Abstract
Glucose-stimulated insulin secretion (GSIS) relies on repetitive, electrical spiking activity of the beta cell membrane. Cyclic activation of voltage-gated potassium channels (Kv) generates an outward, ‘delayed rectifier’ potassium current, which drives the repolarizing phase of each spike and modulates insulin release. Although several Kv channels are expressed in pancreatic islets, their individual contributions to GSIS remain incompletely understood. We take advantage of a naturally occurring cone-snail peptide toxin, Conkunitzin-S1 (Conk-S1), which selectively blocks Kv1.7 channels to provide an intrinsically limited, finely graded control of total beta cell delayed rectifier current and hence of GSIS. Conk-S1 increases GSIS in isolated rat islets, likely by reducing Kv1.7-mediated delayed rectifier currents in beta cells, which yields increases in action potential firing and cytoplasmic free calcium. In rats, Conk-S1 increases glucose-dependent insulin secretion without decreasing basal glucose. Thus, we conclude that Kv1.7 contributes to the membrane-repolarizing current of beta cells during GSIS and that block of this specific component of beta cell Kv current offers a potential strategy for enhancing GSIS with minimal risk of hypoglycaemia during metabolic disorders such as Type 2 diabetes.
Keywords: Conkunitzin-S1, electrical signalling, GSIS, pancreas, potassium channels
INTRODUCTION
Increase in ATP (and/or a decrease in ADP) due to glucose metabolism causes the closure of KATP channels, leading to depolarization and opening of voltage-gated calcium and sodium channels (Ashcroft & Rorsman, 1989). The resulting increase in intracellular calcium triggers the release of insulin granules. Secretion terminates when the beta cell is repolarized by the opening of potassium channels including members of the voltage- (Kv) and calcium-activated (KCa) potassium channel families (Braun et al, 2008; Houamed et al, 2010; Jacobson et al, 2010). Thus, the amount of insulin secreted is directly coupled to the electrical activity of the beta cell, and modulation of the multiple ion channels involved offers different alternatives for the treatment of glucose homeostasis related disorders such as diabetes. Since KATP channels constitute key initiators of glucose-stimulated insulin secretion (GSIS), great effort has been devoted to the study of anti-diabetic drugs like the sulfonylureas, which modulate one of the two molecular components of the KATP channel (Bryan et al, 2005; Proks et al, 2002). However, a problem commonly associated with such drugs is that KATP current inhibition is independent of the basal glucose levels and hypoglycemia is frequently observed (Amiel et al, 2008; Stahl & Berger, 1999).
Representatives of most K channel families have been identified in pancreatic islets, and specifically in beta cells. Nevertheless, the molecular identities of those voltage-gated potassium channels (Kv channels) involved in the regulation of GSIS remain obscure. Biophysical properties, together with dominant-negative knockdown and pharmacological inhibition, suggest that Kv1 channels account for ∼25% of beta cell delayed rectifier currents, whereas Kv2 channels account for ∼60%; moreover, inhibition of these Kv channels specifically enhances GSIS (Herrington et al, 2005, 2006; Herrington, 2007; Macdonald et al, 2001). Kv2.1 has been reported to be involved in the maintenance of fasting blood sugar during the bursts of beta cell insulin secretion between meals (Jacobson et al, 2007), but its widespread expression makes it a difficult pharmacologic target. The kinetics of the beta cell KCa currents (mediated by SK, IK and BK channels) suggest their capability to modulate various aspects of electrical bursting activity, including action potential shape and amplitude. Two recent papers explore the roles of BK and SK channels in detail (Houamed et al, 2010; Jacobson et al, 2010), and the latter report notes the presence of an unidentified non-Kv2.1 component of the delayed rectifier. mRNAs encoding other Kv channels have been detected in human and rhesus monkey beta cells (Hardy et al, 2009; Yan et al, 2004). Kv1.7 message is expressed at relatively low levels, qualitatively consistent with the voltage clamp data, which we present in this paper.
In rodent islets, multiple Kv α-subunits, including Kv1.7, are expressed at high levels (Kalman et al, 1998; Smith et al, 1990), suggesting that these Kv subtypes contribute to the remainder of the beta cell delayed rectifier current. The gene for human Kv1.7 was mapped to chromosome 19q13.3, a region thought to contain a diabetes susceptibility locus (Kashuba et al, 2001), but the specific role of Kv1.7 remained elusive. Previously, we cloned and characterized mouse Kv1.7 (mKv1.7), which can occur in two isoforms (Finol-Urdaneta et al, 2006). Here, we show that currents mediated by the human homologue (hKv1.7, expressed in tsA-201 cells) resemble those of the short isoform of mKv1.7 (Fig 1), consistent with the sequence similarity between their N-termini, whereas a long isoform of hKv1.7 has yet to be described (Bardien-Kruger et al, 2002). Noteworthy for the whole animal experiments in the present study is that the rat ortholog, rKv1.7, has a predicted 98% sequence identity with the mouse long isoform (see Material and Methods section).
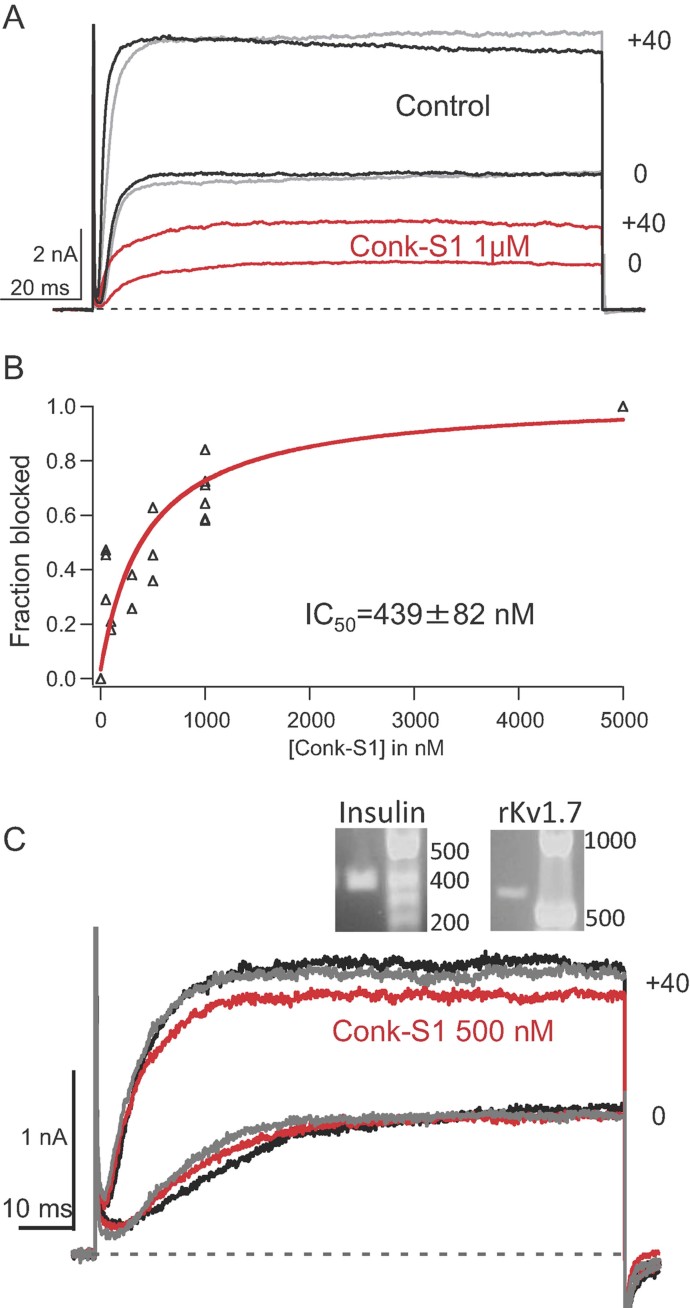
Figure 1. Conkunitzin-S1 blocks Kv1.7 and delayed rectifier currents from isolated rat pancreatic islet cells.
