Abstract
Objective
To compare the different mortality risks between delivery and expectant management in women with gestational diabetes mellitus (GDM).
Study Design
This is a retrospective cohort study that included singleton pregnancies of women diagnosed with GDM delivering at 36-42 weeks gestational age (GA) in California from 1997-2006. A composite mortality rate was developed to estimate the risk of expectant management at each GA incorporating the stillbirth risk during the week of continuing pregnancy plus the infant mortality risk at the GA one week hence.
Results
In women with GDM, the risk of expectant management is lower than the risk of delivery at 36 weeks, (17.4 vs. 19.3 per 10,000), but at 39 weeks, the risk of expectant management exceeds that of delivery (RR 1.8, 95% CI: 1.2 – 2.6).
Conclusion
In women with GDM, infant mortality rates at 39 weeks are lower than the overall mortality risk of expectant management for one week absolute risks of stillbirth and infant death are low.
Keywords: expectant management, gestational diabetes, infant mortality, stillbirth
INTRODUCTION
Gestational diabetes mellitus (GDM) affects 5-7% of pregnancies in the United States, and in 2005 was diagnosed in 5.3% of all California pregnancies.1 This condition is characterized by newly recognized hyperglycemia occurring in pregnancy, and is associated with an elevated risk of macrosomia, shoulder dystocia, hypoglycemia, cesarean delivery, and future maternal Type 2 diabetes, among other maternal and neonatal morbidities.2,3 When O’Sullivan and colleagues first recognized this condition in the 1960s, they observed an increased incidence of stillbirth among women with gestational diabetes who were undiagnosed or suboptimally treated.4 Subsequent early studies and those in the developing world have also demonstrated an increased risk of stillbirth associated with GDM.5,6 As screening, diagnosis and treatment of GDM have become more widespread, however, the association between GDM and perinatal mortality has become less clear. More recent studies in Italy, Israel, and Sweden report a lack of association.7-9 Due to this conflicting evidence, there is continuing controversy about the optimal timing of delivery for women with GDM.3
When considering the optimal time for delivery to improve perinatal outcomes, the risk of stillbirth must be weighed against the risk of neonatal and infant morbidity and mortality. While gestational diabetes has been shown to be associated with macrosomia and neonatal hypoglycemia, this has not consistently been shown to contribute to higher neonatal and infant death rates.8 Although some studies find that macrosomia is independently associated with increased mortality2, other data suggest that neonates born large-for-gestational age actually have a lower post-neonatal death rate when compared with neonates who were average-for-gestational age or small-for gestational age.10,11 Thus, examining only short-term morbidities may not fully account for the true mortality risk associated with GDM.
We have previously demonstrated in a large dataset of California births that using a novel composite mortality calculation to estimate the risk of expectant management at term can be useful in quantifying the risks faced by a pregnant woman and her care provider when trying to determine the optimal time of delivery.12 In this study, we apply this novel methodology to the specific subpopulation of women with GDM to determine their optimal delivery time from a mortality perspective.
MATERIALS AND METHODS
We designed a retrospective cohort study of singleton births to women diagnosed with GDM identified through the California Vital Statistics Birth Certificate Data linked with the California Patient Discharge Data as well as Vital Statistics Death Certificate Data and Vital Statistics Fetal Death File between 1997 and 2006.13 Linkage of data was performed by the California Office of Statewide Health Planning and Development (OSHPD) Healthcare Information Resource Center under the State of California Health and Human Services Agency (HHS). The resultant linked datasets include maternal antepartum and postpartum hospital records for the nine months prior to delivery and one year post delivery as well as birth records and all infant admission and readmissions occurring within the first year of life. Linkage for the mother/baby pair was achieved using the “record linkage number”, a unique alphanumeric encrypted code unique to the mother and the baby. Institutional Review Board (IRB) approval was obtained from the Committee on Human Research at the University of California, San Francisco, the IRB at Oregon Health & Science University, and the California OSHPD and the Committee for the Protection of Human Subjects (CPHS). The reporting of births and deaths in California is nearly 100% complete and the HHS performs rigorous statistical quality checks. Since the linked dataset did not contain potential patient privacy and identification information, informed consent was exempted. Since the dataset did not contain potential patient identification information, informed consent was exempted.
