Abstract
Acid-sensing ion channel 1b (ASIC1b) is expressed in peripheral sensory neurons and has been implicated in nociception. Understanding the modulation of ASIC1b will provide important insight into how ASIC1b contributes to pain sensation. In our previous study, we showed that zinc, an important modulator of pain sensation, reduces rat ASIC1b current. However, rat ASIC1b shows several important differences from its recently identified human homolog. Most noticeably, human ASIC1b (hASIC1b) has a sustained component, which may play a role in persistent pain. Therefore, we tested here the hypothesis that zinc modulates the current properties of hASIC1b. Bath application of zinc suppressed the peak amplitude of hASIC1b currents, with a half-maximum inhibitory concentration of 37 μM. However, zinc did not affect the sustained component of hASIC1b currents. The effect of zinc was independent of pH-dependent activation, steady-state desensitization, and extracellular Ca2+, suggesting noncompetitive mechanisms. Further, we found that extracellular site(s) of the hASIC1b subunit is important for the effect of zinc. Mutating cysteine 196, but not cysteine 309, in the extracellular domain of the hASIC1b abolished the zinc inhibition. These results suggest that, through modulating cysteine196, zinc may have a modulatory role in acute pain.
Keywords: Acid-sensing ion channels, zinc, hASIC1b, patch-clamp, pain
Introduction
The acid-sensing ion channels (ASICs) are voltage-insensitive cation channels that belong to the degenerin/epithelial Na+ channel superfamily [1] and expressed primarily in neurons throughout the central and peripheral nervous system [2-4]. Besides protons, emerging evidence demonstrates that non-proton ligands such as heteromeric Texas coral snake toxin and agmatine can also activate the ASICs under normal pH condition [5,6]. So far, at least seven ASIC subunits encoded by four genes (ASIC1 - ASIC4) have been cloned [4,7,8]. Each ASIC subunit has two hydrophobic transmembrane domains, a large extracellular loop with cysteine-rich residues, and short cytoplasmic N- and C-termini [3,8]. The crystal structure of the chicken ASIC1a channels demonstrates that three individual ASIC subunits associate to form functional channels [9,10].
The ACCN2 gene encodes two splice variants, ASIC1a and ASIC1b [2-4]. ASIC1a is expressed both in the brain and in peripheral sensory neurons [8,11]. In the brain, ASIC1a contributes to synaptic plasticity [12-14] and plays important roles in multiple neurological diseases [15-20]. In the periphery, ASIC1a modulates pain sensation [21,22]. Unlike ASIC1a, ASIC1b is primarily expressed in the periphery sensory neurons [23-25]; however, the physiological and pathological roles of ASIC1b are poorly defined [22,26-28]. Recently, a Texas coral snake toxin, which directly activates both ASIC1a and ASIC1b, produces pain [5]. These results suggest that either ASIC1a or ASIC1b may contribute to nociception and implicate ASIC1 as a potential therapeutic target for pain.
To gain more insight into the physiological role of ASIC1b, we recently studied its modulation by zinc [29], which plays important roles in pain sensation [30]. Zinc exhibits an inhibitory effect on rat ASIC1b channels and cysteine149 located in the extracellular finger domain of rat ASIC1b subunit is responsible for zinc-induced inhibition [29]. Recently, Hoagland et al. [25] identified a human homolog of rodent ASIC1b [23,24]. Rat and human ASIC1b show 95% identity in amino acid sequence and shares similar electrophysiological and pharmacological properties. However, hASIC1b is calcium permeable and displays an acid-dependent sustained current [25]. Given the fact that zinc plays critical roles in physiological processes as well as pathological conditions [31-33], here we tested the zinc effect on hASIC1b channels by using whole-cell patch-clamp recording combined with site-directed mutagenesis in cultured CHO cells expressing hASIC1b subunit. Our results demonstrate that hASIC1b channels are sensitive target of zinc and cysteine 196 in the extracellular domain is responsible for zinc-mediated inhibition.
Materials and methods
Tissue culture and hASIC1b transient expression in Chinese hamster ovary cells
Tissue culture and transfection of Chinese hamster ovary (CHO) cells with various ASIC subunits were described in detail previously [34,35]. Briefly, CHO cells were maintained in standard F12 medium (American Type Culture Collection, Manassas, VA) supplemented with 10% fetal bovine serum at 37°C in a CO2 incubator. Cells were split with trypsin-EDTA, plated on a 35-mm culture dish at 10 to 20 % confluence, and allowed to recover for 1 day at 37°C. At ~70% confluence, cells were transiently transfected with pcDNA 3.1 expression vectors containing hASIC1b cDNA [25] and enhanced green fluorescent protein (eGFP) at a 3:2 molar ratio (Life Technologies, Carlsbad, CA) using X-tremeGENE HP DNA transfection reagent (Roche Diagnostics, Indianapolis, IN). Transfected cells were identified by expression of eGFP and used for electrophysiological recording 2-3 days after transfection. The cDNA of the hASIC1b clone was a gift from Dr. C. Askwith (The Ohio State University, Columbus, OH, USA).
