Abstract
Antibodies to granulocyte-macrophage colony-stimulating factor (GM-CSF) can be induced when GM-CSF is used as an adjuvant to solid tumor vaccination. Neutralizing anti-GM-CSF IgG has been associated with pulmonary alveolar proteinosis (PAP), and secondary PAP has been linked to myeloid leukemia. We studied 69 patients with acute myeloid leukemia, chronic myeloid leukemia and myelodysplastic syndrome, including 19 patients who received GM-CSF with peptide antigen and incomplete Freund's adjuvant in a vaccine trial for the presence or induction of anti-GM-CSF antibodies. Anti-GM-CSF IgG were present in 36 (52%) patients with myeloid leukemia compared to only 1 of 33 (3%) healthy subjects (P=0.008) and in none of 6 patients with lymphoid leukemia (P=0.0001). Antibody titers were unaffected by vaccination. Anti-GM-CSF IgA and IgM were found in 33 and 20% of patients, respectively; IgA from two patients neutralized GM-CSF. Strikingly, while anti-GM-CSF IgG titers were higher in patients with active disease (n=52) versus those in complete remission (n=14, P=0.0009), GM-CSF expression was not increased in either group. These data are first to show that anti-GM-CSF antibodies of multiple isotypes are present in patients with active myeloid leukemia without PAP and may be useful markers of disease activity.
Keywords: acute myeloid leukemia, chronic myeloid leukemia, myelodysplastic syndrome, GM-CSF, autoantibodies
Introduction
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an autocrine and a paracrine cytokine. It stimulates growth, differentiation and function of normal and leukemic myeloid progenitors.1,2 GM-CSF augments both innate and adaptive immunity by facilitating growth and function of neutrophils,3 macrophages,4 monocytes and dendritic cells.5 GM-CSF has also been implicated in leukemogenesis, although altered regulation of GM-CSF expression in myeloproliferative disorders is complex. There is some evidence that GM-CSF is involved in the autocrine or paracrine induction of proliferation of acute myeloid leukemia (AML) cells,1 and chemicals such as hydro-quinones selectively enhance clonogenic responses to GM-CSF in murine and human bone marrow (BM) cells.6 However, GM-CSF also facilitates cell differentiation and maturation, which are usually blocked in AML. Finally, partial and complete deletion of the GM-CSF gene, including deletion of 5q31, occurs in chromosomal abnormalities, which are associated with secondary AML and myelodysplastic syndrome (MDS),7 suggesting a complex role of GM-CSF dysregulation in leukemogenesis.
Antibodies to GM-CSF, while uncommon and of unknown significance, have been detected in patients with autoimmune diseases,8 in neonatal cord blood,9 and in 0.3% of healthy donors (HD).10 Exogenous recombinant human GM-CSF can also elicit both humoral11,12 and cellular13 immune responses to GM-CSF when administrated as a single agent for hematopoietic recovery or as an adjuvant. Humoral response to GM-CSF varies and may depend on patients' immune status, the adjuvant dose, number of vaccinations, route of administration and source of recombinant GM-CSF used for vaccination.11,12 Immunity to GM-CSF is a potential problem, because autoantibodies to GM-CSF are implicated in the pathogenesis of idiopathic pulmonary alveolar proteinosis (PAP) where they inhibit GM-CSF-mediated endocytosis of surfactant in alveoli.14,15 The clinical relevance of induced antibodies is not established; however, recent findings indicate that they may weaken the biological activity of endogenous and pharmacological GM-CSF.16,17 Disis et al.18 showed that subcutaneous co-administration of tumor-derived peptides and GM-CSF was effective in generating tumor-specific cytotoxic lymphocytes in mice, and GM-CSF has been extensively used as an adjuvant in clinical trials of anti-tumor vaccines.19,20 Therefore, an understanding of anti-GM-CSF humoral responses that might occur when GM-CSF is used as an adjuvant in the treatment of leukemia may be critical for successful vaccination.
To assess the extent of humoral immunity to GM-CSF in leukemia patients, we studied patients with chronic myeloid leukemia (CML), AML and MDS at various stages of disease for the evidence of anti-GM-CSF antibodies. To determine whether vaccination using GM-CSF plus incomplete Freund's adjuvant would induce anti-GM-CSF antibodies, we also tested patients with CML, AML and MDS who received PR1 (proteinase 3-derived HLA-A2-restricted) peptide21 vaccine. Although the clinical results of the vaccine trial are not reported here, we show that vaccination does not induce antibodies. Instead, we found preexisting autoantibodies in a large cohort of patients. Moreover, our data show an association between anti-GM-CSF antibodies and active leukemia, which may suggest a role of anti-GM-CSF autoantibodies in myeloid leukemia pathogenesis and may also implicate anti-GM-CSF antibodies as markers of myeloid leukemia progression.
Patients and methods
Patients
All the samples from patients and HD for this study were treated similarly: separated, aliquoted and cryo-preserved (−80 °C), then thawed just before use. Our study population included 19 vaccinated patients (9 AML, 5 CML and 5 MDS), 50 non-vaccinated patients (22 AML, 24 CML and 4 MDS), including 17 pre-vaccine patients (pre-vaccine serum samples were not available for 2 vaccinated patients), 2 patients with acute lymphocytic leukemia, 4 with chronic lymphocytic leukemia and 33 HD. We assessed them for the presence of antibodies to GM-CSF. Vaccinated patients had received PR121 peptide vaccine in a phase I/II clinical trial. Vaccine was administrated subcutaneously by a single injection of PR1 peptide in incomplete Freund's adjuvant (Montanide ISA-51), followed immediately by a second injection of 75 μg of yeast-derivedv GM-CSF (Leukine; Berlex, Seattle, WA, USA) into the same depot area every 3 weeks for three consecutive vaccinations. Peripheral blood (PB) sera of 22 patients (12 AML and 10 CML) and 30 HD, and BM sera from18 patients (6 AML, 6 CML, 2 Hodgkin disease, 2 chronic lymphocytic leukemia, 1 acute lymphocytic leukemia and 1 lymphoma) were used to assess the level of GM-CSF in the circulating blood and in BM fluid, respectively. BM sera were prepared by centrifugation of fresh BM for 20 min at 2000 rpm with collection of supernatant. The PB mononuclear cells from 12 patients and 9 HD were isolated by Ficoll-Histopaque separation22 and used to assess GM-CSF mRNA expression. Patients were distinguished as having active disease (AD: untreated, refractory or relapsed disease) or as being in complete remission at the time of sample collection. This was assessed using standard criteria from the National Cancer Institute. All patients and HD gave written informed consent for sample collection on protocols approved by the MD Anderson Institutional Review Board.
