Abstract
Although bone marrow-derived mesenchymal stem cells (MSCs) are an attractive cell therapy candidate, their potential is limited by poor survival following transplantation. Over-expression of anti-apoptotic heat shock proteins using viral vectors can improve the survival of these cells under stressful conditions in vitro and in vivo. It is also possible to induce heat shock protein expression in many cell types by simply exposing them to a transient, nonlethal elevation in temperature. The response profile of MSCs to such a thermal stress has not yet been reported. Therefore, this study sought to determine the kinetics of thermally induced heat shock protein expression by MSCs in vitro. To determine if heat shock protein expression was a function of thermal stress exposure time, MSCs were exposed to 42°C for 15, 30, 45, and 60 min and were harvested 24 h later. To establish the time-course of heat shock protein expression, MSCs were heat shocked for 60 min and harvested 2, 24, 48, 72, 96, and 120 h later. The cells were then analyzed for Hsp27 and Hsp70 expression by Western blot. Densitometric analysis revealed that exposure to a thermal stress induced expression of both Hsp27 and Hsp70 and that the level of expression was dependant on stress exposure time. Following 60 min of heat stress, both Hsp27 and Hsp70 accumulated maximal expression after 48 h with both proteins returning to constitutive expression levels by 120 h. This study demonstrates that heat shock protein expression can be induced in MSCs by a simple thermal stress.
Keywords: mesenchymal stem cells, heat shock proteins, apoptosis, thermal stress
Introduction
Adult bone marrow-derived mesenchymal stem cells (MSCs) have attracted considerable attention as a potential therapeutic moiety and have been used in encouraging preclinical studies for numerous disease states.1–9 However, one major obstacle that may hinder their clinical translation is the poor survival and viability of MSCs post-transplantation.10, 11 This is thought to occur as cells are exposed to oxidative stress, loss of matrix attachments, nutrient, and serum deprivation and in some cases, are placed in relatively hypoxic, ectopic environment to that in which they usually reside.12–15 Given that MSCs are susceptible, in part, to an apoptotic fate following transplantation,11 it follows that an antiapoptotic strategy may yield improved cell survival thus improving their candidacy for cell transplantation. One such approach may be to over-express antiapoptotic heat shock proteins in the cells prior to transplantation.16–18
Although heat shock proteins are more widely known for their protein chaperone role, literature suggests that members of the Hsp27 and Hsp70 family are important regulators of the signal transduction pathway leading to apoptosis.19 The intrinsic mitochondrial apoptotic pathway is used extensively in response to extracellular cues and internal insults such as DNA damage. Formation of the apoptosome (mitochondrial cytochrome c/Apaf-1/procaspase-9 complex) leads to cleavage and activation of caspase-3, resulting in an ordered dismantling of the cell. Hsp27 can bind cytochrome c, released from the mitochondria to the cytosol, thereby preventing cytochrome-c-mediated interaction of Apaf-1 with procaspase-9.20, 21 Hsp27 has also been shown to associate with procaspase-3 thereby repressing its activation.21, 22 Hsp70 also regulates the intrinsic apoptotic pathway, binding the caspase-recruitment domain of Apaf-1 thereby preventing recruitment of procaspase-9 to the apoptosome complex and blocking the assembly of a functional apoptosome.23, 24 As well as targeting the apoptosome, both Hsp27 and Hsp70 target several other components on the apoptotic pathway. Hsp27 can antagonize Bax-mediated mitochondrial injury,25 as well as modulating the extrinsic death receptor apoptotic pathway by interacting with Daxx thereby blocking Daxx-mediated apoptosis.26 Hsp70 can modulate apoptosis inducing factor activity,27–29 interfere with the Bid-dependent apoptotic pathway via inhibition of JNK,30 inhibit release of Smac, a mitochondrial derived activator of caspases31 and inhibit the death-associated permeabilization of lysosomes.32 Taken together, there is a considerable body of evidence to support the anti-apoptotic role of these heat shock proteins.