Solutions and compounds
Standard extracellular fluid (ECF) contained (mM) 140 NaCl, 5.4 KCl, 2.0 CaCl2, 1.0 MgCl2, 20 HEPES, and 10 glucose (pH 7.4; 320 ~ 330 mOsm). For solutions with pH of 6.0 or lower, MES was used instead of HEPES for more reliable pH buffering [14,29,36]. The pipette solution contained (mM) 140 K-Gluconate, 10 HEPES, 11 EGTA, 2 TEA, 1 CaCl2, 2 MgCl2, and 4 K2ATP (pH 7.2 ~ 7.3; 290 ~ 300 mOsm). Chemicals were purchased from Sigma-Aldrich (St. Louis, MO). A multi-barrel perfusion system (SF-77, Warner Instrument Co., CT) was employed to achieve a rapid exchange of extracellular solutions. For bath application of zinc, zinc was present in the ECF of both pH 7.4 and lower pH (e.g. 6.0). For solution containing different of concentrations of extracellular calcium (e.g. 2, 5 or 10 mM), the same concentration of calcium was present in the ECF of both pH 7.4 and lower pH (e.g. 6.0). For co-application of zinc, zinc was only present in the ECF of lower pH (e.g. 6.0).
Electrophysiological recording in CHO cells
Whole-cell patch-clamp recordings were conducted as described previously [14,29,35]. Patch electrodes, whose resistance ranged from 4 to 6 MΩ when filled with intracellular solution, were constructed from thin-walled borosilicated glass (1.5 mm diameter, WPI, Sarasota, FL) on a two-stage puller (PC-10, Narishige, Tokyo, Japan). Whole-cell currents were triggered by a drop in pH from 7.4 to various levels at a holding potential of -60 mV and recorded using Axopatch 200B amplifiers (Axon CNS, Molecular Devices, Foster City, CA). Data were collected at 2 kHz and digitized at 5 Hz using Digidata 1440 DAC units (Axon CNS, Molecular Devices, Foster City, CA). The on-line acquisition was done using pCLAMP software (Version 10.2, Axon CNS, Molecular Devices, Foster City, CA).
In general, hASIC1b channels were activated by a drop in pH from 7.4 to target levels every 2 min to allow for a complete recovery of the channels from desensitization. During each experiment, a voltage step of –10 mV from the holding potential (-60 mV unless specified otherwise) was applied periodically to monitor the cell capacitance and the access resistance. Recordings in which either the access resistance or the capacitance changed by more than 10% during the experiment were excluded from data analysis.
hASIC1b construct
Human ASIC1b construct in mammalian expression vector (pcDNA 3.1) was kindly provided by Dr. Candice C. Askwith (The Ohio State University, Columbus, OH, USA). Site-directed mutagenesis was performed as described previously [14,29,34,35], using the Quick-Change Site-Directed Mutagenesis system (Stratagene, La Jolla, CA) in accordance with the manufacture’s protocol. The primers were obtained from Integrated DNA Technologies (Coralville, IA). Mutations were confirmed by sequencing.
Data analysis and statistics
All data were analyzed using Clampfit 10.2 software (Axon CNS, Molecular Devices, Foster City, CA). For half-maximum inhibitory concentration (IC50) curves of zinc, pH 6.0-triggered hASIC1b currents with different concentrations of zinc treatment (1, 3, 10, 30, 100, and 300 μM) were normalized to a pH 6.0-triggered hASIC1b current without zinc treatment. The sustained component of hASIC1b current was measured at 5 seconds time point following pH drop (7 seconds duration). Normalized values were fitted to the Hill equation to obtain IC50 values and Hill coefficients. Statistical analyses were carried out using SigmaPlot software (Version10.0.1, Systat). Significant differences between mean values from each experimental group were tested using the Student’s t-test for two groups and one-way analysis of variance (ANOVA) for multiple comparisons. Differences were considered significant if p < 0.05.
Results
Co-application of zinc don’t affect hASIC1b currents
To test whether co-application of zinc with pH drop has any effect on hASIC1b currents, eEGF positive CHO cells expressing hASIC1b subunit were identified and hASIC1b currents were recorded by a drop (7 seconds in duration) in pH from 7.4 to 6.0 under whole-cell voltage clamp configuration. As shown in Figure 1A, co-application of zinc, at concentrations between 1 to 300 μM, did not affect hASIC1b currents both in peak amplitude (Figure 1B) and sustained component (Figure 1C). These data suggest that zinc doesn’t bind to the channel pores to affect hASIC1b activity when they are in the open state.
Figure 1.