Black is control; red, Conk-S1; and grey, wash.
- Whole-cell current traces. Effect of 1 µM Conk-S1 on currents through hKv1.7 channels expressed in tsA-201 cells evoked by depolarization to 0 or 40 mV (Vh = −80 mV). For I–V relationships, see Supporting Information Fig S1.
- Dose–response relation for Conk-S1 block of the long isoform of mKv1.7 channels, expressed in tsA-201 cells (Individual data points are plotted from 19 different cells, and were determined from currents at +40 mV). IC50 = 439 ± 82 nM, (mean ± sem, estimated by the Origin nonlinear least squares fitting routine).
- Rat pancreatic islet cell native Kv currents. Inset: single-cell PCR for insulin and Kv1.7 transcripts (DNA standard in bp). Reduction of whole-cell Kv currents by 500 nM Conk-S1 (Vh = −80 mV). For normalized I–V relationships, see Supporting Information Fig S1.
More importantly, we demonstrate that Kv1.7 channels are physiologically relevant for pancreatic insulin secretion. Furthermore, we identify Conkunitzin-S1 (Conk-S1), as a preferential peptide blocker of Kv1.7, and an experimental tool to dissect the role of Kv1.7 in the regulation of insulin secretion, as well as a possible molecular archetype for the design of new pharmacological agents to control glucose homeostasis.
RESULTS
Conkunitzin-S1 (Conk-S1) blocks expressed Kv1.7 channels and part of the delayed rectifier current in insulin-secreting islet cells
Conk-S1 from the venom of the predatory cone snail Conus striatus is known to block Drosophila shaker channels (Kv1) with high affinity (Bayrhuber et al, 2005). Figure 1A shows potassium currents from human Kv1.7 (hKv1.7) channels expressed in tsA-201 cells, where exposure to 1 µM Conk-S1 produced a >50% reversible block over a voltage range from −20 to +100 mV (see also Supporting Information Fig S1A). Conk-S1 also blocks murine Kv1.7 (mKv1.7) channels with an IC50 of 439 ± 82 nM (Fig 1B), identifying Kv1.7 as a mammalian target of Conk-S1. In contrast, none of 15 other expressed potassium channels, from the sub-families Kv(1-4), eag and slo (high-conductance calcium-activated), were affected by Conk-S1 in the sub-micromolar range (>20-fold lower affinity than for mKv1.7, see Supporting Information Table S1).
mRNA encoding Kv1.7 has been detected in mouse pancreatic islet cells by in situ hybridization (Kalman et al, 1998) and in rat islet cells by single-cell PCR (current work). Whole-cell patch clamp recordings show that 0.5 µM Conk-S1 blocked 18 ± 2% (n = 10) of the total delayed rectifier currents at +40 mV (∼1–1.5 nA) from rat islet cells that contained both insulin and kcna7 transcripts (Fig 1C and Supporting Information Fig S1B). At 0.5–1 µM, Conk-S1 had no effect in other islet cell populations, which typically showed currents with smaller amplitude, more rapid inactivation or lacked detectable levels of insulin mRNA (e.g. Supporting Information Fig S2). These cells include examples of cells that were negative for insulin (6/25 or 24%), from which about half were positive for glucagon (4/6 or 16% of the total). Thus, we conclude that Conk-S1 acts primarily to block Kv1.7-mediated currents in beta cells, which comprise the majority of cells in endocrine regions of the rat pancreas (Elayat et al, 1995).
Conk-S1 block of fluxes through voltage-gated K channels in isolated islets is associated with increased insulin secretion
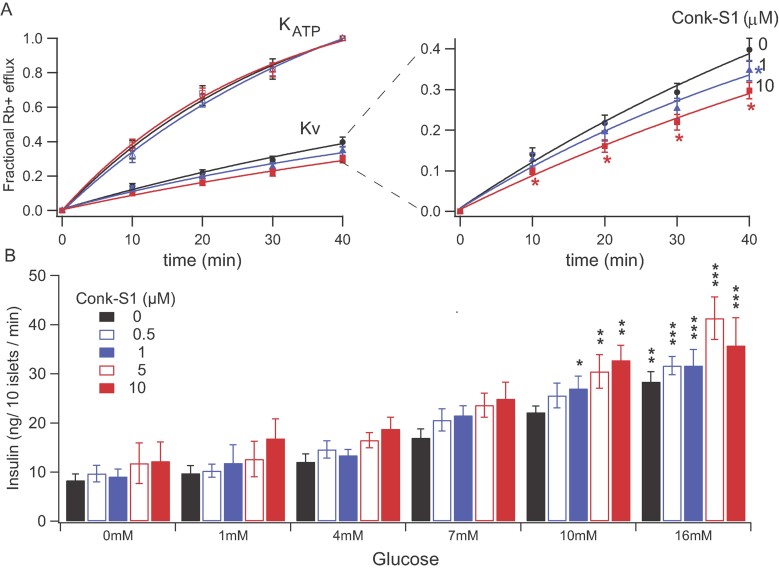
To further explore the functional importance of the small, but consistent Conk-S1-induced decrease in Kv currents, Rb+ effluxes through KATP and Kv channels were measured at different concentrations of Conk-S1 in competent, isolated rat islets. Addition of Conk-S1 significantly reduced the Kv channel-mediated Rb+ efflux, whereas the KATP-mediated response was unaffected (Fig 2A left panel). 10 µM Conk-S1 produced a reduction of ∼25% of the Rb+ efflux at all time points (p < 0.05), while 1 µM inhibited ∼13% of Rb+ effluxes at 40 min (Fig 2A left panel, t = 40 min, p < 0.05).
Figure 2. Conkunitzin-S1 modulates GSIS through block of Kv channels, but not KATP channels (see Research Design and Methods for further details).
- Left panel, Fractional 86Rb+ efflux in the presence and absence of Conk-S1, as a function of time, from representative islet pools. KATP data (circles)—MI (metabolic inhibitor) solution included: 2.5 mg/ml oligomycin, 1 mM 2-deoxyglucose, 10 mM TEA, 10 µM nifedipine and 30 mM KCl, black circles. Kv data (squares)—MI solution included 10 mM d(+)glucose, 1 µM glibenclamide and 30 mM KCl (mean ± sem of 5–8 independent determinations from islets isolated from different animals). Black is control; blue, 1 µM Conk-S1; red, 10 µM Conk-S1. No detectable effect of Conk-S1 on fluxes through KATP channels was observed. Right panel, Expanded presentation of Kv channel fluxes. Conk-S1 significantly inhibits Rb+ fluxes through Kv channels. Data are shown as mean ± sem; n = 5–9 independent determinations from islets isolated from different animals (*denotes a significant difference between Conk-S1 and control, p < 0.05, pair wise t-test at each time point). See Supporting Information Table S2 for individual n and p values.