Women with a diagnosis of GDM were identified using the International Statistical Classification of Diseases and Related Health Problems, revision 9 (ICD-9) codes. ICD-9 codes used for the identification of GDM include: 648.8, 648.80, 648.81, 648.82, 648.83, 648.84. We excluded women with a diagnosis of pre-pregnancy (Type 1 or Type 2) diabetes mellitus using ICD-9 codes: 648.0, 648.01, 648.02, 648.03, and 648.04. These ICD-9 codes were taken from maternal medical records but do not specify how or when the diagnosis of GDM was made. The California Diabetes and Pregancy Program, administered by the state Department of Health oversees the diagnosis and management of most pregnant women with diabetes in the state. During the time frame that these patients were cared for, they recommended at a minimum universal screening of GDM with a 1 hour 50 gram glucose challenge test, followed by a 3 hour 100 gram glucose tolerance test if the screening value is greater than 140mg/dl. Other exclusion criteria were multiple gestations and births with congenital anomalies as determined by diagnosis codes on the birth certificate and the infant’s medical record (ICD-9 codes Q00-Q99).
In this database, length of gestation in days was calculated by subtracting the date of last normal menstrual period (LMP) from the date of birth of the linked infant. If a negative value was obtained, one year was subtracted from the date of LMP and the interval was recomputed. Gestational age was then converted to into weeks and treated as an ordered categorical variable. If date of LMP was missing or was nonsensical, the mother/infant pair was excluded from analysis. For this study, we included births between 36 and 42 completed weeks; 36 weeks GA included births ranging from 36 weeks and 0 days to 36 weeks and 6 days; and 42 weeks GA included births from 42 weeks 0 days to 42 weeks 6 days.
The purpose of this study was to compare the mortality risks (including both stillbirth and infant death) associated with delivery at a given week of gestation, as compared to expectant management (i,e. continuing the pregnancy for another week and then delivering one week later). More specifically, the mortality risk of delivery at a given week was defined as the rate among those neonates born at that week of gestation. The mortality risk of a week of expectant management was defined as the risk of stillbirth over that week plus the mortality risk experienced by infants born in the subsequent week of gestation. This comparison was made at varying gestational ages among women with GDM. Neonatal death (death within 28 days of birth) has typically been the metric included in estimates of perinatal death rates, but recent data demonstrate that term infants who die within the first year of life are more likely to do so in the post-neonatal (age 29 – 365 days of life) period than in the neonatal period.11 Infant mortality has also been shown to vary with GA at term and share many of the same risk factors as stillbirth.14,15 Thus, infant death was examined because of its significant magnitude and persistent association with GA at delivery.
The incidence of stillbirth at a given gestational age was calculated as the number of stillbirths at that gestational age per 10,000 ongoing pregnancies. Infant mortality at each gestational age was calculated as the number of infants born at this gestational age who die within one year of life per 10,000 live births at that same GA. The composite risk of expectant management for one week represents the sum of the probabilities of stillbirth during a given week of gestation plus the probability of infant death when birth occurs the following week. This composite risk of expectant management was then compared to the risk of infant death in the prior week of gestation to compare risk of delivery versus expectant management.
Our calculations rely on the following assumptions:
The risk of infant death has a uniform distribution throughout the week of gestation.
When estimating the risk of delivering at a particular GA, the fetus is not at risk for stillbirth beyond that GA, therefore their mortality risk in that week is equal only to the risk of infant death.
The composite risk associated with expectant management is the sum of the risk of stillbirth during the week of gestation plus the risk of infant death in the following week of gestation.
Statistical calculations were performed with Excel and Stata (version 12, StataCorp, College Station, TX), including simple proportions, relative risks, and 95% confidence intervals (CI). We assumed that the binomial probability distributions of both mortality risks approximated the normal distribution and derived the confidence interval of the composite risk using the sum of the variances of the estimates of infant death and stillbirth. The chi-squared test was performed to compare proportions, two-tailed t-tests were performed to compare means, and a p value of <0.05 was considered statistically significant.
RESULTS
Our dataset included 4,190,953 non-anomalous deliveries from gestational ages of 36 to 42 weeks, including 193,028 deliveries to women with GDM. Women with GDM were more likely to be older, Latina or Asian rather than White or African-American, and carry a diagnosis of chronic hypertension. There was a slight decrease in gestational age at delivery (38.8 vs 39.1 weeks) and a slight increase in birthweight at delivery (3475g vs 3415g). (Table 1)
Table 1.