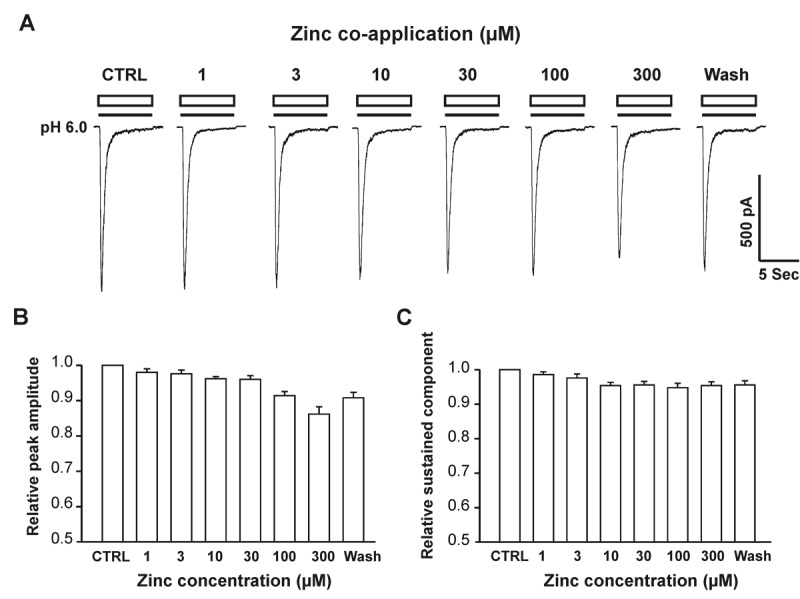
Co-application of zinc doesn’t affect hASIC1b currents in hASIC1b transfected CHO cells. A, Representative traces showing pH-6.0 elicited hASIC1b currents with co-application of different concentrations of zinc in CHO cells. Transient inward hASIC1b currents in CHO cells were recorded with drops in pH from 7.4 to 6.0 in whole-cell configurations at –60 mV. The duration of co-application with pH drops is 7 seconds. B & C, Quantification of relative peak current amplitude (B), and sustained-component (C) of pH 6.0-activated currents with co-application of zinc at different concentrations. Each point represents the average responses of five to eight cells. There are no significant differences between each group (p > 0.05, ANOVA). CTRL represents control.
Pre-application of zinc concentration-dependently suppresses the peak amplitude, but not the sustained component of hASIC1b currents
We next examined the concentration-response relationship on hASIC1b currents by bath application of zinc for 2 minutes before decreasing the pH. As shown in Figure 2A, pre-application of 1 or 3 μM zinc revealed slight decreases in peak amplitude of hASIC1b currents, but these changes were not significant (Figure 2B and 2C). Pre-application of zinc, at concentrations of 10, 30, 100 or 300 μM, however, profoundly and concentration-dependently suppressed peak amplitude, but not sustained component of the hASIC1b currents (Figure 2B and 2C). At 10, 30, 100 and 300 μM, the peak amplitude of hASIC1b currents were reduced by 27%, 46%, 69% and 82%, respectively. The inhibitory effect of zinc on the peak amplitude of hASIC1b currents was rapidly reversed after washout (Figure 1A). The concentration-inhibition curve for zinc is shown in Figure 2B, with an IC50 value of 36.5 ± 1.5 μM and a Hill coefficient of 1.0 ± 0.01. These data indicate that the peak amplitude, but not the sustained component of the hASIC1b current is inhibited by pre-application of zinc in a concentration-dependent manner; and that zinc appears to interact with hASIC1b channels in the closed state.
Figure 2.
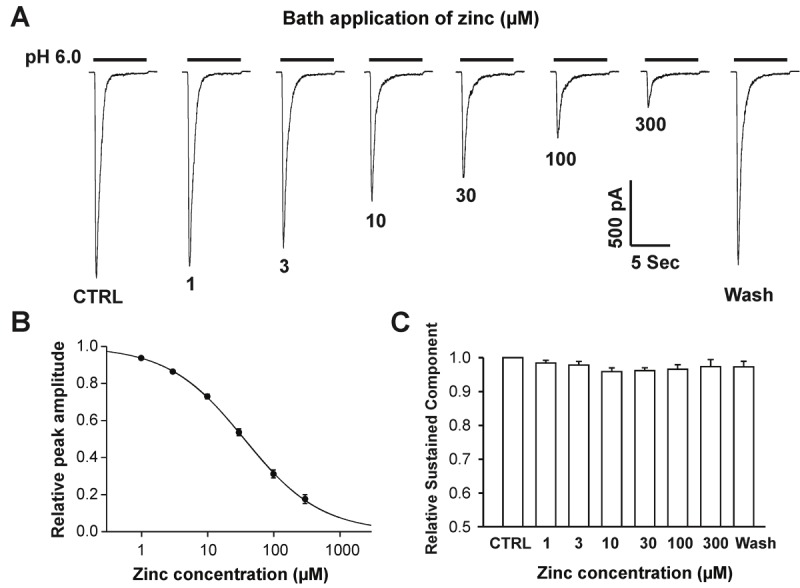
Bath application of zinc suppresses peak amplitude, but not sustained component of hASIC1b currents concentration-dependently in hASIC1b transfected CHO cells. A, Representative traces showing the concentration-dependent blockade of hASIC1b currents triggered by dropping the pH from 7.4 to 6.0 in the presence of different concentrations of zinc. B, Concentration-inhibition curve of pH 6.0-induced currents by bath application of zinc. The IC50 of zinc blockade is 36.45 ± 1.52 μM. Each point represents the average responses of eight cells. C, Quantification of relative sustained-component of pH 6.0-activated currents with bath application zinc at different concentrations. Each point represents the average responses of eight cells. There are no significant differences between each group (p > 0.05, ANOVA). CTRL represents control.