Indeed, the evidence that over-expression of anti-apoptotic heat shock proteins is protective has been reported for many cell types including bone marrow-derived MSCs. Adenoviral mediated over-expression of Hsp20 by MSCs protected against oxidative stress-mediated apoptosis in vitro,18 through Akt activation and increased secretion of growth factors. This increased Hsp20 expression also enhanced the survival of the MSCs following direct cardiac injection in a rat model of myocardial infarction. Similarly, over-expression of Hsp70 in MSCs resulted in higher cell viability under hypoxic conditions in vitro,16 and exhibition of antiapoptotic properties, including an increase in Bcl2 and a reduction of Bax and caspase-3 activity. Importantly, Hsp70 over-expression by the MSCs also resulted in an improved outcome following their transplantation in a rat myocardial infarction model. Lentiviral mediated over-expression of Hsp70 by MSCs has also been reported to enhance MSC survival and increase resistance to apoptosis in vitro under hypoxic and ischemic conditions.17
In addition to viral over-expression systems, a simple physiologically relevant method to increase heat shock protein synthesis and accumulation in cells is to expose the cells to a transient, nonlethal elevation in temperature.33–35 This induces a state of thermotolerance, thus rendering the cells resistant to subsequent otherwise lethal insults. Although documented for many cell types, the response profile of MSCs to such a thermal stress has not been reported. Therefore, this study sought to determine the kinetics of thermal induction of heat shock proteins by MSCs in vitro.
Results and Discussion
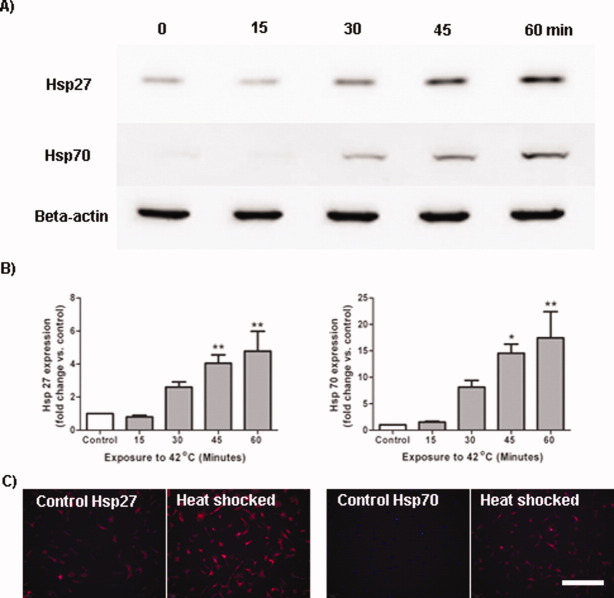
This experiment sought to determine whether exposure to a thermal stress would induce expression of the pro-survival proteins of interest, Hsp27 and Hsp70, in MSCs. MSCs were heat shocked by exposure to 42°C for 0 (i.e., control), 15, 30, 45, and 60 min followed by a 24 h recovery period in the 37°C incubator. Densitometric analysis of the immunoreactive Western blot bands (normalised to beta actin) revealed that MSCs constitutively expressed Hsp27 and relatively low levels of Hsp70 [Fig. 1(A)]. Exposure to a thermal stress induced a significantly increased expression of both Hsp27 (Time, F(4,15) = 7.90; P < 0.05) and Hsp70 (Time, F(4,10) = 17.10, P < 0.05) by MSCs over the 60 min time course compared to control MSCs. In terms of fold change in heat shock protein expression, 60 min exposure to 42°C induced a 4.8 fold increased expression of Hsp27 (Time, F(4,15) = 8.69, P < 0.05) and a 17.4 fold increased expression of Hsp70 (Time, F(4,10) = 9.41, P < 0.05) in MSCs compared with control populations. Corroborating the Western blotting data, immunocytochemistry for Hsp27 and Hsp70 on cells that had been exposed to a thermal stress for 60 min followed by a 24 h recovery period revealed stronger immunopositive staining for both Hsp27 and Hsp70 compared to control cells [Fig. 1(C)].
Figure 1.