Demographic and health characteristics of women without Type 1 or Type 2 diabetes mellitus giving birth in California between 1997 and 2006, with and without gestational diabetes mellitus (GDM).
| Characteristic | Women with GDM (N=193,028) | Women without GDM (N=3,997,925) | p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Maternal Age (years: mean, SD) | 31.4 | 5.8 | 27.7 | 6.2 | <0.001 |
| Ethnicity | <0.001 | ||||
| White | 52,498 | 27.2 | 1,504,878 | 37.7 | |
| African-American | 7,548 | 3.9 | 217,883 | 5.5 | |
| Latino | 94,682 | 49.1 | 1,766,579 | 44.2 | |
| Asian | 35,295 | 18.3 | 443,980 | 11.1 | |
| Other | 2,877 | 1.5 | 59,816 | 1.5 | |
| Preeclampsia | 7,827 | 4.1 | 84,588 | 2.1 | <0.001 |
| Chronic Hypertension | 4,574 | 2.4 | 22,325 | 0.6 | <0.001 |
| GA at delivery (weeks: mean, SD) | 38.8 | 1.4 | 39.1 | 1.4 | <0.001 |
| Birthweight (grams: mean, SD) | 3,475 | 541 | 3,415 | 475 | <0.001 |
| Education (≥12 years) | 71,014 | 43.5 | 1,496,734 | 42.6 | <0.001 |
The risk of stillbirth increased continuously with gestational age in women with and without GDM rising to its highest level at 42 weeks of gestation. The risk of neonatal and infant death displayed a U-shaped curve, highest at 36 weeks and decreasing to a nadir at 39-40 weeks in both the women with and without GDM before increasing again at 41 and 42 weeks. (Table 2, Figure 1).
Table 2.
Mortality Rates in Women With and Without Gestational Diabetes Mellitus (GDM)
| Gestational Diabetes | No Gestational or Pre-gestational Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| GA | Deliveries | Stillbirth/10,000 ongoing pregnancies (95% CI) | Neonatal Death/10,000 live births (95% CI) | Infant Death/10,000 live births (95% CI) | Deliveries | Stillbirth/10,000 ongoing pregnancies (95% CI) | Neonatal Death/10,000 live births (95% CI) | Infant Death/10,000 live births (95% CI) |
| 36 | 10445 | 3.3 (2.6 - 4.2) | 10.6 (5.3 - 19.0) | 19.3 (11.8 - 29.8) | 155597 | 2.1 (2.0 - 2.3) | 9.1 (7.7 - 10.8) | 22.9 (20.6 - 25.4) |
| 37 | 22,157 | 4.1 (3.2 - 5.1) | 6.8 (3.8 - 11.2) | 14.0 (9.5 - 19.9) | 340,239 | 2.2 (2.1 - 2.4) | 6.1 (5.3 - 7.0) | 18.4 (17.0 - 19.9) |
| 38 | 44,487 | 4.2 (3.2 - 5.3) | 3.6 (2.1 - 5.9) | 10.6 (7.8 - 14.1) | 736,413 | 2.9 (2.7 - 3.1) | 3.9 (3.5 - 4.4) | 13.3 (12.5 - 14.2) |
| 39 | 56,085 | 5.7 (4.4 - 7.2) | 3.4 (2.0 - 5.3) | 8.7 (6.5 - 13.2) | 1,105,279 | 3.6 (3.4 - 3.9) | 2.8 (2.5 - 3.1) | 10.7 (10.1 - 11.4) |
| 40 | 37,819 | 5.7 (3.9 - 7.9) | 2.6 (1.3 - 4.9) | 9.5 (6.7 - 13.2) | 981,106 | 4.4 (4.1 - 4.7) | 3.4 (3.1 - 3.8) | 11.6 (10.9 - 12.3) |
| 41 | 15,739 | 8.6 (5.2 - 13.5) | 3.2 (1.0 - 7.4) | 11.5 (6.8 - 18.1) | 510,292 | 6.4 (5.8 - 7.0) | 3.6 (3.1 - 4.2) | 12.8 (11.9 - 13.9) |
| 42 | 6,296 | 9.5 (3.5 - 20.7) | 6.4 (1.7 - 16.3) | 9.5 (3.5 - 20.8) | 168,999 | 11.5 (10.0 - 13.3) | 4.7 (3.7 - 5.8) | 14.0 (12.3 - 15.9) |
| Total | 193,028 | 17.1 (15.3 – 19.1) per 10,000 deliveries | 4.2 (3.3 – 5.2) per 10,000 live births | 10.7 per 10,000 live births (9.3 – 12.3) | 3,997,925 | 12.7 (12.4 – 13.1) per 10,000 deliveries | 3.8 (3.7 – 4.1) per 10,000 live births | 14.0 (12.3 - 15.9) per 10,000 live births |
Figure 1. Infant Death and Stillbirth Rates in Women With and Without Gestational Diabetes Mellitus (GDM).
