Abstract
Aims
Functional mitral regurgitation (FMR) contributes to morbidity and mortality in heart failure (HF) patients. The aim of this study was to determine whether percutaneous mitral annuloplasty could safely and effectively reduce FMR and yield durable long-term clinical benefit.
Methods and results
The impact of mitral annuloplasty (Carillon Mitral Contour System) was evaluated in HF patients with at least moderate FMR. Patients in whom the device was placed then acutely recaptured for clinical reasons served as a comparator group. Quantitative measures of FMR, left ventricular (LV) dimensions, New York Heart Association (NYHA) class, 6 min walk distance (6MWD), and quality of life were assessed in both groups up to 12 months. Safety and key functional data were assessed in the implanted cohort up to 24 months. Thirty-six patients received a permanent implant; 17 had the device recaptured. The 30-day major adverse event rate was 1.9%. In contrast to the comparison group, the implanted cohort demonstrated significant reductions in FMR as represented by regurgitant volume [baseline 34.5 ±11.5 mL to 17.4 ±12.4 mL at 12 months (P < 0.001)]. There was a corresponding reduction in LV diastolic volume [baseline 208.5 ±62.0 mL to 178.9 ±48.0 mL at 12 months (P =0.015)] and systolic volume [baseline 151.8 ±57.1 mL to 120.7 ±43.2 mL at 12 months (P =0.015)], compared with progressive LV dilation in the comparator. The 6MWD markedly improved for the implanted patients by 102.5 ±164 m at 12 months (P =0.014) and 131.9 ±80 m at 24 months (P < 0.001).
Conclusion
Percutaneous reduction of FMR using a coronary sinus approach is associated with reverse LV remodelling. Significant clinical improvements persisted up to 24 months.
Keywords: Mitral regurgitation, Percutaneous mitral annuloplasty, Heart failure, Transcatheter mitral valve repair
Introduction
Functional mitral regurgitation (FMR) is common in systolic heart failure and is increasingly thought to contribute to both the symptomatology and progressive remodelling which accompanies the condition.1,2 Despite its importance, relatively few targeted interventions have focused specifically on the treatment of FMR. Surgical annuloplasty has been shown to reduce New York Heart Association (NYHA) and left ventricular (LV) dimensions, but typically in the context of surgical revascularization.3 Cardiac resynchronization therapy has been shown to cause reverse remodelling4 and improve survival5 in patients in whom the pacing reduces FMR; however, this therapy is not uniformly effective in regard to its effect on ventricular function or FMR per se. Recently, the application of a percutaneous approach directed specifically toward the reduction of FMR using the MitraClip has been reported in high-risk patients. While the study showed both clinical improvement and reverse remodelling, no comparison group was available to evaluate the risk–benefit profile.6
Mitral annuloplasty can be accomplished with the Carillon® Mitral Contour System™ (Cardiac Dimension Inc., Kirkland, WA, USA). A feasibility study with a first-generation implant positioned in the coronary sinus (CS) previously showed both FMR reduction and clinical improvement in exercise tolerance during 6 months.7 The TITAN (Transcatheter Implantation of Carillon Mitral Annuloplasty Device) trial was designed to build upon the foundation of that feasibility study in three ways: (i) a modified device designed to anchor more effectively in the CS was used; (ii) non-implanted patients were followed for 12 months to serve as a comparison group; and (iii) implanted patients were followed for 24 months to assess safety and functional changes further.
Methods
Patient population
Fifty-three patients from seven European centres were included in the trial. Inclusion criteria were dilated ischaemic or non-ischaemic cardiomyopathy; at least moderate (2+) FMR; LV ejection fraction <40%; NYHA class II–IV; 6 min walk distance (6MWD) 150–450 m; and stable heart failure medication regimen (i.e. diuretic, beta-blocker, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for 3 months). Key exclusion criteria were: hospitalization in the past 30 days for intravenous inotrope infusion; severe tricuspid regurgitation; serum creatinine >2.2 mg/dL; significant organic mitral valve pathology; and pacing lead already in the CS.
