Figure 7.
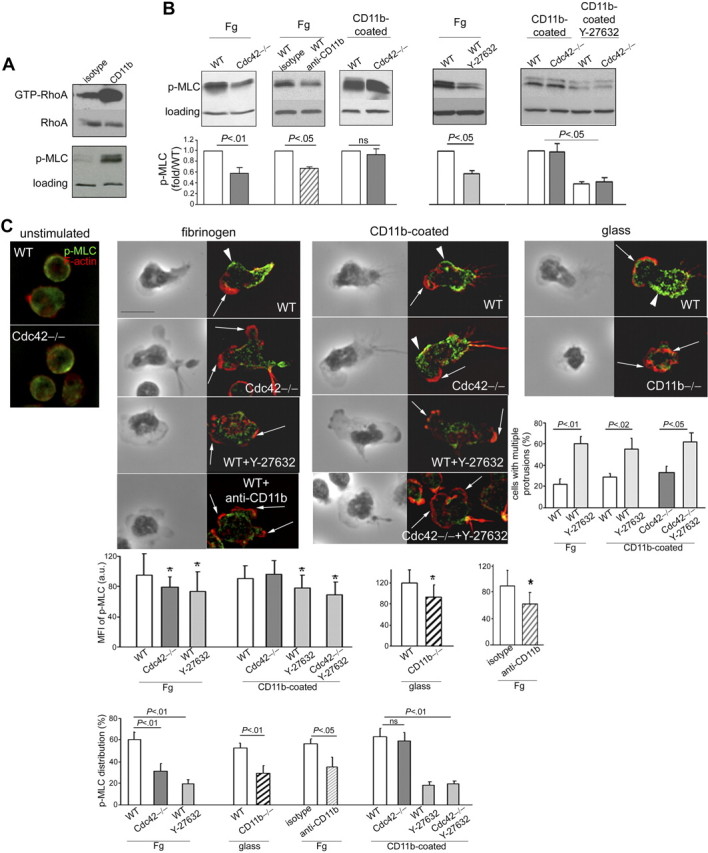
Cdc42 suppresses actin through CD11b-induced p-MLC signaling. (A) WT cells were crosslinked with CD11b or isotype controls for 20 minutes and were analyzed for RhoA activity using the pulldown assay with Rhotekine beads. Total RhoA of cell lysates is used as loading control. The cells were also analyzed for phosphorylated MLC at Ser19 and total p38MAPK as loading control (one blot representative of 3 independent experiments). (B) WT and Cdc42−/− neutrophils with or without Y-27632 treatment were stimulated with fMLP and seeded on fibrinogen or on an anti-CD11b–coated plate for 10 minutes. In addition, WT cells treated with functional anti-CD11b blocking antibody were stimulated with fMLP and on Fg-coated slides. The cells were lysed on plate and analyzed for p-MLC. Histograms represent densitometry analysis compared with WT after normalization to loading (mean ± SD; n = 3 independent experiments). Of note, the experiments with and without Y-27632 were performed independently such that the absolute level of p-MLC is not comparable. (C) Cells, treated or not with Y-27632, were stimulated or not on fMLP and on Fg or glass or anti-CD11b–coated slides for 10 minutes. The cells were stained with anti-p-MLC (in green) and rhodamine-phalloidin (in red). In addition, WT cells treated with anti-CD11b or isotype were plated on fibrinogen and analyzed for F-actin and p-MLC distribution. These later experiments were performed separately. WT cells treated with isotype are not different from nontreated WT cells, and only one image of WT control is shown. The black-and-white pictures represent the phase-contrast images. The images colored in red and green are one x-y view of the z-series analyzed by deconvolution in Volocity. Percentage of cells with p-MLC staining along the lateral sides of the cells is enumerated (mean ± SD; n = 3 independent experiments). The average intensity of fluorescence per area of p-MLC along the sides of the cells was analyzed in Openlab, and the data are shown as histogram (mean ± SD of n = 30-50 cells, from at least 2 independent experiments). Percentage of cells with more than one protrusion (mean ± SD; n = 3 independent experiments); at least 30 cells per experiments were analyzed. *Results that are significantly different from WT (P < .05). Arrows point to actin protrusions. Arrowheads point to p-MLC lateral distribution. Scale bar represents 5 μm. The slides were mounted with Slowfade Gold antifade reagent. Z series of fluorescence images were captured at room temperature using a Leica DMI6000 fluorescence microscope at 63×/1.3 NA objective, with ORCA-ER C4742-95 camera driven by Openlab software and analyzed by deconvolution with Volocity software.