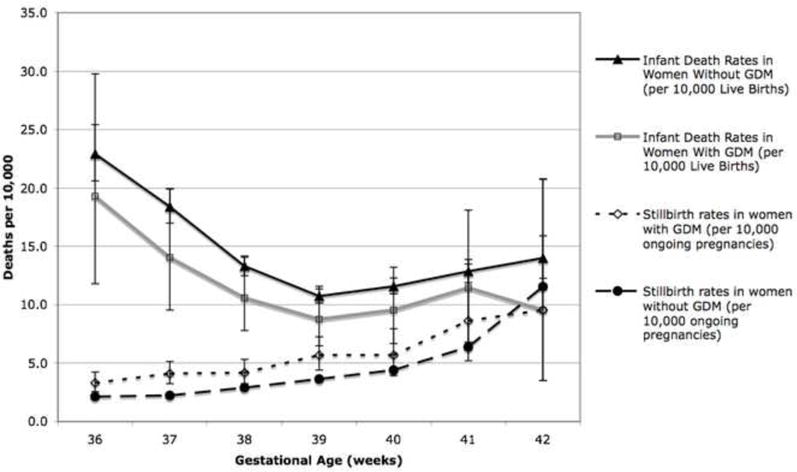
This figure graphically compares the gestational-age specific rates of stillbirth and infant death in women with and without gestational diabetes mellitus (GDM).
The overall risk of stillbirth from 36-42 weeks was higher in women with GDM when compared with women without diabetes (17.1 vs. 12.7 per 10,000 deliveries, RR 1.34 (95% CI 1.2 – 1.5). Stillbirth rates were also examined at each gestational age, and from 36 to 39 weeks, women with GDM had a statistically significant elevated relative risk of stillbirth compared with women without GDM, ranging from RR 1.45 (95% CI 1.1 – 1.9) at 38 weeks to RR 1.84 (95% CI 1.5 – 2.3) at 37 weeks. Although the risk was also higher for women with GDM at 40 and 41 weeks, this did not reach statistical significance (Table 3). At 42 weeks the point estimate of stillbirth was lower for women with GDM compared with women without, although due to the low numbers of women with GDM at this gestational age the confidence intervals were very wide and this result was not statistically significant (9.5 per 10,000 ongoing pregnancies, (95% CI: 3.5 – 20.7 per 10,000) in women with GDM vs. 11.5 per 10,000 ongoing pregnancies, (95% CI 10.0 – 13.3 per 10,000) in women without GDM).
Table 3.
Relative Risks of Stillbirth and Infant Death Comparing Women with and without GDM
| Gestational Age | Relative Risk of Stillbirth (95% CI) | Relative Risk of Infant Death (95% CI) |
|---|---|---|
| 36 | 1.57 (1.2-2.0) | 0.84 (0.54 - 1.32) |
| 37 | 1.84 (1.5 - 2.3) | 0.76 (0.53 - 1.1) |
| 38 | 1.45 (1.1 - 1.9) | 0.80 (0.59 - 1.06) |
| 39 | 1.56 (1.2 - 2.0) | 0.82 (0.61 - 1.08) |
| 40 | 1.29 (0.92 - 1.8) | 0.82 (0.59 - 1.14) |
| 41 | 1.35 (0.85 - 2.13) | 0.89 (0.56 - 1.4) |
| 42 | 0.83 (0.37 - 1.9) | 0.68 (0.3 - 1.5) |
| Overall | 1.34 (1.2 – 1.5) | 0.83 (0.72 – 0.95) |
There was no statistically significant difference in the risk of infant death when stratified by gestational age, (Table 3) although the total risk of infant mortality at gestational ages 36-42 weeks was lower for babies born to women with GDM compared to those born to women without GDM (10.7 vs. 12.9 per 10,000 live births, RR 0.83 (95% CI 0.72 – 0.95)).
When the risk of planned delivery (as quantified by the risk of infant death at a given gestational age) is compared with the risk of expectant management for one week (calculated as the sum of the risk of stillbirth at that given gestational age plus the risk of infant death in those born the following week) in women with GDM, the risk of delivery is higher than expectant management at 36 weeks. The risks of expectant management and delivery were similar at 37 weeks; however, the risk of expectant managed exceeded that of delivery at 38 weeks and beyond (Figure 2). This risk difference is statistically significant at 39 and 40 weeks (RR 1.8 at 39 and 40 weeks, p<0.05). The absolute risk difference can also be calculated and the reciprocal of that risk difference is the “number needed to deliver” (analogous to the number needed to treat): the number of pregnant women who would have to be delivered at 39 or 40 weeks to prevent one excess death. At 39 weeks this would be 1518 women with GDM, while at 40 weeks it would be 1311. (Table 4)
Figure 2. The Mortality Risk of Expectant Management Compared with Delivery in Women with Gestational Diabetes Mellitus (GDM).
