Abstract
The aim of this study was to determine adequate energy doses using specific parameters of LLLT to produce biostimulatory effects on human gingival fibroblast culture. Cells (3 × 104 cells/cm2) were seeded on 24-well acrylic plates using plain DMEM supplemented with 10% fetal bovine serum. After 48-hour incubation with 5% CO2 at 37°C, cells were irradiated with a InGaAsP diode laser prototype (LASERTable; 780 ± 3 nm; 40 mW) with energy doses of 0.5, 1.5, 3, 5, and 7 J/cm2. Cells were irradiated every 24 h totalizing 3 applications. Twenty-four hours after the last irradiation, cell metabolism was evaluated by the MTT assay and the two most effective doses (0.5 and 3 J/cm2) were selected to evaluate the cell number (trypan blue assay) and the cell migration capacity (wound healing assay; transwell migration assay). Data were analyzed by the Kruskal-Wallis and Mann-Whitney nonparametric tests with statistical significance of 5%. Irradiation of the fibroblasts with 0.5 and 3 J/cm2 resulted in significant increase in cell metabolism compared with the nonrradiated group (P < 0.05). Both energy doses promoted significant increase in the cell number as well as in cell migration (P < 0.05). These results demonstrate that, under the tested conditions, LLLT promoted biostimulation of fibroblasts in vitro.
1. Introduction
Tissue healing involves an intense activity of diverse cell types, such as epithelial and endothelial cells, as well as fibroblasts which play a key role in this process [1]. Fibroblasts secrete multiple growth factors during wound reepitelialization and participate actively in the formation of granulation tissue and the synthesis of a complex extracellular matrix after reepitelialization [1]. All these processes directly involve the proliferation and migration capacity to these cells [1]. The use of low-level laser therapy (LLLT) has been proposed to promote biostimulation of fibroblasts and accelerate the healing process [2].
Previous studies have evaluated the effect of LLLT on the proliferation and migration of human gingival fibroblasts as well as other cellular effects and responses, such as protein production and growth factor expression [2–6]. Nevertheless, there is a shortage of studies investigating irradiation parameters capable of promoting biostimulatory effects on fibroblasts in order to establish an ideal irradiation protocol for these cells [7]. Therefore, the aim of this study was to determine the most adequate energy doses using specific parameters of LLLT to produce biostimulatory effects on human gingival fibroblast cultures in an in vitro wound healing model.
2. Material and Methods
2.1. Gingival Fibroblast Cell Culture
All experiments were performed using human gingival fibroblast cell culture (continuous cell line; Ethics Committee 64/99-Piracicaba Dental School, UNICAMP, Brazil). The fibroblast cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), with 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L glutamine (Gibco, Grand Island, NY, USA) in an humidified incubator with 5% CO2 and 95% air at 37°C (Isotemp; Fisher Scientific, Pittsburgh, PA, USA) [8]. The cells were subcultured every 2 days in the incubator under the conditions described above until an adequate number of cells were obtained for the study. The cells (3×104 cells/cm²) were then seeded on sterile 24-well acrylic plates using plain DMEM supplemented with 10% FBS for 48 h.
2.2. LLLT on Fibroblast Culture
The LLLT device used in this study was a near infrared indium gallium arsenide phosphide (InGaAsP) diode laser prototype (LASERTable; 780 ± 3 nm wavelength, 0.04 W maximum power output), which was specifically designed to provide a uniform irradiation of each well (2 cm²) in which cultured cells are seeded [8, 9]. The power loss through the acrylic plate was calculated using a potentiometer (Coherent LM-2 VIS High-Sensitivity Optical Sensor, USA), which was placed inside the culture plate. After this measure, the power loss of the plate was determined as 5%. After that, the power of all diodes was checked and standardized. Therefore, a final power of 0.025 W reached the cultured cells. This standardization was performed as previously described in the literature [8, 9]. For the evaluation of cell metabolism, the radiation originated from the LASERTable was delivered on the base of each 24-well plate with energy doses of 0.5, 1.5, 3, 5, and 7 J/cm², and irradiation times of 40, 120, 240, 400, and 560 s, respectively. The laser light reached the cells on the bottom of each well with a final power of 0.025 W because of the loss of optical power in each well due to the interposition of the acrylic plate. The cells were irradiated every 24 h totalizing 3 applications during 3 consecutive days. The cells assigned to control groups received the same treatment as that of the experimental groups. The 24-well plates containing the control cells were maintained at the LASERTable for the same irradiation times used in the respective irradiated groups, though without activating the laser source (sham irradiation) [8, 9]. Twenty-four hours after the last irradiation (active or sham), the metabolic activity of the cells was evaluated using the MTT assay (described below). Based on cell metabolism results, the two most effective irradiation doses were selected to evaluate the cell number (trypan blue assay), cell migration capacity by using the wound healing assay (qualitative analysis) and the transwell migration assay (quantitative analysis), as described below.
