Abstract
The conformational properties of soluble α-synuclein, the primary protein found in patients with Parkinson's disease, are thought to play a key role in the structural transition to amyloid fibrils. In this work, we report that recombinant 100% N-terminal acetylated α-synuclein purified under mild physiological conditions presents as a primarily monomeric protein, and that the N-terminal acetyl group affects the transient secondary structure and fibril assembly rates of the protein. Residue-specific NMR chemical shift analysis indicates substantial increase in transient helical propensity in the first 9 N-terminal residues, as well as smaller long-range changes in residues 28–31, 43–46, and 50–66: regions in which the three familial mutations currently known to be causative of early onset disease are found. In addition, we show that the N-terminal acetylated protein forms fibrils that are morphologically similar to those formed from nonacetylated α-synuclein, but that their growth rates are slower. Our results highlight that N-terminal acetylation does not form significant numbers of dimers, tetramers, or higher molecular weight species, but does alter the conformational distributions of monomeric α-synuclein species in regions known to be important in metal binding, in association with membranes, and in regions known to affect fibril formation rates.
Keywords: α-synuclein, N-terminal acetylated α-synuclein, NMR, mass spectrometry, aggregation, Parkinson's disease, fluorescence, amyloid fibril
Introduction
Alpha synuclein (αsyn) is a small primarily neuronal protein that is known to make a structural transition to amyloid fibrils in several neurodegenerative diseases. It is a major component of Lewy Bodies in patients with Parkinson's, a disease resulting from a loss of dopaminergic neurons.1, 2 While a large body of evidence over many years has supported the characterization of αsyn as an intrinsically disordered monomer, a recent study by Bartels et al. in which αsyn was isolated from red blood cells, as well as neuronal and non-neuronal cell lines, reported that in its physiological form, αsyn exists as a helical tetramer that is resistant to amyloid formation, and has a mass corresponding to the sole modification of the monomer by an acetyl group.3 Shortly thereafter, a GST recombinant αsyn protein purified from the micellar reagent β-octyl glucoside (BOG), similarly showed the existence of a dynamic αsyn tetramer.4 In response to these papers, Faivet et al. and an assemblage of groups went on to demonstrate that αsyn isolated from rodent and human nervous system tissues and erythrocytes presents as an intrinsically disordered monomer. In this work, Fauvet et al. was the first to address the role of the acetyl group, referred to in the Bartel et al. paper, and showed the acetylated and nonacetylated proteins migrate similarly on nondenaturing gels.5 A follow-up report by Trexler et al. has indicated that recombinant acetylated αsyn (Ac-αsyn) is monomeric under physiological conditions, but that it may display a greater preference for helical structure and higher-order oligomerization states when purified in the presence of BOG.6
It has been demonstrated that soluble and insoluble fractions of brain tissues from patients suffering from Parkinson's and from dementia with Lewy bodies universally contain N-terminal Ac-αsyn.7, 8 While an uncommon modification to prokaryotic proteins, the N-termini of eukaryotic proteins are often processed at the initiating amino acid with the addition of an acetyl group by N-acetyltransferase complexes.9 The role of N-terminal acetylation, however, is poorly understood, but has been suggested to affect the thermal stability of proteins.10, 11 Because N-terminal Ac-αsyn is now believed to be the physiologically relevant species in the brain, it is critically important to characterize the conformational properties and fibrillation kinetics of this protein to understand how acetylation impacts on the mechanism of fibril formation and disease.
Here we present the first direct experimental evidence that N-terminal acetylation affects the secondary structure propensities and kinetics of fibril assembly of Ac-αsyn, relative to the nonacetylated protein. Using NMR, noncovalent electrospray ionization mass spectrometry (ESI-MS), ion mobility spectrometry combined with ESI-MS (ESI-IMS-MS), Thioflavin T (ThT) fluorescence, and electron microscopy (EM), we demonstrate that the 100% N-terminal acetylated recombinant αsyn protein purified under mild physiological conditions presents primarily as a disordered monomer. Our results highlight that N-terminal acetylation impacts on secondary structure propensity in important functional regions including the N-terminal and His-50 metal binding regions12 and the regions of the three familial mutations A30P, E46K, and A53T.13–15 The removal of the positive charge at the N-terminus arising from acetylation thus has short and long-range conformational effects that impact on the distribution of states sampled by the intrinsically disordered αsyn monomer and on the rate of fibril assembly.