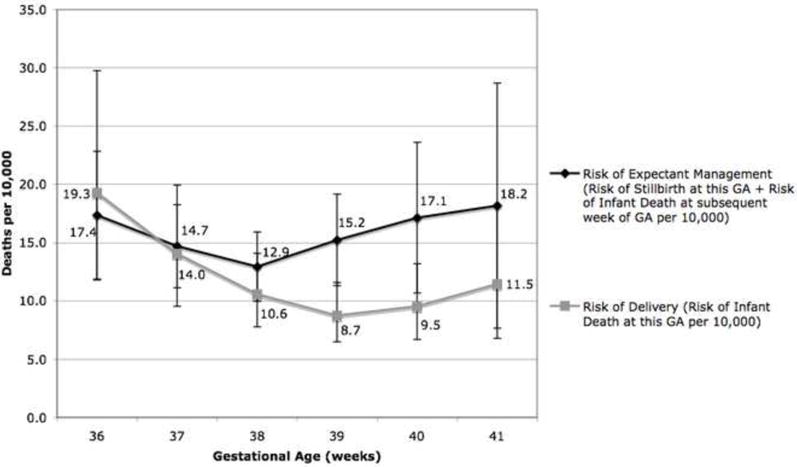
This figure compares the risk of expectant management for one week (calculated as the risk of stillbirth this week plus the risk of infant death at the subsequent week) compared with the risk of delivery (calculated as the risk of infant death at that gestational week) at each gestational age week from 36-41 weeks.
Table 4.
Mortality Risk of Delivery compared with Expectant Management in Women with Gestational Diabetes Mellitus (GDM)
| GA | Mortality Risk of Delivery (Risk of Infant Death per 10,000 Live Births at this GA) (95% CI) | Mortality of Expectant Management per 10,000 (Risk of Stillbirth at this GA + Risk of Infant Death at GA + 1) (95% CI) | Relative Risk of Expectant Management compared with Delivery (95% CI) | Absolute Risk Difference between Delivery and Expectant Management per 10,000 | Number Needed to Deliver at this GA to Prevent Single Excess Death |
|---|---|---|---|---|---|
| 36 | 19.3 (11.8 - 29.8) | 17.4 (11.9 – 22.8) | 0.89 (0.52 - 1.5) | -2.127 | |
| 37 | 14.0 (9.5 - 19.9) | 14.7 (11.1 – 18.2) | 1.0 (0.68 - 1.6) | 0.567 | |
| 38 | 10.6 (7.8 - 14.1) | 12.9 (10.0 – 15.9) | 1.2 (0.84 - 1.8) | 2.255 | 4435 |
| 39 | 8.7 (6.5 - 13.2) | 15.2 (11.3 – 19.1) | 1.8 (1.2 - 2.6)* | 6.588 | 1518 |
| 40 | 9.5 (6.7 - 13.2) | 17.1 (10.7 – 23.6) | 1.8 (1.1 - 3.0)* | 7.626 | 1311 |
| 41 | 11.5 (6.8 - 18.1) | 18.2 (7.6 – 28.7) | 1.5 (0.7 - 3.2) | 6.019 | 1661 |
p<0.05 using chi-square test
COMMENT
Gestational diabetes mellitus is a condition specific to pregnancy with known short- and long-term risks to mother and fetus. In this analysis, contrary to other recent studies, we showed that women with gestational diabetes were more likely than women without diabetes to experience a stillbirth after 35 weeks. This increased risk persisted at all gestational ages except at 42 weeks, likely because few women with GDM receiving prenatal care in California remained undelivered at 42 weeks gestational age. This study is the first to examine the incidence rate of stillbirth by gestational age in women diagnosed with GDM, and such stratification by GA may explain the difference in our findings from other recent studies. Since many women with GDM are delivered by 39 weeks, the overall magnitude of difference in the incidence rate of stillbirth between women with and without GDM may be diminished or even reversed because of prevention of stillbirths that are overall more likely to occur at 40 and 41 weeks gestation. Another important difference between this study and others in the literature with different findings is the baseline characteristics of the study population. Due to the rarity of the adverse events of stillbirth and infant death, it is difficult to design and power a study to show a difference compared with non-diabetic controls or with women receiving treatment compared with those poorly controlled. Our findings could be explained by the increased prevalence of GDM in our population as well as the possibility of more severe or undertreated disease. The prevalence of GDM is much higher our dataset (4.6%) than in Sweden or Italy, (0.8% and 0.9%, respectively).8,9 Also, the Israeli study included only women with diet controlled GDM while this study did not differentiate between those who were only diet controlled and those who required medical therapy; thus, it is likely that our study population could have more severe disease, representing a population at higher risk of stillbirth.7
Although we know that infants born to mothers with gestational diabetes are more likely to be macrosomic, to experience shoulder dystocia, and to have short-term metabolic derangements such as hypoglycemia, hyperbilirubinemia, and polycythemia, our analysis does not demonstrate that these morbidities contribute to an excess risk of infant or neonatal mortality. In fact, in this analysis, it appears that these infants overall are at a lower risk of infant death when compared with babies born to women without GDM. An explanation for this finding may be that more women with GDM were delivered by 40 weeks such that there were fewer babies born to mothers with GDM who were born at 41 and 42 weeks, when infant mortality rates are higher.14,16 It may also be that because the infants born to mothers with known GDM are at higher risk of the short term morbidities listed above that these babies are more likely to undergo more screening and treatment compared to the general population that may experience unexpected neonatal morbidities. However, this analysis was restricted to mortality comparisons only and thus can only hypothesize about the effects on neonatal morbidity seen in these populations.