- Insulin secretion from pools of isolated pancreatic islets (see Materials and Methods section) at different glucose concentrations (0–16 mM), each in the presence of a range of Conk-S1 concentrations (0–10 µM). Data are shown as mean ± sem; n = 3–11 independent determinations in triplicate from islets isolated from different animals. Two-way ANOVA showed a significant dependence of insulin secretion on [Conk-S1] (p = 0.0009) and on [glucose] (p < 0.0001). Bonferroni pairwise comparisons showed significant enhancements of insulin release in the presence of Conk-S1 for both 10 and 16 mM glucose compared to 0 mM glucose (*p < 0.05; **p < 0.01; ***p < 0.001). See Supporting Information Table S3 for additional statistical details, including numbers of independent experiments and probabilities associated with different analyses.
Also, incubation with Conk-S1 enhanced insulin secretion from rat pancreatic islets (Fig 2B). Insulin secretion showed significant dependence on concentrations of both Conk-S1 (p = 0.0009) and glucose (p < 0.0001) based on a two-way ANOVA analysis (see Supporting Information for further details). Thus, Conk-S1 appears to modulate GSIS in pancreatic islets by inhibiting Kv1.7 currents without affecting KATP activity.
A screen for the release of other metabolic hormones (glucagon, pancreatic polypeptide and somatostatin) revealed no significant, systematic effect of Conk-S1 (Supporting Information Fig S3 and Table S4). We detected no leptin release from isolated islets, consistent with the fundamental site of production of leptin being in adipose tissue (Anubhuti & Arora, 2008). Together, these results support the idea that specific, but limited, blockade of beta cell delayed rectifier currents by Conk-S1 can be used to enhance GSIS.
Conk-S1 potentiates electrical bursting activity in islet cells
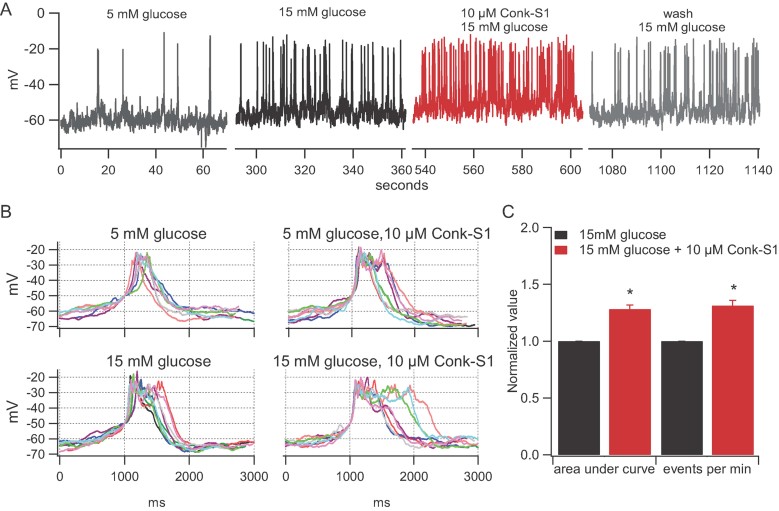
A decrease in Kv currents of pancreatic cells should modulate membrane potential by delaying cell membrane repolarization. As a result, action potential frequency and/or spike amplitude and duration should be measurably affected. Accordingly, the effects of 10 µM Conk-S1 on the electrical activity of isolated islet cells at low and high glucose concentrations (5 and 15 mM, respectively) were investigated. At high glucose concentrations, Conk-S1 generated an increase in firing frequency (∼30%, n = 5, Fig 3A and C) as well as spike or burst broadening (Fig 3B). Due to the heterogeneity of firing patterns in islet cells (Kinard et al, 1999), quantification of effects was done by two methods. First, we determined the total area under the voltage versus time curve with a lower threshold of −75 mV. This analysis revealed a 28.3 ± 3.5% increase in the integrated cell depolarization (change in voltage × time; n = 5; p < 0.05; Fig 3C). Second, spike frequency was determined and found to increase by a similar percentage (Fig 3A and C). Hence, the Conk-S1-sensitive component of Kv current is an important determinant of both spike frequency and action potential shape in beta cells. No statistically significant effect of Conk-S1 was seen at 5 mM glucose [integrated depolarization (p = 0.94); spike frequency (p = 0.34); n = 3 for both parameters] (see Supporting Information Fig S4), though a tendency towards increased secondary bursting within individual spike complexes is clearly visible in the presence of Conk-S1.
Figure 3. Conk-S1 enhances glucose-stimulated increase in action potential firing.
- Action potential firing elicited by glucose (15 mM) stimulation is reversibly accelerated by Conk-S1, but there is little or no effect at low glucose (5 mM)—see text and Supporting Information Fig S4.
- Spike width increases following addition of Conk-S1. Each panel shows 10 spikes in the presence of 5 or 15 mM glucose with and without Conk-S1. For comparison, spikes were aligned at the point crossing −50 mV.
- Quantification of Conk-S1 effect on rat islet cell action potentials; significant increases were observed for both integrated time of depolarization (p = 0.0001), and firing rate (p = 0.0002), n = 5 independent measurements.
Depolarization of beta cells activates L-type calcium channels, leading to a rise in intracellular Ca2+ known to promote insulin granule secretion. The effects of Conk-S1 on spike frequency and action potential duration suggested that intracellular [Ca2+] would also be altered. Qualitative support for this hypothesis is provided by fluorescence measurements of changes in cytoplasmic free Ca2+. Supporting Information Fig S5 shows a 42 ± 12% (n = 14) increase in the Fluo-4 signal reporting the intracellular free [Ca2+], in isolated rat islet cells, in response to Conk-S1 addition. At 15 mM glucose, Conk-S1 enhanced the activity from cells that showed fast Ca2+ oscillations (n = 4, Supporting Information Fig S5 upper panel), as well as for cells that were oscillating slowly or not at all (n = 10, Supporting Information Fig S5 lower panel). Three separate experiments from three different cell preparations showed no changes in Fluo-4 Ca2+ fluorescence when toxin was added at low glucose (0–5 mM), consistent with the idea that Conk-S1 delays cell repolarization and thereby allows increased intracellular calcium accumulation in a glucose-dependent manner, i.e. only during GSIS. Complementary experiments using confocal microscopy showed no measurable Ca2+ response to Conk-S1 applied at 5 mM glucose, but did show a significant 23.7 ± 1.8% (p = 0.006) increment in fluorescence in response to 30 mM KCl depolarization in the presence of Conk-S1 at 15 mM glucose (n = 3 measurements from discrete groups of 5 to 7 cells, each representing a different culture dish—see online Supporting Information). This is consistent with a two-step depolarization: an incremental depolarization by Conk-S1 and an additional depolarization on application of KCl, each associated with an increment in Ca2+ influx.
Conk-S1 enhances GSIS and glucose tolerance in whole animals
To test whether Conk-S1-specific block of Kv1.7 currents can also promote insulin secretion in vivo, oral glucose tolerance tests (OGTT) were performed using healthy, conscious animals. Wistar rats were injected intravenously (i.v.) with saline (control), saline-Conk-S1 (100 nmol/kg) or saline-glibenclamide (0.3 mg/kg, a KATP antagonist interacting with sulfonylurea receptor subunits commonly used for treatment of Type 2 diabetes; Fig 4A). Treatment with Conk-S1 resulted in a transitory increase in insulin release and an attenuation of the transient increase in blood glucose concentration upon subsequent glucose challenge. As expected, glibenclamide injection also resulted in a reduction of the glucose-induced glucose increase. However, a major difference between their effects was that Conk-S1 reduced the glucose levels only transiently and not its steady state level before and after the glucose challenge. Thus, in contrast to glibenclamide, Conk-S1 does not produce hypoglycemia (Fig 4A; note points at t = 0) as expected from the islet data, which indicated that Conk-S1 did not affect KATP-mediated currents (Fig 2A).