Heat shock protein expression in MSCs is dependent on thermal stress exposure time. MSCs were immersed in a 42°C water bath for 15, 30, 45, and 60 min and cells were harvested 24 h later. A: Western blot showing that MSCs expressed both Hsp27 and Hsp70 after thermal stress. B: Densitometric analysis of the Western blots revealed that exposure to a heat stress induced expression of both Hsp27 and Hsp70 with longer exposure times resulting in greater levels of protein expression. *P < 0.05, **P < 0.01 vs. control. C: Immunocytochemistry reveals that following 60 min exposure to a thermal stress, MSCs express greater levels of Hsp27 and Hsp70 compared to untreated controls. Scale bar = 200 μm. These experiments were repeated on three separate occasions with technical replicates within each biological replicate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To determine the duration of heat shock protein expression by MSCs, the cells were heat shocked for 60 min and returned to a 37°C incubator for recovery. Samples were then harvested 2, 24, 48, 72, 96, and 120 h post-thermal stress for Western blot analysis. Results revealed a similar trend in sustenance of heat shock protein expression for both Hsp27 and Hsp70 (Fig. 2). By 2 h post-thermal stress, there was an increase in both Hsp27 and Hsp70 protein expression by the MSCs. This rapid increase in protein levels of heat shock proteins in response to a stress is a characteristic heat shock response.35 By 48 h post-thermal stress, Hsp27 expression levels reached their maximum level and were significantly increased compared with the control population of MSCs, which had not been heat shocked (Time, F(6,13) = 4.35, P < 0.05). By 120 h post-thermal stress, Hsp27 expression was significantly reduced compared with maximal levels seen at 48 h with the expression levels seen at 120 h not significantly different to constitutive control levels. A similar pattern was seen with Hsp70 expression profile. By 48 h post-thermal stress, Hsp70 expression levels reached their maximum and were significantly increased compared to the control population of MSCs which had not been heat shocked (Time, F(6,13) = 5.64, P < 0.05). By 120 h post-thermal stress, Hsp70 expression was significantly reduced compared with maximal levels seen at 48 h with the expression levels at 120 h not significantly different to constitutive levels.
Figure 2.
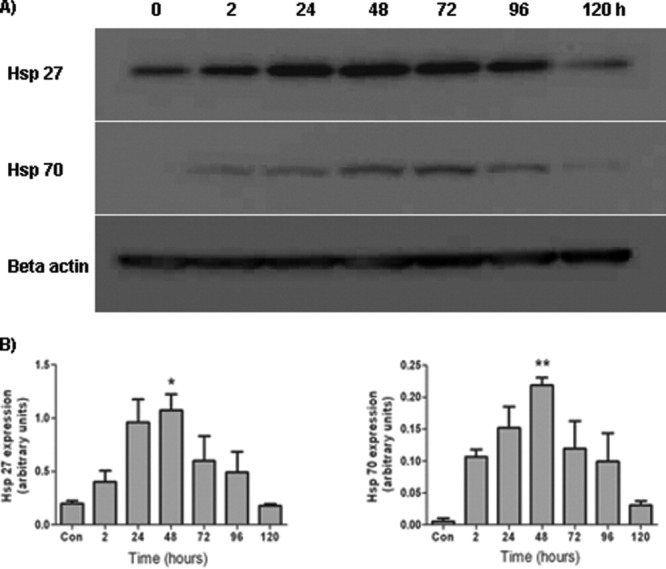
Time-course of heat shock protein expression after heat stress. MSCs were heat shocked for 60 min and harvested 2, 24, 48, 72, 96, and 120 h later. A: Western blot showing the time-course Hsp27 and Hsp70 expression by MSCs after thermal stress. B: Densitometric analysis of the Western blots revealed that protein expression was maximal 48 h following the thermal stress with return to baseline levels within 120 h for both Hsp27 and Hsp70. *P < 0.05, **P < 0.01 vs. control. These experiments were repeated on three separate occasions with technical replicates within each biological replicate.
In summary, this study has shown that Hsp27 and Hsp70 upregulation by MSCs in response to a simple, non-lethal elevation in temperature occurs as a function of the duration of the thermal stress exposure time as well as post-stress recovery time. Since, we7, 11 and other36, 37 have shown that the majority of MSCs die days after transplantation, it is conceivable that the transient (up to 4 days) heat shock protein expression displayed by MSCs following a thermal heat shock may be sufficient to equip the cells to survive the initial apoptotic insults that follow transplantation.
This method could easily be adopted by other researchers investigating the potential of antiapoptotic heat shock protein expression as an approach to improve the survival of MSCs after transplantation in vivo. Moreover, this simple, thermal stress approach may not have the disadvantages associated with viral and nonviral gene transfer techniques thus providing a more widely acceptable approach to heat shock protein expression in these potentially therapeutic cells.
