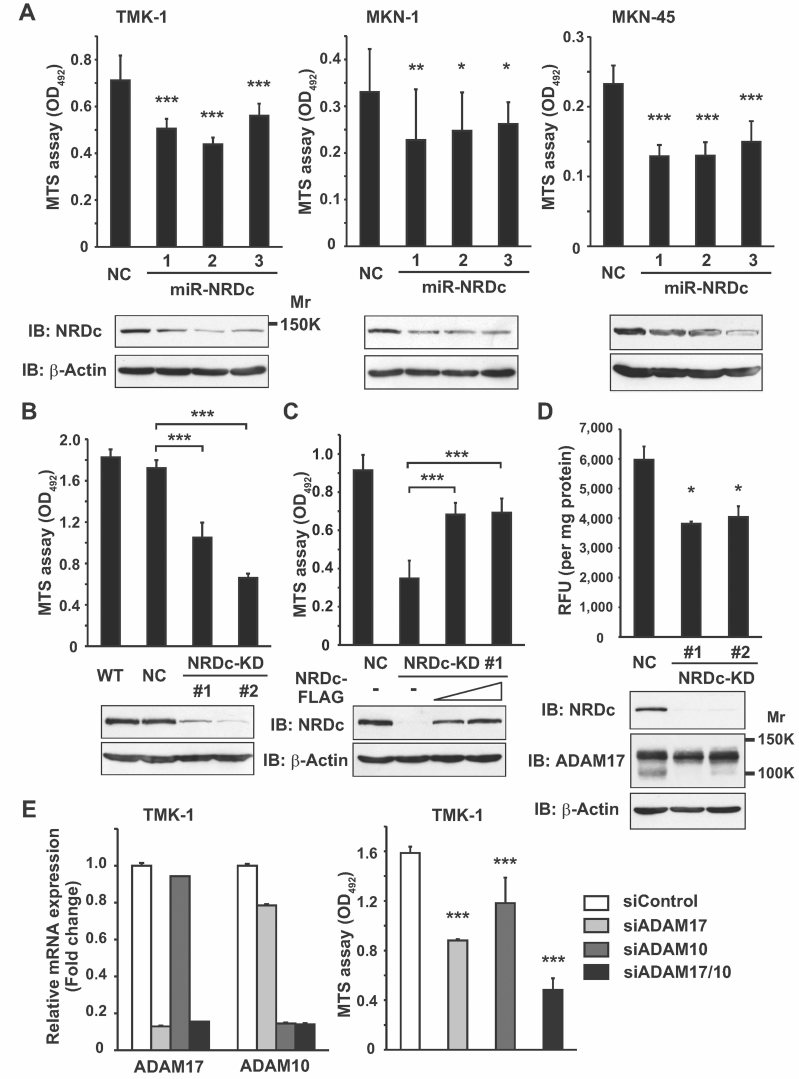
Results of clinical sample analysis suggested that upregulated NRDc plays some roles in gastric cancer development. To address this speculation, we first evaluated the change in cell growth using the MTS assay after suppressing NRDc expression by RNA interference (RNAi) in several gastric cancer cell lines. Indeed, growth of TMK-1, MKN-1 or MKN-45 cells was suppressed when each of the three different NRDc-targeting miRNA constructs was transiently introduced (Fig 2A). For further analyses, we established TMK-1 clones that stably expressed a NRDc-targeting miRNA construct. Both NRDc protein expression and cell growth were decreased in the two-independent clones (NRDc-KD #1 and #2), whereas, the negative control clone (TMK-1 NC) showed a growth rate similar to that of the wild-type (WT) TMK-1 cells (Fig 2B). To exclude off-target effects of RNAi, NRDc was re-expressed in the NRDc-KD #1 cells by transfecting an RNAi-resistant mutant of a NRDc-expressing vector. Certainly, cell growth was well recovered by NRDc re-expression (Fig 2C). These results clearly indicate that gastric cancer cell growth is maintained, at least in part, by endogenous NRDc expression.
Figure 2. Knockdown of NRDc expression attenuates gastric cancer cell growth.
- WT TMK-1, MKN-1 and MKN-45 cells were transiently transfected with the control or miR-NRDc plasmids. At 72 h after transfection, 1.5 or 3.0 × 103 of the cells were seeded in 96-well plates and cultured in the growth medium for 48 h (TMK-1 and MKN-45) or 96 h (MKN-1). The number of viable cells was quantified using the MTS assay (upper panels). Knockdown effects were confirmed by Western blotting (lower panels).
- The MTS assay was performed using the WT, negative control clone (NC), or stable KD TMK-1 clones (NRDc-KD #1 and #2).
- The NRDc-KD #1 TMK-1 cells were transfected with the NRDc-FLAG-expressing plasmids at increasing doses. At 48 h after transfection, the MTS assay was performed in the same manner. Recovery of NRDc expression was confirmed by Western blotting (lower panels).
- Cell lysates were prepared from the indicated clones without using protease inhibitors. TACE activity of each lysate is shown in the upper panel. Lower panels, ADAM17 protein expression was analysed by Western blotting.
- Left panel, siRNA targeting ADAM17, ADAM10 or the combination was introduced into TMK-1 cells. At 72 h after transfection, qRT-PCR was performed. Right panel, after TMK-1 cells were incubated with the indicated siRNA duplex for 48 h, MTS assay was performed for the additional 48 h as described in Fig 2A.
Our previous study revealed that NRDc is coprecipitated with ADAM17 in MKN-45 cells (Nishi et al, 2006). In general, proteolytic removal of the prodomain from the precursor ADAM proteases produces a mature form (Seals & Courtneidge, 2003). We previously reported that direct binding of recombinant NRDc to mature ADAM17 enhances its sheddase activity (Hiraoka et al, 2007, 2008; Nishi et al, 2006). Thus, we investigated the change in ADAM17 sheddase activity after NRDc knockdown in gastric cancer cells by TACE activity assay, the validity of which was verified by recombinant ADAM17 application, treatment with the sheddase (MMPs and ADAMs) inhibitor TAPI-1 (both in vitro and in intact cells) or ADAM17 RNAi (Fig S2A–C of Supporting Information). Indeed, lysates of the NRDc-KD cells showed less TACE activity than those of the control cells (Fig 2D, upper panel). In Western blotting, presumable precursor (130 kDa) and mature (100 kDa) forms of ADAM17 were detected in the TMK-1 cell lysates (Fig S2C of Supporting Information). It is of note that expression of the mature form appeared to be reduced in the NRDc-KD cell, while that of the precursor form remained almost unchanged (Fig 2D, lower panels), suggesting that NRDc is also implicated in the processing of precursor ADAM proteins to mature forms.
To ask whether ADAM protease activities mediate the growth-promoting effect of NRDc, representative ADAM proteases (ADAM17 and ADAM10) were knocked down in TMK-1 cells. Like TAPI-1 treatment (Fig S2D of Supporting Information), cell growth was attenuated by knockdown of either ADAM17 or ADAM10, and combined knockdown showed an additive effect (Fig 2E). These results suggest that NRDc regulates cell growth by enhancing ADAM protease activities.