2.3. Analysis of Cell Metabolism (MTT Assay)
Cell metabolism was evaluated using the methyltetrazolium (MTT) assay [8–10]. This method determines the activity of succinic dehydrogenase (SDH) enzyme, which is a measure of cellular (mitochondrial) respiration and can be considered as the metabolic rate of cells.
Each well with the fibroblasts received 900 μL of DMEM plus 100 μL of MTT solution (5 mg/mL sterile PBS). The cells were incubated at 37°C for 4 h. Thereafter, the culture medium (DMEM; Sigma Chemical Co., St. Louis, MO, USA) with the MTT solution were aspirated and replaced by 700 μL of acidified isopropanol solution (0.04 N HCl) in each well to dissolve the violet formazan crystals resulting from the cleavage of the MTT salt ring by the SDH enzyme present in the mitochondria of viable cells, producing a homogenous bluish solution. Three 100 μL aliquots of each well were transferred to a 96-well plate (Costar Corp., Cambridge, MA, USA). Cell metabolism was evaluated by spectrophotometry as being proportional to the absorbance measured at 570 nm wavelength with an ELISA plate reader (Thermo Plate, Nanshan District, Shenzhen, China) [8, 9]. The values obtained from the three aliquots were averaged to provide a single value. The absorbance was expressed in numerical values, which were subjected to statistical analysis to determine the effect of LLLT on the mitochondrial activity of the cells.
2.4. Viable Cell Counting (Trypan Blue Assay)
Trypan blue assay was used to evaluate the number of cells in the culture after LLLT application. This test provides a direct assessment of the total number of viable cells in the samples as the trypan blue dye can penetrate only porous, permeable membranes of lethally damaged (dead) cells, which is clearly detectable under optical microscopy [11]. The LLLT protocol was undertaken as previously described using energy doses of 0.5 and 3 J/cm². Cell counting was performed in the experimental and control groups 24 h after the last irradiation (active or sham). The DMEM in contact with the cells was aspirated and replaced by 0.12% trypsin (Invitrogen, Carlsbad, CA, USA), which remained in contact with the cells for 10 min to promote their detachment from the acrylic substrate. Then, 50 μL aliquots of this cell suspension were added to 50 μL of 0.04% trypan blue dye (Sigma Aldrich Corp., St. Louis, MO, USA), and the resulting solution was maintained at room temperature for 2 min so that the trypan blue dye could pass through the cytoplasmic membrane of the nonviable cells, changing their color into blue. Ten microliters of the solution were taken to a hemocytometer and examined with an inverted light microscope (Nikon Eclipse TS 100, Nikon Corporation, Tokyo, Japan) to determine the number of total cells and nonviable cells. The number of viable cells was calculated by deducting the number of nonviable cells from the number of total cells [8]. The number of cells obtained in the counting corresponded to n × 104 cells per milliliter of suspension.
2.5. Cell Migration
2.5.1. Wound Healing Assay
The wound healing assay was used because it is a classic method of evaluation in vitro tissue healing assays [12, 13]. After 48 h of cell culture, a sterile 5 mL pipette tip was used to make a straight scratch on the monolayer of cells attached to the acrylic substrate, simulating a wound. Formation of the in vitro wound was confirmed under an inverted microscope (TS 100, Nikon, Tokyo, Japan). The LLLT protocol was undertaken as previously described using energy doses of 0.5 and 3 J/cm². Twenty-four hours after the last irradiation, the cells were fixed in 1.5% glutaraldehyde for 1 h, stained with 0.1% violet crystal for 15 min, and washed twice with distilled water. Wound repopulation was assessed with a light microscope (Olympus BX51, Miami, FL, USA) equipped with a digital camera (Olympus C5060, Miami, FL, USA).