Results and Discussion
To determine the oligomeric status and the conformational properties of Ac-αsyn and to compare this with αsyn, Ac-αsyn was generated from an E.coli coexpression system containing the yeast N-terminal acetyltransferase B(NatB)6, 16 and purified using mild physiological purification conditions (herein described as “mild” purification) that avoid steps involving the application of heat or salting out (herein described as “harsh” conditions) (see Supporting Information for detailed protocols of “mild” and “harsh” purification). Taking into account recent suggestions that harsh purification steps such as boiling can destroy tetramer formation3, 4 or that purification under nonphysiological conditions6 can promote higher-order oligomerization states of αsyn, a mild physiological purification protocol that applies only homogenization and liquid chromatography was adopted for Ac-αsyn in this work. ESI-MS confirms that purified Ac-αsyn coexpressed with this eukaryotic modification system exists as 100% acetylated protein (observed mass 14502.5 Da, expected mass 14502.1 Da) [Fig. 1(A,B)].
Figure 1.
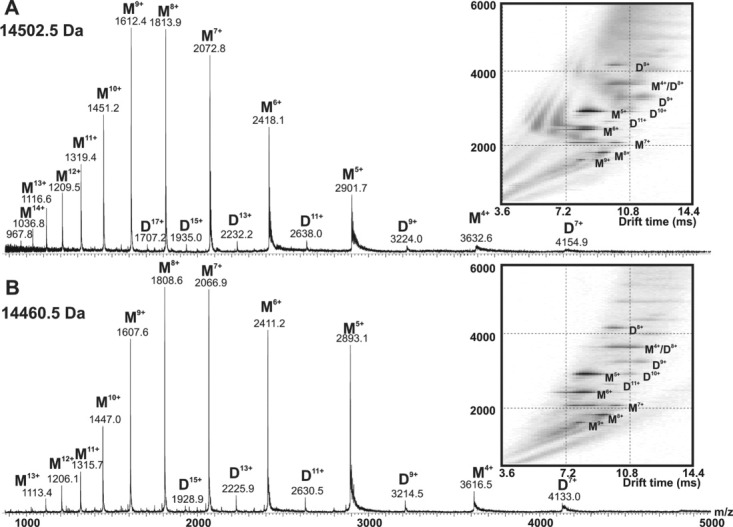
Native ESI-IMS-MS analysis of (A) Ac-αsyn and (B) αsyn, showing both samples to be predominantly monomeric. Ac-αsyn or αsyn (35 μM) were dissolved or buffer exchanged into 165 mM ammonium acetate, pH 7.4. Inset shows the driftscope plot of each sample acquired under conditions optimized for the detection of large noncovalent species (cone voltage 170 V). ESI mass spectra of αsyn purified under “harsh” and “mild” conditions indicate that the purification procedure does not affect the results obtained (not shown).
Investigation of the existence of higher-order oligomeric states in the Ac-αsyn was performed using ESI-MS and ESI-IMS-MS (Fig. 1), along with solution methods including analytical size-exclusion chromatography (SEC) and migration on a native gel (Supporting Information Fig. S1). The latter two methods show that both proteins elute at the same volume as a single peak using SEC and migrate at the same position on the native gel. These data show that the acetylated protein exhibits indistinguishable hydrodynamic dimensions from the nonacetylated protein purified under mild conditions. Furthermore, comparison by native gel, SEC, 1H-15N HSQC and ESI-MS (Supporting Information Fig. S2) of the nonacetylated αsyn purified by harsh or mild purification protocols indicate that they are essentially indistinguishable, consistent with other comparisons made by Fauvet et al.5
ESI-IMS-MS experiments add further information about the oligomerization status of Ac-αsyn, as the population distributions can be obtained quantitatively from these experiments by the ability of ESI-IMS-MS to separate peaks of identical m/z and to quantify their population and conformational properties.17 Comparison of the ESI-IMS-MS spectra of Ac-αsyn and αsyn indicate that acetylation does not perturb the oligomerization status of αsyn, as both αsyn and Ac-αsyn appear predominantly monomeric (∼90–95%), with the presence of a small population of dimeric species (∼5–10%), where the former appears in both more compact and extended forms at physiological pH [Fig. 1(A,B)]. These data are consistent with previous analyses using ESI-MS,18–20 and interchain NMR PRE experiments that have shown weak dimer N- to C-terminal interchain interactions in αsyn under physiological conditions.21 Because of the suggestion in the recent literature that Ac-αsyn purified under micellar BOG conditions can shift the monomer populations toward oligomeric species,6 the possibility of higher-order transient oligomeric species purified under physiological conditions was further probed using a higher cone voltage (170 V) in ESI-IMS-MS, which favors detection of large noncovalent aggregates. These experiments revealed no difference between the oligomeric distribution of acetylated and nonacetylated samples and no evidence for the population of higher-order species, consistent with results obtained by Trexler et al. when purification was performed under physiological conditions.6 While the biophysical techniques inform about the hydrodynamic radii, ESI-IMS-MS provides definite evidence that both proteins are predominantly monomeric in aqueous solution at pH 7.4.
