Abstract
Survival of chronic lymphocytic leukemia (CLL) cells in vivo is supported by the tissue microenvironment, which includes components of the extracellular matrix. Interactions between tumor cells and the extracellular matrix are in part mediated by CD44, whose principle ligand in this respect is hyaluronic acid. Purpose: to evaluate the effect of CD44 engagement on the survival of CLL cells. Experimental Design: CD44 in CLL cells was engaged by anti-CD44 monoclonal antibody, or hyaluronic acid, and the effects of CD44 activation on CLL cell viability and pro-survival pathways were evaluated. Results: engagement of CD44 activated the PI3K/AKT and MAPK/ERK pathways and increased MCL-1 protein expression. Consistent with the induction of these anti-apoptotic mechanisms, CD44 protected CLL cells from spontaneous and fludarabine-induced apoptosis. Leukemic cells of the more aggressive CLL subtype that express unmutated IgVH genes (U-CLL) showed higher CD44 expression than IgVH-mutated CLL (M-CLL) cells, and acquired a greater survival advantage via CD44 activation. Thus, CD44 activation in the tissue microenvironment may contribute to increased MCL-1 protein levels, resistance to apoptosis, and could contribute to the more progressive nature of U-CLL. Furthermore, PI3K or MEK inhibitors as well as obatoclax, an antagonist of MCL-1, blocked the pro-survival effect of CD44. In addition, obatoclax synergized with fludarabine to induce apoptosis of CLL cells. Conclusions: components of the extracellular matrix may provide survival signals to CLL cells through engagement of CD44. Inhibition of MCL-1, PI3K, and MAPK/ERK pathways are promising strategies to reduce the anti-apoptotic effect of the microenvironment on CLL cells.
Introduction
Proliferation and survival of CLL-cells in-vivo is influenced by extrinsic signals which originate primarily in the microenvironment of secondary lymphoid tissues and the bone marrow [1, 2]. When CLL cells are removed from their natural microenvironment and cultured in-vitro, they rapidly undergo apoptosis. The supporting interactions between the microenvironment and the neoplastic cells are complex and multi-factorial. Some of these interactions are cell-cell contact dependent, while others are mediated through chemokines, growth factors and possibly through extracellular matrix components. Considerable clinical heterogeneity exists, and the presence or absence of somatic mutations in the immunoglobulin heavy chain variable regions (IgVH) of the clonal cells separates patients into two major prognostic subgroups. Typically, patients with unmutated -IgVH (U-CLL) genes have a more aggressive clinical course compared to the subgroup with mutated IgVH (M-CLL)[3, 4]. ZAP70, a non-receptor tyrosine kinase primarily involved in T-cell receptor signal transduction, is preferentially expressed in the U-CLL subtype and confers prognostic information similar to Ig mutation status [5, 6].[7] CLL cells of the UCLL/ZAP70 positive subtype appear to respond better to stimulation through different pathways including the B-cell receptor and chemokine signaling than M-CLL cells [8-10].
The interaction between normal or malignant cells and the extracellular matrix is in part mediated through CD44. CD44 is a type I trans-membrane glycoprotein, whose principal ligand is thought to be glycosaminoglycan hyaluronic acid (HA)[11]. CD44 can also interact with numerous other extracellular matrix components including osteopontin, fibronectin, laminin, and collagen [12]. The CD44 molecule is encoded by a single gene but displays extensive size heterogeneity due to alternative splicing and post-translational modifications [13]. The CD44 form that lacks all variable exons is considered the standard form (CD44), while CD44v denotes splice variants that incorporate additional exons, giving rise to a larger molecule with additional extracellular domains that may change affinity to possible ligands or co-receptors [12, 14]. The intracellular domain is shared by all CD44 isoforms. In CLL, the main variant is the standard CD44 form, while CD44v are only weakly expressed in a relatively small proportion of cells [15]. Several reports suggested that high CD44 expression is an adverse prognostic factor associated with inferior clinical outcome in CLL [16, 17].
CD44 signaling and its downstream effects are multifaceted and may depend on the expressed CD44 isoform, the specific ligand, the cell type, and interactions with other transmembrane signaling components [14]. On one hand, CD44 is an adhesion receptor that binds to extracellular matrix and regulates cell migration, homing, and engraftment[18]. On the other hand CD44 activation can induce[19, 20] or protect[21, 22] from apoptosis. Notably, the cytoplasmic domain of CD44 lacks apparent catalytic activity and its ability to transduce intracellular signals depends on interactions with co-receptors or the assembly of an intracellular signaling complex [14].
Here we address the role of CD44 in the pathogenesis of CLL. We show that CD44 engagement protects CLL cells from spontaneous and fludarabine-induced apoptosis through activation of the PI3K/AKT and MAPK/ERK pathways resulting in increased levels of MCL-1. We find higher CD44 expression and a stronger anti-apoptotic effect of CD44 activation in UCLL cells. Our results identify the PI3K/AKT, MAPK/ERK pathways and MCL-1 as rationale therapeutic targets to overcome the prosurvival effect of the microenvironment on CLL cells.
Material and Methods
Reagents
Antibodies included: mouse antihuman CD44 monoclonal antibody (clone BU75) and murine IgG2 (isotype control) from Ancell Corporation (Bayport, MN), fluorescein isothiocyanate (FITC) conjugated antihuman-CD44 standard from AbD Serotec (Raleigh, NC), FITC-conjugated antimurine IgG1 and Phycoerythrin (PE)-conjugated CD19 from BD Pharmingen (San Jose, CA), anti-BCL-XL, phospho-Akt (Ser473), ERK1/2, phospho-ERK1/2 (Thr202/Tyr204) from Cell Signaling (Beverly, MA). Akt, MCL-1, BCL-2, PARP-1 antibodies from Santa Cruz Biotechnology, Inc (Santa Cruz. CA) and anti-γ-Tubulin from Sigma (St. Louis, MO). 9-β-D-arabinofuranosyl-2-fluoroadenine (fludarabine) and wortmannin were purchased from Sigma (St. Louis, MO), PD98509 from Calbiochem (Gibbstown, NJ) and obatoclax was obtained from Geminex (Ontario, Canada). MitoTracker Red CMXRos and MitoTracker Green FM was were obtained from Invitrogen Corporation (Carlsbad, CA).
Patient samples and cell purification
After obtaining informed consent, blood samples were collected from treatment naïve patients fulfilling the standard morphologic and immunophenotypic criteria for B-CLL (NCI study 97-C-0178; www.clinicaltrials.gov identifier: NCT00019370) or obtained by leukaphresis from normal donors (Department of transfusion medicine, NIH). Peripheral blood mononuclear cells were isolated by density-gradient centrifugation over Lymphocyte Separation Medium (ICN Biomedicals, Aurora, OH). Cells used were either fresh or from viably frozen samples. Viably frozen cells were kept in fetal calf serum (FCS) containing 10% dimethyl sulfoxide and stored in liquid nitrogen. Before use, frozen cells were thawed and cultured at 37°C, 5% CO2 in RPMI media (Mediatech Inc, Herndon, VA) supplemented with 10% FCS, penicillin, streptomycin and glutamine.