Analyses of gene and protein expression profiles after stable suppression of NRDc in gastric cancer cells
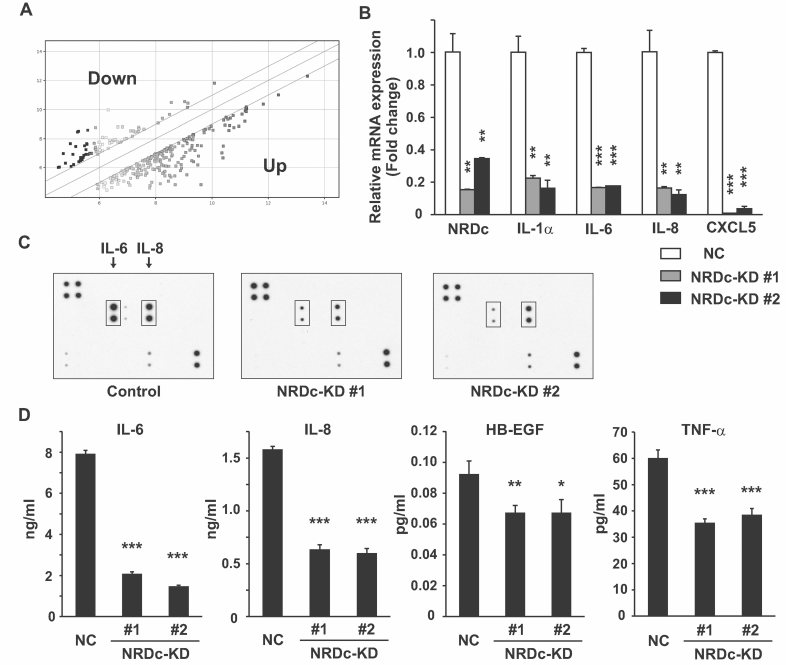
To elucidate the molecular mechanism by which NRDc regulates gastric cancer cell growth, we first compared gene expression profiles between the control and NRDc-KD #1 TMK-1 cells by DNA microarray (Fig 3A). Representative genes critically affected by NRDc knockdown are shown in Table 1. Among these genes, several cytokines such as IL1A and IL6 were found to be downregulated in the NRDc-KD cells. qRT-PCR was performed to confirm the findings of the microarray experiment. In the NRDc-KD cells, IL-1α, IL-6, IL-8 and CXCL5 mRNA levels were markedly decreased compared with the control cells (Fig 3B). Similar results were obtained when the NRDc-targeting miR vector was transiently introduced into TMK-1 or MKN-45 cells or when TMK-1 cells were treated with TAPI-1 (Fig S3A and B of Supporting Information).
Figure 3. Changes in gene and protein expression profiles after knockdown of NRDc expression in gastric cancer cells.
- Gene expression analysis by microarray. Total RNA was extracted from the NC or NRDc-KD #1 TMK-1 cells and subjected to microarray analysis using the Affymetrix GeneChip Human Gene 1.0 ST Array. The scatter plot shows the genes upregulated (bottom right) or downregulated (top left) more than twofold in the NRDc-KD cells against the control cells.
- Total RNA was extracted from the NC or NRDc-KD TMK-1 clones, and qRT-PCR was performed for the indicated genes.
- Protein expression profile in CM from the control or stable NRDc-KD TMK-1 clones was screened using the antibody array. Positions of IL-6 and IL-8 are indicated.
- Concentrations of the indicated proteins in CM from each stable clone were quantified by ELISA. Results are shown as mean ± SD of triplicate samples.
Table 1.
A partial gene list of gene expression analysis
| Gene symbol | Description | Fold changea |
|---|---|---|
| CCNA1 | Cyclin A1 | −8.262 |
| CXCL5 | Chemokine (C–X–C motif) ligand 5 | −4.518 |
| NRD1 | Nardilysin (N-arginine dibasic convertase) | −2.935 |
| FGF7 | Fibroblast growth factor 7 | −2.874 |
| IL1A | Interleukin 1, alpha | −2.689 |
| S100A8 | S100 calcium binding protein A8 | −2.665 |
| IL6 | Interleukin 6 (Interferon, beta 2) | −2.638 |
| SPON2 | Spondin 2, extracellular matrix protein | 2.015 |
| CCL5 | Chemokine (C–C motif) ligand 5 | 2.110 |
| IRF9 | Interferon regulatory factor 9 | 2.979 |
| TGFBR1 | Transforming growth factor, beta receptor I | 3.934 |
| IFI6 | Interferon, alpha-inducible protein 6 | 6.328 |
| OCLN | Occludin | 8.338 |
Global mRNA expression levels in the NRDc-KD #1 TMK-1 cells were compared with those in the NC TMK-1 cells using the Affymetrix GeneChip Human Gene 1.0 ST Array. Representative genes, whose expression was upregulated or downregulated more than twofold, are shown.
NRDc-KD #1 TMK-1 versus NC TMK-1 cells.
Next, we prepared conditioned medium (CM) from the control or stable NRDc-KD TMK-1 clones under a serum-free condition and compared the protein secretion profiles using a cytokine antibody array. Consistent with the decreased mRNA levels, signals of IL-6 and IL-8 were suppressed in CM from the NRDc-KD clones (Fig 3C). Reduced secretion of IL-6 and IL-8 was confirmed quantitatively by ELISA (Fig 3D). We also analysed the secretion of HB-EGF and TNF-α proteins because our previous reports demonstrated that processing and secretion of these proteins is promoted by NRDc (Hiraoka et al, 2008; Nishi et al, 2006). Indeed, secretion of both ligands in CM was decreased in the NRDc-KD cells than in the control cells (Fig 3D). Relatively small differences in HB-EGF secretion can be attributed to the heparin binding capacity of the cleaved HB-EGF; a significant amount of HB-EGF might bind to cell surface heparan sulphate proteoglycan.