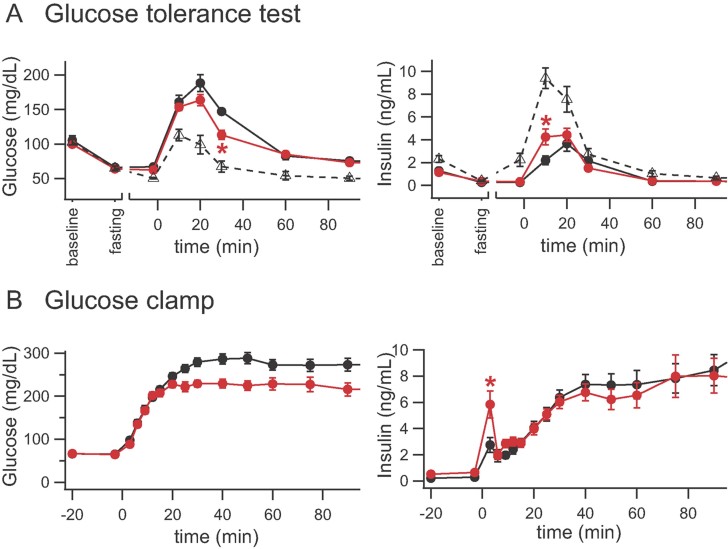
Figure 4. Conk-S1 modulates glucose levels (left panels) and insulin secretion (right panels) in vivo in conscious and pithed rats.
- Glucose tolerance test in conscious rats. Conk-S1 and glibenclamide blunt the spike in plasma glucose following oral glucose challenge 1 g/kg. Symbols: Conk-S1 (red filled circles, 100 nmol/kg i.v. 130 min before the glucose challenge); glibenclamide (black open triangles 0.3 mg/kg i.v. 10 min before glucose challenge); controls (black filled circles). Asterisks, *p < 0.05 for comparison of Conk-S1 with controls, at the indicated time. For a complete listing of numbers of independent experiments, and p values for comparisons at all time points, see Supporting Information Table S5.
- Glucose clamp using pithed rats. Influence of Conk-S1 on glucose and insulin levels during glucose clamp (8.99 mg/min; i.v.). Conk-S1: red filled circles, 100 nmol/kg i.v. as a bolus 120 min before glucose clamp, plus 100 nmol/kg as a maintenance dosage within 4 h; controls: black filled circles; asterisks, *denote p < 0.05 for comparison of Conk-S1 with controls. A complete listing of numbers of independent experiments, and p values for comparisons at all time points, is given in Supporting Information Table S6.
Despite the fact that Kv1.7 has been reported to be present in skeletal and heart muscle (Finol-Urdaneta et al, 2006), there were no discernable deleterious side effects of Conk-S1 treatment on animals during and after the in vivo experiments. We did not observe seizure activity or deaths. Thus, we have no evidence of significant cardiovascular or neurological side effects at the doses used. Blood glucose levels did not change significantly in the period from 90 to 240 min after the glucose challenge, during which the fast was maintained (unpublished observations). After that, food was again provided, and blood glucose of all animals returned to normal, pre-fasting levels within 24 h.
To test for a possible direct central nervous system-induced regulation or adaptation during Conk-S1 treatment, glucose was continuously infused into pithed rats, and the blood glucose and insulin levels were measured (‘glucose clamp’, see Material and Methods section). This protocol provides a constant rate of infusion of glucose without experimenter-imposed feedback control on the blood glucose concentration. The glucose-induced increases in blood glucose were identical during the first ∼15 min of glucose infusion for control and Conk-S1-treated groups (Fig 4B). In the Conk-S1-treated animals, the rising phase terminated earlier, decreasing the time to reach half-maximal glucose by ∼50% and yielding a significantly reduced steady state level of blood glucose. With Conk-S1 present, the maximal glucose concentration was attained in <20 min, while for control animals, the glucose concentration peaked at ∼40 min after the start of glucose infusion (Fig 4B left panel). Attenuation of the rise in glucose followed the significant spike in blood insulin induced by Conk-S1 infusion (Fig 4B right panel). In the presence of Conk-S1, insulin release increased transiently only during the first 3 min of glucose clamp; soon after, it became indistinguishable from control values. Consistent with the OGTT experiments, no effect on basal glucose levels was observed. Blood pressure and heart rate during these experiments were unaffected by Conk-S1 (Supporting Information Fig S6). These results demonstrate that, during constant glucose infusion, i.v. administration of Conk-S1 affects blood glucose levels only by enhancement of the initial phase of insulin release. In pithed rats, only the initial phase of insulin release was modulated by Conk-S1, suggesting that later changes in glucose levels depended on peripheral, but insulin-independent, regulatory mechanisms.
DISCUSSION
The present work shows that Conk-S1 enhances GSIS via Kv channel modulation. Moreover, our results identify Conk-S1 as a specific blocker of Kv1.7 and indicate that Kv1.7 activity contributes actively to the control of GSIS in pancreatic beta cells. In agreement with the idea that Kv channels specifically modulate membrane potential during electrical bursting activity of beta cells, no statistically significant effects of Conk-S1 were observed at lower glucose concentrations, at which action potentials were infrequent. Accordingly, Conk-S1 did not reduce blood glucose prior to glucose stimulation in OGTT and thus, hypoglycemia was not associated with Conk-S1 administration as it is with commonly used sulfonylurea drugs like glibenclamide. Meanwhile, similar to glibenclamide, Conk-S1 reduces blood glucose during oral glucose administration.
The variety of pancreatic ion channels and cell types
The most prominent Kv channel in beta cells is Kv2.1 [e.g. see (Jacobson & Philipson, 2007)]. When expressed in Xenopus oocytes, these channels are not affected by Conk-S1, and in accordance with this, Conk-S1 application to beta cells never reduced the total delayed rectifier K current amplitude by more than ∼20% (Fig 1C). Most likely, Conk-S1 specifically reduces currents mediated by Kv1.7 homo- or hetero-tetrameric channels. This likely causes a reduction of the Rb+ efflux, increased insulin secretion from isolated rat islets (Fig 2), as well as the observed changes in islet bursting (Fig 3) and in vivo effects (Fig 4). The action of Conk-S1 may result from preferential targeting of islet-specific heteromeric Kv channels. A similar explanation was proposed by Jacobson et al for the pharmacological complexity of residual delayed rectifier current in mice lacking Kv2.1 (Jacobson et al, 2007). In particular, we suggest that Kv1.7 is a critical element of Conk-S1's target. This is consistent with preliminary experiments, which show that Conk-S1 (and other peptides) can discriminate among different heteromeric constructs (Fig 5, and see following paragraph). In addition, this might also account for the lack of observed side effects from actions of Conk-S1 on other tissues where Kv1.7 transcripts have been found. Such a scenario has recently been proposed as a basis for the specific cardioprotective action of κ-conotoxin RIIIK (Chen et al, 2010).