CD19 enrichment
Peripheral blood mononuclear cells were magnetically labeled using a cocktail of biotinylated CD2, CD14, CD16, CD36, CD43, and CD235a antibodies (Miltenyi Biotec Inc, Auburn, CA) After washing, the cells were incubated with anti-biotin microbeads (Miltenyi Biotec Inc, Auburn, CA) and separated on magnetic cell separation (MACS) column (Miltenyi Biotec Inc, Auburn, CA) according to the manufactures’ instructions. In the indicated experiments, only purified samples containing CD19+ cells with purity of more than 97% (monitored by flow cytometry) have been used.
Cell stimulation
Stimulation with anti-CD44 antibody was performed as previously reported.[23] Briefly, CLL cells (5×106/ml) were incubated with anti-CD44 antibody (BU75, 10μg/ml) or isotype control antibody (anti-mouse IgG2, 10μg/ml) for 30 minutes. The cells were washed, incubated with secondary goat anti-mouse antibody (1μg/ml) and cultured at 37°C for the indicated time periods.
Flow Cytometry
To detect surface CD44 expression, cells were stained with isotype control anbtibodies, or CD44-FITC and CD19-PE antibodies. 5 μL of the antibodies were added to 5×105 cells and incubated for 30 minutes on ice. Samples were washed with PBS/1% FCS and assayed on a FC500 flow cytometer (Coulter). To detect apoptosis after CD44 activation, the MitoTracker staining protocol was used as previously described.[24] Briefly, cultured cells were stained with 200 nM of MitoTracker Green FM and MitoTracker Red CMXRos, incubated at 37°C for 30 min in dark and immediately assayed by flow cytometry. The viability of CLL cells incubated in the presence of hyaluronic acid was assessed by DiOC6 (3,3’ dihexylocarbocyanine iodide) (Sigma) staining protocol. Briefly, DiOC5 was added to 1×106 cells to a final concentration of 6pg/ml. Then, Cells were incubated at 37°C for 20 minutes, washed twice with PBS and immediately analyzed by flow cytometry.
Hyaluronic acid coating
24-well plates were incubated at 4°C for 18 h with the indicated concentration of hyaluronic acid in PBS. To remove unbound hyaluronic acid, the plates were washed twice with PBS. To block non-HA coated sites, the coated plates were treated with 1% bovine serum albumin (BSA) for 60 minutes at 37°C.
Western blot analysis
CLL cells were lysed in extraction buffer containing 1% NP40 in the presence of anti-phosphatase and protease inhibitors. Protein concentration was determined by Bradford assay. Proteins were separated on a SDS-acrylamide gel, transferred to nitrocellulose membranes and subsequently subjected to immunoblot analysis using appropriate antibodies. Immunoreactive antigen was recognized by using horseradish peroxidase-labeled anti-IgG antibodies (Amersham, Piscataway, NJ), and blots were developed by chemiluminescence (Termo Scientific, Rockford, IL).
IgVH gene analysis
Amplification of the IgVH gene was performed as described.[6] In brief: 500 ng mRNA was used to generate oligo-dT primed cDNA using Superscript (Invitrogen, Carlsbad, CA). cDNA was amplified by polymerase chain reaction (PCR) using a mixture of 5' oligonucleotides specific for each leader sequence of the VH1 to VH7 IgVH families as forward primers and either a 3' oligonucleotide complementary to the consensus sequence of the joining region or the constant region of the IgM locus as reverse primers. PCR was performed in 50 μL reactions with Taq polymerase (Sigma, St Louis, MO) and 20 pmol of each primer. Products were purified (MinElute PCR Purification Kit; Qiagen, Valencia, CA) and sequenced directly with the appropriate 3' oligonucleotide using Big Dye Terminator and analyzed using an automated DNA sequencer (Applied Biosystems, Foster City, CA). Nucleotide sequences were aligned to the V-Base sequence directory (http://www.mrc-cpe.cam.ac.uk). Sequences with 2% or less deviation from any germ line IgVH sequence were considered unmutated.
Quantitative RT-PCR
5 μL mRNA (0.5 ng/μL) per reaction was used for quantitative reverse transcriptase (RT)–PCR using Taqman reagents (Applied Biosystems) and analyzed in real time on an ABI Prism 7700 (Applied Biosystems). All samples were run in triplicates. Amplification of the sequence of interest was compared with a reference probe (β-2-microglobulin) and normalized against a standard curve of cell line mRNA. The primers and probes for β-2-microglobulin and MCL-1 were purchased from Applied Biosystems.
MTT assays and synergy calculations
Cytotoxicity assays were performed with the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide) reagent (Chemicon, Temecula, CA). Five hundred thousand CLL cells resuspended in AIM V medium (Invitrogen) were plated per well in flat bottomed 96-well plates and exposed to serial doubling concentrations of drug for 72 hours. For the last 6 hours, 0.5 mg/ml MTT was added before also adding 10% SDS with 0.01 M HCl. After incubation overnight at 37°C, absorbance was measured at the wavelengths of 570 nm (test) and 650 nm. The difference between the absorbance measurements at test and reference wavelengths was used to fit a dose-response curve, and the necessary drug concentration to kill 50% of the cells, the IC50, was calculated by non-linear regression using Prism 4.0 (GraphPad Software, San Diego, CA). Vehicle-treated cells (0.1% DMSO) served as controls. Synergy between compounds was calculated with CalcuSyn software (Biosoft, Ferguson, MO) according to the method described by Chou and Talalay [25].
Statistical analysis
Unpaired and paired T-tests were used to assess differences in means of two groups for CD44 expression and cell viability. A P value<0.05 was considered significant.