Autocrine IL-6-STAT3 signalling, maintained by NRDc, promotes gastric cancer cell growth
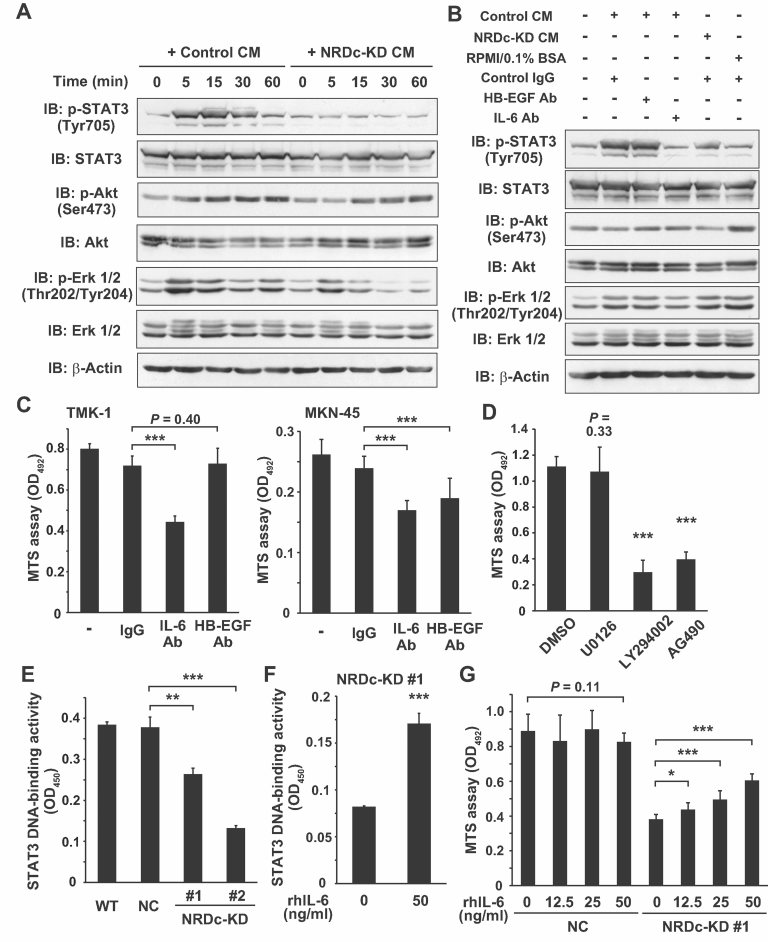
Based on these results, we hypothesized that NRDc stimulates the secretion of some humoral factors by enhancing ADAM protease activity, which in turn promotes cell growth via activation of specific signalling cascades. To address this issue, we treated serum-starved WT TMK-1 cells with CM from the control or NRDc-KD cells, and time-dependent activation of representative signalling pathways was analysed. Control CM treatment induced rapid phosphorylation of STAT3 (Tyr705) and Erk 1/2 (Thr202/Tyr204) and relatively delayed Akt phosphorylation (Ser473); however, STAT3 was hardly phosphorylated by NRDc-KD CM treatment (Fig 4A). Erk phosphorylation was also reduced with NRDc-KD CM treatment, whereas, the extent of Akt phosphorylation was similar to that with control CM treatment (Fig 4A). IL-6 induces tyrosine phosphorylation of STAT3 through activation of JAK kinases (Heinrich et al, 1998), but HB-EGF also has the potential to promote STAT3 phosphorylation by binding to and activating the EGF receptor (EGFR; Sartor et al, 1997; Zhong et al, 1994). To determine the ligand responsible for STAT3 phosphorylation, CM was preincubated with anti-HB-EGF or IL-6 neutralizing antibody before cell treatment. CM preincubated with anti-IL-6 antibody failed to phosphorylate STAT3, whereas, preincubation with anti-HB-EGF antibody had little effect (Fig 4B). We also analysed the activation status of gp130, a coreceptor for IL-6 family cytokines such as IL-6 or IL-11, using an ELISA kit specific for tyrosine-phosphorylated gp130. Phospho-/total gp130 ratio was slightly decreased or almost unaltered in NRDc-KD cells (Fig S4A of Supporting Information).
Figure 4. NRDc regulates gastric cancer cell growth through activation of autocrine IL-6–STAT3 signalling.
- Confluent monolayers of WT TMK-1 cells were serum starved overnight and incubated with CM from the control NC TMK-1 (control CM) or NRDc-KD #1 cells (NRDc-KD CM) for the indicated time periods. Cell lysates were prepared and probed with the indicated antibodies.
- Control CM, NRDc-KD CM or RPMI containing 0.1% bovine serum albumin (BSA) was incubated at 37°C for 1 h with 10 µg/ml HB-EGF neutralizing antibody, 2 µg/ml IL-6 neutralizing antibody, control isotype IgG or combinations of the antibodies (total amounts of IgG were adjusted to 10 µg/ml). Serum-starved TMK-1 cells were treated with preincubated CM for 10 min as indicated. Each cell lysate was probed with the indicated antibodies.
- TMK-1 (left panel) or MKN-45 (right panel) cells were cultured in the growth medium containing 10 µg/ml HB-EGF neutralizing antibody, 2 µg/ml IL-6 neutralizing antibody or control isotype IgG for 24 h, and the MTS assay was performed.
- TMK-1 cells were treated with 10 µM U0126, 50 µM LY294002, 50 µM AG490 or 0.05% DMSO (as a control) for 24 h, and the number of viable cells was quantified using the MTS assay.
- Nuclear extracts were prepared from the indicated cells. DNA-binding activity of STAT3 to its consensus sequence was analysed using the TransAM DNA-binding ELISA kit. Results are shown as mean ± SD of triplicate assays.
- The NRDc-KD #1 cells were incubated with vehicle (PBS containing 0.1% BSA) or 50 ng/ml recombinant human IL-6 protein for 1 h, followed by STAT3 DNA-binding ELISA.
- The NC or stable NRDc-KD #1 TMK-1 cells were treated with the indicated concentrations of IL-6 protein or vehicle. The MTS assay was performed after incubation for 24 h.
The above results suggest that NRDc stimulates autonomous IL-6–STAT3 signalling. To investigate whether this signalling is involved in the regulation of cell growth, we performed the experiments using neutralizing antibodies. While anti-HB-EGF antibody had little effect on the growth of TMK-1 cells, treatment with anti-IL-6 neutralizing antibody markedly suppressed the growth of both TMK-1 and MKN-45 cells (Fig 4C), suggesting the implication of autocrine IL-6 signalling in the growth regulation of gastric cancer cells. Furthermore, we tested small molecule signal transduction inhibitors for the MAPK, PI3K–Akt and JAK–STAT pathways. As shown in Fig 4D, AG490 (a JAK inhibitor) treatment strongly suppressed the growth of TMK-1 cells to the same extent as LY294002 treatment (a PI3K inhibitor). However, to our surprise, U0126 (a MEK 1/2 inhibitor) had little effect on cell growth, although phosphorylation of Erk 1/2 proteins almost disappeared in Western blotting (data not shown). This indicates that JAK–STAT3 and PI3K–Akt signalling pathways play a crucial role in maintaining gastric cancer cell growth.
Next, we investigated whether STAT3 transcriptional activity might be changed after NRDc knockdown. Nuclear extracts from the NRDc-KD clones had a reduced binding activity of STAT3 to oligonucleotides containing the STAT binding consensus sequence (Fig 4E), which was restored by recombinant IL-6 treatment (Fig 4F). Furthermore, recombinant IL-6 treatment restored the cell growth of the NRDc-KD cells in a dose-dependent manner, whereas, the growth of the control cells remained almost unchanged (Fig 4G and Fig S4B of Supporting Information). This recovery of cell growth by IL-6 treatment seems to be partial, suggesting the involvement of other humoral factors downstream of NRDc. These results clearly demonstrate that NRDc stimulates autocrine IL-6 secretion from gastric cancer cells, which activates JAK–STAT3 signalling, thereby, promoting cell growth.
NRDc maintains intrinsic NF-κB signalling by enhancing ectodomain shedding of TNF-α
We found that autonomous IL-6–STAT3 signalling mediates the regulation of gastric cancer cell growth by NRDc. As described above, NRDc stimulates ectodomain shedding of various ligands and cell surface receptors, but IL-6 is a soluble protein which is not a direct target for ectodomain shedding. We suspected the involvement of soluble IL-6 receptor (sIL-6R), which is generated either by ectodomain shedding of a membrane-bound form of IL-6R or alternative splicing of IL-6R mRNA, and activates gp130–JAK–STAT signalling by forming a complex with IL-6 (Jones et al, 2001). However, sIL-6R secretion was not decreased in the NRDc-KD cells (Fig S4C of Supporting Information). Interestingly, mRNA expressions of both the full-length (FL) form of IL-6R and the splice variant (SV) form coding sIL-6R were increased in NRDc-KD cells (Fig S4D of Supporting Information), raising the possibility that upregulated transcription of IL-6R, including SV form, compensates the reduced efficiency of IL-6R shedding.