We cannot exclude the fact that confounding may play a role in the elevated risk estimates for stillbirth seen in the women with GDM. From our dataset, we are only able to examine maternal age and race, which have been previously demonstrated to be associated with GDM as well as increased stillbirth risk.3, 14, 17 As expected, women with GDM are more likely to be older and of Latino or Asian descent. Also as expected, the highest rate of stillbirth was seen in the African-American population, which is less likely to have GDM compared with the other ethnic groups. (data not shown). Due to the limitations of the dataset and the rarity of the outcomes, we are unable to quantify the magnitude of these potential confounders, which should be a focus of future research.
There is substantial controversy in the literature and in clinical practice regarding the optimal time to deliver a woman with GDM to minimize the risks to her fetus. The American Diabetes Association recommends delivery at 38 weeks while the American College of Obstetricians and Gynecologists does not recommend routine delivery before 40 weeks.3,18 Multiple observational studies and a single randomized controlled trial did show decreased macrosomia and shoulder dystocia with delivery at 38 weeks, but none of these studies were powered to examine stillbirth or infant death.19,20 There has been increasing concern about the excess morbidities seen in neonates delivered electively before 39 weeks,21,22 and these results have been extrapolated to include women with GDM. The limitation of the studies that focus on neonatal morbidities by GA at delivery is that they do not take into consideration the risks faced by the fetus while still in utero. However, at least one recent study has suggested that the policy of limiting pre-39 week non-indicated labor induction was associated with an increased rate of stillbirth in the 37th and 38th week of gestation.23 Our methodology, which compares expectant management with planned delivery is useful as it more closely approximates the decision faced by a woman with GDM and her provider, in simultaneously weighing the various risks to the baby whether in utero or after birth. Another strength of our study is the heterogeneous study population in California, which is racially/ethnically and socioeconomically diverse; our study findings can be more validly applied to a broad range of populations.
When we examined the population of women with GDM, the risk of expectant management carried a higher risk of mortality than the risk of delivery at 39 and 40 weeks. The absolute risk between expectant management and delivery is low, however, and based on these figures, the “number needed to deliver” to prevent one excess death at 39 or 40 weeks of gestation is 1500 and 1300, respectively. Although our data do show a potential mortality benefit at 38 weeks, this difference is not statistically significant, and the “number needed to deliver” at this gestational age is much higher, 4435. The widespread current obstetrical practice of inducing labor at 39-40 weeks for women with gestational diabetes is designed to lower the risk of macrosomia and shoulder dystocia along with decreasing stillbirth. Our analysis would suggest that 39 weeks may be the most appropriate GA at which to plan delivery in order to decrease infant mortality.
While the current study is novel in its examination of both stillbirth and infant death risks, it is not without limitations. One of our study limitations is that we do not have access to the medical records of these women and thus cannot comment on the degree to which poor gylcemic control due to late diagnosis or suboptimal treatment contributes to these risk estimates, or the extent to which other comorbidities are present and may play a role. As previous research has demonstrated that perinatal mortality rates decrease with improved glycemic control, perhaps the optimal time for delivery for women with poor glycemic control is actually earlier, while those with excellent control could be managed expectantly beyond 39 weeks of gestation.24, 25 We also cannot rule out significant selection bias where the women who had more severe GDM were also those most likely to have early iatrogenic deliveries, avoiding stillbirth as an outcome but inflating the infant death rate due to prematurity. This may play a role in the elevated infant death risk seen in the population of infants delivered at 36 weeks who may have had more severe co-morbidities that necessitated a late preterm delivery. We note, however, that the infant death rates at all gestational ages are higher in the non-diabetic group compared with the gestational diabetics and that the stillbirth rate increases with gestational age in both groups. This suggests that early delivery does not account for a substantial decrease in the stillbirth rate and that had these women not been delivered earlier, the difference in mortality at later gestational ages might be even more pronounced.