Study design
The TITAN study was a prospective, non-randomized, non-blinded, multicentre trial. Patients in whom the device was implanted then acutely recaptured for clinical indications (i.e. transient coronary compromise or <1 grade FMR reduction) underwent follow-up assessments identical to the implanted cohort (baseline, 1, 6, and 12 months) and served as a comparison group. The implanted cohort underwent further safety and functional assessments up to 24 months.
Echocardiographic assessments included regurgitant volume, effective regurgitant orifice area (EROA), vena contracta, FMR jet area relative to left atrial area (MRJA/LAA), overall FMR grade, annular septal–lateral diameter, LV dimensions, and LV ejection fraction. An echo core lab provided hands-on training of all sonographers prior to study initiation, and performed quality control throughout the study. The core lab followed The American Society of Echocardiography criteria to analyse measures of FMR and LV dimensions.8 The MRJA/LAA was determined from apical two- and four-chamber windows at standard Nyquist limits, vena contracta from optimized parasternal long axis windows, and EROA and regurgitant volume from apical windows using a Nyquist limit of 30–40 cm/s. LV ejection fraction was determined from the modified Simpson formula.
Clinical outcome measures included NYHA classification, 6MWD, and quality of life (QOL) assessed by the Kansas City Cardiomyopathy Questionnaire. Patients were provided with instructions about the 6MWD test, but received no encouragement during the test.
Safety was defined as the 30-day composite of death, myocardial infarction, cardiac perforation, device embolization, or surgery for device failure.
An independent clinical events committee and a data and safety monitoring board had trial oversight. Data management and statistical analysis was undertaken by an independent data management group. The study complies with the Declaration of Helsinki. The protocol incorporated feedback from the Food and Drug Administration regarding trial size, clinical study methods, inclusion/exclusion criteria, and independent trial oversight to ensure that the feasibility trial was appropriately designed. The protocol was approved by the institutional ethics committee at each site, and informed written consent was obtained from all patients (or their legal representatives).
Device description and procedure overview
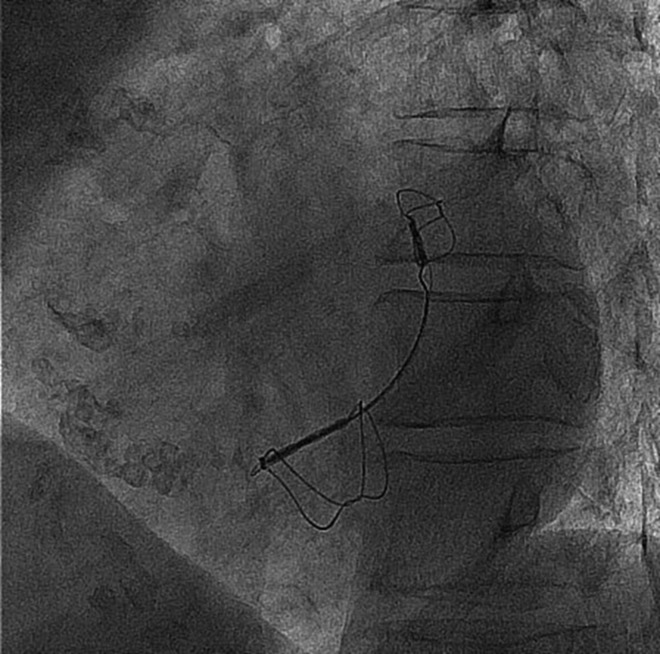
The Carillon device is a fixed-length double anchor implant with mirror-image hoop-shaped helical anchors (Figure 1). For the procedure, patients were either sedated or anaesthetized depending on site preference. The CS was cannulated from the jugular vein with a 9F delivery catheter. A distal anchor, oversized relative to the venous dimensions to provide stable anchoring, was then deployed deep in the CS/great cardiac vein. Next, traction was placed on the delivery system to plicate the periannular tissue. The degree of traction was guided by both fluoroscopic and echocardiographic assessments. Once tissue plication was optimized, the proximal anchor was deployed near the CS ostium. Because the circumflex artery crosses the CS, angiography was performed to assess coronary flow and determine whether device recapture was warranted. Further details of the procedure have been described previously.7
Figure 1.