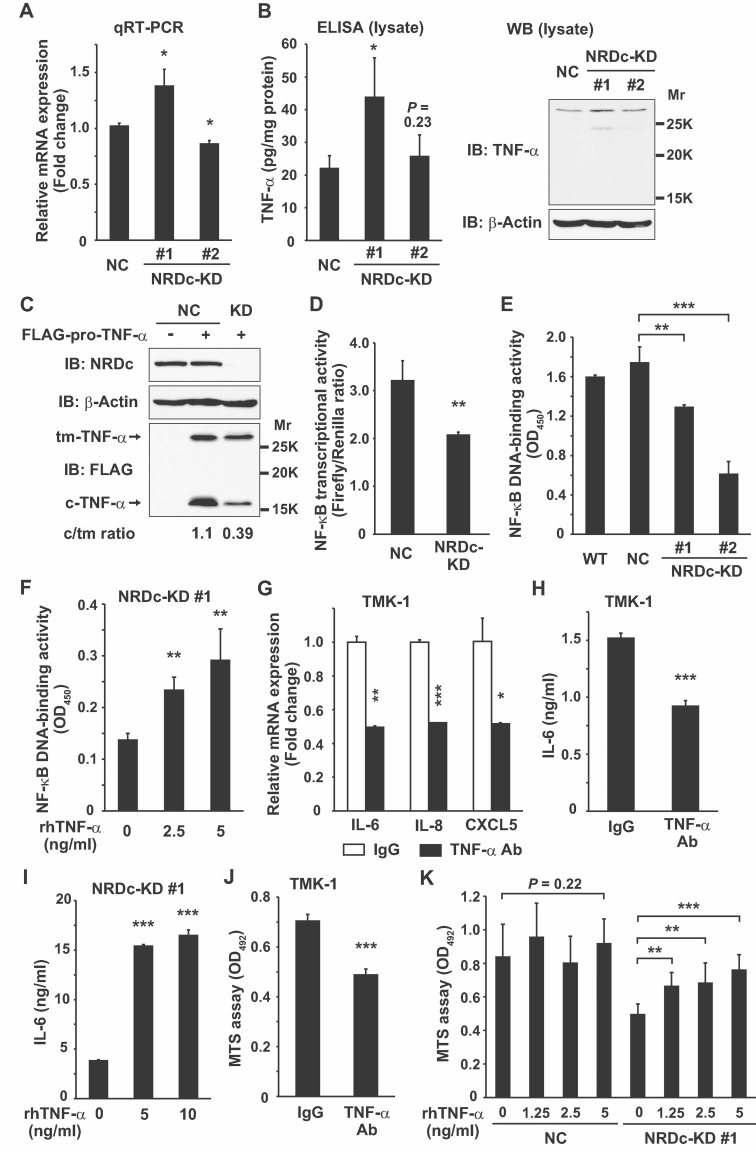
On the other hand, NF-κB transcriptional factors are key molecules regulating IL-6 transcription (Libermann & Baltimore, 1990). NF-κB activity in cancer cells is maintained, at least in part, by TNF-α secreted by the cancer cell itself (Balkwill, 2009). Therefore, we hypothesized that NRDc is necessary for the effective ectodomain shedding of TNF-α, which leads to constitutive expression of the IL6 gene through NF-κB activation in gastric cancer cells. The TNFA gene is translated to a type II single-span transmembrane precursor (pro-TNF-α), which is cleaved outside of the plasma membrane by proteases such as ADAM17, producing a C-terminal soluble TNF-α ligand (sTNF-α) and an N-terminal cytosolic remnant (Black et al, 1997; Moss et al, 1997). As shown in Fig 3D, sTNF-α secretion into CM was reduced in the NRDc-KD cells. One possibility is that mRNA or protein expression of pro-TNF-α is decreased by NRDc RNAi; however, mRNA level of TNF-α was slightly increased or almost unchanged by transient knockdown of NRDc (Fig S5A in Supporting Information). Likewise, the NRDc-KD stable clones showed increased (KD #1) or similar (KD #2) TNF-α mRNA expression compared with the control cells (Fig 5A). Similar expression change was observed at the protein level when the cell lysates were subjected to ELISA (Fig 5B, left panel). This ELISA kit seems to recognize pro-TNF-α rather than sTNF-α in the cell lysates, because Western blotting analysis using an antibody binding to the ectodomain of TNF-α demonstrated a 27 kDa band (presumably corresponding to pro-TNF-α) in the same lysates, while sTNF-α (17 kDa) was not detected (Fig 5B, right panel). Furthermore, post-translational processing of the overexpressed TNF-α protein was analysed. When the expressing vector of FLAG-pro-TNF-α was introduced into TMK-1 cells, two bands were detected in the cell lysates by anti-FLAG antibody; the more slowly migrating band (27 kDa) is the transmembrane precursor, and the more rapidly migrating band (16 kDa) corresponds to the cytosolic remnant (Fig 5C). The expression ratio of the cytosolic remnant form to the transmembrane precursor in the NRDc-KD cells was decreased compared with that in the control cells (Fig 5C). Taken together, these results indicate the implication of NRDc in the processing of pro-TNF-α to a mature ligand.
Figure 5. Reduced ectodomain shedding of TNF-α and attenuated NF-κB activity in NRDc-KD gastric cancer cells.
- TNF-α mRNA level in the indicated stable cells was analysed by qRT-PCR.
- Left panel, TNF-α protein expression in the cell lysates was quantified by ELISA. Right panels, same lysates were subjected to Western blotting using anti-TNF-α antibody recognizing the extracellular domain of TNF-α.
- FLAG-pro-TNF-α (or equal amount of the empty vector) was expressed in the NC or NRDc-KD #1 TMK-1 cells as indicated. At 36 h after transfection, the cells were lysed and aliquots were probed with the indicated antibodies. tm-, transmembrane precursor; c-, N-terminal cytosolic remnant.
- NF-κB transcriptional activity was analysed by luciferase assay in the NC or NRDc-KD #1 TMK-1 cells transfected with pNF-κB-Luc and pRL-TK reporter genes.
- Nuclear extracts were prepared from the indicated cells, and DNA-binding activity of NF-κB p65 in each nuclear extract was measured.
- The NRDc-KD #1 cells were stimulated with the increasing doses of recombinant TNF-α protein for 1 h. Then NF-κB DNA-binding activity was analysed.
- TMK-1 cells were cultured in the presence of 2 µg/ml control IgG or anti-TNF-α neutralizing antibody for 24 h, and qRT-PCR was performed for the indicated genes.
- IL-6 protein secretion into CM was quantified by ELISA after TMK-1 cells were treated with 2 µg/ml control IgG or anti-TNF-α neutralizing antibody for 48 h.
- ELISA quantified the IL-6 concentration in CM from the NRDc-KD #1 cells incubated with the indicated concentrations of recombinant TNF-α for 48 h.
- Cell viability was analysed with the MTS assay after TMK-1 cells were incubated with the indicated antibodies for 24 h.
- The NC or NRDc-KD #1 TMK-1 cells seeded in 96-well plates were incubated with the increasing doses of TNF-α or vehicle for 24 h, followed by analysis using the MTS assay.