Another limitation is that our dataset determines gestational age by LMP alone. Studies show that pregnancies that are dated based on LMP alone rather than clinical judgment or ultrasound have less accurate gestational age assignations, and these pregnancies are more likely to be classified as “post-term.”26-29 Women with gestational diabetes are more likely to be obese and to be of lower socioeconomic status, factors associated with incorrectly recalled LMPs and more specifically, of cycles longer than 28 days.30 This bias, while more prevalent in the GDM group compared with the non-GDM group, should otherwise be distributed evenly among the stillbirth and infant death populations. This misclassification would bias the result towards the null since many “term” infants (at 37-40 wks) would be classified instead as “postterm” (41 and 42 wks) making the pregnancies at these different gestational ages seem more similar than they actually are. Thus, if such misclassification was occurring, our threshold designation of the gestational age at which the GDM patients were having higher risk of the combined fetal and infant death outcome would only be earlier.
Our study suggests that there exists a mortality benefit in delivering women with gestational diabetes at 39 weeks instead of continuing with expectant management. We are cautious when making clinical recommendations from this type of observational data alone, and we acknowledge the absence of neonatal and maternal morbidity in these calculations. We cannot comment on the impact on short-term neonatal outcomes, cesarean delivery rate, maternal complications, and cost to the health care system that a policy of inducing women with GDM at 39 weeks compared with 40 weeks would incur and further research should explore these potential repercussions. Because the absolute risks of stillbirth and infant death are so low, an increase in short-term neonatal morbidities such as NICU admissions associated with a policy of early delivery may have a public health ramification that overshadows any small mortality benefit. Specifically, randomized clinical trials need to be considered in order to determine the best management of term pregnancy for women with GDM that consider both morbidity and mortality as outcomes. Until prospective studies can be performed, this type of risk assessment demonstrated in our study may prove to be very useful in helping women with GDM and their care providers determine the optimal gestational age for delivery.
Acknowledgments
Financial Disclosure: Dr. Cheng is supported by the National Institute of Child Health and Human Development, Grant # HD01262, as a Women’s Reproductive Health Research Scholar
Footnotes
This research is scheduled to be presented as an oral presentation at the 32nd Annual Meeting of the Society for Maternal Fetal Medicine, February 11, 2012.
Disclosure: None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maternal, Child, and Adolescent Health Program/Epidemiology, Assessment and Program Developement. Gestational diabetes mellitus. Sacramento, CA: California Department of Publis; 2008. [Google Scholar]
- 2.Mitanchez D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010 Dec;36(6 Pt 2):617–27. doi: 10.1016/j.diabet.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational Diabetes. Obstet Gynecol. 2001 Sep;98(3):525–38. [PubMed] [Google Scholar]
- 4.O’Sullivan JB, Gellis SS, Dandrow RV, Tenney BO. The potential diabetic and her treatment in pregnancy. Obstet Gynecol. 1966 May;27(5):683–9. [Google Scholar]
- 5.Pettitt DJ, Knowler WC, Baird HR, Bennett PH. Gestational diabetes: Infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care. 1980 May-Jun;3(3):458–64. doi: 10.2337/diacare.3.3.458. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt MI, Duncan BB, Reichelt AJ, Branchtein L, Matos MC, Costa e Forti A, et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. 2001 Jul;24(7):1151–5. doi: 10.2337/diacare.24.7.1151. [DOI] [PubMed] [Google Scholar]
- 7.Karmon A, Levy A, Holcberg G, Wiznitzer A, Mazor M, Sheiner E. Decreased perinatal mortality among women with diet-controlled gestational diabetes mellitus. Int J Gynaecol Obstet. 2009 Mar;104(3):199–202. doi: 10.1016/j.ijgo.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Fadl HE, Ostlund IK, Magnuson AF, Hanson US. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med. 2010 Apr;27(4):436–41. doi: 10.1111/j.1464-5491.2010.02978.x. [DOI] [PubMed] [Google Scholar]