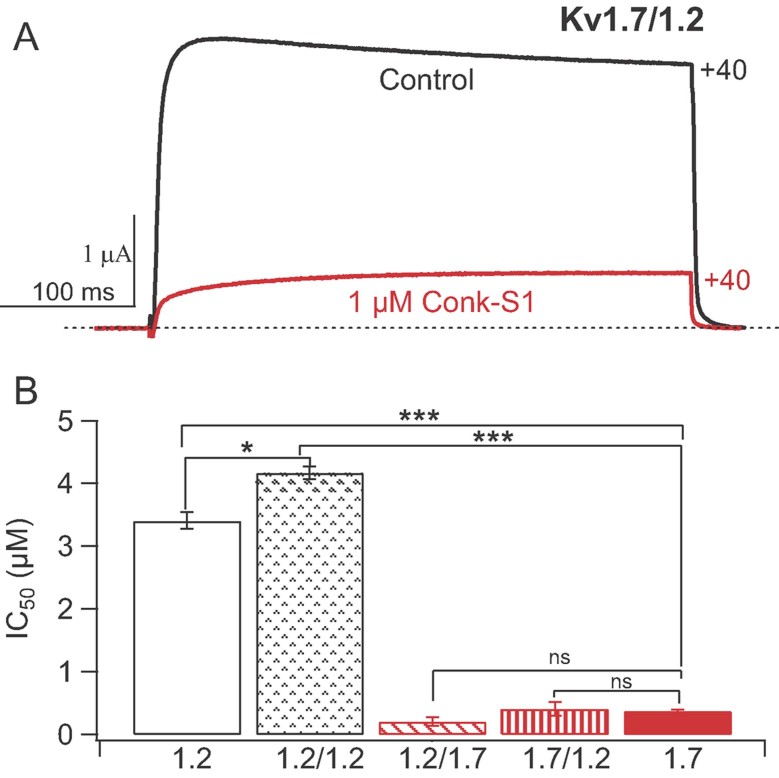
Figure 5. Conk-S1 strongly inhibits heteromeric Kv channels incorporating Kv1.7 α-subunits.
- Two-electrode voltage clamp current traces showing Conk-S1 (1 µM) block of currents resulting from expression of Kv1.7/1.2 dimers in Xenopus oocytes.
- IC50s for inhibition of Kv1.2 and 1.7 homotetrameric channels, as well as dimer of dimers formed from Kv1.2/1.2, Kv1.2/1.7 and Kv1.7/1.2. Numbers of independent determinations for the IC50s were: Kv1.2 (4), Kv1.2/1.2 (3), Kv1.2/1.7 (4), Kv1.7/1.2 (3) and Kv1.7 (4). The IC50 for the Kv1.7 homotetramer differed strongly from both the Kv1.2 homotetramer (p = 0.0002) and the Kv1.2/1.2 dimer of dimers (p = 0.0001), but did not differ significantly from values for the mixed dimers: Kv1.2/1.7 (p = 0.054) and Kv1.7/1.2 (p = 0.73). There was a modest difference between IC50s for the Kv1.2 homotetramer and the Kv1.2/1.2 dimer of dimers (p = 0.008). Overall, the presence of two Kv1.7 α-subunits (or domains), assembled with Kv1.2, was sufficient to yield high affinity block by Conk-S1.
We have recently verified that Conk-S1 is capable of preferentially blocking heteromeric Kv channels containing Kv1.7 α-subunits as opposed to other heteromers of the forms Kv1.2/Kv1.x or Kv1.x/Kv1.2 (x being 1–6). Figure 5 illustrates block of channels formed after expression of Kv1.2-1.7 or Kv1.7-1.2 dimers. Presumably, these assemble as dimer-of-dimers, 4-domain channels, and both of these channel constructs are blocked with IC50s approximating that of the homotetrameric Kv1.7 channels, formed by expression of only monomeric Kv1.7 α-subunits. Thus, regardless of the order of linkage in the dimer, Conk-S1 effectively targets Kv1.7 domains in these heteromeric constructs.
A recent, detailed analysis of gene transcripts and beta cell lineage revealed significant inhomogeneity in the expression patterns of pancreatic hormone transcripts even in ‘fully committed’ beta cells (Katsuta et al, 2010). However, given that insulin is the main hormone secreted by glucose-stimulated stably committed beta cells, the expected dominant action of Conk-S1 would be to modulate insulin secretion, as we observed. Our present data reveal that block of a small component of the beta cell Kv current by Conk-S1 is an effective mechanism to modulate beta cell electric activity. These changes are immediately mirrored by changes in insulin secretion as evidenced by the isolated islet and in vivo data. Thus, our results support a mechanism for a specific enhancement of glucose-dependent insulin secretion by modulating a particular, limited component of the native beta cell Kv currents.
To place our results in a broader context, we underline the fact that the pancreas is an enormously complex integrator of varied signals relevant to the maintenance of metabolic homeostasis. A large variety of ion channels contribute to the function of this signalling network. Recently studied examples include members of the transient receptor potential (Trp) and ether-a-go-go related (hrg) channel families. The three groups of Trp channels (Trp-C, -M, and -V), represented by at least seven different individual channels, are widely present in pancreata of different species and in different pancreatic cell lines (Hiriart & Aguilar-Bryan, 2008). Trp-M3 acts as an ionotropic steroid receptor which can stimulate insulin secretion from β-cells (Wagner et al, 2008). Human erg (herg) channels are present in both α- and β-cells (Hardy et al, 2009; Rosati et al, 2000). In the latter extensive study, inhibition of herg channels was shown to have mechanistically distinct, but physiologically complementary, functions to enhance insulin secretion from beta cells and inhibit glucagon secretion from α-cells. Finally, influences of calcium-activated and Kv channels have been carefully explored (Houamed et al, 2010; Jacobson et al, 2010).
Therapeutic possibilities
Each pancreatic ion channel that is discovered offers new insight into the intricacies of glucose regulation, and perhaps, opens new pharmacological possibilities. In the case of Kv1.7, such an opportunity is underscored by our whole-animal data, which demonstrate enhancement of GSIS by Conk-S1 without alteration of basal glucose levels or induction of apparent side effects. It is possible that an unobtrusive Kv1.7 component escaped detection in the experiments of Jacobson and co-workers (Jacobson et al, 2010), where about 10% of delayed rectifier current in Kv1.4−/− cells persisted in the presence of simultaneously applied, high doses of preferential blockers of Kv2.1 and Kv1.3. Our screening data suggest that the relatively high concentrations of Conk-S1 used in our islet and whole animal experiments would be sufficient to block almost all Kv1.7-mediated current, but, at most, a small fraction of current through other channel types, which is consistent with the lack of side effects.
There are, of course, limitations to the conclusions that can be drawn from our results. Clearly, Conk-S1 can modulate GSIS, and a varied array of evidence points to a role for Kv1.7 as a mediator of Conk-S1's action. Despite the use of several complementary approaches at molecular, cellular, tissue and whole animal levels, we cannot conclude absolutely that Kv1.7 is the sole molecular target of Conk-S1. In future studies, inducible knockdown of Kv1.7-perhaps strategically driven by the Ins2 promoter (Katsuta et al, 2010)—will offer further tests of conclusions and hypotheses derived from our results. Rigorous performance of such experiments would include in-context identification of Kv1.7 protein by antibodies, which enable not just detection of Kv1.7 monomers, but also identification of other Kv1 α-subunits with which Kv1.7 may co-assemble. Unfortunately, the antibodies available for Kv1.7 detection/labelling have, to date, proved inadequate for this task.