2.5.2. Transwell Migration Assay
The capacity of human gingival fibroblasts to migrate through a cell permeable membrane was assessed using 6.5 mm-diameter transwell chambers (Corning Costar, Cambridge, MA, USA) with polycarbonate membrane inserts (8 μm pore size) [14]. The chambers were placed in 24-well plates containing 1 mL of plain DMEM per well. The cells were seeded onto the upper compartment of the chamber (1.5 × 104 cells/cm²) and incubated at 37°C for 48 h. After this period, the LLLT protocol was undertaken as previously described using energy doses of 0.5 and 3 J/cm². Twenty-four hours after the last irradiation (active or sham), the cells that had migrated through the membrane to the lower compartment of the chamber were fixed in 1.5% glutaraldehyde for 1 h, incubated with 0.1% violet crystal dye for 15 min, and washed twice with distilled water. After the last wash, the stained cells were viewed under a light microscope (Olympus BX51, Miami, FL, USA) equipped with a digital camera (Olympus C5060, Miami, FL, USA) and photomicrographs from three randomly chosen fields were taken at ×10 magnification for counting the number of migrated cells using the image-analysis J 1.45S software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). Two samples of each group were evaluated and the experiment was performed in triplicate.
2.6. Analysis of Migrated Cells by Scanning Electron Microscopy (SEM)
Part of the specimens used in the transwell migration assay was also used for the analysis of the cells by SEM. Twenty-four hours after the last irradiation (active or sham), the culture medium was aspirated and the transwell inserts were fixed in 1 mL of 2.5% glutaraldehyde in PBS for 2 h. Then, the glutaraldehyde solution was aspirated and the cells adhered to the transwell inserts were washed with PBS and distilled water two consecutive times (5 min each) and then dehydrated in a series of increasing ethanol concentrations (30, 50 and 70%, one time for 30 min each; 95 and 100%, two times for 60 min each) and covered 3 times with 200 μL of 1,1,1,3,3,3-hexamethyldisilazane (HMDS; Sigma Aldrich Corp., St. Louis, USA) [8]. The transwell inserts were stored in a desiccator for 24 h, sputter-coated with gold, and the morphology of the surface-adhered cells was examined with a scanning electron microscope (JMS-T33A scanning microscope, JEOL, Tokyo, Japan).
2.7. Statistical Analysis
Data from MTT, Trypan blue and Transwell assay had a nonnormal distribution (Kolmogorov-Smirnov, P < 0.05) and were analyzed by the Kruskal-Wallis and Mann-Whitney nonparametric tests. A significance level of 5% was set for all analyses.
3. Results
3.1. Analysis of Cell Metabolism (MTT Assay)
Data from SDH production by human gingival fibroblast cultures (MTT assay) after LLLT, according to the energy dose are presented in Table 1.
Table 1.
Succinate dehydrogenase enzyme (SDH) production by human gingival fibroblasts detected by the MTT assay according to the energy dose used in the low-level laser therapy.
| Energy dose (J/cm2) | MTT (%) |
|---|---|
| 0 (control) | 100 (96–104) C∗ |
| 0.5 | 111 (110–113) B |
| 1.5 | 94 (92–97) D |
| 3 | 117 (113–119) A |
| 5 | 95 (81–108) CD |
| 7 | 92 (91–96) D |
Values expressed as medians of SDH production (P25–P75) (n = 12). ∗Same letters indicate no statistically significant difference (Mann-Whitney, P > 0.05).
Regarding the energy dose of 5 J/cm² no statistically significant difference between the irradiated group and the nonirradiated control group was observed (P > 0.05). Conversely, irradiation of the fibroblast cultures with doses of 0.5 J/cm² and 3 J/cm² resulted in 11% and 17% increases in cell metabolism, respectively, differing significantly from the control group (P < 0.05). The cells irradiated with 1.5 J/cm² and 7 J/cm² presented the lowest metabolic rate compared with the nonirradiated control group (6% and 8% decrease, resp., P < 0.05).
3.2. Viable Cell Counting (Trypan Blue Assay)
The number of viable cells (%) after LLLT application, according to the energy dose, is presented in Table 2.
Table 2.
Number of viable cells (%) detected by the trypan blue assay, according to the energy doses used in the low-level laser therapy.
| Energy dose (J/cm2) | Number of viable cells (%) |
|---|---|
| 0 (control) | 100 (95–104) B∗ |
| 0.5 | 133 (112–175) A |
| 3 | 168 (149–181) A |
Values expressed as medians of SDH production (P25–P75) (n = 8). ∗Same letters indicate no statistically significant difference (Mann-Whitney, P > 0.05).