Fluoroscopic image of an implanted Carillon device illustrates the coronary sinus position along the posterior mitral annulus. Tissue plication is maintained between the great cardiac vein anchor and the coronary sinus anchor.
Statistical analysis
For the primary safety endpoint, the P-value was calculated as the difference between groups for the total proportion of patients who experienced a major adverse event by 30 days. For comparison of baseline characteristics, ordinal measures were assessed using the mean score Cochran–Mantel–Haenszel test, nominal scale measures were assessed using the generalized Cochran–Mantel–Haenszel test, and continuous measures were assessed using the t-test. For intragroup comparisons, the Friedman test was used to assess change through time in repeated measures for categorical variables, and repeated measure analysis of variance (ANOVA) adjusted for baseline for continuous variables. For between-group comparisons, the P-values were computed for comparing the difference between two groups by repeated measure ANOVA for change from baseline to 1-, 6-, and 12-month follow-ups, adjusted for baseline. Intragroup comparisons of echocardiographic data from baseline to 6 and 12 months were assessed using a paired t-test.
Results
Demographics and implant procedure
Patient characteristics are presented in Table 1. There were no significant differences in demographics, echocardiographic measures, or cardiovascular history between implanted and non-implanted patients.
Table 1.
Baseline patient characteristics
| Intent-to-treat population (n =53) | Permanent implant population (n =36) | Device recaptured population (n =17) | P-value | |
|---|---|---|---|---|
| Demographic factors | ||||
| Age (years) | 62.44 ±12.69 (53) | 62.37 ±12.67 (36) | 62.59 ±13.11 (17) | 0.954 |
| Gender | ||||
| Male | 77.4% (41/53) | 75.0% (27/36) | 82.4% (14/17) | 0.730 |
| Female | 22.6% (12/53) | 25.0% (9/36) | 17.6% (3/17) | |
| NYHA class | 3.0 ±0.24 (53) | 3.1 ±0.23 (36) | 2.9 ±0.24 (17) | 0.105 |
| 6 min walk distance (m) | 314 ±77.9 (53) | 302 ±73.6 (36) | 338 ±83.4 (17) | 0.124 |
| Echocardiographic characteristics | ||||
| Baseline MR grade | 0.532 | |||
| Moderate (2+) | 17.0% (9/53) | 19.4% (7/36) | 11.8% (2/17) | |
| Moderate–severe (3+) | 56.6% (30/53) | 55.6% (20/36) | 58.8% (10/17) | |
| Severe (4+) | 26.4% (14/53) | 25.0% (9/36) | 29.4% (5/17) | |
| LVEDD (cm) | 6.7 ±0.82 (53) | 6.6 ±0.85 (36) | 6.7 ±0.77 (17) | 0.695 |
| LVESD (cm) | 5.8 ±0.95 (53) | 5.8 ±1.01 (36) | 5.7 ±0.80 (17) | 0.779 |
| LVEDV (mL) | 217.8 ±75.2 (53) | 208.5 ±62.0 (36) | 237.4 ±96.8 (17) | 0.271 |
| LVESV (mL) | 160.1 ±70.3 (53) | 151.8 ±57.1 (36) | 177.7 ±91.9 (17) | 0.296 |
| LVEF (%) | 28.1 ±7.56 (53) | 28.66 ±7.49 (36) | 26.99 ±7.80 (17) | 0.457 |
| Cardiovascular history | ||||
| Ischaemic aetiology to HF | 64.2% (34/53) | 66.7% (24/36) | 58.8% (10/17) | 0.760 |
| HF admission in past 12 months | 77% (41/53) | 75% (27/36) | 82% (14/17) | 0.730 |
| History of ICD | 15.1% (8/53) | 16.7% (6/36) | 11.8% (2/17) | 1.00 |
| History of diabetes | 20.8% (11/53) | 16.7% (6/36) | 29.4% (5/17) | 0.301 |
Gender, MR grade, and measures of cardiovascular history are assessed as % (proportion). All other measures are reported as mean ±SD (n).