Methods
Cell isolation and characterization
Isolation and characterization of the MSCs has been described in more detail elsewhere.38 In brief, MSCs were isolated from the bone marrow of 8–12 week old Sprague Dawley rats and subsequently cultured in complete medium composed of 44.5% Alpha MEM, 44.5% F12, (Gibco, The Netherlands), 10% FBS (ThermoScientific Hyclone, Fisher, Ireland), 1% penicillin/streptomycin (Sigma-Aldrich, Ireland), and incubated at 37°C in 5% CO2 at 90% humidity. The MSCs were characterized by differentiation to adipogenic, chondrogenic, and osteogenic lineages. The MSCs were also analyzed by flow cytometry for surface markers associated with MSCs including the presence of CD71 and CD172, and the absence of CD45.
Heat shock protocol
MSCs were seeded at 3 × 104 cells cm−2 in culture vessels and allowed to adhere overnight. Flask necks were wrapped with Parafilm® and the flasks were heat sealed into plastic bags. Flasks were submerged in a water bath set at 42°C for up to 60 min. Following treatment, the waterproof protection was removed and the cells were returned to a 37°C incubator.
Immunocytochemistry
Immunocytochemistry was performed for rat Hsp27 and rat Hsp70 on paraformaldehyde fixed cells adhered to poly-l-lysine coated plastic culture vessels. Nonspecific binding was blocked by incubating the cells in blocking solution for 1 h at room temperature (1% bovine serum albumin, 5% normal goat serum, 0.3% Triton-X 100, 0.01% sodium azide in TBS, Sigma-Aldrich, Ireland) followed by overnight incubation at room temperature in primary antibody (rabbit anti-Hsp25, 1:500; rabbit anti-Hsp70, 1:500, Stressgen) diluted in 1% BSA-TBS with 0.01% sodium azide. Cells were washed three times with TBS followed by 3 h incubation with an appropriate fluorophore-labeled secondary antibody (Alexa Fluor 546, goat anti-rabbit, 1:200, Invitrogen, UK) diluted in TBS with 1% normal goat serum. Cell nuclei were counterstained by incubation with 4′-6-diamidino-2-phenylindole (DAPI, 1 μg ml−1) for 5 min. Immunolabeled cells were viewed using an Olympus IX81 fluorescent microscope (Olympus UK, London, UK).
Western blotting
Cells pellets were lysed using 2% sodium dodecyl sulfate (SDS) containing a protease inhibitor cocktail (1 μl ml−1; Sigma-Aldrich, Ireland). The extracts (10 μg protein) were subjected to SDS polyacrylamide gel electrophoresis (SDS–PAGE) on a 12% acrylamide gel and transferred onto nitrocellulose membranes. After blocking in 5% nonfat milk in TBS containing 0.05% Tween-20, blots were incubated with rabbit polyclonal antibodies to Hsp27 (1:500; Stressgen), Hsp70 (1:500; Stressgen), and mouse monoclonal antibody to beta actin (1:10,000; Sigma-Aldrich, Ireland). Horseradish peroxidase-conjugated secondary antibodies (1:10,000) were obtained from Pierce. The protein bands were detected by the SuperSignal West Pico Chemiluminescent Substrate (Pierce), according to the manufacturer's protocol.
Statistical analyses
All data are expressed as mean ± s.e.m. Data was analyzed by one-way ANOVA followed by a post-hoc Newman Keuls.
Acknowledgments
This project was funded by the Irish Health Research Board.