Zinc inhibition of hASIC1b is independent of pH activation and steady-state desensitization
To determine whether inhibition of hASIC1b channels by bath application of zinc is pH- dependent, we produced pH concentration-response curves before and after bath (pre- plus co-) application of 50 μM zinc. Bath application of 50 μM zinc inhibited hASIC1b currents induced by pH drops from 7.4 to 6.5, 6.0, 5.0, 4.0, and 3.0 to 42.9 ± 2.7%; 45.1 ± 2.8 %, 45.5 ± 2.3%, 48.1 ± 2.6%, and 49.0 ± 3.1% of the control value, respectively (Figure 3A and 3B). The degree of zinc to inhibit hASIC1b currents induced by drops in pH from 7.4 to 6.0 did not differ significantly from the level of inhibition observed with other pH values (p > 0.05; ANOVA). These results suggest that the degree of inhibition of hASIC1b currents by bath application of zinc does not depend on the pH-dependent activation of the hASIC1b channels.
Figure 3.
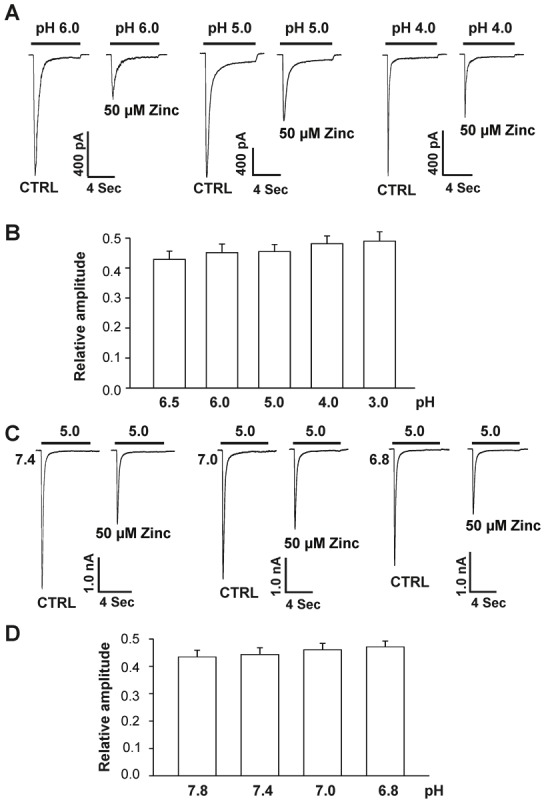
Zinc inhibition of hASIC1b currents in hASIC1b transfected CHO cells is independent of pH activation and steady-state desensitization. A, Original current traces showing inhibitory effects of 50 μM zinc with pretreatment on the pH-dependent activation of hASIC1b currents in CHO cells. The starting pH for all experiments was 7.4, the activating pH is indicated by bars above the trace and CHO cells were clamped at –60 mV. B, Quantification of relative peak amplitude of hASIC1b current inhibition by bath application of 50 μM zinc. Each point represents the average response of six to nine cells. There are no significant differences between different groups in the presence of 50 μM zinc (p > 0.05, ANOVA). C, Original current traces showing inhibitory effects of 50 μM zinc with pretreatment on steady-state desensitization of ASIC1b currents in CHO cells. Steady-state desensitization was induced by various conditioning pH values between 7.4 and 6.8 for ~6 min before application of pH 5.0. CHO cells were clamped at –60 mV. D, Quantification of relative peak amplitude of hASIC1b current inhibition on steady-state desensitization by pre-applied 50 μM zinc at various conditioning pH values (between 7.8 to 6.8). hASIC1b current was evoked by application of pH 5.0. Each point represents the average response of five to nine cells. There are no significant differences between each group in the presence of 50 μM zinc (p > 0.05, ANOVA). CTRL represents control.
To further explore the mechanism underlying the inhibition of the hASIC1b current by bath application of zinc, we determined the effect of 50 μM zinc on steady-state desensitization of hASIC1b. CHO cells expressing hASIC1b subunit were incubated in extracellular solutions at various conditioning pH values between 7.8 and 6.8 for ~6 min before the hASIC1b currents were activated by a drop in pH to 5.0. As shown in Figure 3C and 3D, bath application of 50 μM zinc inhibited hASIC1b currents induced by pH drops from different conditioning values to 5.0. The capacity of zinc to inhibit hASIC1b currents triggered by drops in pH to 5.0 from different conditioning values did not differ significantly (p>0.05; ANOVA). These results demonstrate that the degree of inhibition of hASIC1b currents by bath application of zinc is independent of the steady-state desensitization of the hASIC1b channels. Taken together, these data suggest that a noncompetitive mechanism is responsible for zinc inhibition.