HF, heart failure; ICD, implantable cardioverter defibrillator; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; MR, mitral regurgitation; NYHA, New York Heart Asssociation.
Of the 53 patients who were fully qualified and analysed in the intent-to-treat analysis, 36 underwent permanent device implantation and 17 had the device recaptured. Transient coronary compromise was the indication for recapture in eight patients; <1 grade FMR reduction prompted recapture in nine patients. The mean CARILLON implant time was 38 ±9 min. Coronary arteries were crossed by the implant in 34 of 53 patients (64%); coronary artery compromise precluded permanent device placement in only 8 of 53 patients (15%).
Echocardiographic measures of functional mitral regurgitation and cardiac structure
For implanted patients, there was a significant reduction in the septal–lateral annular diameter between baseline (3.9 ±0.4 cm) and 1 month (3.5 ±0.4 cm) (P < 0.001). This reduction was persistent throughout the 12-month follow-up. In contrast, there was no reduction in diameter noted in the comparison group.
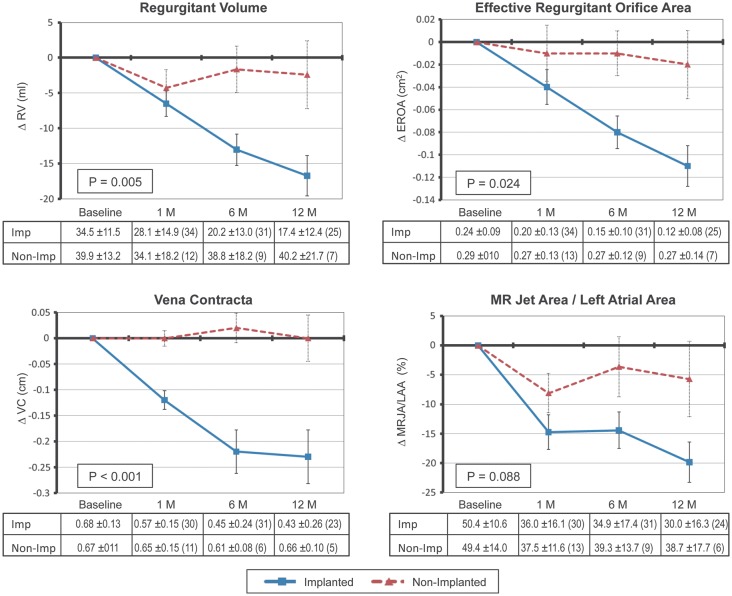
Percutaneous mitral annuloplasty was associated with a significant decrease in quantitative measures of FMR. For the implanted cohort, there was a significant reduction in regurgitant volume (P < 0.001), EROA (P < 0.001), vena contracta (P < 0.001), and MRJA/LAA (P < 0.001) from baseline up to 12 months as shown in Figure 2. The average reduction from baseline to 12 months ranged up to 50% for both EROA and regurgitant volume. The graphs in Figure 2 compare the implanted cohort with the non-implanted cohort. A statistically significant difference was noted between the two cohorts, with a continued decrease of FMR up to 12 months being noted only in the implanted cohort.
Figure 2.
Echocardiographic changes in functional mitral regurgitation severity between implanted (n =36) and non-implanted (n =17) patients as assessed by regurgitant volume (RV), effective regurgitant orifice area (EROA), vena contracta (VC), and mitral regurgitation jet area/left atrial area (MRJA/LAA). P-values were computed by comparing the difference between the two groups from baseline to 12 months. The number of patients at each time point is noted in parentheses in the tables corresponding to each plot.