Results
CD44 expression differs between prognostically distinct CLL subtypes
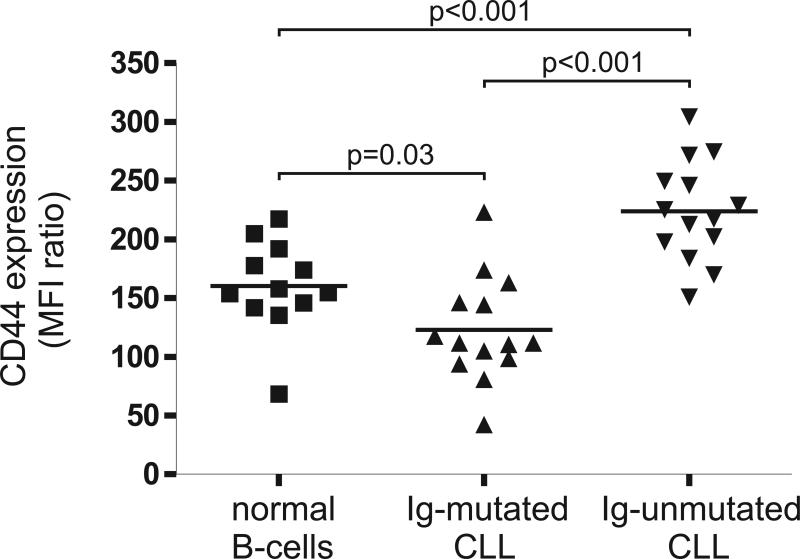
High expression of CD44 on CLL cells has been associated with adverse clinical features. However, the correlation between CD44 expression and the more recently defined prognostic subtypes of CLL and in particular with IgVH mutational status or ZAP70 expression has not been described. Using flow-cytometry, we quantified CD44 expression in CLL-cells and in B-lymphocytes obtained from healthy donors. Surface CD44 was detected on all CLL cells as well as on normal B-cells. The degree of CD44 expression was highly variable among different CLL samples and correlated with IgVH mutational status (Figure 1). To quantify the expression of CD44 we calculated the ratio between the mean fluorescent intensity (MFI) of CD44 staining divided by the MFI of the corresponding isotype staining. The expression of CD44 was significantly higher in U-CLL cells than in M-CLL cells (MFI ratio 224 ±43 to 122 ±44, respectively, p<0.0001) or in normal B-cells (MFI ratio 160 ±39, p=0.0006). In contrast, MCLL-cells had lower CD44 expression than normal B-cells (p=0.003).
Figure 1. CD44 cell surface expression on CLL subtypes and normal B-cells.
Surface CD44 expression was quantified by flow cytometry and is shown as the ratio between the MFI of CD44 divided by the MFI of the isotype control (MFI ratio). U-CLL-cells (n=14), M-CLL-cells (n=14) and normal B-cells (n=12) are compared by T-test.
CD44 induces homotypic aggregation and protects CLL-cells from spontaneous apoptosis
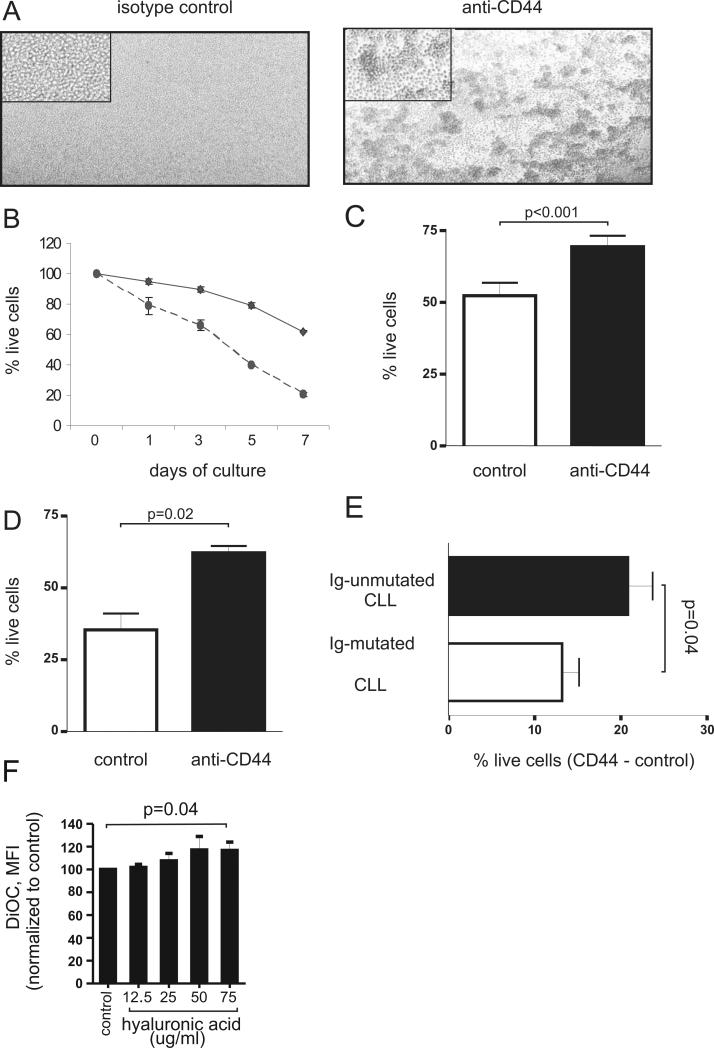
To investigate the effect of CD44 signaling on CLL cells, we first stimulated PBMCs from CLL patients with a monoclonal antibody that binds to the extracellular domain of CD44. CD44 engagement triggered homotypic aggregation of the CLL cells, which is a common effect of various exogenous stimuli that activate cells or modulate cell adhesion.[26] CLL cells aggregated within minutes and clustered into clumps containing large numbers of cells (Figure 2A). These clumps were characterized by strong cell-cell interactions and were difficult to dissociate. As expected, the induction of homotypic aggregation was temperature dependent and completely blocked at 4°C, consistent with the requirement of intracellular signaling for the aggregation to occur. These data indicate that the monoclonal antibody against CD44 acts as an agonist and can trigger an intracellular signal.
Figure 2. CD44 induces homotypic aggregation and protects CLL cells from spontaneous apoptosis.
Peripheral blood mononuclear cells of CLL patients (5×106/ml) were incubated with isotype control antibody (anti-mouse IgG2, 10μg/ml) or anti-CD44 antibody (BU75, 10μg/ml) for 30 minutes, washed and incubated with secondary anti-mouse antibody (1μg/ml). (A) After 60 minutes of incubation formation of homotypic aggregation was studied under a light microscope. (B-D) The percentages of live and apoptotic CLL cells after CD44 activation were measured by flow cytometry using Mito-tracker staining. (B) The viability of CD44 stimulated and control CLL cells (performed in duplicates) on day 0, 3, 5 and 7 of PBMC culture (one of 3 experiments with similar results is shown). The relative viability normalized to the day 0 measurement is shown. (C) Mean viability of CD44 stimulated and isotype treated control cells after 3 days of PBMC culture (n=20, comparison by paired T-test). (D) CD44 activation protects purified CLL cells from apoptosis. PBMC of CLL patients were subjected to negative selection, yielding >97% pure CD19/CD5 positive cells. After 3 days of culture the viability of the purified CLL samples with or without CD44 stimulation was determined (n=5, comparison by paired T-test). (E) Cell viability of CLL samples with or without CD44 stimulation after 3 days of culture according to mutational status (n=10 each subgroup). Shown is the mean and standard deviation of cells specifically protected from apoptosis by CD44 activation (% live cells with CD44 stimulation - % live cells in the control; unpaired T-test). (F) Increase in viability of CLL cells cultured for 96 hours in wells coated with increasing concentrations of hyaluronic acid. Shown is the mean and standard deviation % increase in DiOC6 intensity of cells grown on hyaluronic acid coated wells compared to cells grown in untreated wells (mean and standard deviation of 5 independent experiments; P=0.037 for linear trend, P=0.04 for highest concentration compared to control).