We then examined the ability of NRDc to activate intrinsic TNF-α–IKK–NF-κB signalling. When TMK-1 cells were treated with NRDc-KD CM as in Fig 4A, IκB-α was gradually dephosphorylated in a time-dependent manner, whereas, cells treated with control CM had the ability to maintain IκB-α phosphorylation (Fig S5B of Supporting Information). Furthermore, we investigated the change in NF-κB transcriptional activities after NRDc gene silencing. In a luciferase reporter gene experiment, the NRDc-KD cells showed decreased NF-κB transcriptional activity (Fig 5D). More specifically, DNA binding of NF-κB to its consensus sequence was analysed by the DNA-binding ELISA with an anti-p65 (a subunit of NF-κB) antibody. Treatment of the WT TMK-1 cells with an IKK inhibitor BAY-117082 strongly suppressed the p65 DNA-binding activity, indicating that a high level of NF-κB transcriptional activity is maintained in gastric cancer cells, even under a steady-state culture condition (Fig S5C of Supporting Information). Both transient and stable RNAi against NRDc resulted in the reduced DNA-binding activity of p65 (Fig 5E and Fig S5D of Supporting Information), and recombinant TNF-α treatment restored the impaired NF-κB activity in the NRDc-KD cells in a dose-dependent manner (Fig 5F).
To explore whether endogenously secreted TNF-α is involved in cytokine expression and maintenance of cell growth, TMK-1 cells were cultured with anti-TNF-α neutralizing antibody. We found that IL-6, IL-8 and CXCL5 mRNA expressions, as well as IL-6 secretion in CM, were suppressed by TNF-α neutralizing antibody treatment (Fig 5G and H). On the other hand, recombinant TNF-α treatment induced IL-6 expression in the NRDc-KD cells both at the mRNA and proteins levels (Fig 5I and Fig S5E of Supporting Information). Furthermore, incubation with anti-TNF-α neutralizing antibody suppressed the growth of TMK-1 and MKN-45 cells (Fig 5J and Fig S5F of Supporting Information), while recombinant TNF-α protein treatment restored the cell growth of the two-independent NRDc-KD clones (Fig 5K and Fig S5G of Supporting Information). These results demonstrate that intrinsic IL-6–STAT3 activity is mediated, at least in part, by autocrine TNF-α–IKK–NF-κB signalling in gastric cancer cells, and NRDc promotes sTNF-α secretion by enhancing ectodomain shedding of pro-TNF-α rather than by upregulating TNFA gene expression.
ADAM proteases such as ADAM17 also mediate the ectodomain shedding of TNF receptors (p55 TNF-R1 and p75 TNF-R2), which promotes the clearance of TNFRs from the cell surface, and increased secretion of soluble forms of TNFRs (sTNFRs) may attenuate the TNF signalling by competing with the cell surface receptors (Edwards et al, 2008; McDermott et al, 1999). Secretion of sTNF-R1 was decreased in the NRDc-KD cells, while TNF-R1 protein expression in cell lysates was almost unaffected (Fig S6A and B of Supporting Information). These results suggest that reduced shedding of TNFRs might partially compensate the attenuated TNF-α–TNFR signalling in NRDc-KD cells, but further investigation is required.
NRDc and ADAM proteases regulate expression of growth-related and anti-apoptotic genes downstream of STAT3 and NF-κB
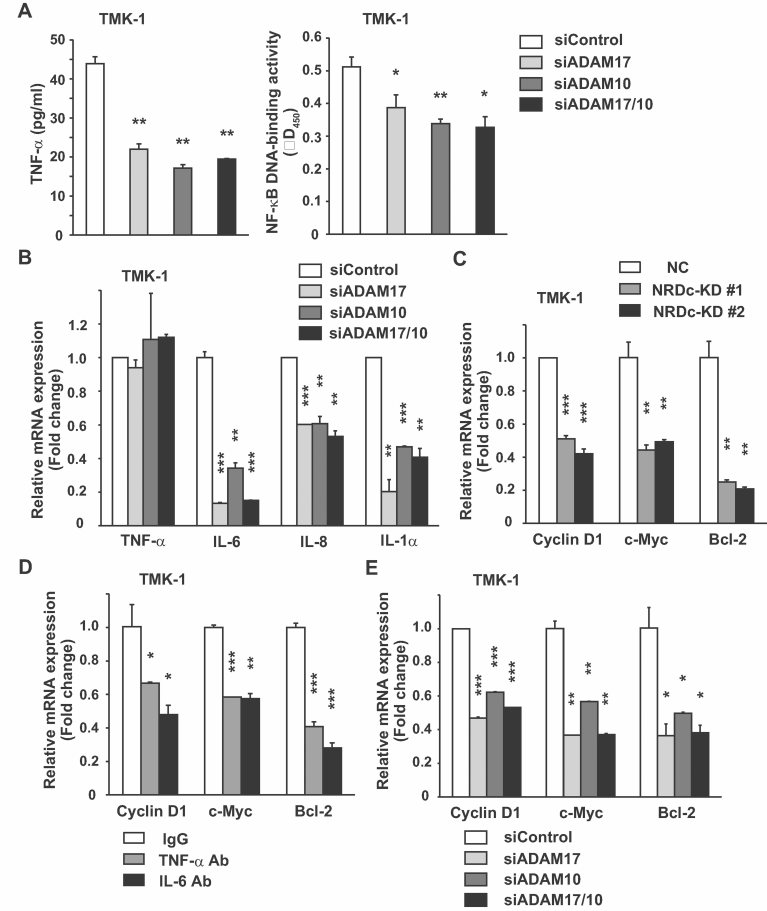
Our data suggest that NRDc activates intrinsic cytokine signalling and resulting cancer cell growth by enhancing sheddase activity of ADAM proteases. As in the case of NRDc RNAi, knockdown of ADAM17 or ADAM10 attenuated both sTNF-α secretion into CM and NF-κB transcriptional activity in TMK-1 cells (Fig 6A). Furthermore, gene silencing of these ADAM proteases suppressed the mRNA expressions of IL-6, IL-8 and IL-1α, while TNF-α mRNA remained almost unaltered (Fig 6B).
Figure 6. NRDc and ADAM proteases upregulate growth-related and anti-apoptotic genes downstream of STAT3 and NF-κB in gastric cancer cells.
- Left panel, TMK-1 cells were transfected with control siRNA or siRNA targeting ADAM17, ADAM10 or their combination. At 48 h after transfection, medium was changed to RPMI containing 0.1% BSA. CM was prepared after the cells were cultured for additional 48 h, and TNF-α in CM was quantified by ELISA. Right panel, NF-κB p65 DNA-binding activity was measured at 72 h after each siRNA was introduced into TMK-1 cells.
- At 72 h after TMK-1 cells were transfected with each siRNA, qRT-PCR was performed for the indicated genes.
- qRT-PCR analyses for cyclin D1, c-Myc and Bcl-2 mRNA levels in the stable TMK-1 clones.
- A subconfluent monolayer of TMK-1 cells was treated with 2 µg/ml control IgG, anti-TNF-α neutralizing antibody, or anti-IL-6 neutralizing antibody for 24 h. Then, mRNA levels were measured by qRT-PCR.
- mRNA levels of the indicated genes in TMK-1 cells were quantified by RT-PCR after siRNA-mediated knockdown of ADAM17, ADAM10 or both genes for 72 h.