Zinc inhibition does not change with increased extracellular Ca2+ concentration
To determine whether zinc-induced inhibition of hASIC1b currents is affected by changes in the concentration of extracellular Ca2+, increasing the extracellular Ca2+ concentration from 2 to 5 or 10 mM (in the ECF of both pH 7.4 and 6.0) had an inhibitory effect on hASIC1b currents, as shown in Figure 4A. However, the capacity of bath application of 50 μM zinc to inhibit hASIC1b currents triggered by a drop in pH from 7.4 to 6.0 was not significantly affected by increasing the extracellular Ca2+ concentration from 2 to 5 or 10 mM (Figure 4B; p > 0.05). The percentile of inhibition on hASIC1b currents by bath application of 50 μM zinc was 39.7 ± 4.5%, 44.7 ± 3.5%, and 46.1 ± 3.6% in the presence of 2.0, 5.0 and 10 mM Ca2+, respectively (Figure 4B). The failure of extracellular Ca2+, at concentrations between 2.0 and 10 mM, to significantly impact zinc-mediated inhibition of hASIC1b currents, in conjunction with the capacity of Ca2+ to inhibit hASIC1b currents, suggests that zinc and calcium inhibit hASIC1b by binding to distinct sites on the hASIC1b subunit, and that they do not compete with one another for the binding sites.
Figure 4.
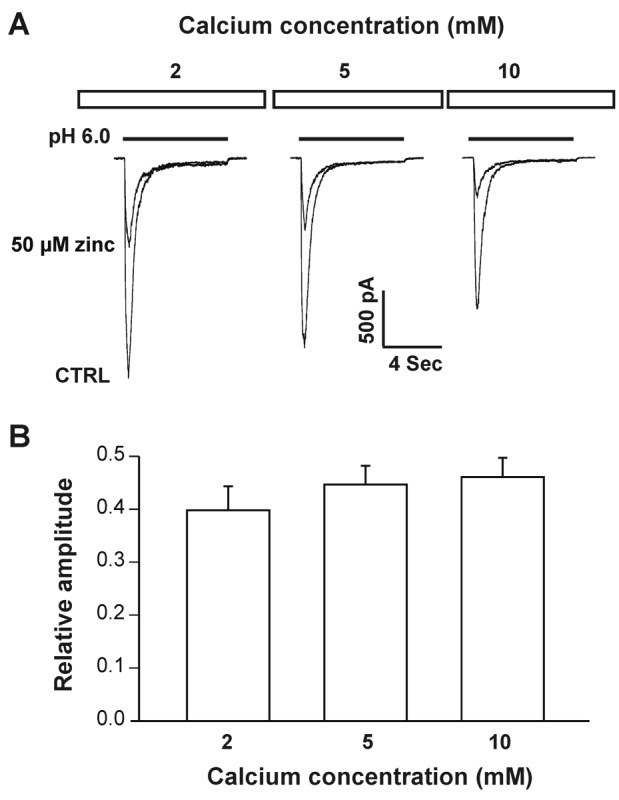
Zinc inhibition of hASIC1b currents in hASIC1b transfected CHO cells is independent of calcium. A, Original current traces showing inhibitory effect of pre-applied zinc on hASIC1b currents in the presence of 2, 5 and 10 mM Ca2+. hASIC currents were activated by a drop in pH from 7.4 to 6.0 at a membrane potential of -60 mV. B, Quantification of relative peak amplitude of zinc inhibition at different concentrations of Ca2+. Each point represents the average response of five cells. There are no significant differences between different groups (p > 0.05, ANOVA). CTRL represents control.
Zinc-mediated inhibition is via extracellular domain of hASIC1b subunit
To determine whether zinc blocks hASIC1b by binding to extracellular or intracellular domains, we included zinc at a concentration of 50 μM in the recording pipette before testing the effect of extracellular zinc in the bath solution. After formation of whole-cell configurations, zinc was allowed to diffuse from the recording pipette into cells for 20 min. Once a stable hASIC1b current was recorded, 50 μM zinc was then added to the bath solution to examine whether it could still inhibit the hASIC1b current. As shown in Figure 5A and 5B, under conditions in which intracellular solution contained 50 μM, addition of 50 μM zinc in the extracellular solution still suppressed hASIC1b currents (Figure 4A and 4B; **p < 0.01). The extent of inhibition by extracellular zinc in the presence of intracellular zinc is similar to that without intracellular zinc (Figure 5C and 5D). These data suggest that zinc inhibits hASIC1b currents by binding to the extracellular domain(s) of the channel.
Figure 5.