Residue-specific analysis of Ac-αsyn by NMR was next pursued to enable the secondary structure propensities of Ac-αsyn and αsyn monomers to be compared (Fig. 2). Backbone assignments by triple resonance experiments confirmed that Ac-αsyn is acetylated on the N-terminal residue Met-1 (Supporting Information Fig. S3). An overlay of the 1H-15N spectrum of Ac-αsyn and αsyn at pH 7.4, shows that the two proteins share a high degree of similarity, except at the first nine N-terminal residues [Fig. 2(A)]. Both Ac-αsyn and αsyn display narrow chemical shift dispersion, characteristic of a predominantly unfolded protein, consistent with analyses using far UV CD (Supporting Information Fig. S4). Acetylation of the N-terminus results in the appearance of the Met-1 and Asp-2 resonances in the Ac-αsyn 1H-15N HSQC spectrum possibly because of changes in hydrogen exchange rates arising from the modification at Met-1. Additionally, acetylation results in up-field shifting of residues observable in both spectra in the region of the first nine N-terminal residues, [inset Fig. 2(A)] further supporting the site of acetylation as Met-1 and demonstrating the extent to which N-terminal acetylation alters the conformational properties of the polypeptide chain.
Figure 2.
A. 1H–15N HSQC spectra of Ac-αsyn (magenta) versus αsyn (blue) at 15°C. The changes in the first nine residues of Ac-αsyn are indicated in the spectrum. The Δδ (ppm) for the inset is calculated by the expression ((ΔH)2 + (0.159*Δ15N)2)½22(inset). B. Top: Schematic of αsyn divided into N-terminal, NAC and C-terminal regions. Middle: SSP analysis of Ac-αsyn (magenta) and αsyn (blue) using 13Cα and 13Cβ chemical shifts as input and a five residue sliding window with Zhang et al. random coil references.2313C assignments for Ac-αsyn were obtained from a 350 μM doubly labeled protein in PBS buffer at 15°C and 13C assignments for αsyn were used from a previous report.24 Bottom: Differences of SSP (ΔSSP = SSP(Ac-αsyn) – SSP(αsyn)) between Ac-αsyn and αsyn with boxes shown at positions of familial mutations. 1H-15N HSQC comparison of αsyn purified under mild and harsh conditions indicate that they are very similar (Supporting Information Fig. S2).
The secondary structure propensity of Ac-αsyn was next examined by analyzing NMR chemical shift perturbations and was compared with that of αsyn [Fig. 2(B)]. A number of methods have been developed to determine the secondary structural propensities of unfolded proteins.25–27 Here we use secondary structure propensity scores (SSP)26 which represent ensemble-averaged values over a distribution of states to obtain the secondary structure propensities of both Ac-αsyn and αsyn. Paralleling the chemical shift deviations observed in 1H-15N HSQC spectra [Fig. 2(A)], increased SSP values up to 0.3 are observed for the first 12 residues in the N-terminal region of Ac-αsyn. These values are significantly larger than those observed previously for αsyn and mutants of this protein24, 28–30 and represent a significant stabilization of transient helix and hence a redistribution of the structural ensemble sampled by the monomeric protein within the N-terminal region [Fig. 2(B)]. Longer-range perturbations, although small, are observed, in other regions of the N-terminus in the regions between residues 28–31, 43–46, 50–66 and are marked by a decrease in β-sheet propensity in Ac-αsyn [Fig. 2(B)]. By contrast, the NAC and C-terminal regions [Fig. 2(B)] remain relatively unperturbed by acetylation. The change of secondary structure propensities arising from acetylation may relate to important structural and functional properties of the protein. Specifically, changes are observed at Met-1, Asp-2, and His-50, the high affinity copper binding regions,12, 31–33 and at the three familial mutants A30P, E46K, and A53T associated with Parkinson's disease that affect the rate of fibril formation.34, 35 Together, the results reveal that N-terminal acetylation of αsyn does not by itself cause the intrinsically disordered protein (IDP) to self-assemble into tetrameric or other higher oligomeric forms, however, marked short range and subtle long-range effects of N-terminal acetylation are observed on the disordered monomer.