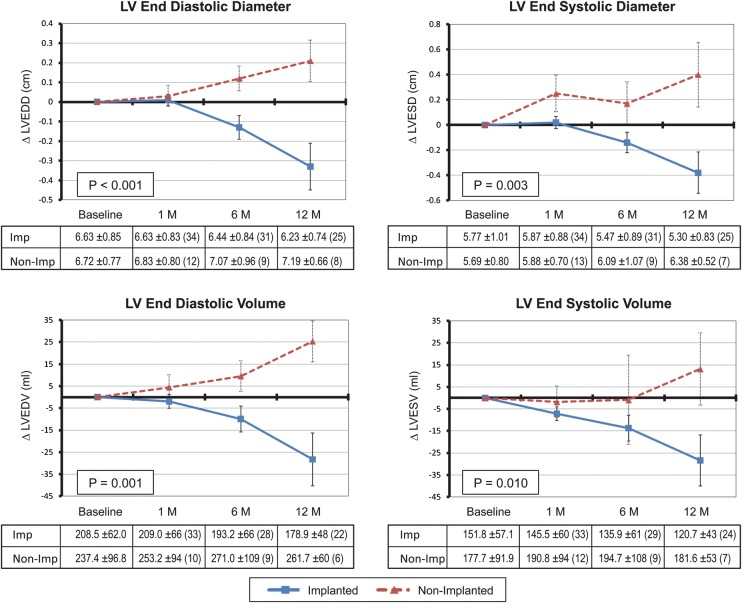
The effect of FMR reduction on LV size is shown in Figure 3. For the implanted cohort, there was a significant reduction in LV end-diastolic diameter (P =0.004), LV end-systolic diameter (P =0.005), LV end-diastolic volume (P =0.015), and LV end-systolic volume (P =0.015) between baseline and up to 12 months. Reverse remodelling was noted in the implanted group, compared with continued ventricular enlargement in the non-implanted group.
Figure 3.
Echocardiographic changes in left ventricular (LV) dimensions between implanted (n =36) and non-implanted (n =17) patients assessed during systole and diastole. P-values were computed by comparing the difference between the two groups from baseline to 12 months. The number of patients at each time point is noted in parentheses in the tables corresponding to each plot.
To complement the intent-to-treat analysis, a separate analysis of the 25 implanted patients who had paired data at both 6 and 12 months is shown in Table 2. Mean reduction in LV end-systolic volume was 19% at 12 months. Eight of 25 patients had a >10% reduction in LV end-systolic volume at 12 months. FMR reduction at 12 months ranged from three grades for three patients, two grades for five patients, one grade for 12 patients, and less than one grade for five patients.
Table 2.
Effect of mitral annuloplasty on funtional mitral regurgitation and left ventricular structure for implanted patients
| Parameter | Baseline | 6 months |
12 months |
||
|---|---|---|---|---|---|
| Mean ±SD | Mean ±SD | P-value | Mean ±SD | P-value | |
| Mitral regurgitation | |||||
| EROA (cm2) | 0.23 ±0.07 | 0.15 ±0.1 | <0.0001 | 0.12 ±0.08 | <0.0001 |
| Regurgitant volume (mL) | 34 ±10 | 20 ±13 | <0.0001 | 17 ±12 | <0.0001 |
| Regurgitant fraction (%) | 60 ±18 | 34 ±21 | <0.0001 | 31 ±23 | <0.0001 |
| Vena contracta (cm) | 0.67 ±0.13 | 0.45 ±0.25 | 0.0001 | 0.43 ±0.26 | 0.0002 |
| MRJA to LAA | 49 ±9 | 33 ±15 | <0.0001 | 30 ±16 | <0.0001 |
| LV structure | |||||
| LVEDV (mL) | 209 ±62 | 193 ±66 | 0.05 | 179 ±48 | 0.03 |
| LVESV (mL) | 150 ±60 | 132 ±50 | ≤0.02 | 121 ±43 | 0.02 |
| LVEF (%) | 29 ±7 | 31 ±8 | 0.11 | 33 ±8 | 0.05 |
Analysis was limited to implanted patients with paired data at both 6- and 12-month follow-up (n =25)
EROA, effective regurgitant orifice area; LAA, left atrial area; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MRJA, mitral regurgitation jet area; SD, standard deviation.