Cell lines
GM-CSF-dependent human erythroleukemia cell line TF-123 (ATCC, Manassas, VA, USA) was used to evaluate the neutralizing activity of anti-GM-CSF antibodies. Cells were maintained in ATCC complete media supplemented with 10% fetal bovine serum and 10 μg ml−1 GM-CSF. The human 5637 cell line (ATCC), which secrets GM-CSF, was used as a positive control for GM-CSF mRNA expression, as described previously.24 The 5637 cells were maintained in complete media supplemented with 10% fetal bovine serum.
Cytokines and antibodies
GM-CSF (see above) was used to assess the anti-GM-CSF antibodies. Interleukin (IL)-3 (BD Pharmingen, San Diego, CA, USA) and interferon-γ (IFN)-γ (InterMune, Brisbane, CA, USA), were used to test the specificity of antibodies to GM-CSF. Neutralizing monoclonal antibody (MAb) to human GM-CSF (MAb 215, R&D Systems, Minneapolis, MN, USA) and mouse immunoglobulin (Ig) G1 (Becton Dickinson, San Jose, CA, USA) were used as positive and negative controls, respectively, for enzyme-linked immunosorbent assay (ELISA), western blotting and neutralization assays. MAb 3092 to human GM-CSF (Endogene, Rockford, IL, USA) was used to assess the epitope specificity of antibodies in a competitive binding assay. Horseradish peroxidase (HRP)-conjugated antibodies to human IgG, IgA, IgM, IgE (Sigma Chemical Corporation, St Louis, MO, USA) and HRP-conjugated goat anti-mouse antibody (Caltag Laboratories, Burlingame, CA, USA) were used for anti-GM-CSF isotype analysis in western blotting and ELISA.
ELISA
The titer of anti-GM-CSF IgG was assessed as described previously.14 Briefly, 96-well plates coated with GM-CSF (2.5 μg ml−1) were incubated for 1 h at 37 °C with purified Igs or sera samples diluted (1:400) with phosphate-buffered saline, washed and incubated for 30 min with HRP-conjugated anti-human IgG, washed again and stained with tetramethylbenzi-dine substrate (BD Pharmingen). To subtract nonspecific binding due to different concentrations of IgG in the patients' samples, parallel ELISA was performed with bovine serum albumin-coated plates. A GM-CSF-specific IgG signal for each sample was normalized to the paired bovine serum albumin-specific IgG signal. Human intravenous Ig (IVIg; Carimune; ZLB Bioplasma Inc., Glendale, CA, USA), previously shown to contain anti-GM-CSF IgG,10 was used to prepare a standard for ELISA. This standard, aliquoted and cryo-preserved, was used to determine anti-GM-CSF IgG concentrations throughout this study. The results were expressed in relative units (RU) corresponding to the anti-GM-CSF activity in 1 μg of commercial IVIg. The GM-CSF-binding activity of IVIg was confirmed by an inhibition assay as described previously.10 To confirm GM-CSF specificity of antibodies, ELISA for IgG antibodies to recombinant yeast-derived IL-3 or IFN-γ was performed under the same conditions. All samples were assayed in duplicate, and each experiment was repeated twice. The serum GM-CSF concentration was determined by using a human GM-CSF ELISA OptEIA Set (BD Pharmingen). To test whether anti-GM-CSF antibodies interfered the determination of GM-CSF by ELISA, GM-CSF standard was preincubated for 1 h with sera containing anti-GM-CSF antibodies, then GM-CSF titers were measured as above.
Western blotting for anti-GM-CSF IgG, IgA and IgM
Granulocyte-macrophage colony-stimulating factor (5 μg per well) was resolved on 15% SDS-polyacrylamide gel electrophoresis and transferred to Hybond-C nitrocellulose membrane (Amersham Bioscience, Piscataway, NJ, USA). The membrane was stripped, incubated with blocking buffer containing skimmed milk (5%; Bio-Rad Laboratories, Hercules, CA, USA) in phosphate-buffered saline with 0.01% Tween-20 and then incubated for 2 h with analyzed serum, diluted 1:500 with blocking buffer, washed and probed with HRP-conjugated antibodies to human Igs. Bands were developed with a chemiluminescence system ECL (Amersham).
Immunoglobulin purification
Protein G Sepharose fast flow, agarose-immobilized anti-human IgM antibodies (both from Sigma) and immobilized jacalin (Pierce, Rockford, IL, USA) were used for affinity chromatography of IgG, IgM and IgA, respectively, all in accordance with manufactures' manuals. Purified fractions were dialyzed against phosphate-buffered saline, concentrated and analyzed for GM-CSF binding by ELISA. Ig isotype was confirmed by western blotting.