Comparison among the energy doses revealed that irradiation of the human gingival fibroblast cultures with 0.5 J/cm² and 3 J/cm² increased the number of viable cells by 31% and 66%, respectively, differing significantly from the control (P < 0.05), but without statistically significant difference between each other (P > 0.05).
3.3. Fibroblast Migration
3.3.1. Wound Healing Assay
The analysis of the monolayer of human gingival fibroblasts after irradiation of the “in vitro wound” showed more intense cell migration, with consequent better coverage of the substrate (wound repopulation) (Figure 1).
Figure 1.
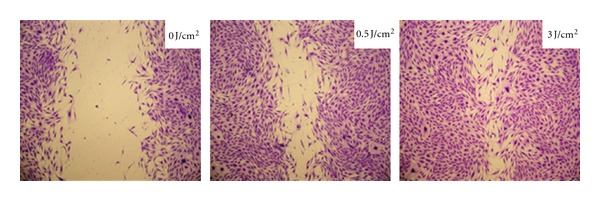
Photomicrographs showing human gingival fibroblast cultures seeded in 24-well plates after LLLT. The control group exhibits a large cell-free area on acrylic surface. The group irradiated with 0.5 J/cm² exhibits cell proliferation and migration, with consequent reduction of the “in vitro wound” size. The group irradiated with 3.0 J/cm² presented more intense cell proliferation and migration, resulting in almost complete closure of the “in vitro wound.”
3.3.2. Transwell Assay
Data from the transwell assay after LLLT, according to the energy dose are, presented in Table 3.
Table 3.
Cell migration (%) by the transwell assay, according to the energy dose used in the low-level laser therapy.
| Energy dose (J/cm2) | Cell migration (%) |
|---|---|
| 0 (control) | 100 (91–107) B∗ |
| 0.5 | 118 (109–123) A |
| 3 | 120 (116–122) A |
Values expressed as medians of SDH production (P25–P75) (n = 6). ∗Same letters indicate no statistically significant difference (Mann-Whitney, P > 0.05).
Comparison among the energy doses revealed that irradiation of the human gingival fibroblast cultures with 0.5 J/cm² and 3 J/cm² increased cell migration by 16% and 18%, respectively, differing significantly from the control (P < 0.05), but without statistically significant difference between each other (P > 0.05).
3.4. Analysis of Migrated Cells by Scanning Electron Microscopy (SEM)
The SEM analysis of the transwell inserts, which complemented the viable cell counting by the trypan blue assay, revealed that the fibroblasts were capable of migrating through the transwell membrane. The cells obtained from human gengiva did not change their morphology after been submitted to LLLT (Figure 2).
Figure 2.
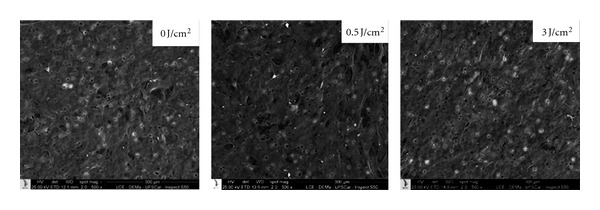
SEM micrograph showing cells with normal morphology that migrated through the transwell membrane. SEM ×500.
4. Discussion
Different LLLT modalities have been used for diverse treatments in the health fields. In Dentistry, LLLT has been widely investigated and indicated for accelerating the healing process, especially in the treatment of ulcerative oral mucosa lesions [15, 16].
Several in vitro studies have evaluated the effect of LLLT on healing [7, 17]. Nevertheless, current research involving irradiation of cell cultures has not yet established the irradiation patterns specific for the different cell lines. Establishing the ideal irradiation parameters and techniques is mandatory for the development of sequential studies that can determine the potential biostimulatory effect of LLLT on oral mucosa cells, such as keratinocytes and fibroblasts, which are directly involved in the local healing process.
In the present study, the metabolic activity of human gingival fibroblast cultures after LLLT with different energy doses was evaluated to determine the adequate doses to produce biostimulatory effects on these cells in vitro. The results for SDH production showed that the 0.5 and 3 J/cm² doses increased cell metabolism. Therefore, these two most effective irradiation doses were selected to evaluate the number of viable cells as well as the cell migration capacity. The increase of SDH production after irradiation of gingival fibroblasts has also been observed by Damante et al. [18], using a similar laser prototype to the one used in the present study. In the same way as in the present study, the SDH production results also served as guide for subsequent experiments that evaluated the expression of growth factors by cultured fibroblasts.