Measures of exercise capacity and quality of life
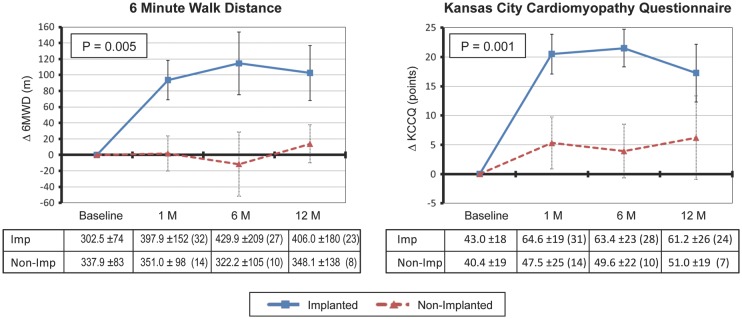
Functional improvements in exercise performance and QOL were apparent by 1 month in the implanted cohort. A significant difference between groups was observed for both 6MWD and QOL at 1 year (Figure 4).
Figure 4.
Comparison of clinical outcome measures between implanted (n =36) and non-implanted (n =17) patients assessed by 6 min walk distance (6MWD) and Kansas City Cardiomyopathy Questionnaire (KCCQ). P-values were computed by comparing the difference between the two groups from baseline to 12 months. The number of patients at each time point is noted in parentheses in the tables corresponding to each plot.
Two year data from the implanted cohort showed that the 6MWD improvement was sustained [302 ±74 m (n =36) at baseline to 406 ±180 m (n =23) at 12 months and 450 ±99 m (n =15) at 24 months (P = 0.005)]. Changes in NYHA class were concordant with the change in 6MWD [3.1 ±0.23 (n =36) at baseline to 2.1 ±0.64 (n =25) at 12 months and 2.1 ±0.74 (n =19) at 24 months (P < 0.001)].
Safety
The 30-day major adverse event rate in the overall intent-to-treat population was 1.9% (Table 3). There were eight deaths in the implanted group (22.2%) and four deaths in the non-implanted group (23.5%). The periprocedural death resulted from contrast-induced renal failure; the remaining deaths in the comparator group were all of cardiac aetiology. The causes of death for implanted patients were urosepsis, haemorrhagic stroke, renal failure, pneumonia, car accident, HF exacerbation, and unknown (n =2). The two myocardial infarctions in the implanted cohort were adjudicated to be not device related based on arteriograms showing no change in circumflex flow in one patient, and a left anterior descending artery lesion in the second patient.
Table 3.
Cumulative major adverse event rate
| Event | 30 days |
12 months |
||
|---|---|---|---|---|
| Incidence | Device related | Incidence | Device related | |
| Death | 1.9% (1/53) | 0% | 22.6% (12/53) | 0% |
| Implanted | 0% (0/36) | 22.2% (8/36) | ||
| Non-implanted | 6% (1/17) | 23.5% (4/17) | ||
| Myocardial infarction | 0% (0/53) | 0% | 4% (2/53) | 0% |
| Cardiac perforation | 0% (0/53) | 0% | 0% (0/53) | 0% |
| Device embolization | 0% (0/53) | 0% | 0% (0/53) | 0% |
| Surgery due to device | 0% (0/53) | 0% | 0% (0/53) | 0% |
| Overall MAE rate | 1.9% (1/53) | 0% | 26.4% (14/53) | 0% |
MAE, major adverse event.
Safety assessments of implanted patients between 12 and 24 months revealed a further three deaths due to HF exacerbation and unknown causes (n =2). None was device related.