GM-CSF neutralization assays
Granulocyte-macrophage colony-stimulating factor-neutralizing activity of antibodies was determined as described previously.25 Briefly, TF-1 cells (2×104 cells per well) were incubated in complete media in 96-well tissue culture plates with GM-CSF (2.5 ng ml−1) and antibody (250 μgml−1). After incubation at 37 °C and 5% CO2 for 48 h, proliferation of TF-1 cells was assessed using MTT assay (Roche, Indianapolis, IN, USA). Inhibition of TF-1 proliferation was estimated according to the method of Uchida.15 Neutralizing antibody concentrations at 50% growth inhibition (IC50) were obtained from dose-dependent growth inhibition curves. To demonstrate GM-CSF-specific neutralizing activity of antibodies, rescue of TF-1 cell proliferation with IL-3 (10 ng ml−1) was performed. A competition assay with epitope-specific MAb 309215,26 was used to test epitope specificity of antibodies.15 Briefly, the GM-CSF-binding activity of MAb 3092 in the presence of GM-CSF-binding Igs from patients was measured by direct ELISA. Additionally, the influence of MAb 3092 on binding of patient-derived IgG, IgA and IgM to GM-CSF was tested. Briefly, plates coated with GM-CSF (100 μgml−1) were preincubated with 50 μl competitive antibody for 30 min at 37 °C, and then 50 μl of tested antibody was added. After a 30-min incubation, plates were washed and developed with corresponding HRP-conjugated secondary antibody.
RNA isolation and reverse transcription-PCR
Total RNA was isolated from thawed PB mononuclear cells using STAT-60 isolation reagent (Tel-Test, Friendswood, TX, USA). RNA (1 μg) was used as a template for synthesis of corresponding cDNA by reverse transcription according to an previously published protocol.27 For PCR, GM-CSF specific primers and conditions were used as described,24 and 36 cycles of amplification were performed. The relative quantity of GM-CSF mRNA for each sample was estimated as a percentage of GM-CSF relative to β-actin control.
Statistical analysis
Data were presented as mean±s.e.m. The Mann–Whitney t-test was used to assess non-parametric data between groups, and P<0.05 was considered significant. Spearman correlation for non-parametric data with P<0.05 was used to evaluate correlations within the groups, and Z>3 with P<0.05 was used to exclude outliers. Receiver-operator characteristic curves with a 95% confidence interval and P<0.05, were used to distinguish between patient samples with or without antibodies to GM-CSF. A cutoff value allowing a higher specificity was chosen. GraphPad Prism 4.0b (Graph Pad Software Inc., San Diego, CA, USA) software was used for statistical analysis.
Results
Anti-GM-CSF IgG are present in serum of patients with AML, CML and MDS
We examined sera from 50 non-vaccinated patients (22 AML, 24 CML and 4 MDS), 19 vaccinated patients (9 AML, 5 CML and 5 MDS), 6 patients with non-myeloid leukemia and 33 HD, for the presence of anti-GM-CSF antibodies. As shown in Figure 1a, we found increased titers of anti-GM-CSF IgG (RU ml−1) in both vaccinated (53–15.6) and non-vaccinated patients (48.2±8.4) with myeloid leukemia compared to control groups of HD (6.4±1.7) and patients with lymphoid leukemia (8.5±1.7). Anti-GM-CSF IgG was detected using a cutoff value of 26.5RUml−1, calculated as described in Patients and methods, in 29 of 50 (58%) patients with myeloid leukemia compared to only 1 of 33 (3%) HD (P<0.0001) and none of the 6 patients with lymphoid leukemia (P=0.001) (Figure 1a). The absence of binding to the control proteins IL-3 and IFN-γ confirmed antibody specificity for GM-CSF (data not shown).
Figure 1.
High titers of anti-GM-CSF IgG are present in myeloid leukemia patients and are unaffected by vaccination with GM-CSF as an adjuvant. (a) Serum anti-GM-CSF IgG titers were evaluated by ELISA in vaccinated (n=19) and non-vaccinated (n=50) patients with myeloid leukemia and in a control group of patients with lymphoid leukemia (n=6) and HD (n=33). The results are the means of two experiments performed in duplicate; horizontal lines are column means. (b) Serum anti-GM-CSF IgG were evaluated in vaccinated patients (n=17) before the first vaccination and 3 weeks after the third vaccination. The lines connect results at different time points for each individual. Relative unit (RU): anti-GM-CSF reactivity of 1 μg IVIg. HD, healthy donors; Igs, immunoglobulins; ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte-macrophage colony-stimulating factor.
We sought to determine whether anti-GM-CSF antibodies were elicited in AML, CML and MDS patients enrolled on a vaccination trial using GM-CSF as an adjuvant. As shown in Figure 1b, anti-GM-CSF IgG were found in 7 of 17 (41%) patients prior to vaccination. However, unlike the results in solid tumor patients,12,28 vaccination of leukemia patients with adjuvant GM-CSF did not increase the titer of anti-GM-CSF in the 7 patients with preexisting antibodies (P=0.18), and no anti-GM-CSF antibodies were induced in the 12 patients who did not have preexisting antibodies (Figure 1b).
High titer of anti-GM-CSF IgG correlates with AD
We found no significant differences in the disease-associated incidence of anti-GM-CSF antibodies in AML, CML or MDS patients (62, 56 and 50%, respectively) or in the disease-associated mean titers (RU ml−1) of anti-GM-CSF IgG (51±16, 47±11 and 54±29, respectively). Similarly, the antibody titer did not correlate with patient age (P=0.49) or gender (P=0.88). In 12 patients for whom total IgG levels were available from the clinical laboratory, there was no significant correlation between total serum IgG level and anti-GM-CSF IgG titer (R2=0.53; P=0.08), which suggests specific effect of anti-GM-CSF antibodies.
Since there is evidence that GM-CSF might be involved in the autocrine or paracrine growth of leukemia,2,29 we considered the possibility that antibodies might interrupt this process, which might therefore slowdown leukemia growth. We compared anti-GM-CSF titers in patients with AD to those in patients in complete remission. We studied 52 patients with AD, and because remission samples were not available from these patients, we studied an additional 14 AML and CML patients who were in complete remission. To our surprise, we found that the titer (RU ml−1) of anti-GM-CSF IgG was higher in patients with AD compared to patients in complete remission (58.9±9.2 versus 19.5±5.6, respectively, P=0.0009), as shown in Figure 2. These results suggest a role of anti-GM-CSF antibodies in pathogenesis, or as a marker of disease activity.