While N-terminal acetylation has been shown to increase helicity in peptides,36 the data presented here represent the first investigation of acetylation at the N-terminus of a full-length IDP. The increased helicity in Ac-αsyn can be rationalized by stabilization of the helix macrodipole,37 where removal of the α-amino positive charge upon acetylation is favorable to the overall dipole moment of the helix that this IDP transiently samples. The acetyl group is also known to form a highly favorable helix N-cap, in which the acetyl-carbonyl group interacts favorably with unsatisfied hydrogen bond donors in the N-terminal turn of the helix.38, 39
The fibrillation properties of Ac-αsyn and αsyn were also examined using ThT fluorescence to provide macroscopic information about the role of N-terminal acetylation in modifying the efficiency of fibril nucleation and elongation. The normalized ThT fluorescence of Ac-αsyn and αsyn at pH 7.4 exhibit typical sigmoidal curves for fibril assembly (Supporting Information Fig. S5). The lag times of the samples were highly variable, thereby ruling out analysis of the role of N-terminal acetylation in fibril nucleation. The elongation rates are slower in the acetylated versus nonacetylated asyn proteins for all experimental trails. For both the lag times and elongation rates we note that the aggregation kinetics are sensitive to the purification methods [Fig. 3(A)]. The change in fibrillation rate may result from stabilization of the N-terminal region of the protein by acetylation, or changes in secondary structure propensities at residues 50–66, which have been shown previously to have significant effects upon the kinetics of fibril formation,24, 28, 40 or both. Physicochemical changes resulting from acetylation may affect electrostatic and hydrophobic interactions, resulting in alterations in transient long-range contacts between the highly charged C-terminal region and the helix stabilized N-terminal region. This redistribution of states may have effects upon fibril formation.
Figure 3.
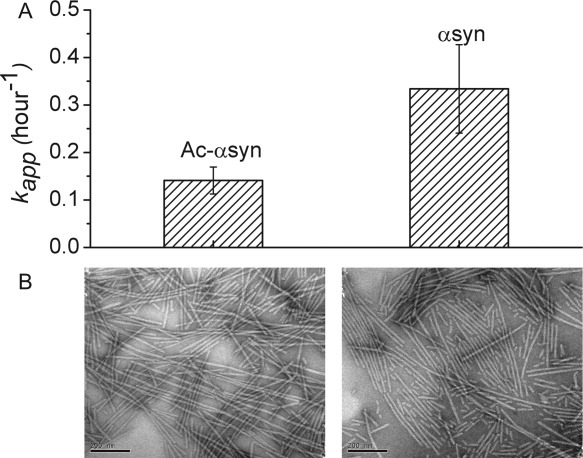
A. The apparent rate of fibril elongation of Ac-αsyn and αsyn performed at 37°C with 600 rpm shaking at a protein concentration of ∼150 μM measured using ThT fluorescence. Both proteins formed fibrils within 230 h. B. Negatively stained electron micrographs of the end products of fibril formation of Ac-αsyn (left) and αsyn (right). The scale bar is 200 nm.
This work presents the first NMR structural characterization of N-terminal Ac-αsyn and illustrates the effect of N-terminal acetylation on the fibrillation rates of the protein. We demonstrate conclusively using ESI-IMS-MS that the equilibrium states that are sampled in the Ac-αsyn are primarily monomer and a small population of dimer. NMR data support the view that both Ac-αsyn and αsyn exist at pH 7.4 as intrinsically disordered monomers, and further shows that N-terminal acetylation results in significant stabilization of transient helical propensity in the first 9 residues of the protein along with longer range changes in secondary structure between residues 50–66 and around the three familial mutants A30P, E46K, and A53T. These regions represent important functional regions associated with metal ion binding and with familial mutants that affect aggregation rates. We show that N-terminal acetylation not only changes the distribution of the intrinsically disordered monomeric conformers within the structural ensemble of αsyn, but that this cotranslational modification disfavors fibril formation, presumably caused by the conformational redistribution of the monomeric protein.
Materials and Methods
Protein expression and purification
Expression of acetylated protein was achieved by coexpressing the αsyn plasmid and the NatB plasmid in E. coli. using a procedure similar to that described by Trexler et al.6 The NatB plasmid was kindly gifted by Dr. Daniel Mulvihill. Acetylated αsyn was purified only under “mild” physiological conditions and nonacetylated αsyn protein was purified under both “mild” physiological and “harsh” conditions for comparison (see Supporting Information for detailed descriptions of Ac-αsyn expression and “harsh” and “mild” purification protocols).