Nine patients were observed to have a fracture of an anchor wire. The fracture was located at the proximal end of the CS anchor in seven of nine patients, and at the distal end of the CS and great cardiac vein anchors in two patients. After fracture, the device position remained unchanged within the CS. A review of adverse events confirmed no link between fracture and observed events. The reduction in FMR was maintained in all but one of these patients.
Discussion
The objective of the TITAN trial was to assess the haemodynamic and clinical significance of treating FMR percutaneously in HF patients using the Carillon Mitral Contour System. Follow-up of patients in whom the device was recaptured during the index procedure provided a non-randomized, non-blinded comparison group. Device implantation was shown to be safe, with no major adverse event attributable to the annuloplasty device. In contrast to the comparison group, implanted patients displayed a significant reduction in the degree of FMR that was associated with reverse remodelling. There was sustained benefit in clinical parameters of NYHA class and 6MWD up to 24 months.
The major adverse event rate in TITAN is less than reported for both surgical and other percutaneous approaches to treating FMR. For patients with an LV ejection fraction between 21% and 30%, surgical mitral repair is associated with 5.5% mortality and 24% combined morbidity/mortality at 30 days.9 The percutaneous MitraClip device was recently reported to have a 15% 30-day major adverse event rate.10 The advantage of a CS approach is that it provides a right-sided conduit to treat FMR, allowing a straightforward procedure and early hospital discharge.
The observation of wire fractures shows that the nitinol device was exposed to forces not anticipated from prior pre-clinical experience. Fractures were subtle to identify on chest X-ray, were not associated with any adverse events, and thus went undetected. A post-hoc review of all images from the previously reported AMADEUS trial identified two out of 30 patients with fracture, neither of whom had adverse events.7 The lack of clinical events associated with wire fracture suggests that the device may have been sufficiently incorporated into the CS by a process of vascular remodelling, as has been previously reported.11
The TITAN data complement recent reports from the cardiac resynchronization therapy (CRT) literature showing that CRT response relates to reductions in FMR and is associated with both reverse remodelling and improved survival.4,5 The data from percutaneous mitral repair technologies suggest that FMR reduction alone is an important contributor to clinical improvement and reverse remodelling. The mean reduction in LV end-systolic volume of 12% at 6 months and 19% at 12 months in the TITAN trial is also important since previous studies show that a decrease in LV end-systolic volume of >10% following CRT is a strong predictor of lower long-term mortality and HF events.12 The observation of a significant change in LV end-systolic volume in patients with only mild FMR in the Liang4 study is not totally surprising given the dynamic nature of FMR.13 Percutaneous therapies that demonstrate the proper risk–benefit profile may also be able to treat HF patients earlier in their disease course.
While this study does provide a comparator group with which to evaluate the haemodynamic and clinical significance of treating FMR, the lack of a randomized and blinded comparator also remains the primary limitation of the study. As such, a randomized trial comparing intervention with a medically managed control group is warranted.
Conclusions
This study demonstrates that percutaneous CS-based mitral annuloplasty can significantly and safely reduce FMR severity in HF patients, resulting in significant LV reverse remodelling over 12 months and improved measures of clinical outcome over 24 months.
Funding
Cardiac Dimensions, Inc., Kirkland, WA, USA.
Conflicts of interest: the interventional cardiology investigators (T.S., M.H., U.C.H., J.S., J.L., and J.F.) as well as the echocardiographers from the core lab (J.C.W., A.M.S., and S.D.S.) have no relationship with industry in connection with the submitted article. A clinical trial agreement defined the compensation with respect to the clinical trial. T.F. no financial relationship with Cardiac Dimensions. D.M.K. and W.C.L. are consultants to the company with limited stock options. S.L.G. and D.G.R. are both employees of the company (with stock options) as well as practising physicians at the University of Washington (Cardiology) and Seattle Childrens Hospital (Pediatric Emergency Medicine), respectively.