Engagement of CD44 prevented CLL cells from undergoing spontaneous apoptosis and extended the survival of leukemic cells in-vitro. A survival advantage for CD44 stimulated cells was apparent as early as 24 hours after stimulation and increased further with prolonged culture (Figure 2B). We chose 72 hours of culture to quantify the effect of CD44 stimulation in a larger number of samples. This time point appeared ideal because on average, 50% of unstimulated CLL cells remained viable after 3 days of culture. All samples with CD44 stimulation showed significantly better viability than control samples (Figure 2C). On average, CD44 stimulated CLL cells had a 46% increase (range 7% – 181%) in viability over the corresponding unstimulated control cells (n=20, p<0.0001). All these measurements were done in peripheral blood mononuclear cells (PBMNC) from CLL patients containing a high proportion of leukemic cells, typically in excess of 90%. Nevertheless, a small number of non B-lymphocytes that also expressed CD44 were present. Thus, in order to exclude any possibility that the pro-survival effect of CD44 was not directly generated in the tumor cells, we isolated the leukemic cells through negative selection yielding samples containing more than 97% pure CLL cells. In these purified CLL cells, we again found that stimulation of CD44 increased the viability in all samples tested on average by 104 ±49 % (n=5, p=0.02, Figure 2D), which equals the average survival increase of 103 ±30% in the matching PBMC samples. These results show that the protective effect is directly mediated by CD44 activation in the leukemic cells and independent of additional cells.
Considering that U-CLL cells had higher CD44 expression than M-CLL cells, we determined whether the higher CD44 expression could translate into increased CD44 signaling and enhanced protection from apoptosis. Cell viability in PBMCs after 3 days of culture without CD44 stimulation was comparable between M-CLL (56 ±19% live cells, n=10) and U-CLL cells (46 ±21% live cells, n=10; p=0.2). To estimate the number of cells specifically protected from apoptosis by CD44 stimulation, we subtracted the % live cells in the control from the % live cells in the CD44 stimulated cells (Figure 2E). While all samples gained a survival advantage, the effect was more prominent for U-CLL than mutated-CLL with 21 ±9% compared to 13 ±6% of cells, respectively, that were rescued from apoptosis by CD44 activation (p=0.04, Figure 2E). This translates into a relative increase in viability compared to unstimulated control cells of 65% for U-CLL cells but of only 26% for M-CLL cells, indicating a more potent anti-apoptotic effect of CD44 engagement in the former subtype.
Having shown a pro-survival effect of CD44 engagement using monoclonal antibodies, we wished to test whether a physiologic ligand of CD44 would have the same effect. To this end, we evaluated the viability of CLL cells cultured on hyaluronic acid coated plates. In these experiments, CLL cells were incubated in wells coated with hyaluronic acid at increasing concentrations. After 96 hours of culture, CLL cell viability increased in a dose dependent manner (P=0.037 for linear trend). At the highest HA concentration cell viability increased by 20% compared with cells cultured in the absence of HA (P=0.04; Figure 2F).
CD44 activates the PI3K/AKT and MAPK/ERK pathways and increases MCL-1 protein expxression
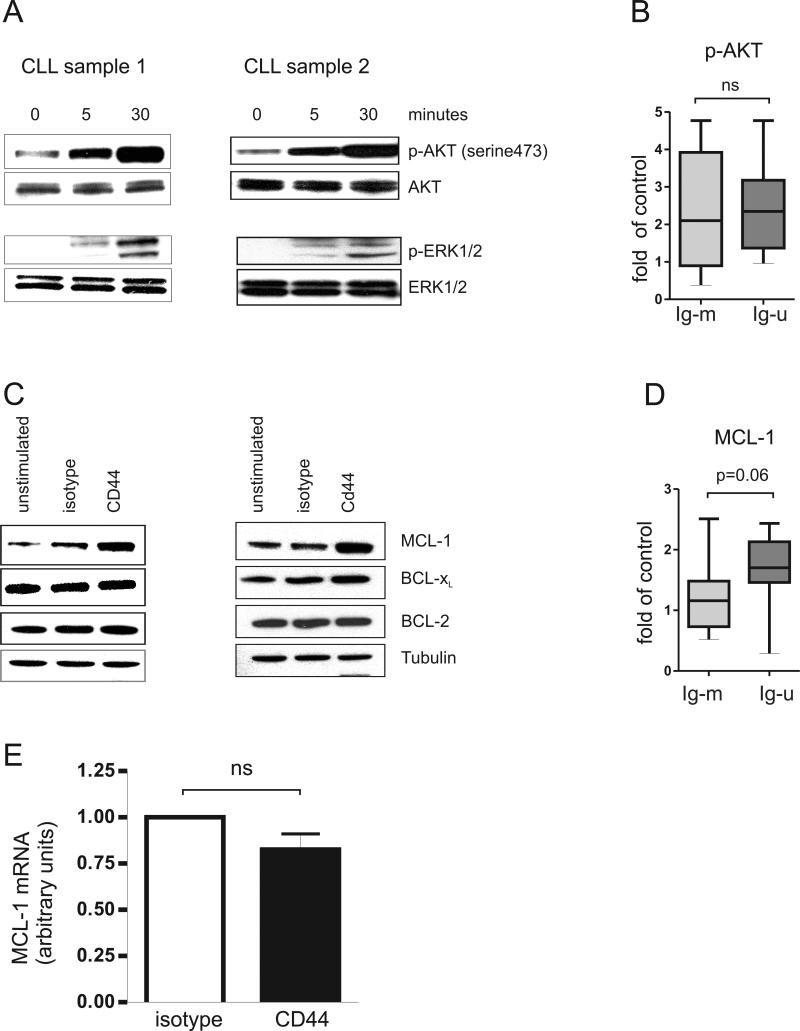
We next investigated the effect of CD44 activation on the PI3K/AKT and MAPK/ERK pathways, which have been reported to be activated by CD44 in solid tumor cell lines. CD44 engagement on CLL cells was followed by a prompt and strong increase of AKT phosphorylation and activation of ERK1/2 (Figure 3A). We validated AKT activation in an extended cohort of M-CLL and U-CLL samples. In both subtypes, a majority of samples showed increased AKT phosphorylation which on average reached 2.3-fold compared to control (p=0.0002) There was no significant difference between the CLL subtypes (p=0.4, Figure 3B).