Our study points strongly to Kv1.7 as a functionally significant molecular contributor to the beta cells delayed rectifier current, by a combination of (i) screening of Conk-S1 action on homo- and hetero-tetrameric Kv channels of known composition, (ii) confirmation of both Kv1.7 and insulin gene transcripts in individual cells for which Conk-S1 inhibits a limited fraction of the delayed rectifier current, (iii) Conk-S1 enhancement of electrical activity and GSIS in islets and (iv) Conk-S1 potentiation of both GSIS and glucose regulation in whole animals.
Finally, the identification of Conk-S1 as a specific blocker of Kv1.7 highlights the potential of cone snail venom peptides as a rich source for a wide variety of specific pharmacological tools (Terlau & Olivera, 2004). Since Conk-S1 affects glucose-mediated insulin secretion without affecting basal glucose levels, our results identify delayed rectifier K currents as a potential target for the treatment of metabolic diseases like Type 2 diabetes. In general, substances, which specifically interact with minor components of voltage-activated K currents from pancreatic beta cells, provide a wide safety margin enhancing their potential value as therapeutic agents. Specifically, targeting of Kv1.7, which represents only a small fraction of the delayed rectifying K+ channels and is primarily active upon membrane depolarization, has the advantage of lessening potential side effects compared to KATP channel inhibitors. This expectation is in agreement with both our observations on rat islets and our in vivo data.
MATERIALS AND METHODS
Experimental methods are outlined below, and further details are provided in the online Supporting Information. Animal experiments performed in Canada, Germany, and the United States were conducted according to the guidelines of the Canadian Council of Animal Care, NIH, as well as the guidelines for the care and use of laboratory animals and authorized by the local regulatory authority (Ministerium für Landwirtschaft, Umwelt und ländliche Räume des Bundeslandes Schleswig-Holstein).
Research design
We set out to test the specificity of the conopeptide inhibitor, Conk-S1, under voltage clamp using 16 different K channels expressed either in Xenopus oocytes or in mammalian cells. We established that Conk-S1 is >20-fold more potent in blocking Kv1.7 (Fig 1) than the next most susceptible channel, Kv1.2 (Supporting Information Table S1), and showed no measurable action against most other channels tested. Subsequently, we used Conk-S1 to test for a contribution of Kv1.7 to the control of GSIS, in isolated cells, dissociated islets and whole animals. For β-cells isolated from rat pancreatic islets, identified as insulin-producing by single-cell PCR (but also see (Katsuta et al, 2010)), we attribute the Conk-S1-sensitive fraction (∼18%) of the total delayed rectifier current to channels containing Kv1.7 monomer(s). In intact, isolated rat islets, nearly saturating concentrations of Conk-S1 reduced Kv channel-mediated rubidium efflux by a similar fraction, and reduces insulin secretion in a glucose-dependent manner. In parallel, islet cells under current clamp show increased action firing activity at high, but not at low, glucose. Finally, we observed the effects of Conk-S1 on glucose and insulin levels in conscious (by OGTT) and pithed rats (under glucose “clamp”).
Cloning and expression of Kv1.7
KCNA7 RNA was amplified with one-step RT-PCR (Advantage RT-PCR kit, Invitrogen) with human heart total RNA as template. Mouse Kv1.7 cloning (mKv1.7 long form, 98% sequence identity with the predicted sequence for rat Kv1.7) has been described by Finol-Urdaneta et al (Finol-Urdaneta et al, 2006). For electrophysiological studies in X. laevis oocytes, full length constructs were sub-cloned into the expression vector pSGEM (Liman et al, 1992). For expression in tsA-201 cells, Kv1.7 constructs were sub-cloned in pTracer-CMV2.
Conkunitzin-S1
Highly purified recombinant Conk-S1 was produced as described in Bayrhuber et al (Bayrhuber et al, 2006). Conk-S1 purity was indistinguishable from 100% by mass spectrometry.
The Conk-S1 structure comprises a twisted double loop backbone, held by disulphide links between the C-terminal helical section and both the N-terminal (C7-C57) and the middle (C32-C53) section of the peptide backbone (Bayrhuber et al, 2005). The sequence of Conk-S1 is as follows:
| 1 | 10 | 20 | 30 |
| KDRPSLCDLPADSGSGTKAEKRIYYNSARK | |||
| 31 | 40 | 50 | 60 |
| QCLRFDYTGQGGNENNFRRTYDCQRTCLYT | |||
Electrophysiology
Screening of Conk-S1 effects on Kv1.1–Kv1.7, Kv2.1, Kv2.2, Kv3.1, Kv3.2, Kv3.4, hKv4.2, reag1 and reag2 was performed by two microelectrode voltage clamp (TEVC) in Xenopus oocytes (Supporting Information Table S1). In addition, a series of ‘test of principle’ experiments were performed to assay the ability of Conk-S1 to block channels expressed from cRNA encoding the following dimeric constructs: homomeric Kv1.2/1.2, and heteromeric forms in the two possible orders of linkage, Kv1.2/1.7 and Kv1.7/1.2.
Whole-cell patch clamp (Axopatch 200B, Molecular Devices Corp. Sunnyvale, CA, USA) was used to record currents from tsA cells expressing human Kv1.7 α-subunits, or from dissociated islet cells (24–48 h after transfection or primary culture). After recording, individual islet cells were lysed, and single-cell RT-PCR was used to test for transcripts of Kv1.7, insulin and glucagon. Further details are provided in the Supporting Information.
Current clamp recordings from partially dissociated islet cells (see below) were performed using a MultiClamp 700A Microelectrode Amplifier (Molecular Devices Corp., Sunnyvale, CA, USA) using and the nystatin-perforated patch configuration (Horn & Marty, 1988) at 28–33°C. Data summarized in the Results (e.g. Fig 3) were obtained from surface cells of mildly trypsinized islets. The activity patterns illustrated in Fig 3, and in Supporting Information Fig S4, are representative of the vast majority of our recordings, and hence, such records were used for analysis. Because action potential frequency and morphology depend on species (Pedersen, 2010), and factors including the temperature, size of cell clusters and cell-to-cell coupling (Smolen et al, 1993), firing frequencies and integrated depolarizations were normalized for our analysis of Conk-S1 action. Nonetheless, the activity which we observed was similar to that observed in numerous other studies.
Islet isolation and measurements of 86Rb+ efflux and insulin release
Pancreatic islets were isolated from adult male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) as previously described (Remedi et al, 2004). 86Rb+ efflux was assayed by replacing the bathing solution with Ringer's solution and metabolic inhibitor (MI) plus 0, 1 or 10 µM Conk-S1. Fluxes through KATP and Kv channels were estimated separately by use of appropriate blockers of other channels, and ionic content was adjusted to maintain the transmembrane voltage at ∼0 mV. Insulin release into the bathing medium was measured at different glucose concentrations, with and without Conk-S1, at 37°C, using Rat Insulin radioimmunoassay according to the manufacturer's protocol (Millipore, St. Charles, MO). Within each experiment, triplicate determinations were done for each set of conditions. Numbers of experiments are indicated in the figure legends. More details are provided in references (Remedi et al, 2004, 2006) and the online appendix.