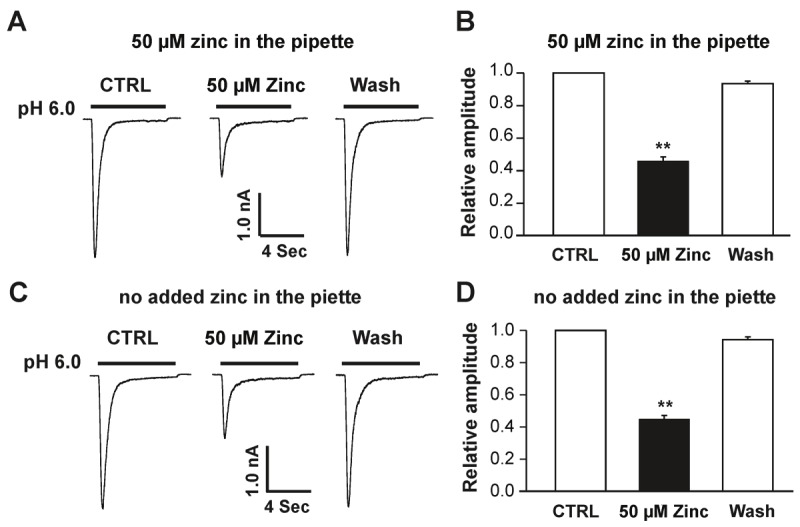
Extracellular zinc is responsible for inhibition of the hASIC1b current in hASIC1b transfected CHO cells. A, Representative traces showing that inhibition of hASIC1b currents by bath application of 50 μM zinc (extracellular zinc) was not affected by inclusion of 50 μM zinc in the pipette solution (i.e. intracellular zinc). hASIC1b currents were activated by a drop in pH from 7.4 to 6.0. B, Quantification of relative peak amplitude of zinc inhibition in the presence of 30 μM zinc in the pipette. Each point represents the average response of six cells. C, Original traces showing that inhibition of hASIC1b currents by bath application of 50 μM zinc (extracellular zinc) under no added zinc in the pipette. hASIC1b currents were triggered by a drop in pH from 7.4 to 6.0. D, Quantification of relative peak amplitude of zinc inhibition under no added zinc in the pipette. Each point represents the average response of five cells. Asterisk indicates values significantly different from the control, t-test, **p < 0.01. CTRL represents control.
Zinc-mediated inhibition involves cysteine 196, but not cysteine 309, in the extracellular domain of the hASIC1b subunit
Recently, we found that cysteine149 located in the extracellular finger domain of rat ASIC1b subunit is critical for zinc inhibition [29]. Cysteine 149 in rat ASIC1b is comparable to cysteine 196 in hASIC1b. Cysteine 309, another cysteine residue in extracellular domain of hASIC1b subunits, was randomly chosen as a control. Cysteine, as a non-charged residue, was replaced with alanine (A), which is also non-charged residue. Site-directed mutagenesis studies were performed to identify whether these residues are responsible for zinc inhibition. Two mutants (hASIC1b-C196A and hASIC1b-C309A) were generated, in which alanine was substituted for each of the two cysteines listed above. Similar to wild-type hASIC1b, hASIC1b-C309A currents were inhibited by bath application of zinc in a concentration-dependent manner in CHO cells expressing hASIC1b-C309A mutants (Figure 6A). A detailed concentration-response curve is presented in Figure 6C, and the IC50 was determined to be 31.4 ± 1.3 μM. In cells expressing hASIC1b-C196A mutants, however, hASIC1b-C196A currents were not significantly suppressed by zinc application at any of the concentrations tested (Figure 6B, 6D). Taken together, these data suggest that C196, but not C309, at the extracellular domain of the hASIC1b subunit is critical for zinc-mediated inhibition.
Figure 6.
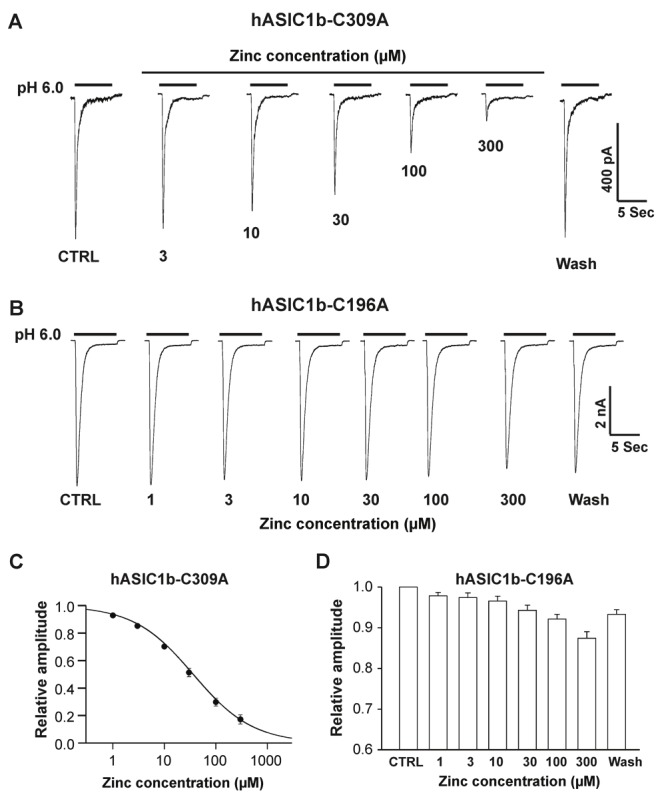
hASIC1b-C196A, but not hASIC1b-C309 mutant abolished the zinc inhibition. A, Representative traces showing the concentration-dependent blockade of hASIC1b-C309A mutant currents triggered by dropping the pH from 7.4 to 6.0 in the presence of different concentrations of zinc. B, Representative traces showing that hASIC1b-C196A mutation abolished the zinc inhibition in the presence of different concentrations of zinc with bath application. C, Concentration-inhibition curve of pH 6.0-induced currents on hASIC1b-C309A mutant by bath application of zinc. The IC50 of zinc blockade is 31.4 ± 1.3 μM. Each point represents the average responses of five cells. D, Quantification of relative amplitude of pH 6.0-activated currents on hASIC1b-C196A mutant with bath application of zinc at different concentrations. Each point represents the average responses of six to eight cells. There are no significant differences among all groups p > 0.05, ANOVA). CTRL represents control.