Figure 3. CD44 activates the PI3K/AKT and MAPK/ERK pathways and increased MCL-1 expression.
CLL-cells were incubated with anti-CD44 antibody (BU75, 10μg/ml) or with isotype control antibody (anti-mouse IgG2, 10μg/ml) for 30 minutes, washed and then cross-linked with secondary goat anti-mouse antibody (1μg/ml). (A) Cell lysates (>90% CLL cells) were obtained at baseline and after 5 and 30 minutes of stimulation. The blots were probed with anti-phospho-Akt (ser473) and anti-phospho-ERK1/2 (Thr202/Tyr204) antibody as indicated. Equal protein loading was confirmed by probing for total AKT and ERK1/2. (B) An extended cohort of both M-CLL and U-CLL samples (n=20), showed increased AKT phosphorylation on average of 2.37±1.3 fold compared to control (p=0.0002). shown are Box and Whisker plots for densitomtery quantification of fold change increase in phospho-Akt (ser473) levels, after 30 minutes of CD44 engagement, both in M-CLL and U-CLL cells compared to control and normalized to total AKT (M-CLL samples n= 9, mean=2.45±1.57 Vs. U-CLL samples n= 11, mean=2.31±1.11 One-tailed T-test p=0.4).(C) Western blots of cell lysates obtained 24 hours after indicated stimulation were probed with antibodies against MCL-1, BCL-XL, BCL-2, and tubulin (loading control). (D) An extended cohort of both M-CLL and U-CLL samples (n=22), showed an increased MCL-1 expression on average of 1.45±0.66 fold compared to control (p=0.004). Shown are Box and Whisker plots for densitomtery quantification of fold change increase in MCL-1 levels, 30 minutes after CD44 engagement, both in M-CLL and U-CLL cells compared to control and normalized to tubulin (M-CLL n=10, mean=1.2±0.6, Vs. U-CLL=12, mean=1.65±0.66. One-tailed T-test p=0.06). (E) mRNA levels of MCL-1 by quantitative PCR after 6 hours of stimulation. The mean and standard deviation from 5 independent experiments is shown.
In order to determine whether expression of BCL-2 family members could be directly regulated by CD44, we evaluated changes in the protein expression of MCL-1, BCL-XL and BCL-2, all of which have been shown to play a role in protecting CLL cells from apoptosis.[27-30] We detected higher MCL-1 protein levels in CLL cells stimulated by CD44 than in cells exposed to isotype control antibody for 24 hours (Figure 3C). The increase in MCL-1 was confirmed in an extended cohort of M-CLL and U-CLL samples. Irrespective of the CLL subtype, MCL-1 protein levels increased on average by 1.45 fold after CD44 activation compared to control (p=0.004). Consistent with a more potent pro-survival effect in U-CLL, MCL-1 expression showed a trend for increased levels in U-CLL than in M-CLL after CD44 activation (p=0.06, Figure 3D). Also among M-CLL samples only one of ten showed a 2-fold increase, while 5 of 12 U-CLL samples showed at least a 2-fold increase in MCL-1 protein expression after CD44 engagement. MCL-1 mRNA levels were unaffected by CD44 stimulation (control/CD44 stimulated expression 0.82, p=0.1, Figure 3E). The higher MCL-1 protein expression in the absence of increased transcription is consistent with known translational and post-translation effects of PI3K/AKT and MAPK/ERK signaling. In contrast, BCL-2 protein expression was not affected, and BCL-XL was increased in only one of 5 samples after CD44 stimulation (Figure 3C).
PI3K and MEK inhibitors block the protective effect of CD44 on leukemic cell survival
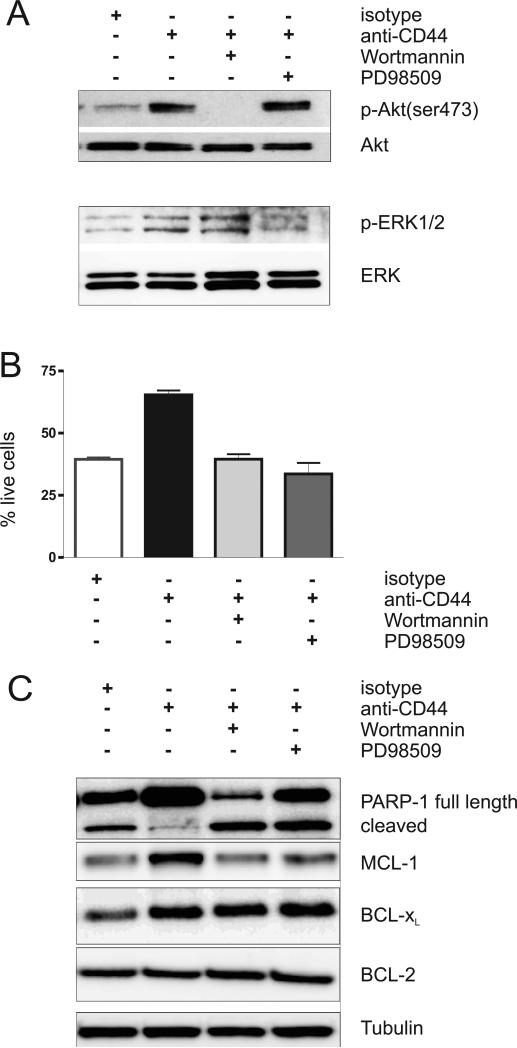
Having shown that CD44 activation induced activation of the PI3K/AKT and MEK signal transduction pathways and protected CLL cells from apoptosis, we wished to evaluate whether specific inhibitors directed against these signal transduction pathways could inhibit the pro-survival effect of CD44. Untreated CLL cells or CLL cells pre-incubated with either wortmannin (100nM) or PD98509 (50μM) for 30 minutes were stimulated with CD44, and activation of signal transduction pathways and cell viability were compared. As expected, wortmannin blocked the phosphorylation of AKT in response to CD44 ligation and PD98509 prevented ERK1/2 activation (Figure 4A). Next we determined the effect on CLL cell viability. As shown previously (Figure 2C), CD44 activation increased cell viability, and this effect was completely blocked by either wortmannin or PD98509 (Figure 4B). The effect of these inhibitors on the expression on anti-apoptotic proteins is shown in Figure 4C. PARP1- cleavage indicates the degree of apoptosis in the samples after 24 hours of treatment. Decreased PARP-1 cleavage after CD44 treatment correlated with the protective effect of CD44 against spontaneous apoptosis. Again this protection was abrogated by both wortmannin and PD98509. Likewise the CD44 induced increase in MCL-1 protein was blocked by the inhibitors. In contrast, there was no effect on BCL-2 levels.