The paper explained
PROBLEM
Voltage-gated potassium (Kv) channels are membrane-embedded proteins, which open and close in response to changes in the voltage across the surface membrane. In electrically active, insulin-secreting beta cells of the pancreas, Kv channels help to terminate the electrical spikes, which trigger insulin secretion in response to increased glucose levels, but the specific roles of different Kv channels remain unclear. In this study, we use a cone-snail venom peptide, Conkunitzin-S1 (Conk-S1), which shows a strongly selective inhibitory action among even closely related Kv channels, to explore the functional role of a distinct component of beta cell Kv activity. We associate the inhibitory action of Conk-S1 with presence of the particular channel protein Kv1.7 and examine its effect on insulin release from isolated pancreatic islets and in intact animals.
RESULTS
Conk-S1 specifically inhibits homotetrameric Kv1.7 channels, as well as heteromeric channels, which contain Kv1.7, indicating that Conk-S1 action is directed against the Kv1.7 alpha subunit. Conk-S1 inhibits part of the Kv channel activity (∼15–20%) and potentiates glucose-stimulated insulin secretion in pancreatic islets, as well as enhances insulin secretion and increases glucose tolerance in vivo in rats.
IMPACT
We provide the first detailed analysis of the role the specific channel protein Kv1.7 in pancreatic function. Our results indicate a circumscribed role for Kv1.7 in regulating pancreatic insulin secretion. Conk-S1's actions suggest the possibility of an intrinsically limited enhancement of glucose regulation by targeted inhibition of Kv1.7.
Also in the Supporting Information, we describe a screen for the possible release, from isolated islets, of additional metabolic hormones including glucagon, pancreatic polypeptide, somatostatin and leptin.
Whole animal studies—in vivo oral glucose tolerance tests and pithed rats—glucose clamp
Male Wistar rats (∼300 g; Charles River, Sulzfeld, Germany) were used for all in vivo and pithed rat experiments.
Rats, fasted for 16 h before OGTT. OGTT were performed using untreated, as well as Conk-S1-treated (100 nmol/kg i.v. 130 min prior to glucose challenge), and glibenclamide–treated (glibenclamide: 0.3 mg/kg i.v. 10 min pre-glucose challenge) animals (Muller et al, 2007). Considering bodyweight and an intravascular distribution of Conk-S1, the plasma concentration was estimated to be about 1–2 µM.
Glucose clamp experiments employed the pithed rat preparation, which is well established as a model for peripheral cardiovascular regulation, given that central neural reflex mechanisms have been eliminated (Gillespie & Muir, 1967; Zhang et al, 1993). We used it in order to remove possible direct neural influences on pancreatic function. Glucose (8.99 mg/min, i.v.) was infused, and blood samples were periodically withdrawn for the determination of glucose (using glucose sensors, Ascensia® ELITE XL, Bayer), and insulin (RIA, RI-13K®, Linco, USA). Blood pressure was monitored via arterial catheters (Muller et al, 2007), and was averaged over a 1 min period before starting the glucose infusion, and 3, 30 and 120 min afterwards.
Statistical analysis
In general, summary data are expressed as mean ± standard error. Two tailed t-tests were used to evaluate the significance of the difference between means (Gossett, 1958). One-way and two-way ANOVA, followed by a Bonferroni post hoc test of pairwise comparisons (GraphPad Prism version 5.0d for Mac, GraphPad Software, San Diego California USA, http://www.graphpad.com) were used to test the significance of effects of Conk-S1 applied to islets exposed to particular glucose concentrations. Unless otherwise stated, differences between groups, or trends within a treatment group were taken to be significant of the probability of the observation occurring due to chance was p < 0.05.
Details of Materials and Methods, plus Tables S1 through S6, and Figs S1 through S6, are provided in the Supporting Information.
Acknowledgments
We thank Dr. Wayne Giles for use of facilities for some of the islet and beta cell experiments, Dr. Michael Colicos for the use of his calcium imaging setup, and Dr. Gerald Zamponi for the use of his Zeiss LSM 510 confocal microscope. The technical assistance of Catherine Diao, Mona Honemann, Marie-Luise Stolte, Beate Lembrich, Kamila Sabagh, Ann-Kathrin Brückner and Yvonne Laukat is greatly appreciated. Dr. Andrew P. Braun kindly provided the bSlo and mSlo cDNA. We are grateful to Dr. Willem Wildering for discussions on the statistical analysis, and for independently checking some of the calculations. This work was supported by the Canadian Institutes of Health Research MOP-10053 (RJF); the Heart & Stroke Foundation of Alberta, NWT & Nunavut (EP); NIH DK69445 (CN); Max Planck Society (SB). RJF was a Medical Scientist of the Alberta Heritage Foundation for Medical Research. HT was supported in part by the BioFuture Prize of the German Ministry of Education and Research.
Supporting Information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
RKFU, MSR, WR, CGN, HT, RJF conceived and designed the experiments; RKFU, MSR, WR, RBC, NS, EP, RJF performed experiments and analysed data; SB, NS, CGN, HT contributed analysis tools and reagents; RKFU, MR, CGN, RJF, HT wrote the paper; All authors edited the paper.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in Type 2 diabetes. Diab Med. 2008;25:245–254. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anubhuti V, Arora S. Leptin and its metabolic interactions: an update. Diabetes Obes Metab. 2008;10:973–993. doi: 10.1111/j.1463-1326.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Bardien-Kruger S, Wulff H, Arieff Z, Brink P, Chandy KG, Corfield V. Characterisation of the human voltage-gated potassium channel gene, KCNA7, a candidate gene for inherited cardiac disorders, and its exclusion as cause of progressive familial heart block I (PFHBI) Eur J Hum Genet. 2002;10:36–43. doi: 10.1038/sj.ejhg.5200739. [DOI] [PubMed] [Google Scholar]
- Bayrhuber M, Vijayan V, Ferber M, Graf R, Korukottu J, Imperial J, Garrett JE, Olivera BM, Terlau H, Zweckstetter M, et al. Conkunitzin-S1 is the first member of a new Kunitz-type neurotoxin family. Structural and functional characterization. J Biol Chem. 2005;280:23766–23770. doi: 10.1074/jbc.C500064200. [DOI] [PubMed] [Google Scholar]
- Bayrhuber M, Graf R, Ferber M, Zweckstetter M, Imperial J, Garrett JE, Olivera BM, Terlau H, Becker S. Production of recombinant Conkunitzin-S1 in Escherichia coli. Protein Expr Purif. 2006;47:640–644. doi: 10.1016/j.pep.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and K(ATP) channels. Curr Pharm Des. 2005;11:2699–2716. doi: 10.2174/1381612054546879. [DOI] [PubMed] [Google Scholar]
- Chen P, Dendorfer A, Finol-Urdaneta RK, Terlau H, Olivera BM. Biochemical characterization of kappaM-RIIIJ, a Kv1.2 channel blocker: evaluation of cardioprotective effects of kappaM-conotoxins. J Biol Chem. 2010;285:14882–14889. doi: 10.1074/jbc.M109.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186:629–637. [PMC free article] [PubMed] [Google Scholar]