Figure 2.
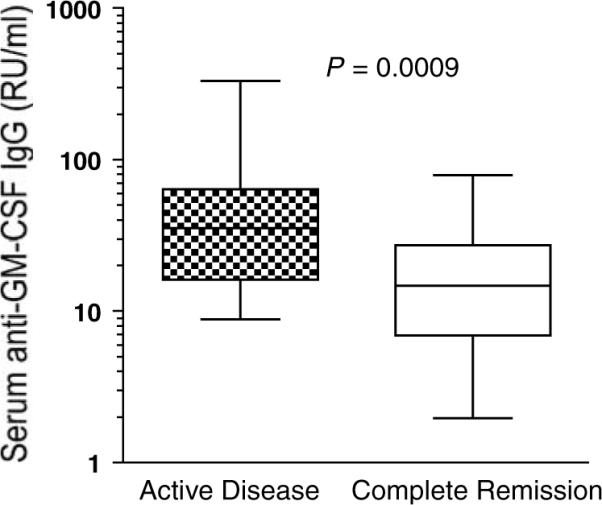
High titer of anti-GM-CSF IgG correlates with active disease. Anti-GM-CSF IgG titers were evaluated by ELISA in patients with active leukemia (n=52) and in patients in clinical remission (n=14). Both groups included vaccinated and non-vaccinated patients. Each sample was studied in duplicate. Boxes indicate column medians, 25 and 75th percentile; whiskers indicate the columns' largest and smallest values. Relative unit (RU): anti-GM-CSF reactivity of 1 μg IVIg. ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte-macrophage colony-stimulating factor.
IgA and IgM isotypes of anti-GM-CSF antibodies are present in addition to IgG
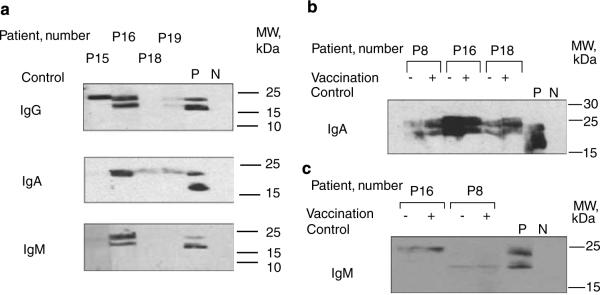
Because of the high prevalence of IgG antibodies to GM-CSF in patients with myeloid leukemia who had AD, we sought to characterize the breadth of this immune response. Using immunoblotting techniques, we analyzed sera from 18 vaccinated patients for anti-GM-CSF IgG, IgA, IgM and IgE. Western blotting confirmed the ELISA results (Table 1) showing anti-GM-CSF IgG in 6 of 18 patients (Figure 3a, anti-IgG probe). Interestingly, anti-GM-CSF IgA and IgM were present in 5 of 15 (33%) patients and in 3 of 15 (20%) patients, respectively (Figure 3a, anti-IgA and anti-IgM probes); however, none of the 18 patients had detectable anti-GM-CSF IgE (data not shown). Although patient P16 exhibited high titers of anti-GM-CSF IgG, IgM and IgA, there was no association between the presence of any particular isotype and the presence of any other isotype (Table 1). Similar to IgG titers, there was no significant change in anti-GM-CSF IgA or IgM titers after vaccination (Figures 3b and c). Anti-GM-CSF IgA and IgM have not been previously described in HD, vaccine patients or in patients with primary PAP,14 which suggests a unique role for these antibodies in leukemia patients.
Table 1.
Anti GM-CSF Igs in vaccinated patients by ELISA and western blot
| UPN | Diagnosis | Anti-GM-CSF IgG titer in ELISAa (RU ml−1) | Anti GM-CSF Igs in western blotb |
||
|---|---|---|---|---|---|
| IgG | IgA | IgM | |||
| P1 | AML | 3 | − | − | − |
| P2 | CML | 120 | ++ | − | − |
| P3 | AML | 3 | − | − | − |
| P4 | CML | 16 | − | − | − |
| P5 | CML | 48 | ++ | − | − |
| P6 | MDS | 15 | − | − | – |
| P7 | AML | 23 | − | + | − |
| P8 | AML | 20 | − | + | + |
| P9 | AML | 18 | − | − | − |
| P10 | AML | 21 | − | − | − |
| P11 | MDS | 121 | − | − | − |
| P13 | AML | 23 | + | − | + |
| P14 | MDS | 22 | − | N/D | N/D |
| P15 | CML | 192 | ++ | − | − |
| P16 | AML | 245 | +++ | +++ | +++ |
| P17 | CML | 40 | ++ | N/D | N/D |
| P18 | MDS | 48 | − | ++ | − |
| P19 | MDS | 59 | + | + | − |
| P20 | AML | 266 | N/D | N/D | N/D |
Abbreviations: AML, acute myeloid leukemia; CML, chronic myeloid leukemia; ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte-macrophage colony-stimulating factor; Igs, immunoglobulins; RT-PCR, reverse transcription-polymerase chain reaction; MDS, myelodysplastic syndrome.
ELISA was performed for anti-GM-CSF IgG only.
The number of (+) reflects the band's intensity on western blot, (−) no visible band on western blot, (N/D) western blot was not performed because the limited quantity of serum.
Figure 3.
Multiple isotypes of anti-GM-CSF antibodies are present in a subset of patients with AML and CML and are not induced by vaccine. (a) GM-CSF was resolved in 15% SDS-PAGE into three species with molecular weights of 19.5, 16.8 and 15.5 kDa. Western blotting with sera of four representative vaccinated patients (P15, P16, P18 and P19) and consequent probing with antibodies specific to human IgG, IgA and IgM revealed corresponding subclasses of anti-GM-CSF Igs. Antibody MAb 215 (anti-human GM-CSF) and mouse IgG1 were used as positive (P) and negative (N) controls, respectively. (b) Anti-GM-CSF IgA and (c) anti-GM-CSF IgM were present in patients' sera before (−) and after (+) vaccination. ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte-macrophage colony-stimulating factor; Igs, immunoglobulins; MAb, monoclonal antibody; SDS-PAGE, SDS-polyacrylamide gel electrophoresis.