In the present study, a significant increase in the number of viable cells that presented normal morphological characteristics (SEM analysis) was observed after LLLT using doses of 0.5 and 3 J/cm2. These results confirm those of previous laboratory investigations in which LLLT with the same wavelength as that of the present study (780 nm) increased the proliferation of gingival fibroblasts [19, 20]. Kreisler et al. [2] also reported increase of fibroblast cell culture in vitro after direct and consecutive low level laser irradiations. The mechanism by which LLLT can promote biostimulation and induce proliferation of different cell types remains a controversial subject [20, 21]. Some authors [21, 22] claim that this mechanism is derived from light absorption by the enzyme cytochrome c oxidase in the cells, which participates in the cascade of oxidative respiration. Eells et al. [23] demonstrated the increase in the production of this enzyme after different LLLT application of cell cultures. It has also been suggested that the mechanism of cell proliferation induced by LLLT might be derived from the activation of singling pathways, such as the MAPK and PI3K/Akt pathways, which control both cell proliferation and regulation of gene expression [21, 24].
Fibroblast cell migration and proliferation are essential events for tissue healing and are directly related with its success [1, 3]. In the present study, the effect of LLLT on the capacity of gingival fibroblast migration, using two energy doses capable of increasing cell metabolism (0.5 and 3 J/cm²), was evaluated qualitatively, by the wound healing assay, and quantitatively, by the transwell migration assay. Both methodologies demonstrated that LLLT was able to increase the migration capacity of fibroblasts and the quantitative analysis of the results revealed no significant difference between the energy doses. These results are in accordance with those of previous investigations [7, 17], but studies using the transwell migration method to evaluate the LLLT on cell cultures are still scarce. This methodology is relevant because it measures the number of cells that can pass through the transwell membrane inserts, demonstrating their migration capacity after stimulation by LLLT.
Diverse mechanisms are involved in cell migration during tissue healing, including expression and secretion of growth factors [1]. Previous studies demonstrated that LLLT may cause positive effects on cells by increasing growth factor expression, which could be a form of action of specific laser parameters on cell migration [2, 25]. A recent study of our research group demonstrated that LLLT had a biostimulatory effect on epithelial cells in vitro by increasing their metabolic activity, number of viable cells and expression of growth factors [8]. In the present paper, the biostimulation of human gingival fibroblast cultures by LLLT with consequent increase in the number of viable cells and cell migration capacity demonstrates the efficacy of specific laser parameters and irradiation technique on the healing process. In addition, the obtained results are supportive to those of previous in vivo studies in which acceleration of the healing process was observed after LLLT [15, 16, 26], but the limitations of an in vitro experiment should be considered.
In conclusion, the findings of the present study demonstrated that the preset laser parameters in combination with the sequential irradiation technique caused biostimulation, proliferation, and migration of human gingival fibroblast cultures. These encouraging laboratory outcomes should guide forthcoming studies involving tissue irradiation with laser and its effects on in vivo tissue healing.
Acknowledgments
The authors acknowledge the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP (Grants: 2009/54722-1 and BP.DR: 2009/52326-1) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Grant: 301029/2010-1) for the financial support.