Figure 4. The anti-apoptotic effect of CD44 on CLL-cells is blocked by PI3K/AKT or MAPK/ERK inhibitors.
CLL cells were pre-incubated either with the PI3K inhibitor, wortmannin (100nM), or with the MEK inhibitor, PD98509 (50μM), for 30 minutes. Then cells were incubated with either an isotype control antibody (anti-mouse IgG2, 10μg/ml) or a CD44 activating antibody (BU75, 10μg/ml) for 30 minutes, washed and incubated with secondary anti-mouse antibody (1μg/ml). (A, C) Cell lysates (>90% CLL cells) were obtained at baseline and at 30 minutes after stimulation and were then subjected to Western blot analysis. (A) Activation the AKT and ERK. (B) Cell viability by MitoTracker staining. The mean and standard deviation of 3 independent experiments is shown (mean % live cells: isotype 39 ±1%, CD44 stimulated 65 ±3%, CD44 stimulated plus Wortmannin 40 ±3%, CD44 stimulated plus PD98509 34 ±8%, p<0.0.02 for comparisons of CD44 stimulated to control or drug treated cells, p>0.25 for comparisons of isotype treated to CD44 stimulated cells in the presence of inhibitors). (C) Expression of BCL-2 family members.
CD44 signaling protects CLL cells from apoptosis induced by fludarabine, whereas obatoclax reverses the prosurvival effect of CD44 and can synergize with fludarabine
A role of microenvironment mediated signals in the induction of chemotherapy resistance has been suggested. We were therefore particularly interested to test whether CD44 activation could contribute to chemotherapy resistance in CLL. We exposed cells for 3 days to fludarabine at previously determined IC50 concentrations either in the presence of isotype control or CD44 activating antibody (Figure 5A). Fludarabine killed approximately one third of the cells in the presence of isotype antibody while this effect was almost completely antagonized by CD44 activation.
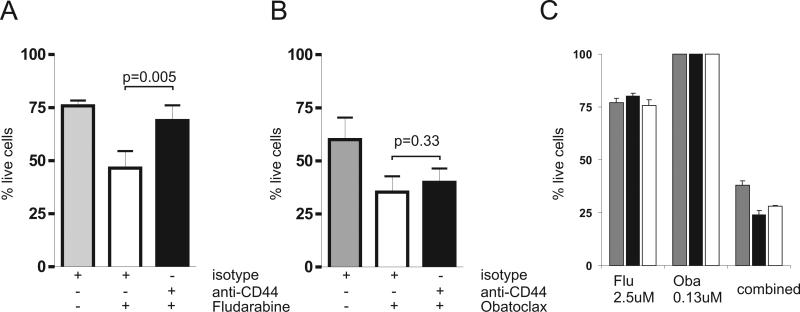
Figure 5. CD44 stimulation inhibits apoptosis induced by fludarabine but not by obatoclax and obatoclax can sensitize cells to fludarabine.
(A, B) The effect of CD44 stimulation on drug sensitivity. CLL cells stimulated by anti-CD44 or isotype control antibody were exposed to the indicated drugs and cell viability was determined after 3 days of culture by MitoTracker staining. The mean and standard deviation of 5 independent experiments with 4 different CLL samples are shown. (A) 0.5 uM fludarabine was added. Cell viability was for isotype treated cells 76 ±6%, for isotype and fludarabine treated cells 47 ±18% and for CD44 stimulated and fludarabine treated cells 69 ±16% (p=0.005, by paired T-test). (B) 0.5 uM of obatoclax was added. Cell viability was for isotype treated cells 62 ±21%, for isotype and obatoclax treated cells 39 ±16% and for CD44 stimulated and obatoclax treated cells 41±14% (p=0.33 by paired T-test). (C). Obatoclax synergizes with fludarabine. CLL cells from 3 patients (represented by differently shaded bars) were exposed to serial dilutions of fludarabine, obatoclax or a combination of the 2 drugs at a fixed molar ratio of 20:1. Cell viability was quantified by MTT assay after 72 hours. The mean cell viability of triplicates and the standard deviation of the mean estimate are shown for 2.5 uM fludarabine (Flu), 0.13 uM obatoclax (Oba) and the combination of 2.5 uM fludarabine with 0.13 uM obatoclax (combined). The combination index calculated by the method of Chou and Talalay was 0.38, 0.24, and 0.27 at IC50 and 0.39, 0.26 and 0.43 at IC75 for the 3 patients, respectively.
MCL-1 that we found to be increased by CD44 activation has been shown to inhibit drug induced apoptosis [31, 32]. Recently, agents that can antagonize the prosurvival effect of MCL-1 have been developed, and one such agent, obatoclax, has successfully completed phase I testing in CLL [33]. We determined the effect of obatoclax against CLL PBMC using MTT assays after 3 days of drug exposure. IC50 concentrations for obatoclax in these assays typically ranged between 0.5uM and 2uM. In the absence of CD44 activation, obatoclax at 0.5uM reduced cell viability on average by 37%. In contrast to what we observed with fludarabine treated cells, the pro-apoptotic effect of obatoclax could not be blocked by CD44 activation, resulting in decreased viability of obatoclax treated cells irrespective of the presence of CD44 activating antibody (Figure 5B).
Next, we tested whether obatoclax could synergize with fludarabine. Using MTT assays we determined the effect of each drug alone and of the combination of fludarabine and obatoclax combined at a molar ratio of 20:1 (Figure 5C). We found greatly enhanced killing of the combined drugs, even when obatoclax was used at a concentration that by itself had no effect on cell viability. To confirm synergy we calculated the combination index (CI) according to the method described by Chou and Talalay.[25] For all three patients, the CI values at the IC50 concentration were <0.5 indicating the presence of a strong synergistic effect between obatoclax and fludarabine.