- Finol-Urdaneta RK, Struver N, Terlau H. Molecular and functional differences between heart mKv1.7 channel isoforms. J Gen Physiol. 2006;128:133–145. doi: 10.1085/jgp.200609498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JS, Muir TC. A method of stimulating the complete sympathetic outflow from the spinal cord to blood vessels in the pithed rat. Br J Pharmacol Chemother. 1967;30:78–87. doi: 10.1111/j.1476-5381.1967.tb02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett WS. New tables for testing the significance of observations. In: Pearson ES, Wishart J, editors. “Student's” Collected Papers. Cambridge: University Press; 1958. pp. 115–120. [Google Scholar]
- Hardy AB, Fox JE, Giglou PR, Wijesekara N, Bhattacharjee A, Sultan S, Gyulkhandanyan AV, Gaisano HY, Macdonald PE, Wheeler MB. Characterization of Erg K+ channels in alpha- and beta-cells of mouse and human islets. J Biol Chem. 2009;284:30441–30452. doi: 10.1074/jbc.M109.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J. Gating modifier peptides as probes of pancreatic beta-cell physiology. Toxicon. 2007;49:231–238. doi: 10.1016/j.toxicon.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Herrington J, Sanchez M, Wunderler D, Yan L, Bugianesi RM, Dick IE, Clark SA, Brochu RM, Priest BT, Kohler MG, et al. Biophysical and pharmacological properties of the voltage-gated potassium current of human pancreatic beta-cells. J Physiol. 2005;567:159–175. doi: 10.1113/jphysiol.2005.089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J, Zhou YP, Bugianesi RM, Dulski PM, Feng Y, Warren VA, Smith MM, Kohler MG, Garsky VM, Sanchez M, et al. Blockers of the delayed-rectifier potassium current in pancreatic beta-cells enhance glucose-dependent insulin secretion. Diabetes. 2006;55:1034–1042. doi: 10.2337/diabetes.55.04.06.db05-0788. [DOI] [PubMed] [Google Scholar]
- Hiriart M, Aguilar-Bryan L. Channel regulation of glucose sensing in the pancreatic beta-cell. Am J Physiol Endocrinol Metab. 2008;295:E1298–E1306. doi: 10.1152/ajpendo.90493.2008. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houamed KM, Sweet IR, Satin LS. BK channels mediate a novel ionic mechanism that regulates glucose-dependent electrical activity and insulin secretion in mouse pancreatic beta-cells. J Physiol. 2010;588:3511–3523. doi: 10.1113/jphysiol.2009.184341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DA, Philipson LH. Action potentials and insulin secretion: new insights into the role of Kv channels. Diabetes Obes Metab. 2007;9:89–98. doi: 10.1111/j.1463-1326.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammala CE, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 2007;6:229–235. doi: 10.1016/j.cmet.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DA, Mendez F, Thompson M, Torres J, Cochet O, Philipson LH. Calcium-activated and voltage-gated potassium channels of the pancreatic islet impart distinct and complementary roles during secretagogue induced electrical responses. J Physiol. 2010;588:3525–3537. doi: 10.1113/jphysiol.2010.190207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman K, Nguyen A, Tseng-Crank J, Dukes ID, Chandy G, Hustad CM, Copeland NG, Jenkins NA, Mohrenweiser H, Brandriff B, et al. Genomic organization, chromosomal localization, tissue distribution, and biophysical characterization of a novel mammalian Shaker-related voltage-gated potassium channel, Kv1.7. J Biol Chem. 1998;273:5851–5857. doi: 10.1074/jbc.273.10.5851. [DOI] [PubMed] [Google Scholar]
- Kashuba VI, Kvasha SM, Protopopov AI, Gizatullin RZ, Rynditch AV, Wahlestedt C, Wasserman WW, Zabarovsky ER. Initial isolation and analysis of the human Kv1.7 (KCNA7) gene, a member of the voltage-gated potassium channel gene family. Gene. 2001;268:115–122. doi: 10.1016/s0378-1119(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Katsuta H, Akashi T, Katsuta R, Nagaya M, Kim D, Arinobu Y, Hara M, Bonner-Weir S, Sharma AJ, Akashi K, et al. Single pancreatic beta cells co-express multiple islet hormone genes in mice. Diabetologia. 2010;53:128–138. doi: 10.1007/s00125-009-1570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinard TA, de VG, Sherman A, Satin LS. Modulation of the bursting properties of single mouse pancreatic beta-cells by artificial conductances. Biophys J. 1999;76:1423–1435. doi: 10.1016/S0006-3495(99)77303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Macdonald PE, Ha XF, Wang J, Smukler SR, Sun AM, Gaisano HY, Salapatek AM, Backx PH, Wheeler MB. Members of the Kv1 and Kv2 voltage-dependent K(+) channel families regulate insulin secretion. Mol Endocrinol. 2001;15:1423–1435. doi: 10.1210/mend.15.8.0685. [DOI] [PubMed] [Google Scholar]
- Muller H, Schweitzer N, Johren O, Dominiak P, Raasch W. Angiotensin II stimulates the reactivity of the pituitary-adrenal axis in leptin-resistant Zucker rats, thereby influencing the glucose utilization. Am J Physiol Endocrinol Metab. 2007;293:E802–E810. doi: 10.1152/ajpendo.00650.2006. [DOI] [PubMed] [Google Scholar]
- Pedersen MG. A biophysical model of electrical activity in human β-cells. Biophys J. 2010;99:3200–3207. doi: 10.1016/j.bpj.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51:S368–S376. doi: 10.2337/diabetes.51.2007.s368. [DOI] [PubMed] [Google Scholar]
- Remedi MS, Koster JC, Markova K, Seino S, Miki T, Patton BL, McDaniel ML, Nichols CG. Diet-induced glucose intolerance in mice with decreased beta-cell ATP-sensitive K+ channels. Diabetes. 2004;53:3159–3167. doi: 10.2337/diabetes.53.12.3159. [DOI] [PubMed] [Google Scholar]
- Remedi MS, Rocheleau JV, Tong A, Patton BL, McDaniel ML, Piston DW, Koster JC, Nichols CG. Hyperinsulinism in mice with heterozygous loss of K(ATP) channels. Diabetologia. 2006;49:2368–2378. doi: 10.1007/s00125-006-0367-4. [DOI] [PubMed] [Google Scholar]
- Rosati B, Marchetti P, Crociani O, Lecchi M, Lupi R, Arcangeli A, Olivotto M, Wanke E. Glucose- and arginine-induced insulin secretion by human pancreatic beta-cells: the role of HERG K(+) channels in firing and release. FASEB J. 2000;14:2601–2610. doi: 10.1096/fj.00-0077com. [DOI] [PubMed] [Google Scholar]
- Smith PA, Bokvist K, Arkhammar P, Berggren PO, Rorsman P. Delayed rectifying and calcium-activated K+ channels and their significance for action potential repolarization in mouse pancreatic beta-cells. J Gen Physiol. 1990;95:1041–1059. doi: 10.1085/jgp.95.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen P, Rinzel J, Sherman A. Why pancreatic islets burst but single beta cells do not. The heterogeneity hypothesis. Biophys J. 1993;64:1668–1680. doi: 10.1016/S0006-3495(93)81539-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Berger W. Higher incidence of severe hypoglycaemia leading to hospital admission in Type 2 diabetic patients treated with long-acting versus short-acting sulphonylureas. Diab Med. 1999;16:586–590. doi: 10.1046/j.1464-5491.1999.00110.x. [DOI] [PubMed] [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Yan L, Figueroa DJ, Austin CP, Liu Y, Bugianesi RM, Slaughter RS, Kaczorowski GJ, Kohler MG. Expression of voltage-gated potassium channels in human and rhesus pancreatic islets. Diabetes. 2004;53:597–607. doi: 10.2337/diabetes.53.3.597. [DOI] [PubMed] [Google Scholar]
- Zhang J, Pfaffendorf M, van Zwieten PA. Positive inotropic action of angiotensin II in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:658–663. doi: 10.1007/BF00166950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.