Acknowledgements
The authors appreciate the contributions of the following individuals: Krzysztof Bartus, Jacek Myc, Wojciech Zajdel, Janusz Konstanty-Kalandyk, Boguslaw Kapelak, Olga Jerzykowska, Piotr Kalmucki, and Hubertus Degen.
References
- 1.Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction heart failure. Am J Cardiol. 2003;91:538–543. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 2.DiBiase L, Auricchio A, Mohanty P, Bai R, Kautzner J, Pieragnoli P, Regoli F, Sorgente A, Spinucci G, Ricciardi G, Michelucci A, Natale A. Impact of cardiac resynchronization therapy on the severity of mitral regurgitation. Europace. 2011;13:829–838. doi: 10.1093/europace/eur047. [DOI] [PubMed] [Google Scholar]
- 3.Fattouch K, Guccione F, Sampognaro R, Panzarella G, Corrado E, Navarra E, Calvaruso D, Ruvolo G. Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278–285. doi: 10.1016/j.jtcvs.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Liang YJ, Zhang Q, Fung J, Chan JYS, Yip GWK, Lam YY, Yu CM. Impact of reduction in early- and late-systolic functional mitral regurgitation on reverse remodeling after cardiac resynchronization therapy. Eur Heart J. 2010;31:2359–2368. doi: 10.1093/eurheartj/ehq134. [DOI] [PubMed] [Google Scholar]
- 5.VanBommel RJ, Marsan NA, Delgado V, Borleffs CJW, van Rijnsoever EPM, Schalij MJ, Bax JJ. Cardiac resynchronization therapy as a therapeutic option in patients with moderate–severe functional mitral regurgitation and high operative risk. Circulation. 2011;124:912–919. doi: 10.1161/CIRCULATIONAHA.110.009803. [DOI] [PubMed] [Google Scholar]
- 6.Franzen O, vanderHeyden J, Schlüter Baldus S, Schillinger W, Butter C, Hoffman R, Corti R, Pedrazzini G, Swaans MJ, Neuss M, Rudolph V, Sürder D, Grünenfelder J, Eulenburg C, Reichenspurner H, Meinertz T, Auricchio A. MitraClip therapy in patients with end-stage systolic heart failure. Eur J Heart Fail. 2011;13:569–576. doi: 10.1093/eurjhf/hfr029. [DOI] [PubMed] [Google Scholar]
- 7.Schofer J, Siminiak T, Haude M, Herrman JP, Vainer J, Wu JC, Levy WC, Mauri L, Feldman T, Kwong RY, Kaye DM, Duffy SJ, Tubler T, Degen H, Brandt MC, Van Bibber R, Goldberg S, Reuter DG, Hoppe UC. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON mitral annuloplasty device European Union study. Circulation. 2009;120:326–333. doi: 10.1161/CIRCULATIONAHA.109.849885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 9.Haan CK, Cabral CI, Conetta DA, Coombs LP, Edwards FH. Selecting patients with mitral regurgitation and left ventricular dysfunction for isolated mitral valve surgery. Ann Thorac Surg. 2004;78:820–825. doi: 10.1016/j.athoracsur.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Feldman T, Foster E, Glower D, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Wiegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 11.Maniu CV, Patel JB, Reuter DG, Meyer DM, Edwards WD, Rihal CS, Redfield MM. Acute and chronic reduction of functional mitral regurgitation in experimental heart failure by percutaneous mitral annuloplasty. J Am Coll Cardiol. 2004;44:1652–1661. doi: 10.1016/j.jacc.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 12.Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 13.Yamano T, Nakatani S, Kanzaki H, Toh N, Amaki M, Tanaka J, Abe H, Hasegawa T, Sawada T, Matsubara H, Kitakaze M. Exercise-induced changes of functional mitral regurgitation in asymptomatic or mildly symptomatic patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2008;102:481–485. doi: 10.1016/j.amjcard.2008.03.086. [DOI] [PubMed] [Google Scholar]