References
- 1.Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontology 2000. 2000;24(1):127–152. [PubMed] [Google Scholar]
- 2.Kreisler M, Christoffers AB, Al-Haj H, Willershausen B, D’Hoedt B. Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers in Surgery and Medicine. 2002;30(5):365–369. doi: 10.1002/lsm.10060. [DOI] [PubMed] [Google Scholar]
- 3.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. Low-level laser therapy for wound healing: mechanism and efficacy. Dermatologic Surgery. 2005;31(3):334–340. doi: 10.1111/j.1524-4725.2005.31086. [DOI] [PubMed] [Google Scholar]
- 4.Saygun I, Karacay S, Serdar M, Ural AU, Sencimen M, Kurtis B. Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers in Medical Science. 2008;23(2):211–215. doi: 10.1007/s10103-007-0477-3. [DOI] [PubMed] [Google Scholar]
- 5.Skopin MD, Molitor SC. Effects of near-infrared laser exposure in a cellular model of wound healing. Photodermatology Photoimmunology and Photomedicine. 2009;25(2):75–80. doi: 10.1111/j.1600-0781.2009.00406.x. [DOI] [PubMed] [Google Scholar]
- 6.Hakki SS, Bozkurt SB. Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers in Medical Science. 2012;27(2):325–331. doi: 10.1007/s10103-010-0879-5. [DOI] [PubMed] [Google Scholar]
- 7.Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomedicine and Laser Surgery. 2010;28(supplement 1):S3–S40. doi: 10.1089/pho.2010.2771. [DOI] [PubMed] [Google Scholar]
- 8.Basso FG, Oliveira CF, Kurachi C, Hebling J, Costa CA. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. doi: 10.1007/s10103-012-1057-8. Lasers in Medical Science. In press. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira CF, Basso FG, Lins EC, et al. In vitro effect of low-level laser on odontoblast-like cells. Laser Physics Letters. 2011;8(2):155–163. [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Wiegand C, Hipler U. Methods for the measurement of cell and tissue compatibility including tissue regeneration process. GMS Krankenhaushygiene Interdisziplinär. 2008;3(1):1863–5245. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoang AM, Oates TW, Cochran DL. In vitro wound healing responses to enamel matrix derivative. Journal of Periodontology. 2000;71(8):1270–1277. doi: 10.1902/jop.2000.71.8.1270. [DOI] [PubMed] [Google Scholar]
- 13.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 14.Cáceres M, Romero A, Copaja M, Díaz-Araya G, Martínez J, Smith PC. Simvastatin alters fibroblastic cell responses involved in tissue repair. Journal of Periodontal Research. 2011;46(4):456–463. doi: 10.1111/j.1600-0765.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- 15.Chor A, de Azevedo AM, Maiolino A, Nucci M. Successful treatment of oral lesions of chronic lichenoid graft-vs.-host disease by the addition of low-level laser therapy to systemic immunosuppression. European Journal of Haematology. 2004;72(3):222–224. doi: 10.1046/j.0902-4441.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 16.Abramoff MMF, Lopes NNF, Lopes LA, et al. Low-level laser therapy in the prevention and treatment of chemotherapy-induced oral mucositis in young patients. Photomedicine and Laser Surgery. 2008;26(4):393–400. doi: 10.1089/pho.2007.2144. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff LD, Bounkeo JM, Brannon WM, et al. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomedicine and Laser Surgery. 2004;22(3):241–247. doi: 10.1089/1549541041438623. [DOI] [PubMed] [Google Scholar]
- 18.Damante CA, De Micheli G, Miyagi SPH, Feist IS, Marques MM. Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts. Lasers in Medical Science. 2009;24(6):885–891. doi: 10.1007/s10103-008-0582-y. [DOI] [PubMed] [Google Scholar]
- 19.Almeida-Lopes L, Rigau J, Zângaro RA, Guidugli-Neto J, Jaeger MMM. Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers in Surgery and Medicine. 2001;29(2):179–184. doi: 10.1002/lsm.1107. [DOI] [PubMed] [Google Scholar]
- 20.AlGhamdi KM, Kumar A, Moussa NA. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers in Medical Science. 2011;27(1):237–249. doi: 10.1007/s10103-011-0885-2. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. Journal of Biomedical Science. 2009;16, article 4 doi: 10.1186/1423-0127-16-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. Journal of Photochemistry and Photobiology B. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Eells JT, Henry MM, Summerfelt P, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Xing D, Gao X, Wu S. Low-power laser irradiation promotes cell proliferation by activating PI3K/Akt pathway. Journal of Cellular Physiology. 2009;219(3):553–562. doi: 10.1002/jcp.21697. [DOI] [PubMed] [Google Scholar]
- 25.Azevedo LH, De Paula Eduardo F, Moreira MS, De Paula Eduardo C, Marques MM. Influence of different power densities of LILT on cultured human fibroblast growth: a pilot study. Lasers in Medical Science. 2006;21(2):86–89. doi: 10.1007/s10103-006-0379-9. [DOI] [PubMed] [Google Scholar]
- 26.Lagan KM, Clements BA, McDonough S, Baxter GA. Low intensity laser therapy (830 nm) in the management of minor postsurgical wounds: a controlled clinical study. Lasers in Surgery and Medicine. 2001;28(1):27–32. doi: 10.1002/1096-9101(2001)28:1<27::AID-LSM1013>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]