Previous studies reported that STAT3 stimulates transcription of several growth-promoting and anti-apoptotic genes such as those for cyclin D1, c-Myc and Bcl-2 family proteins (Catlett-Falcone et al, 1999; Kiuchi et al, 1999; Leslie et al, 2006; Yu et al, 2009). These genes are also positively regulated by NF-κB in cancer cells (Yu et al, 2009). Therefore, we investigated whether expression of these genes is changed after NRDc knockdown. As shown in Fig 6C, mRNA expression of each gene was attenuated in the NRDc-KD TMK-1 clones. A similar change was observed in MKN-45 cells in which NRDc expression was transiently suppressed (Fig S7A of Supporting Information). To confirm these results, qRT-PCR was performed after NRDc was re-expressed in the NRDc-KD cells. mRNA levels of these growth-related genes, as well as those of NF-κB-regulated cytokines, were recovered by introducing the NRDc-FLAG-expressing plasmid in a dose-dependent manner (Fig S7B of Supporting Information). To ask whether autocrine secretion of TNF-α and IL-6 mediates the regulation of growth-related genes, TMK-1 cells were cultured with anti-TNF-α or IL-6 neutralizing antibody and qRT-PCR was performed. Treatment with either antibody suppressed the mRNA level of cyclin D1, c-Myc and Bcl-2 (Fig 6D). Similar change was observed by ADAM17/ADAM10 siRNA or TAPI-1 treatment (Fig 6E and Fig S7C of Supporting Information). These results indicate that endogenous secretion of TNF-α and IL-6, which are maintained by NRDc and ADAM proteases, works in concert to induce expressions of growth-promoting and anti-apoptotic genes.
NRDc is essential for tumour formation by gastric cancer cells in a xenograft model
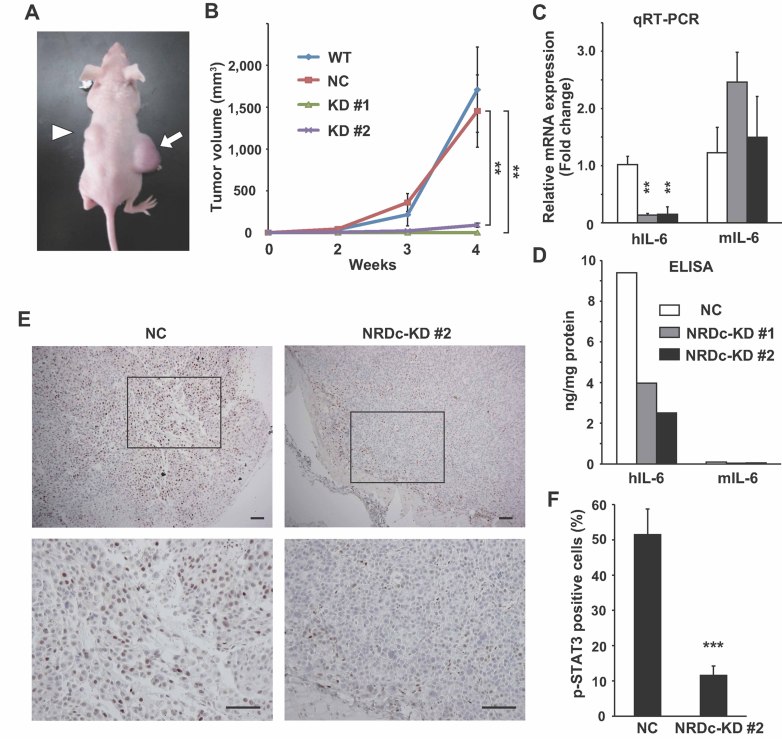
Finally, we examined the roles of NRDc in tumour development after xenotransplantation of gastric cancer cells. The WT, NC or stable NRDc-KD TMK-1 cells were subcutaneously injected into the flanks of nude mice, and tumour volume was measured routinely up to 4 weeks after inoculation (Fig 7A). The WT and control stable TMK-1 cells showed a time-dependent growth of a similar rate, whereas, the growth of the NRDc-KD clones remained attenuated within the observed period (Fig 7B).
Figure 7. NRDc is essential for tumour growth of xenotransplanted gastric cancer cells.
- The WT or each stable TMK-1 cells were injected subcutaneously into the flanks of nude mice. The photograph shows the representative tumours grown from the NC (arrow) and NRDc-KD #2 cells (arrowhead) at 4 weeks after inoculation.
- Tumour volume of each xenograft calculated at the indicated time points is plotted. Combined results of two-independent experiments are shown as mean ± SEM with total n = 8 per each clone.
- qRT-PCR was performed using RNA extracted from tumours at 4 weeks after xenotransplantation. Data represent mean ± SEM of three-independent tumours of each clone.
- Human or mouse IL-6 protein concentration in the indicated tumours was quantified using species-specific ELISA kits.
- Tumour tissues at 4 weeks after xenografting were processed for immunohistochemical analysis with anti-phspho-STAT3 antibody. Representative microphotographs of tumours from the NC (left panels) or KD #2 TMK-1 cells (right panels) are shown. Lower panels are magnified photographs of the boxed areas. Scale bar, 100 µm.
- Ratios of the tumour cells expressing p-STAT3 in the peripheral regions of the grown tumours are shown. Values represent mean ± SD from randomly selected tumour fields (n = 10).
We addressed whether IL-6–STAT3 signalling is also activated in this model. A qRT-PCR experiment showed suppressed human IL-6 mRNA expression in tumours from the NRDc-KD clones, whereas, mouse IL-6 expression was not decreased (Fig 7C). It is of note that human IL-6 was decreased in the NRDc-KD tumours also at the protein level, while mouse IL-6 protein level was about 100-fold lower than that of human IL-6 (Fig 7D). Microscopically, xenografted tumours showed expansive rather than invasive growth, encapsulated by fibrous tissue. Phospholyrated STAT3 (p-STAT3) was detected by immunohistochemical analysis in tumour cells located in the peripheral region beneath the fibrous capsule, and ratio of p-STAT3-positive cells in tumours from the NRDc-KD cells was significantly smaller than those from the control cells (Fig 7E and F). These results indicate that NRDc expression is essential for tumour growth in a xenograft model. And given that a previous report demonstrated that mouse IL-6 lacks activities on human cells (Chiu et al, 1988), it is suggested that STAT3 activation by IL-6 secreted from the tumour cells, not from the stromal cells, is involved in the promotion of tumour growth in this model.
DISCUSSION
In this report, we addressed the roles of NRDc in gastric cancer biology. We found that NRDc expression was elevated in the epithelium of gastric cancer tissues, and NRDc knockdown attenuated gastric cancer cell growth both in vitro and in a xenograft model. During the submission of this manuscript, Choong et al reported that NRDc is highly expressed in invasive breast cancer tissue and promotes breast cancer cell growth (Choong et al, 2011). Taken together with our results, the possibility is raised that NRDc regulates tumour cell growth in a variety of human cancers. Our clinical sample analyses suggested that NRDc might be useful not only for pathological diagnosis but also as a serum biomarker for gastric cancer, although prospective control studies are needed for further elucidation.