Discussion
CLL cells depend on cell extrinsic signals for survival. Here we identified CD44 as a survival molecule in CLL that not only protects tumor cells from spontaneous apoptosis, but also, can confer resistance to fludarabine. Our findings in CLL are consistent with studies showing that activation of CD44, either via natural ligands or through a antibody mediated dimerization, can promote cell survival and induce drug resistance in different cell types [21, 34-37]. However, it is crucial to determine the effect of CD44 activation for each tumor type separately, as this molecule can mediate opposing cell fate decisions depending on the cell type and has been shown to induce apoptosis in thymic lymphomas and in myeloid leukemia cells [19, 20, 38]. In vivo, the most likely ligand for CD44 is hyaluronic acid, a ubiquitous component of the extracellular matrix. Consistent with this view, we found that either hyaluronic acid or specific activation of CD44 in leukemic CLL cells is sufficient to protect cells from apoptosis in vitro. In mouse xenograft models, expression of CD44 in tumor cells has been associated with increased tumorigenicity [39, 40]. This tumor promoting effect was absent in cells transfected with a mutant CD44 that is unable to bind to hyaluronic acid. Further supporting the crucial role of CD44 receptor–ligand interactions in vivo is the tumor suppressive effect of soluble CD44 fusion proteins that can inhibit growth or even induce apoptosis of tumor grafts [39-41]. In addition, CD44 could function as a co-stimulatory receptor in vivo contributing and or synergizing with activating signals from the microenvironment. For example, CD44 has been identified as an essential component of a CD44-CD74 receptor complex that mediates prosurvival effects of the macrophage migration inhibitory factor (MIF) on B-cells [22].
We and others found that CD44 expression levels on CLL cells are quite variable between patients. Previous studies reported high CD44 expression in patients with diffuse bone marrow infiltration, advanced clinical stage, more rapid disease progression and inferior overall survival [16, 42]. We now show that CD44 expression differs between CLL subtypes. Specifically, CD44 expression was on average twice as high in cells of the more rapidly progressive U-CLL CLL subtype than in M-CLL cells. Tumor cells from both subtypes showed reduced spontaneous apoptosis after CD44 stimulation. However, U-CLL cells gained a more significant survival advantage with a 65% improved viability of CD44 stimulated cells over unstimulated cells; this compares to a modest 26% increase in viability for the M-CLL cells. The observation that cells with higher CD44 expression gain a more pronounced survival effect suggests a dose response relationship of CD44 signaling and is consistent with enhanced tumorigenicity of cells transfected with CD44 [39, 40]. A competing but not mutually exclusive explanation could be that U-CLL cells, which typically express ZAP70, appear to have a somewhat more responsive signal transduction network that leads to stronger B-cell receptor and chemokine signaling[8, 10] that could also contribute to enhanced CD44 signaling.
To determine the mechanism involved in the anti-apoptotic effect of CD44 on CLL cells we focused on the PI3K/AKT and MAPK/ERK pathways, two major intracellular signaling pathways with prominent roles in leukemia that are involved in cell survival in response to growth factors, matrix adhesion and oncogene transformation (reviewed in[43]), and that have been reported to be activated by CD44 in solid tumor and lymphoma cell lines [21, 44, 45]. We found that both the PI3K/AKT and MAPK/ERK pathways are activated in CLL cells following CD44 stimulation. While the PI3K/Akt pathway is constitutively active in CLL cells, different exogenous stimuli derived from the tissue microenvironment including engagement of the B-cell receptor [46], CD40 ligand [47], stroma-derived factor-1, and CXCL13[48] have been shown to augment intracellular signaling and promote cell survival. Phosphorylation of Akt and ERK1/2 was rapidly apparent after CD44 stimulation and could be blocked by the PI3K inhibitor wortmannin and the MEK inhibitor, PD98059, respectively. Both inhibitors also effectively antagonized the anti-apoptotic effect of CD44 activation. We also found that stimulation of CD44 lead to an increase in MCL-1 levels through a post-transcriptional mechanism. This is in agreement with a recent study showing that forced expression of a constitutively active mutant of Akt is sufficient to increase MCL-1 protein levels without affecting MCL-1 mRNA transcription [46]. ERK1/2 on the other hand, has been shown to phosphorylate MCl-1 at Thr163, resulting in reduced MCL-1 protein degradation [49]. MCL-1 is a central survival factor for CLL cells [32, 46, 50] and appears to be the common survival molecule regulated by several different signaling pathways that include BCR stimulation [46], CD40 ligand [51], BAFF [29], APRIL [29], VEGF [52], and stroma cell contact [50]. Consistent with the activation of pathways in the microenvironment that lead to increased MCL-1 proteins levels, Smit and colleagues reported higher expression of MCL-1 protein but not mRNA in CLL cells obtained from lymph nodes compared to cells from the peripheral blood [51].
Increasingly, a picture is emerging that CLL cells are opportunistic cells that can use various signaling pathways to enhance cell survival [53]. Some of these pathways are tumor cell specific such as BCR signaling through a cognate antigen, while others are more general such as cytokines and chemokine pathways. Intriguingly, our data indicates that interactions of CD44 with the amorphous building blocks of the microenvironment can be sufficient to induce survival signals. How then can one best target these survival mechanisms? The convergence of many extracellular signals onto the PI3K/AKT and MAPK/ERK pathways makes these excellent candidates for intervention and the development of clinical grade inhibitors is advancing. A common target of many survival pathways is MCL-1, which is emerging as a key survival switch in CLL. To test whether inhibition of MCL-1 could block the anti-apoptotic effect of CD44 signaling we used obatoclax, a small molecule that binds to the BH3 groove of BCL-2 family members and potently inhibits MCL-1 [54, 55]. Obatoclax has been found to be well tolerated and have some clinical activity in heavily pretreated patients with CLL. These are encouraging results as the main application for obatoclax is expected to be in combination with chemotherapy. Here, we report that obatoclax strongly synergizes with fludarabine and that it can overcome the protective effect of the microenvironment, which is a well known mechanism contributing to fludarabine resistance [56]. Targeting the hyaluronic acid-CD44 axis directly may also become feasible using soluble CD44 constructs or specific antagonists of hyaluronic acid, which have been found to synergize with cytotoxic therapy in pre-clinical models [57].
Statement of Translational Relevance.
Survival of chronic lymphocytic leukemia (CLL) cells in vivo is supported by the tissue microenvironment. Interactions between tumor cells and the extracellular matrix are, in part, mediated by CD44, a receptor for hyaluronic acid. We show that CD44 engagement protects CLL cells from spontaneous and fludarabine-induced apoptosis. The anti-apoptotic effect is mediated through activation of the PI3K/Akt and MAPK/ERK pathways and increased MCL-1 protein levels. PI3K or MEK inhibitors as well as obatoclax, an antagonist of MCL-1, blocked the pro-survival effect of CD44. Furthermore, obatoclax sensitized CLL cells to fludarabine resulting in a synergistic drug effect. Our findings emphasize the therapeutic potential of PI3K/AKT or MAPK/ERK inhibitors and obatoclax for combination chemotherapy approaches that overcome the supportive effect of the tissue microenvironment on CLL cell survival and drug resistance.