Biophysical characterization
NMR assignments, ThT fluorescence and TEM experiments were performed as described previously.24 Native gel, SEC and CD procedures are described along with Supporting Information Figures S1 and S2, respectively. ESI-IMS-MS experiments were performed using similar procedures to Smith et al.17 αsyn proteins (35 μM) were dissolved or buffer exchanged into 165 mM ammonium acetate, pH 7.4 and the sampling cone voltage was varied from native conditions (30 V) to conditions that allow for the detection of large noncovalent species (170 V).
Acknowledgments
The authors thank Elizabeth Rhoades for helpful discussions and for the suggestion of the NatB co-expression system to make acetylated α-synuclein; Vikas Nanda for access to CD; and Mr. Valentin Starovoytov for assistance with TEM images.
Glossary
Abbreviations
- αsyn
alpha synuclein
- Ac-αsyn
acetylated αsyn
- BOG
β-octyl glucoside
- EM
fluorescence electron microscopy
- ESI-IMS-MS
ion mobility spectrometry combined with ESI-MS
- ESI-MS
noncovalent electrospray ionization mass spectrometry
- IDP
intrinsically disordered protein
- NatB
N-acetyltransferase B
- SEC
analytical size-exclusion chromatography
- ThT
Thioflavin T
Supplementary material
Additional Supporting Information may be found in the online version of this article.
References
- 1.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 2.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA. Alpha-synuclein in the central nervous system and from erythrocytes, mammalian cells and E. coli exists predominantly as a disordered monomer. J Biol Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trexler AJ, Rhoades E. N-terminal acetylation is critical for forming alpha-helical oligomer of alpha-synuclein. Protein Sci. 2012;21:601–605. doi: 10.1002/pro.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 8.Ohrfelt A, Zetterberg H, Andersson K, Persson R, Secic D, Brinkmalm G, Wallin A, Mulugeta E, Francis PT, Vanmechelen E, Aarsland D, Ballard C, Blennow K, Westman-Brinkmalm A. Identification of novel alpha-synuclein isoforms in human brain tissue by using an online NanoLC-ESI-FTICR-MS method. Neurochem Res. 2011;36:2029–2042. doi: 10.1007/s11064-011-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325:595–622. doi: 10.1016/s0022-2836(02)01269-x. [DOI] [PubMed] [Google Scholar]
- 10.Arnesen T. Towards a functional understanding of protein N-terminal acetylation. PLoS Biol. 2011;9:e1001074. doi: 10.1371/journal.pbio.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polevoda B, Sherman F. Nalpha-terminal acetylation of eukaryotic proteins. J Biol Chem. 2000;275:36479–36482. doi: 10.1074/jbc.R000023200. [DOI] [PubMed] [Google Scholar]
- 12.Bisaglia M, Tessari I, Mammi S, Bubacco L. Interaction between alpha-synuclein and metal ions, still looking for a role in the pathogenesis of Parkinson's disease. Neuromol Med. 2009;11:239–251. doi: 10.1007/s12017-009-8082-1. [DOI] [PubMed] [Google Scholar]
- 13.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 14.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 15.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 16.Johnson M, Coulton AT, Geeves MA, Mulvihill DP. Targeted amino-terminal acetylation of recombinant proteins in E. coli. PLoS One. 2010;5:e15801. doi: 10.1371/journal.pone.0015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DP, Radford SE, Ashcroft AE. Elongated oligomers in beta2-microglobulin amyloid assembly revealed by ion mobility spectrometry-mass spectrometry. Proc Natl Acad Sci USA. 2010;107:6794–6798. doi: 10.1073/pnas.0913046107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein SL, Liu D, Wyttenbach T, Bowers MT, Lee JC, Gray HB, Winkler JR. Alpha-synuclein: stable compact and extended monomeric structures and pH dependence of dimer formation. J Am Soc Mass Spectrom. 2004;15:1435–1443. doi: 10.1016/j.jasms.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Natalello A, Benetti F, Doglia SM, Legname G, Grandori R. Compact conformations of alpha-synuclein induced by alcohols and copper. Protein. 2011;79:611–621. doi: 10.1002/prot.22909. [DOI] [PubMed] [Google Scholar]