Anti-GM-CSF IgA antibodies are weakly neutralizing
To evaluate the potential biological significance of anti-GM-CSF autoantibodies, we tested the GM-CSF-binding fractions of IgG (n=5), IgA (n=3) and IgM (n=3) isolated from patients' sera for their ability to inhibit proliferation of the GM-CSF-dependent TF-1 cell line. IgG, IgA and IgM fractions that were isolated from patient P3 showed no GM-CSF-binding activity and were used as controls. Using purified Igs (from 50 to 250 μg) to neutralize GM-CSF (2.5 ng), we found that IgG (n=5) (Figure 4) and IgM (n=3) (data not shown) did not inhibit GM-CSF-dependent proliferation of TF-1 cells. In contrast, IgA from patient P16 neutralized GM-CSF with IC50=30 μgml−1 compared to IC50=2 μgml−1 for control neutralizing antibody (MAb 215) (Figure 4). In addition, there was only minimal neutralizing activity of IgA from patient P18. Thus, IgG and IgA from patient P16 recognized unique antigenic determinants on GM-CSF. Furthermore, anti-GM-CSF IgG and IgM from leukemia patients are non-neutralizing, whereas some anti-GM-CSF IgA antibodies are neutralizing.
Figure 4.
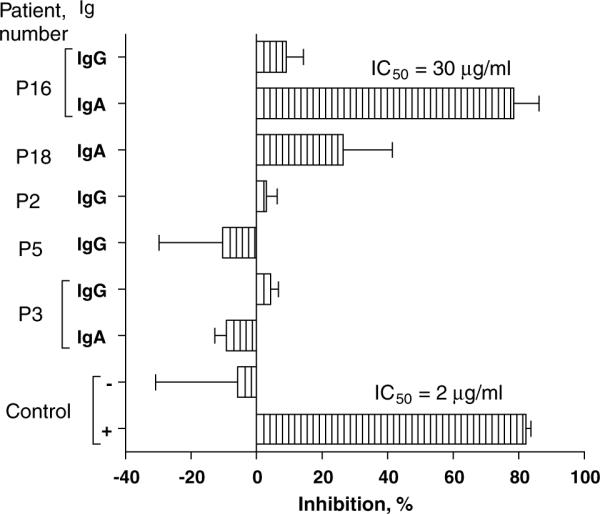
Anti-GM-CSF IgA neutralizes GM-CSF-dependent proliferation of TF1 cells. GM-CSF-reactive Igs were purified from patients' sera by affinity chromatography, as indicated in Patients and methods. Igs from patient P3, which do not bind GM-CSF, were likewise purified for control. Cells were cultured for 48 h in the presence of GM-CSF (2.5 ng ml−1) and Igs (250 μg ml−1). At the end of incubation, MTT assay was performed. GM-CSF neutralizing antibody MAb 215 and murine IgG1 were used as positive (+) and negative (−) controls, respectively. The results are shown as means±s.e.m. in one of three experiments performed in triplicate. ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte-macrophage colony-stimulating factor; Igs, immunoglobulins; MAb, monoclonal antibody.
Patients with primary idiopathic PAP have neutralizing anti-GM-CSF IgG, and an epitope within GM-CSF has been identified in some patients.15 To explore the epitope specificity of the antibodies in the leukemia patients, we tested the antibodies for their ability to compete with MAb 3092 because this antibody has been shown to bind amino-acid residues 78–94, close to the functional domain of GM-CSF,26 and because neutralizing anti-GM-CSF IgG from PAP patients can also inhibit this binding.15 However, in leukemia patients with anti-GM-CSF antibodies, binding of both IgG (n=4) and IgM (n=2) to GM-CSF was not affected by the addition of 50 ng antibody 3092 to serum (P>0.5), as measured by ELISA at serial dilutions of sera from 1:400 to 1:3200. In contrast, antibody 3092 inhibited the GM-CSF binding of non-purified IgA and IgM from patient P16 by 10% (P=0.005) and 7% (P=0.007), respectively, suggesting partial cross-recognition of GM-CSF functional domain, which is also consistent with the growth-inhibitory properties of these antibodies against TF-1 cells.
Anti-GM-CSF antibody titer does not correlate with GM-CSF expression
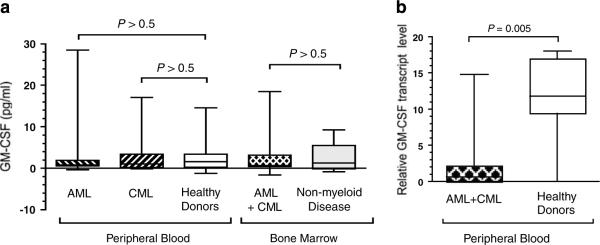
The neutralizing and non-neutralizing antibodies to GM-CSF can be induced after administration of GM-CSF.16 We hypothesized, therefore, that in patients with active leukemia, abnormally high concentrations of GM-CSF could induce autoantibodies to GM-CSF. To test our hypothesis, we assessed concentrations of serum GM-CSF (pg ml−1) in both AML (3.5±2.5, n=12) and CML (2.8±1.6, n=10) patients with AD and found it to be similar to that of healthy controls (2.5±0.6, n=30; P>0.05) (Figure 5a). The serum concentration of GM-CSF was below the lowest detectable titer (0.75 pg ml−1) in 9 (41%) leukemia patients and 13 (43%) HD (P>0.05). Similarly, the GM-CSF level in the BM of patients with myeloid leukemia was not elevated (3.2±1.7, n=12) and was comparable to that of patients without myeloid leukemia (2.2±1.5, n=6; P>0.05) (Figure 5a). Furthermore, serum anti-GM-CSF IgG titers did not correlate with serum GM-CSF levels in leukemia patients (Spearman r=0.08, P=0.72, n=22) nor in HD (Spearman r=−0.28, P=0.13, n=30).