- 9.Lapolla A, Dalfra MG, Bonomo M, Parretti E, Mannino D, Mello G, et al. Gestational diabetes mellitus in Italy: A multicenter study. Eur J Obstet Gynecol Reprod Biol. 2009 Aug;145(2):149–53. doi: 10.1016/j.ejogrb.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Wassimi S, Wilkins R, Mchugh NG, Xiao L, Simonet F, Luo ZC. Association of macrosomia with perinatal and postneonatal mortality among First Nations people in Quebec. CMAJ. 2011 Feb 22;183(3):322–6. doi: 10.1503/cmaj.100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HY, Chauhan SP, Ward TC, Mori N, Gass ET, Cisler RA. Aberrant fetal growth and early, late, and postneonatal mortality: An analysis of Milwaukee births, 1996-2007. Am J Obstet Gynecol. 2011 Mar;204(3):261.e1–261.e10. doi: 10.1016/j.ajog.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 12.Caughey AB, Rosenstein MG, Cheng YW, Ward C, Nicholson J. The risk of perinatal death stratified by gestational age: A novel methodologic approach. Obstet Gynecol. 2011 Jan;204:S234–5. [Google Scholar]
- 13.California Department of Health Services. Center for health statistics. Birth cohort public use file, 1999-2003. Sacramento, CA: 2006. [Google Scholar]
- 14.Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: A period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011 Jun;117(6):1279–87. doi: 10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GC, Pell JP, Dobbie R. Risk of sudden infant death syndrome and week of gestation of term birth. Pediatrics. 2003 Jun;111(6 Pt 1):1367–71. doi: 10.1542/peds.111.6.1367. [DOI] [PubMed] [Google Scholar]
- 16.Bruckner TA, Cheng YW, Caughey AB. Increased neonatal mortality among normal-weight births beyond 41 weeks of gestation in california. Am J Obstet Gynecol. 2008 Oct;199(4):421.e1–421.e7. doi: 10.1016/j.ajog.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol. 2006 Sep;195(3):764–70. doi: 10.1016/j.ajog.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004 Jan;27(Suppl 1):S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 19.Kjos SL, Henry OA, Montoro M, Buchanan TA, Mestman JH. Insulin-requiring diabetes in pregnancy: A randomized trial of active induction of labor and expectant management. Am J Obstet Gynecol. 1993 Sep;169(3):611–5. doi: 10.1016/0002-9378(93)90631-r. [DOI] [PubMed] [Google Scholar]
- 20.Witkop CT, Neale D, Wilson LM, Bass EB, Nicholson WK. Active compared with expectant delivery management in women with gestational diabetes: A systematic review. Obstet Gynecol. 2009 Jan;113(1):206–17. doi: 10.1097/AOG.0b013e31818db36f. [DOI] [PubMed] [Google Scholar]
- 21.Clark SL, Miller DD, Belfort MA, Dildy GA, Frye DK, Meyers JA. Neonatal and maternal outcomes associated with elective term delivery. Am J Obstet Gynecol. 2009 Feb;200(2):156.e1–156.e4. doi: 10.1016/j.ajog.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 22.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009 Jan 8;360(2):111–20. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrenthal DB, Hoffman MK, Jiang X, Ostrum G. Neonatal outcomes after implementation of guidelines limiting elective delivery before 39 weeks of gestation. Obstet Gynecol. 2011 Nov;118(5):1047–55. doi: 10.1097/AOG.0b013e3182319c58. [DOI] [PubMed] [Google Scholar]
- 24.Bassaw B, Ataullah I, Roopnarinesingh S, Sirjusingh A. Diabetes in pregnancy. Int J Gynaecol Obstet. 1995 Jul;50(1):5–9. doi: 10.1016/0020-7292(95)02390-x. [DOI] [PubMed] [Google Scholar]
- 25.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005 Jun 16;352(24):2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 26.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the united states: Temporal trends and variability in indices of perinatal outcomes. Paediatr Perinat Epidemiol. 2007 Sep;21(Suppl 2):22–30. doi: 10.1111/j.1365-3016.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 27.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked california livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007 Sep;21(Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 28.Pearl M, Wier ML, Kharrazi M. Assessing the quality of last menstrual period date on california birth records. Paediatr Perinat Epidemiol. 2007 Sep;21(Suppl 2):50–61. doi: 10.1111/j.1365-3016.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 29.Savitz DA, Terry JW, Jr, Dole N, Thorp JM, Jr, Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002 Dec;187(6):1660–6. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008 Nov;22(6):587–96. doi: 10.1111/j.1365-3016.2008.00965.x. [DOI] [PubMed] [Google Scholar]