- 20.Frimpong AK, Abzalimov RR, Uversky VN, Kaltashov IA. Characterization of intrinsically disordered proteins with electrospray ionization mass spectrometry: conformational heterogeneity of alpha-synuclein. Proteins. 2010;78:714–722. doi: 10.1002/prot.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu KP, Baum J. Detection of transient interchain interactions in the intrinsically disordered protein alpha-synuclein by NMR paramagnetic relaxation enhancement. J Am Chem Soc. 2010;132:5546–5547. doi: 10.1021/ja9105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liokatis S, Dose A, Schwarzer D, Selenko P. Simultaneous detection of protein phosphorylation and acetylation by high-resolution NMR spectroscopy. J Am Chem Soc. 2010;132:14704–14705. doi: 10.1021/ja106764y. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Neal S, Wishart DS. RefDB: a database of uniformly referenced protein chemical shifts. J Biomol NMR. 2003;25:173–195. doi: 10.1023/a:1022836027055. [DOI] [PubMed] [Google Scholar]
- 24.Kang L, Wu KP, Vendruscolo M, Baum J. The A53T mutation is key in defining the differences in the aggregation kinetics of human and mouse alpha-synuclein. J Am Chem Soc. 2011;133:13465–13470. doi: 10.1021/ja203979j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Jardetzky O. Probability-based protein secondary structure identification using combined NMR chemical-shift data. Protein Sci. 2002;11:852–861. doi: 10.1110/ps.3180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh JA, Singh VK, Jia Z, Forman-Kay JD. Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci. 2006;15:2795–2804. doi: 10.1110/ps.062465306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilloni C, De Simone A, Vranken WF, Vendruscolo M. Determination of secondary structure populations in disordered States of proteins using nuclear magnetic resonance chemical shifts. Biochemistry. 2012;51:2224–2231. doi: 10.1021/bi3001825. [DOI] [PubMed] [Google Scholar]
- 28.Bussell R, Jr, Eliezer D. Residual structure and dynamics in Parkinson's disease-associated mutants of alpha-synuclein. J Biol Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 29.Sung YH, Eliezer D. Residual structure, backbone dynamics, and interactions within the synuclein family. J Mol Biol. 2007;372:689–707. doi: 10.1016/j.jmb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rospigliosi CC, McClendon S, Schmid AW, Ramlall TF, Barre P, Lashuel HA, Eliezer D. E46K Parkinson's-linked mutation enhances C-terminal-to-N-terminal contacts in alpha-synuclein. J Mol Biol. 2009;388:1022–1032. doi: 10.1016/j.jmb.2009.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Structural characterization of copper(II) binding to alpha-synuclein: insights into the bioinorganic chemistry of Parkinson's disease. Proc Natl Acad Sci USA. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung YH, Rospigliosi C, Eliezer D. NMR mapping of copper binding sites in alpha-synuclein. Biochim Biophys Acta. 2006;1764:5–12. doi: 10.1016/j.bbapap.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Binolfi A, Lamberto GR, Duran R, Quintanar L, Bertoncini CW, Souza JM, Cervenansky C, Zweckstetter M, Griesinger C, Fernandez CO. Site-specific interactions of Cu(II) with alpha and beta-synuclein: bridging the molecular gap between metal binding and aggregation. J Am Chem Soc. 2008;130:11801–11812. doi: 10.1021/ja803494v. [DOI] [PubMed] [Google Scholar]
- 34.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 35.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabartty A, Doig AJ, Baldwin RL. Helix capping propensities in peptides parallel those in proteins. Proc Natl Acad Sci USA. 1993;90:11332–11336. doi: 10.1073/pnas.90.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fairman R, Shoemaker KR, York EJ, Stewart JM, Baldwin RL. Further studies of the helix dipole model: effects of a free alpha-NH3+ or alpha-COO- group on helix stability. Proteins. 1989;5:1–7. doi: 10.1002/prot.340050102. [DOI] [PubMed] [Google Scholar]
- 38.Doig AJ, Chakrabartty A, Klingler TM, Baldwin RL. Determination of free energies of N-capping in alpha-helices by modification of the Lifson-Roig helix-coil therapy to include N- and C-capping. Biochemistry. 1994;33:3396–3403. doi: 10.1021/bi00177a033. [DOI] [PubMed] [Google Scholar]
- 39.Aurora R, Rose GD. Helix capping. Protein Sci. 1998;7:21–38. doi: 10.1002/pro.5560070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uversky VN, Eliezer D. Biophysics of Parkinson's disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