Acknowledgments
Research support: This research was supported by the Intramural Research Program of the NIH, the National Cancer Institute and the National, Heart, Lung and Blood Institute.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 4.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 5.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 6.Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 7.Aguayo A, Kantarjian H, Manshouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]
- 8.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–4614. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 9.Deaglio S, Vaisitti T, Aydin S, et al. CD38 and ZAP-70 are functionally linked and mark CLL cells with high migratory potential. Blood. 2007 doi: 10.1182/blood-2007-06-094029. [DOI] [PubMed] [Google Scholar]
- 10.Richardson SJ, Matthews C, Catherwood MA, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL). Blood. 2006;107:3584–3592. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 11.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 12.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 15.Zarcone D, De Rossi G, Tenca C, et al. Functional and clinical relevance of CD44 variant isoform expression on B-cell chronic lymphocytic leukemia cells. Haematologica. 1998;83:1088–1098. [PubMed] [Google Scholar]
- 16.De Rossi G, Zarcone D, Mauro F, et al. Adhesion molecule expression on B-cell chronic lymphocytic leukemia cells: malignant cell phenotypes define distinct disease subsets. Blood. 1993;81:2679–2687. [PubMed] [Google Scholar]
- 17.Eisterer W, Bechter O, Soderberg O, et al. Elevated levels of soluble CD44 are associated with advanced disease and in vitro proliferation of neoplastic lymphocytes in B-cell chronic lymphocytic leukaemia. Leuk Res. 2004;28:1043–1051. doi: 10.1016/j.leukres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 19.Artus C, Maquarre E, Moubarak RS, et al. CD44 ligation induces caspase-independent cell death via a novel calpain/AIF pathway in human erythroleukemia cells. Oncogene. 2006;25:5741–5751. doi: 10.1038/sj.onc.1209581. [DOI] [PubMed] [Google Scholar]
- 20.Maquarre E, Artus C, Gadhoum Z, Jasmin C, Smadja-Joffe F, Robert-Lezenes J. CD44 ligation induces apoptosis via caspase- and serine protease-dependent pathways in acute promyelocytic leukemia cells. Leukemia. 2005;19:2296–2303. doi: 10.1038/sj.leu.2403944. [DOI] [PubMed] [Google Scholar]
- 21.Fujita Y, Kitagawa M, Nakamura S, et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002;528:101–108. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- 22.Gore Y, Starlets D, Maharshak N, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- 23.Nakano K, Saito K, Mine S, Matsushita S, Tanaka Y. Engagement of CD44 up-regulates Fas ligand expression on T cells leading to activation-induced cell death. Apoptosis. 2007;12:45–54. doi: 10.1007/s10495-006-0488-8. [DOI] [PubMed] [Google Scholar]
- 24.Poot M, Pierce RH. Detection of changes in mitochondrial function during apoptosis by simultaneous staining with multiple fluorescent dyes and correlated multiparameter flow cytometry. Cytometry. 1999;35:311–317. doi: 10.1002/(sici)1097-0320(19990401)35:4<311::aid-cyto3>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Klaus GG, Holman M, Hasbold J. Properties of mouse CD40: the role of homotypic adhesion in the activation of B cells via CD40. Eur J Immunol. 1994;24:2714–2719. doi: 10.1002/eji.1830241121. [DOI] [PubMed] [Google Scholar]
- 27.Dancescu M, Rubio-Trujillo M, Biron G, Bron D, Delespesse G, Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. J Exp Med. 1992;176:1319–1326. doi: 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francia di Celle P, Mariani S, Riera L, Stacchini A, Reato G, Foa R. Interleukin-8 induces the accumulation of B-cell chronic lymphocytic leukemia cells by prolonging survival in an autocrine fashion. Blood. 1996;87:4382–4389. [PubMed] [Google Scholar]
- 29.Nishio M, Endo T, Tsukada N, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106:1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Fuente MT, Casanova B, Moyano JV, et al. Engagement of alpha4beta1 integrin by fibronectin induces in vitro resistance of B chronic lymphocytic leukemia cells to fludarabine. J Leukoc Biol. 2002;71:495–502. [PubMed] [Google Scholar]
- 31.Petlickovski A, Laurenti L, Li X, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105:4820–4827. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 32.Pepper C, Lin TT, Pratt G, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008 doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien SM, Claxton DF, Crump M, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayroldi E, Cannarile L, Migliorati G, Bartoli A, Nicoletti I, Riccardi C. CD44 (Pgp-1) inhibits CD3 and dexamethasone-induced apoptosis. Blood. 1995;86:2672–2678. [PubMed] [Google Scholar]
- 35.Allouche M, Charrad RS, Bettaieb A, Greenland C, Grignon C, Smadja-Joffe F. Ligation of the CD44 adhesion molecule inhibits drug-induced apoptosis in human myeloid leukemia cells. Blood. 2000;96:1187–1190. [PubMed] [Google Scholar]
- 36.Bates RC, Edwards NS, Burns GF, Fisher DE. A CD44 survival pathway triggers chemoresistance via lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res. 2001;61:5275–5283. [PubMed] [Google Scholar]
- 37.Lakshman M, Subramaniam V, Rubenthiran U, Jothy S. CD44 promotes resistance to apoptosis in human colon cancer cells. Exp Mol Pathol. 2004;77:18–25. doi: 10.1016/j.yexmp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Guy R, Yefenof E, Naor D, Dorogin A, Zilberman Y. CD44 co-stimulates apoptosis in thymic lymphomas and T cell hybridomas. Cell Immunol. 2002;216:82–92. doi: 10.1016/s0008-8749(02)00505-1. [DOI] [PubMed] [Google Scholar]
- 39.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sy MS, Guo YJ, Stamenkovic I. Inhibition of tumor growth in vivo with a soluble CD44-immunoglobulin fusion protein. J Exp Med. 1992;176:623–627. doi: 10.1084/jem.176.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eistere W, Hilbe W, Stauder R, Bechter O, Fend F, Thaler J. An aggressive subtype of B-CLL is characterized by strong CD44 expression and lack of CD11c. Br J Haematol. 1996;93:661–669. doi: 10.1046/j.1365-2141.1996.d01-1704.x. [DOI] [PubMed] [Google Scholar]
- 43.Steelman LS, Abrams SL, Whelan J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 44.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 45.Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276:46024–46030. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- 46.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 47.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–1400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 48.Ticchioni M, Essafi M, Jeandel PY, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007 doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 49.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen IM, Kitada S, Leoni LM, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- 51.Smit LA, Hallaert DY, Spijker R, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109:1660–1668. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- 52.Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104:788–794. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- 53.Dal-Bo M, Bertoni F, Forconi F, et al. Intrinsic and extrinsic factors influencing the clinical course of B-cell chronic lymphocytic leukemia: prognostic markers with pathogenetic relevance. J Transl Med. 2009;7:76. doi: 10.1186/1479-5876-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burger M, Hartmann T, Krome M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 57.Gilg AG, Tye SL, Tolliver LB, et al. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008;14:1804–1813. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]