Figure 5.
GM-CSF expression is reduced in non-vaccinated patients with AML. (a) GM-CSF concentration in peripheral blood (12 AML, 10 CML and 30 HD) and bone marrow (12 AML plus CML and 6 non-myeloid malignancies) was evaluated by ELISA. GM-CSF concentration in each sample was calculated as a mean of three independent measurements performed in duplicate. (b) GM-CSF mRNA expression in peripheral blood of patients with myeloid leukemia (n = 12) and HD (n = 9) was evaluated by RT-PCR and subsequent PCR with GM-CSF RNA-specific primers. For both (a) and (b), the boxes are the column medians, 25 and 75th percentiles; whiskers are the largest and smallest values in each group. AML, acute myeloid leukemia; CML, chronic myeloid leukemia; HD, healthy donors; ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte-macrophage colony-stimulating factor; RT-PCR, reverse transcription-PCR.
We considered the possibility that falsely low serum GM-CSF might result from either presence of GM-CSF binding antibodies, which can interfere with GM-CSF determination by ELISA, or from absorption by leukemia cells of GM-CSF that is over-produced by blasts or other hematopoietic cells. However, the addition of up to 10% of patients' sera (n=10) containing anti-GM-CSF antibodies did not reduce the measured GM-CSF concentration (data not shown). Finally, PB mononuclear cells of 12 myeloid leukemia patients and 9 HD showed that GM-CSF mRNA expression was lower in leukemia patients than in HD (2.4±1.4 versus 12.3±1.9; P=0.005) (Figure 5b). Therefore, these data show that anti-GM-CSF antibodies did not result from GM-CSF overexpression.
Discussion
In this study, we found that IgG autoantibodies to GM-CSF were present in more than half of the patients with myeloid leukemia and MDS, irrespective of disease category. We also found a strong correlation between the presence of anti-GM-CSF IgG and disease activity (P=0.0009), which suggests the clinical relevance of anti-GM-CSF IgG in leukemia pathogenesis and for disease monitoring. Furthermore, we showed that, in addition to IgG, IgA and IgM anti-GM-CSF isotypes were present in patients with myeloid leukemia, and IgA showed GM-CSF-neutralizing activity.
Contrary to our expectations, high titers of anti-GM-CSF antibodies neither correlate with a high concentration of GM-CSF in sera from PB or BM, nor with the type of therapy each patient received, which included patients who received a combination of yeast-derived GM-CSF and incomplete Freund's adjuvant as part of a vaccine trial. Other investigators have used a similar dose of Escherichia coli-derived GM-CSF, but a different dose schedule (75–80 μg day−1 for 4 consecutive days rather than every 3 weeks), and 16% of their patients developed non-neutralizing IgG antibodies.12 This suggests the possibility of different dose schedules, different origin of recombinant antigen (bacterial versus eukaryotic source) or differences between leukemia and solid tumor patients as factors that predispose to the development of anti-GM-CSF antibodies. The origin of the immunity to GM-CSF and the consequences of this immunity in leukemia patients is not clear, although the important role of GM-CSF in normal differentiation of myeloid progenitors together with the differentiation arrest of leukemia blasts6 suggest there might be a role of anti-GM-CSF antibodies in leukemia pathogenesis.
The biological role of anti-GM-CSF antibodies in myeloid leukemia has not been thoroughly explored. Although neutralizing antibodies to GM-CSF are present in patients with primary PAP,14,30,31 and secondary PAP has been linked to AML,32,33 CML34 and MDS,35,36 the prevalence of these antibodies among leukemia patients and their biological link to myeloid leukemia is unknown. Kitamura et al.14 showed that anti-GM-CSF IgG, but not IgM or IgA, are present in patients with PAP and may be pathogenic in this disease. Neutralizing autoantibodies in patients with PAP recognize the functional domain of GM-CSF,15 located between residues 78 and 94, which has been shown to mediate the binding of GM-CSF to its receptor.26 In contrast, anti-GM-CSF IgG has not been found in patients with secondary PAP, although only a few patients have been studied. Interestingly, we found a high prevalence of anti-GM-CSF antibodies only in patients with myeloid leukemia and MDS (not in patients with lymphoid leukemia), but none of these patients had PAP. In addition to IgG, anti-GM-CSF IgA and IgM were also present in some patients. Significantly, none of the anti-GM-CSF IgG bound to the GM-CSF functional domain nor neutralized its activity. In contrast, IgA antibodies neutralized GM-CSF. Because IgA is a principal isotype in the secretions of mucosal epithelia, neutralizing IgA activity could prevent GM-CSF function in immune responses against respiratory and gut infections in patients. Although one of the two patients had pneumonia, studies with larger numbers of patients will be necessary to confirm any association. Also, studies of GM-CSF-neutralizing IgA in secondary PAP have not been performed. Our data suggest that these antibodies should be investigated in patients with secondary PAP.
Although our data showed there was no neutralizing activity of GM-CSF by IgG and IgM, it is possible that such antibodies might inactivate GM-CSF by other immunological mechanisms in vivo, such as by facilitating phagocytosis or by altering the serum half-life of GM-CSF. The clinical relevance of induced antibodies12,16 is not yet established; however, recent findings indicate that such antibodies are associated with accelerated pharmacokinetic clearance and decreased effectiveness of exogenous GM-CSF.16,17 Similarly, anti-GM-CSF antibodies in leukemia patients might weaken the biological activity of endogenous and pharmacological GM-CSF. Conversely, mechanisms whereby antibodies facilitate the activity of GM-CSF have also been suggested,37 including those that propose a cytokine-carrier function,38 protection from proteolysis or an effect on the normal biodistribution of GM-CSF.16,37 Therefore, it is possible that high titers of anti-GM-CSF antibodies in some leukemia patients might influence myeloid development. Unfortunately, limiting quantities of available patients material did not allow us to test these possibilities.