With regard to the regulation of cancer cell growth by NRDc, we demonstrated that the central function of NRDc is to enhance ectodomain shedding of TNF-α in cooperation with ADAM proteases. Many recent works have uncovered the involvement of zinc metalloproteinases in cancer development, among which MMPs and ADAMs are representative families (Blobel, 2005; Egeblad & Werb, 2002). ADAM proteases promote ectodomain shedding of multiple growth factors and cytokines, which modulates extracellular signalling cascades in the tumour microenvironment. In fact, our study demonstrated that siRNA-mediated knockdown of ADAM17 or ADMA10 in gastric cancer cells resulted in decreased transcription of several cytokines and growth-related genes and attenuated cell growth, indicating that ADAM proteases play roles in maintaining cell growth by activating intrinsic cytokine signalling. Multiple signalling pathways may be modulated by ADAMs. EGFR signalling is a representative and well-investigated example; ADAM proteases shed several EGFR ligands, such as HB-EGF, transforming growth factor-α and amphiregulin, thereby, transactivating EGFR signalling and leading to increased tumour cell proliferation and migration (Blobel, 2005). However, in our experiments EGFR signalling seems to be less involved in the growth regulation of gastric cancer cells by ADAMs. Instead, increased TNF-α signalling mediates the growth-promoting activities of NRDc and ADAMs.
We found that increased TNF-α secretion by gastric cancer cells resulted in sequential activation of IKK–NF-κB and IL-6–JAK–STAT3 signalling, which promotes cell growth via enhanced transcription of growth-promoting genes. JAK–STAT3 cascade is known to be one of the key signalling pathways in gastric cancer development. Previous reports showed that STAT family transcriptional factors, particularly STAT3, are persistently activated not only in tumour cells but also in cancer-associated inflammatory cells, and maintain a tumour-promoting inflammatory microenvironment in various cancers (Yu et al, 2009). We previously reported that STAT3 is constitutively activated in human gastric cancer cells and promotes cell growth (Kanai et al, 2003; Kanda et al, 2004). Mice experiments using the gp130Y757F knock-in mouse model revealed that STAT3 hyperactivation resulted in spontaneous gastric tumourigenesis (Judd et al, 2004; Tebbutt et al, 2002). In this mouse model, IL-11 rather than IL-6 was responsible for the activation of gp130-STAT3 signalling and resulting gastric tumourigenesis (Ernst et al, 2008). On the other hand, the present study demonstrated that IL-6 was highly secreted from the human gastric cancer cells, while IL-11 secretion was hardly detected (Fig 3C). Furthermore, our experiments using a neutralizing antibody or recombinant protein demonstrated the implication of autocrine IL-6 signalling in STAT3 activation in human gastric cancer cells. Whether IL-6 or IL-11 is responsible for the STAT3 activation in gastric tumour cells may differ between species, or even among individual human gastric cancers.
IL-6 has been demonstrated to be involved in tumour development, especially in inflammation-associated carcinogenesis models. Naugler et al demonstrated that IL-6 produced by Kupffer cells promotes chemical-induced hepatocarcinogenesis in diethylnitrosamine-treated mice (Naugler et al, 2007). In a colitis-associated cancer model induced by administration of azoxymethane and DSS, STAT3 activation by IL-6 is essential for epithelial cell survival during colitis and proliferation of tumour-initiating cells (Bollrath et al, 2009; Grivennikov et al, 2009). In those reports, it seems that IL-6, which is produced by stromal or immune cells and stimulates epithelial cells in a paracrine manner, has a pivotal role in the early stage of inflammation-associated carcinogenesis. On the other hand, autocrine IL-6 signalling has been reported to promote tumour cell growth in lung and breast cancer cells (Gao et al, 2007; Sansone et al, 2007). Taken together with our results, these results suggest that once transformed by genetic or epigenetic alterations, tumour cells may acquire the ability to upregulate and secrete IL-6, thereby, promoting tumour cell growth in an autonomous manner.
In terms of the link between inflammation and cancer, NF-κB is another key transcriptional factor. In many cancers, NF-κB is constitutively activated both in cancer cells and cancer-associated immune cells, and activated NF-κB promotes transcription of proinflammatory and prosurvival (anti-apoptotic) genes, which are associated with tumour development and progression (Karin, 2006). Many extrinsic and intrinsic mechanisms are known to contribute to increased NF-κB activity in cancer cells, and TNF-α constitutively secreted by tumours cells may be involved as an intrinsic factor (Balkwill, 2009). It is noteworthy that concomitant activation of STAT3 and NF-κB is a characteristic feature observed in many tumour cells, and these transcriptional factors share many target genes for cell proliferation and anti-apoptosis (Yu et al, 2009). Our experiments illustrated that stimulation of endogenous secretion of TNF-α by NRDc and ADAM proteases keeps both NF-κB and STAT3 in active states, which is required for maintaining cancer cell growth.
Of note, growth of the NRDc-KD cells seems to be suppressed more strongly in a xenograft model than in vitro culture. A possible explanation is that interactions between xenotransplanted tumour and stromal cells, which are required for proper tumour growth (Egeblad et al, 2010), are also attenuated because of the decreased secretion of some humoral factors from the NRDc-KD cancer cells. For example, our data revealed that expression of two types of CXC chemokines – IL-8 and CXCL5 – are also positively regulated by NRDc via autocrine TNF-α–NF-κB signalling. These chemokines have been reported to be upregulated in gastric cancer cells (Kitadai et al, 1998; Park et al, 2007), and chemokines play a role in tumour development by promoting leucocyte infiltration, angiogenesis, tumour cell migration and growth (Balkwill, 2004). Given that restoration of cell growth of the NRDc-KD cells by recombinant IL-6 treatment remained limited (Fig 4G), NF-κB-regulated cytokines or chemokines other than IL-6 might be involved in cell growth regulation also in vitro.
The paper explained
PROBLEM
Gastric cancer (also called as stomach cancer) is one of the leading causes of cancer-related deaths worldwide. Gastric cancer arises from the inner lining of the stomach, infiltrates into the deeper layers of the gastric wall and eventually spreads to regional lymph nodes, liver, lungs and peritoneum. Localized gastric cancer is potentially curable by surgical resection. However, because early gastric cancer is usually asymptomatic, many patients are diagnosed as having gastric cancer when the disease has progressed to advanced stages. Palliative chemotherapy may be performed for advanced gastric cancer. In addition to the conventional cytotoxic drugs (e.g. 5-fluorouracil), monoclonal antibodies or small molecule inhibitors targeting specific signal cascades such as EGFR signalling have been developed recently, but the prognosis of gastric cancer patients with distant metastases remains poor. Thus, further investigations are required for elucidating molecular mechanisms associated with gastric cancer development.
RESULTS
Nardilysin (NRDc), a zinc peptidase of the M16 family, promotes ectodomain shedding of the precursor forms of various proteins such as growth factors and cytokines. We investigated if NRDc is implicated in the gastric cancer development. We found that NRDc concentration was elevated in the serum of gastric cancer patients. Immunohistochemical analysis showed that NRDc was highly expressed in the parenchyma of gastric cancer tissues. RNAi-mediated knockdown of NRDc, as well as of ADAM17 or ADAM10, reduced expression of multiple cytokines and cell growth in gastric cancer cells. NRDc enhances the sheddase activitiy of ADAM proteases, which promotes the ectodomain shedding of TNF-α. Sequential activation of TNF-α–NF-κB and IL-6–STAT3 signalling cascades drives tumour cell proliferation by upregulating growth-promoting genes downstream of NF-κB and STAT3.
IMPACT
Our clinical data suggest that NRDc is a candidate biomarker for the gastric cancer diagnosis. Previous reports showed that NF-κB and STAT3 are crucial procarcinogenic transcriptional factors, especially in the inflammation-associated carcinogenesis. Our data indicate that upregulated NRDc expression maintains the persistent coactivation of NF-κB and STAT3 in gastric cancer cells, thereby, promoting cancer cell growth. Therefore, NRDc and ADAM proteases, as well as TNF-α–NF-κB and IL-6–STAT3 signalling axes, seem to be novel and potential therapeutic targets for gastric cancer treatment.