Discussion
Zinc has been implicated as a key endogenous molecule that plays a critical role in physiological as well as pathological conditions [31-33]. More recently, zinc has been reported to reduce pain in animal models [30,37]. Because of its potential importance in diseases, the effect of zinc in voltage- and ligand-gated ion channels has been extensively studied [38-40]. For example, four residues of extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors [41]. Consistent with this finding, Nozaki et al. recently found that zinc alleviates pain behavior through high-affinity binding to the NMDA receptor NR2A subunit by using NR2A-H128S knock-in mice [30]. Moreover, zinc also inhibits voltage-gated K+ channel in olfactory neurons [42]. Here, we showed that zinc inhibits hASIC1b through modulating cysteine 196 in the extracellular domain of the channel. Collectively, these data demonstrate that zinc affects a wide variety of ion channels. The effect of zinc on physiology or pathology may depend upon the exact paradigm.
Finding zinc regulates ASIC1 is interesting, because both ASIC1a and ASIC1b are expressed in sensory neurons that receive nociceptive input [23,24,43]. More importantly, ASIC1 channels have been implicated in pain sensation [5,23]. For example, Psalmotoxin 1, a peptide extracted from the South American tarantula Psalmopoeus cambridgei, has profound analgesic properties against thermal, mechanical, chemical, inflammatory and neuropathic pain in rodents. It exerts its action by blocking ASIC1a, suggesting that ASIC1a channels contribute to pain modulation [44]. More recently, Bohlen et al. [14] found that injection MitTX, a venom isolated from Texas coral snake, into the hindpaw of wild-type mice produces robust pain-related behavior, which are reduced by deleting the ASIC1 but not ASIC3 gene. Further, in CHO cells expressing homomeric ASIC1a and ASIC1b subunits, MitTX can trigger robust inward currents in both ASIC1a and ASIC1b channels under normal pH value (7.4). These findings suggest that predominant ASIC1 channels contribute to coral snake toxin-evoked nociception [5]. Taken together, all these results suggest that ASIC1 plays a critical role in pain sensation. It will be interesting to test whether zinc, a potent inhibitor of ASIC1 channels, can combat pain induced by MixTX or other paradigms.
Unlike rodent ASIC1b, hASIC1b has significant calcium permeability and exhibits a small sustained current in response to a drop in pH [25]. The physiological significance of the sustained current is unclear but has been implicated in persistent pain [45,46]. Besides hASIC1b, ASIC3 also displays a sustained component [7,46]. However, unlike hASIC1b, zinc inhibits both peak and sustained component of rat ASIC3 channels [47]. These results suggest that mechanisms regulating zinc susceptibility are different between ASIC1b and ASIC3.
Although further study are needed to understand how zinc has differential effects on the acute and sustained components, our data suggest that zinc inhibits hASIC1b currents in the closed state because pre-application, but not co-application of zinc inhibits ASIC1b current. In addition, zinc-mediated inhibition of hASIC1b channels is independent of pH activation, steady-state desensitization and extracellular Ca2+, suggesting noncompetitive mechanisms.
The effects of zinc on ASICs in native neurons are complex due to the fact that these channels may consist of combinations of different ASIC subunit, and the fact that zinc has differential effects on different ASIC subunits [48,49]. For example, zinc potentiates homomeric ASIC2a and heteromeric ASIC1a/2a, but inhibits homomeric ASIC1b and ASIC3 channels with low-affinity [29,47,50]; while zinc inhibits homomeric ASIC1a and heteromeric ASIC1a/2a channels with high-affinity [51]. Since ASICs can function as homomeric and heteromeric channels, these data suggest that the effect of zinc on acid-activated currents depends upon subunit combination [51]. In addition, heteromerization may also regulate the spatial location of ASIC channels [52], which is another important variable that can influence the functional outcome of heteromeric channels. These results indicate that the effect of zinc on ASICs is complex, and is determined by the exact stoichiometry of the channel.