References
- 1.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 2.Bouchez G, Sensebe L, Vourc'h P, Garreau L, Bodard S, Rico A, Guilloteau D, Charbord P, Besnard JC, Chalon S. Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson's disease. Neurochem Int. 2008;52:1332–1342. doi: 10.1016/j.neuint.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. J Neuroimmunol. 2009;216:39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, Perez-Polo JR, Yang K. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res. 2005;80:611–619. doi: 10.1002/jnr.20494. [DOI] [PubMed] [Google Scholar]
- 6.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Moloney TC, Rooney GE, Barry FP, Howard L, Dowd E. Potential of rat bone marrow-derived mesenchymal stem cells as vehicles for delivery of neurotrophins to the Parkinsonian rat brain. Brain Res. 2010;1359:33–43. doi: 10.1016/j.brainres.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Rooney GE, McMahon SS, Ritter T, Garcia Y, Moran C, Madigan NN, Flugel A, Dockery P, O'Brien T, Howard L, Windebank AJ, Barry FP. Neurotrophic factor-expressing mesenchymal stem cells survive transplantation into the contused spinal cord without differentiating into neural cells. Tissue Eng Part A. 2009;15:3049–3059. doi: 10.1089/ten.TEA.2009.0045. [DOI] [PubMed] [Google Scholar]
- 9.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 10.Coyne TM, Marcus AJ, Reynolds K, Black IB, Woodbury D. Disparate host response and donor survival after the transplantation of mesenchymal or neuroectodermal cells to the intact rodent brain. Transplantation. 2007;84:1507–1516. doi: 10.1097/01.tp.0000288185.09601.4d. [DOI] [PubMed] [Google Scholar]
- 11.Moloney TC, Dockery P, Windebank AJ, Barry PB, Howard L, Dowd E. Survival and immunogenicity of mesenchymal stem cells from the green fluorescent protein transgenic rat in the adult rat brain. Neurorehabil Neural Repair. 2010;24:645–656. doi: 10.1177/1545968309357745. [DOI] [PubMed] [Google Scholar]
- 12.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 13.Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- 14.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 16.Chang W, Song BW, Lim S, Song H, Shim CY, Cha MJ, Ahn DH, Jung YG, Lee DH, Chung JH, Choi KD, Lee SK, Chung N, Jang Y, Hwang KC. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27:2283–2292. doi: 10.1002/stem.153. [DOI] [PubMed] [Google Scholar]
- 17.McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O'Toole D, O'Brien T. Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res Ther. 2011;2:12. doi: 10.1186/scrt53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, Kranias EG, Wang Y, Fan GC. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27:3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22:9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- 20.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 21.Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr. 2001;9:195–201. doi: 10.3727/000000001783992605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, Weichselbaum R, Kufe D, Kharbanda S. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19:1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- 23.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 24.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 25.Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283:12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurbuxani S, Schmitt E, Cande C, Parcellier A, Hammann A, Daugas E, Kouranti I, Spahr C, Pance A, Kroemer G, Garrido C. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene. 2003;22:6669–6678. doi: 10.1038/sj.onc.1206794. [DOI] [PubMed] [Google Scholar]
- 28.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 29.Ruchalski K, Mao H, Singh SK, Wang Y, Mosser DD, Li F, Schwartz JH, Borkan SC. HSP72 inhibits apoptosis-inducing factor release in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol. 2003;285:C1483–1493. doi: 10.1152/ajpcell.00049.2003. [DOI] [PubMed] [Google Scholar]
- 30.Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22:3415–3424. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, Burger R, Munshi N, Ohtake Y, Saxena S, Anderson KC. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- 32.Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, Weber E, Multhoff G, Rohde M, Jaattela M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP, Orrenius S. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:hpmotc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Diller KR, Aggarwal SJ. Kinetics study of endogenous heat shock protein 70 expression. J Biomech Eng. 2003;125:794–797. doi: 10.1115/1.1632522. [DOI] [PubMed] [Google Scholar]
- 36.Isakova IA, Baker K, DuTreil M, Dufour J, Gaupp D, Phinney DG. Age- and dose-related effects on MSC engraftment levels and anatomical distribution in the central nervous systems of nonhuman primates: identification of novel MSC subpopulations that respond to guidance cues in brain. Stem Cells. 2007;25:3261–3270. doi: 10.1634/stemcells.2007-0543. [DOI] [PubMed] [Google Scholar]
- 37.Rossignol J, Boyer C, Thinard R, Remy S, Dugast AS, Dubayle D, Dey ND, Boeffard F, Delecrin J, Heymann D, Vanhove B, Anegon I, Naveilhan P, Dunbar GL, Lescaudron L. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J Cell Mol Med. 2009;13:2547–2558. doi: 10.1111/j.1582-4934.2008.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rooney GE, Moran C, McMahon SS, Ritter T, Maenz M, Flugel A, Dockery P, O'Brien T, Howard L, Windebank AJ, Barry FP. Gene-modified mesenchymal stem cells express functionally active nerve growth factor on an engineered poly lactic glycolic acid (PLGA) substrate. Tissue Eng Part A. 2008;14:681–690. doi: 10.1089/tea.2007.0260. [DOI] [PubMed] [Google Scholar]