In summary, the present study clearly indicates that the regulation of intrinsic TNF-α–NF-κB and IL-6–STAT3 axes by NRDc and ADAM proteases is a key mechanism for the maintenance of gastric cancer cell growth, although further investigation is required to uncover additional molecular pathways, especially with regard to the tumour development in vivo. The mechanism by which NRDc is upregulated in gastric cancer also remains to be elucidated.
MATERIALS AND METHODS
Clinical sample analysis
Blood serum and surgically resected specimens of gastric cancer tissues were obtained from patients admitted to Kyoto University Hospital, and written informed consent was obtained from each patient. Clinical stage of gastric cancer was classified according to the UICC-TNM staging system. NRDc protein concentration in the serum of the control patients (who had not been proven to have any malignant disorder, n = 17) or patients diagnosed as having gastric cancer (stage I/II, n = 4; stage III/IV, n = 25) was quantified by ELISA. Surgically resected gastric cancer tissues (n = 22; male 14 and female 8) were processed for immunohistochemical analysis with anti-NRDc antibody.
Gene expression microarray analysis
Gene expression profiles were compared between the control and NRDc-KD TMK-1 cells using the Affymetrix GeneChip Human Gene 1.0 ST Array (Affymetrix, Santa Clara, CA) with the protocol of the Whole Transcript Sense Target Labeling Assay. Detailed protocols were described in Supporting Information. The microarray data from this publication have been submitted to the NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE29114.
Statistical analysis
If not mentioned particularly, graph data are shown as mean ± SD, and statistical significance was determined by unpaired Student's t-test. p < 0.05 was considered to be statistically significant, and the p-value of each experiment was described as follows: *p < 0.05, **p < 0.01 and ***p < 0.001.
For more detailed Materials and Methods see the Supporting Information.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (21229009, 23300117 and 23590937), Grant-in-Aid for Challenging Exploratory Research (23659154), Grant-in-Aid for Scientific Research on Innovative Areas (23117519), Grant-in-Aid for Young Scientists (22790641) and Research Fellowship for Young Scientists (K. Kan) from the Japan Society for Promotion of Science (JSPS). This study was also supported by Health and Labour Sciences Research Grants for Research on Intractable Diseases, and Research on Hepatitis from the Ministry of Health, Labour and Welfare, Japan. We thank the financial supports by Research Foundation of Translational Research Center at Kyoto University Hospital, Takeda Science Foundation and the Daiichi Sankyo Sponsored Research Program.
Supporting Information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
TS and EN conceived the project; HK, HS and EN designed the experiments and analysed the data; KKan performed the mice experiments; HK and YH constructed the plasmids; MO carried out the confocal imaging; KKaw and YS provided and analysed the surgically resected specimens; KM and MKu carried out the ELISA of serum samples; KKan, HK, SI, YN, MN, RAK and MKa performed the other experiments; KKan, HK, HS and EN wrote the paper; TK and TC supervised the project.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Brandl K, Sun L, Neppl C, Siggs OM, Le Gall SM, Tomisato W, Li X, Du X, Maennel DN, Blobel CP, et al. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci USA. 2010;107:19967–19972. doi: 10.1073/pnas.1014669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, Hartmann D, Cichy J, Gavrilova O, Schreiber S, et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010;207:1617–1624. doi: 10.1084/jem.20092366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CP, Moulds C, Coffman RL, Rennick D, Lee F. Multiple biological activities are expressed by a mouse interleukin 6 cDNA clone isolated from bone marrow stromal cells. Proc Natl Acad Sci USA. 1988;85:7099–7103. doi: 10.1073/pnas.85.19.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong LY, Lim SK, Chen Y, Loh MC, Toy W, Wong CY, Salto-Tellez M, Shah N, Lim YP. Elevated NRD1 metalloprotease expression plays a role in breast cancer growth and proliferation. Genes Chromosomes Cancer. 2011;50:837–847. doi: 10.1002/gcc.20905. [DOI] [PubMed] [Google Scholar]
- Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Ohno M, Yoshida K, Okawa K, Tomimoto H, Kita T, Nishi E. Enhancement of alpha-secretase cleavage of amyloid precursor protein by a metalloendopeptidase nardilysin. J Neurochem. 2007;102:1595–1605. doi: 10.1111/j.1471-4159.2007.04685.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Yoshida K, Ohno M, Matsuoka T, Kita T, Nishi E. Ectodomain shedding of TNF-alpha is enhanced by nardilysin via activation of ADAM proteases. Biochem Biophys Res Commun. 2008;370:154–158. doi: 10.1016/j.bbrc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J. 1997;321:265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospital V, Chesneau V, Balogh A, Joulie C, Seidah NG, Cohen P, Prat A. N-arginine dibasic convertase (nardilysin) isoforms are soluble dibasic-specific metalloendopeptidases that localize in the cytoplasm and at the cell surface. Biochem J. 2000;349:587–597. doi: 10.1042/0264-6021:3490587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Kanai M, Konda Y, Nakajima T, Izumi Y, Kanda N, Nanakin A, Kubohara Y, Chiba T. Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK–ERK-dependent pathway. Oncogene. 2003;22:548–554. doi: 10.1038/sj.onc.1206109. [DOI] [PubMed] [Google Scholar]
- Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ, et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93–100. [PMC free article] [PubMed] [Google Scholar]
- Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, et al. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006;66:2544–2552. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Gadina M, Karenko L, Pettersson T, et al. Germline mutations in the extracellular domains of the 55kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nishi E, Prat A, Hospital V, Elenius K, Klagsbrun M. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J. 2001;20:3342–3350. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi E, Hiraoka Y, Yoshida K, Okawa K, Kita T. Nardilysin enhances ectodomain shedding of heparin-binding epidermal growth factor-like growth factor through activation of tumor necrosis factor-alpha-converting enzyme. J Biol Chem. 2006;281:31164–31172. doi: 10.1074/jbc.M601316200. [DOI] [PubMed] [Google Scholar]
- Ohno M, Hiraoka Y, Matsuoka T, Tomimoto H, Takao K, Miyakawa T, Oshima N, Kiyonari H, Kimura T, Kita T, et al. Nardilysin regulates axonal maturation and myelination in the central and peripheral nervous system. Nat Neurosci. 2009;12:1506–1513. doi: 10.1038/nn.2438. [DOI] [PubMed] [Google Scholar]
- Park JY, Park KH, Bang S, Kim MH, Lee JE, Gang J, Koh SS, Song SY. CXCL5 overexpression is associated with late stage gastric cancer. J Cancer Res Clin Oncol. 2007;133:835–840. doi: 10.1007/s00432-007-0225-x. [DOI] [PubMed] [Google Scholar]
- Pierotti AR, Prat A, Chesneau V, Gaudoux F, Leseney AM, Foulon T, Cohen P. N-arginine dibasic convertase, a metalloendopeptidase as a prototype of a class of processing enzymes. Proc Natl Acad Sci USA. 1994;91:6078–6082. doi: 10.1073/pnas.91.13.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CI, Dziubinski ML, Yu CL, Jove R, Ethier SP. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978–987. [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.