Acknowledgements
This work was supported by startup funds from University of South Alabama (X.M.Z), National Institutes of Health grant R21DA31259, American Heart Association Scientist Development Grant 0735092N, University of Missouri Research Board and University of Missouri-Kansas City School of Medicine Start-up Funds (X.P.C). We thank Dr. Candice C. Askwith from Ohio State University for the human ASIC1b construct.
References
- 1.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Review. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 2.Krishtal O. The ASICs: signaling moleculars? modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 3.Wemmie JA, Price MP, Welsh MJ. Acidsensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Grunder S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- 5.Bohlen CJ, Chesler AT, Sharif-Naeini R, Medzihradszky KF, Zhou S, King D, Sánchez EE, Burlingame AL, Basbaum AI, Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. 2011;479:410–414. doi: 10.1038/nature10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68:61–72. doi: 10.1016/j.neuron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton- gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 9.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Aº resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 10.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 13.Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci USA. 2006;103:16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-Glycosylation of Acid-Sensing Ion Channel 1a Regulates Its Trafficking and Acidosis-Induced Spine Remodeling. J Neurosci. 2012;32:4080–4091. doi: 10.1523/JNEUROSCI.5021-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wemmie JA, Price M, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011;31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pignataro G, Cuomo O, Esposito E, Sirabell R, Di Renzo G, Annunziato L. ASIC1a contributes to neuroprotection elicited by ischemic preconditioning and postconditioning. Int J Physiol Pathophysiol Pharmacol. 2011;3:1–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- 23.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron specific, proton-gated ion channel. Proc Natl Acad Sci USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 25.Hoagland EN, Sherwood TW, Lee KG, Walker CJ, Askwith CC. Identification of a calcium permeable human acid-sensing ion channel 1 transcript variant. J Biol Chem. 2010;285:41852–41862. doi: 10.1074/jbc.M110.171330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu XP, Xiong ZG. Physiological and pathological functions of Acid-sensing ion channels in the central nervous system. Curr Drug Targets. 2012;13:263–271. doi: 10.2174/138945012799201685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 28.Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Q, Inoue K, Wu X, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Cysteine 149 in the extracellular finger domain of acid-sensing ion channel 1b subunit is critical for zinc-mediated inhibition. Neuroscience. 2011;193:89–99. doi: 10.1016/j.neuroscience.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nozaki C, Vergnano AM, Filliol D, Ouagazzal AM, Le Goff A, Carvalho S, Reiss D, Gaveriaux-Ruff C, Neyton J, Paoletti P, Kieffer BL. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat Neurosci. 2011;14:1017–10122. doi: 10.1038/nn.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 32.Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 33.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 34.Chu XP, Close N, Saugstad JA, Xiong ZG. ASIC1a-specific modulation of acid-sensing ion channels in mouse cortical neurons by redox reagents. J Neurosci. 2006;26:5329–5339. doi: 10.1523/JNEUROSCI.0938-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jing L, Jiang YQ, Jiang Q, Wang B, Chu XP, Zha XM. The interaction between the first transmembrane domain and the thumb of ASIC1a is critical for its N-glycosylation and trafficking. PLoS One. 2011;6:e26909. doi: 10.1371/journal.pone.0026909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience. 2009;162:55–66. doi: 10.1016/j.neuroscience.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Júnior WB, Alexandre-Moreira MS, Alves MA, Perez-Rebolledo A, Parrilha GL, Castellano EE, Piro OE, Barreiro EJ, Lima LM, Beraldo H. Analgesic and anti-inflammatory activities of salicylaldehyde 2-chlorobenzoyl hydrazone (H2LASSBio-466), salicylaldehyde 4-chlorobenzoyl hydrazone (H2LASSBio-1064) and their zinc(II) complexes. Molecules. 2011;16:6902–6915. doi: 10.3390/molecules16086902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 39.Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 40.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 41.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000;25:683–694. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 42.Seebungkert B, Lynch JW. A common inhibitory binding site for zinc and odorants at the voltage-gated K+ channel of rat olfactory receptor neurons. Eur J Neurosci. 2001;14:353–362. doi: 10.1046/j.0953-816x.2001.01646.x. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gélot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007;10:943–945. doi: 10.1038/nn1940. [DOI] [PubMed] [Google Scholar]
- 45.Dubé GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honoré P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Q, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Inhibitory regulation of acid-sensing ion channel 3 by zinc. Neuroscience. 2010;169:574–583. doi: 10.1016/j.neuroscience.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 48.Chu XP, Papasian CJ, Wang JQ, Xiong ZG. Modulation of acid-sensing ion channels: molecular mechanisms and therapeutic potential. Int J Physiol Pathophysiol Pharmacol. 2011;3:288–309. [PMC free article] [PubMed] [Google Scholar]
- 49.Xu TL, Xiong ZG. Dynamic regulation of acid-sensing ion channels by extracellular and intracellular modulators. Curr Med Chem. 2007;14:1753–1763. doi: 10.2174/092986707781058977. [DOI] [PubMed] [Google Scholar]
- 50.Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276:35361–35367. doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- 51.Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