The surprisingly high number of myeloid leukemia patients with high titers of anti-GM-CSF antibodies suggested that aberrant antigen expression of GM-CSF in the PB or BM of these patients might have induced an immune response. Cellular and humoral responses to normal proteins that are overexpressed in leukemia has been demonstrated in patients with various types of leukemia.39 We have reported that cytotoxic T lymphocytes specific for a PR1 peptide are present in patients with CML and that they contribute to cytogenetic remission.40 Similarly, both cellular and humoral immunity to WT-1 has been associated with overexpression of WT-1 protein in patients with AML.41 In contrast, humoral immunity to GM-CSF is not associated with overexpression of GM-CSF, as shown in the present study. Because there are conflicting reports within the literature2,42,43 of GM-CSF expression in leukemia patients, we analyzed protein expression in both PB and BM, and correlated this with RNA transcript numbers. All of these measurements were in agreement and showed GM-CSF expression to be low compared to controls. Interestingly, high anti-GM-CSF antibody titers correlated with active disease, while patients that were in remission had low antibody titers. While additional studies are needed to confirm our finding and to address the physiological significance of the antibodies in patients with active leukemia, the antibodies do not appear to be related to PAP.
In summary, we identified anti-GM-CSF antibodies of IgG, IgA and IgM isotypes in a high percentage of myeloid leukemia patients. Importantly, the titers of anti-GM-CSF IgG correlated with disease activity. Because the titers of GM-CSF antibodies were high despite low expression of GM-CSF in patients with advanced leukemia, our data suggest an alternate mechanistic role in the pathogenesis of the disease. In addition, this study suggests that anti-GM-CSF titers may be useful in disease monitoring. Future prospective studies examining the presence of anti-GM-CSF antibodies will help establish the relevance of these findings in diagnosis and prognosis.
Acknowledgements
We thank Maria Tanguma for the collection of patients' samples. This study was supported in part by grants from the National Institutes of Health (Grant CA81247, C49639 and CA100271 to JJM).
References
- 1.Gasson JC. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- 2.Young DC, Griffin JD. Autocrine secretion of GM-CSF in acute myeloblastic leukemia. Blood. 1986;68:1178–1181. [PubMed] [Google Scholar]
- 3.Weisbart RH, Golde DW, Clark SC, Wong GG, Gasson JC. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985;314:361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- 4.Metcalf D. Molecular control of granulocyte and macrophage production. Prog Clin Biol Res. 1985;191:323–337. [PubMed] [Google Scholar]
- 5.Vasilijic S, Colic M, Vucevic D. Granulocyte-macrophage colony stimulating factor is an anti-apoptotic cytokine for thymic dendritic cells and a significant modulator of their accessory function. Immunol Lett. 2003;86:99–112. doi: 10.1016/s0165-2478(02)00295-x. [DOI] [PubMed] [Google Scholar]
- 6.Irons RD, Stillman WS. Cell proliferation and differentiation in chemical leukemogenesis. Stem Cells. 1993;11:235–242. doi: 10.1002/stem.5530110311. [DOI] [PubMed] [Google Scholar]
- 7.Le Beau MM, Westbrook CA, Diaz MO, Larson RA, Rowley JD, Gasson JC, et al. Evidence for the involvement of GM-CSF and FMS in the deletion (5q) in myeloid disorders. Science. 1986;231:984–987. doi: 10.1126/science.3484837. [DOI] [PubMed] [Google Scholar]
- 8.Meager A, Wadhwa M, Bird C, Dilger P, Thorpe R, Newsom-Davis J, et al. Spontaneously occurring neutralizing antibodies against granulocyte-macrophage colony-stimulating factor in patients with autoimmune disease. Immunology. 1999;97:526–532. doi: 10.1046/j.1365-2567.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revoltella RP, Laricchia Robbio L, Liberati AM, Reato G, Foa R, Funaro A, et al. Antibodies binding granulocyte-macrophage colony stimulating factor produced by cord blood-derived B cell lines immortalized by Epstein-Barr virus in vitro. Cell Immunol. 2000;204:114–127. doi: 10.1006/cimm.2000.1704. [DOI] [PubMed] [Google Scholar]
- 10.Svenson M, Hansen MB, Ross C, Diamant M, Rieneck K, Nielsen H, et al. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood. 1998;91:2054–2061. [PubMed] [Google Scholar]
- 11.Wadhwa M, Skog AL, Bird C, Ragnhammar P, Lilljefors M, Gaines-Das R, et al. Immunogenicity of granulocyte-macrophage colony-stimulating factor (GM-CSF) products in patients undergoing combination therapy with GM-CSF. Clin Cancer Res. 1999;5:1353–1361. [PubMed] [Google Scholar]
- 12.Ullenhag G, Bird C, Ragnhammar P, Frödin JE, Strigård K, Österborg A, et al. Incidence of GM-CSF antibodies in cancer patients receiving GM-CSF for immunostimulation. Clin Immunol. 2001;99:65–74. doi: 10.1006/clim.2000.4999. [DOI] [PubMed] [Google Scholar]
- 13.McNeel DG, Schiffman K, Disis ML. Immunization with recombinant human granulocyte-macrophage colony-stimulating factor as a vaccine adjuvant elicits both a cellular and humoral response to recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1999;93:2653–2659. [PubMed] [Google Scholar]
- 14.Kitamura T, Tanaka N, Watanabe J, Uchida K, Kanegasaki S, Yamada Y, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–1098. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 16.Ragnhammar P, Wadhwa M. Neutralising antibodies to granulocyte-macrophage colony stimulating factor (GM-CSF) in carcinoma patients following GM-CSF combination therapy. Med Oncol. 1996;13:161–166. doi: 10.1007/BF02990843. [DOI] [PubMed] [Google Scholar]
- 17.Eisenblatter M, Stahl-Hennig C, Kuate S, Stolte N, Jasny E, Hahn H, et al. Induction of neutralising antibodies restricts the use of human granulocyte/macrophage colony stimulating factor for vaccine studies in rhesus macaques. Vaccine. 2004;22:3295–3302. doi: 10.1016/j.vaccine.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES, et al. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 19.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–154. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 20.Schiffman K, Disis ML. HER2/neu peptide-based vaccines, with GM-CSF as an adjuvant, in patients with advanced-stage HER2/neu-expressing cancers. Clin Lung Cancer. 2000;2:74–77. doi: 10.3816/clc.2000.n.021. [DOI] [PubMed] [Google Scholar]
- 21.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 22.Molldrem JJ, Lee PP, Wang C, Champlin RE, Davis MM. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low-frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer Res. 1999;59:2675–2681. [PubMed] [Google Scholar]
- 23.Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- 24.Harris RJ, Pettitt AR, Schmutz C, Sherrington PD, Zuzel M, Cawley JC, et al. Granuloctye-macrophage colony-stimulating factor as an autocrine survival factor for mature normal and malignant B lymphocytes. J Immunol. 2000;164:3887–3893. doi: 10.4049/jimmunol.164.7.3887. [DOI] [PubMed] [Google Scholar]
- 25.Wadhwa M, Bird C, Fagerberg J, Gaines-Das R, Ragnhammar P, Mellstedt H, et al. Production of neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies in carcinoma patients following GM-CSF combination therapy. Clin Exp Immunol. 1996;104:351–358. doi: 10.1046/j.1365-2249.1996.11704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanakura Y, Cannistra SA, Brown CB, Nakamura M, Seelig GF, Prosise WW, et al. Identification of functionally distinct domains of human granulocyte-macrophage colony-stimulating factor using monoclonal antibodies. Blood. 1991;77:1033–1043. [PubMed] [Google Scholar]
- 27.Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–2534. [PubMed] [Google Scholar]
- 28.Wadhwa M, Mellstedt H, Small E, Thorpe R. Immunogenicity of GM-CSF products in cancer patients following immunostimulatory therapy with GM-CSF. Dev Biol (Basel) 2003;112:61–67. [PubMed] [Google Scholar]
- 29.Vellenga E, Young DC, Wagner K, Wiper D, Ostapovicz D, Griffin JD. The effects of GM-CSF and G-CSF in promoting growth of clonogenic cells in acute myeloblastic leukemia. Blood. 1987;69:1771–1776. [PubMed] [Google Scholar]
- 30.Tanaka N, Watanabe J, Kitamura T, Yamada Y, Kanegasaki S, Nakata K. Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte-macrophage colony stimulating factor. FEBS Lett. 1999;442:246–250. doi: 10.1016/s0014-5793(98)01668-8. [DOI] [PubMed] [Google Scholar]
- 31.Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–579. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 32.Gacouin A, Le Tulzo Y, Suprin E, Briens E, Bernard M, Camus C, et al. Acute respiratory failure caused by secondary alveolar proteinosis in a patient with acute myeloid leukemia: a case report. Intensive Care Med. 1998;24:265–267. doi: 10.1007/s001340050563. [DOI] [PubMed] [Google Scholar]
- 33.Birsak CA, van Rossem RN, Nijhuis-Heddes JM, Maartense E. Pulmonary alveolar proteinosis: a complication in patients with hematologic malignancy. Neth J Med. 2000;56:193–197. doi: 10.1016/s0300-2977(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Luaces M, Lafuente A, Martin MP, Mateos P, Ojeda E, Hernandez-Navarro F. Haematopoietic transplantation in pulmonary alveolar proteinosis associated with chronic myelogenous leukaemia. Bone Marrow Transplant. 1997;20:507–510. doi: 10.1038/sj.bmt.1700915. [DOI] [PubMed] [Google Scholar]
- 35.Shoji N, Ito Y, Kimura Y, Nishimaki J, Kuriyama Y, Tauchi T, et al. Pulmonary alveolar proteinosis as a terminal complication in myelodysplastic syndromes: a report of four cases detected on autopsy. Leuk Res. 2002;26:591–595. doi: 10.1016/s0145-2126(01)00178-3. [DOI] [PubMed] [Google Scholar]
- 36.Ohnishi T, Yamada G, Shijubo N, Takagi-Takahashi Y, Itoh T, Takahashi H, et al. Secondary pulmonary alveolar proteinosis associated with myelodysplastic syndrome. Intern Med. 2003;42:187–190. doi: 10.2169/internalmedicine.42.187. [DOI] [PubMed] [Google Scholar]
- 37.Klein B, Brailly H. Cytokine-binding proteins: stimulating antagonists. Immunol Today. 1995;16:216–220. doi: 10.1016/0167-5699(95)80161-8. [DOI] [PubMed] [Google Scholar]
- 38.Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 39.Rusakiewicz S, Molldrem JJ. Immunotherapeutic peptide vaccination with leukemia-associated antigens. Curr Opin Immunol. 2006;18:599–604. doi: 10.1016/j.coi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 41.Elisseeva OA, Oka Y, Tsuboi A, Ogata K, Wu F, Kim EH, et al. Humoral immune responses against Wilms tumor gene WT1 product in patients with hematopoietic malignancies. Blood. 2002;99:3272–3279. doi: 10.1182/blood.v99.9.3272. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman DC, Baer MR, Gao XZ, Wang ZQ, Preisler HD. Enhanced expression of the granulocyte-macrophage colony stimulating factor gene in acute myelocytic leukemia cells following in vitro blast cell enrichment. Blood. 1988;72:1329–1332. [PubMed] [Google Scholar]
- 43.Bi S, Gao X, Devemy E, Chopra H, Venugopal P, Raza A, et al. Cytokine production by in vitro processed and unprocessed haematopoietic cells. Cytokine. 2000;12:1124–1128. doi: 10.1006/cyto.1999.0631. [DOI] [PubMed] [Google Scholar]