Abstract
Rose bengal (RB) has been utilized as a photodynamic agent for the targeted killing of cancer cells. Recent data suggest that intralesional RB alone may be effective in chemoablating locoregional and metastatic melanomas. The ability of RB to induce direct and bystander melanoma cell death led to the speculation that it may be similarly effective in the treatment of other neoplasms. The objective of this study was to determine whether RB can limit the growth, or kill, ovarian cancer cells in vitro. Ovarian carcinoma cells with or without a germline BRCA1 mutation were cultured with up to 800 μM RB for one hour or four days, after which their ability to proliferate was assessed using the MTT assay. Control cells included an embryonic kidney cell line transformed with adenovirus, and normal human fibroblasts. Ovarian cancer cells exhibited significant dose-dependent suppression of growth in response to RB; this suppression was similar to that seen with carboplatin. RB treated ovarian cancer cells appeared rounded, shrunken, and damaged. RB also inhibited the growth of kidney tumor cells but was much less effective in slowing the growth of normal human fibroblasts suggesting that RB-mediated growth suppression might be tumor cell specific. Ovarian cancer cells treated with RB displayed a significant increase in apoptosis that peaked at approximately four times the levels seen in untreated control cells. Furthermore, RB exposure resulted in the intracellular generation of reactive oxygen species (ROS) at levels that were significantly greater than in untreated cells and similar to levels seen in cells treated short term with H2O2. These data suggest that RB may not only suppress ovarian cancer cell growth but also induce their apoptotic cell death, justifying the further investigation of the effects of RB in an animal model of ovarian cancer.
Keywords: Rose bengal, ovarian cancer, BRCA1, MTT assay, apoptosis, reactive oxygen species
Introduction
Rose bengal (RB), an anionic water soluble xanthene dye, has been used for many years by eye care professionals to assess damage to the cornea and conjunctiva [1,2]. Its ability to be converted to singlet oxygen when irradiated with green light led to its use as a photodynamic sensitizer for killing both microorganisms and cancer cells [3-7]. It has also been reported that cancer cells pre-exposed to RB could be lysed when later exposed to ultrasound [8,9]. Relevant to this study, RB alone was also shown to be toxic to cancer cells and to preferentially enter cancer, but not normal, cells. Intralesional RB was reported to chemoablate locoregional and metastatic melanomas [10,11]. In a phase I study of 20 subjects with stage III‐IV melanoma, a single injection of RB (10% in saline, designated PV-10 by Provectus Pharmaceuticals) into a total of 114 lesions was well tolerated, and yielded a durable objective response (OR) in 40% of subjects and locoregional disease control in 75% of subjects. Furthermore, 15% of subjects achieved an OR in their bystander lesions (43 lesions) [12]. In more recent phase II testing in 80 subjects, this RB formulation was shown to induce a complete or partial response, or stabilize disease in 49% of the 80 enrolled subjects [12] following intralesional injection. In addition, 33% of 21 subjects with evaluable bystander lesions achieved a CR in these lesions, along with 10% and 14% in the PR and SD groups, respectively [13].
The ability of RB to induce both direct and bystander melanoma cell death led to the speculation that it may be similarly effective in the treatment of other intractable cancers. The objective of this study was to determine whether there is justification in pursuing such an approach for the treatment of ovarian cancer. While accounting for only three percent of cancers in women, ovarian cancer is the fifth most common cause of cancer death in women behind lung, breast, colorectal, and pancreatic cancers [14]. There has been a decline of less than one percent in its incidence over the past twenty years and, despite the development of new treatment approaches, its mortality rate has remained relatively unchanged [15]. These statistics, coupled with the fact that peritoneal dissemination of ovarian cancer makes it particularly hard to surgically eradicate necessitate the development of new treatment methodologies. The appeal of RB is that it represents a biocompatible compound that appears to exhibit an immune bystander effect that could be of great benefit in the treatment of ovarian carcinosis. As a first step in the evaluation of this approach, we determined the effects of RB on human ovarian cancer cells in vitro.
Materials and methods
Reagents
RB was purchased from Sigma Chemical Co. (catalogue #R3877-5G). The Vibrant MTT Cell Proliferation Assay Kit (catalogue#: V13154), purchased from Invitrogen (Grand Island, NY) was used to evaluate the responses of human ovarian cancer, transformed kidney, and normal fibroblast cell lines to various doses of RB. Incubation of cells with carboplatin (Sigma; catalogue #C2538-100MG), a chemotherapeutic drug known to be toxic to ovarian cancer cells, served as a positive control for suppressed cell growth. The TiterTACS™ 96-well Apoptosis Detection Kit from R&D Systems (Minneapolis, MN; catalogue #TA600) was used to detect apoptotic cell death in ovarian carcinoma cells. Whether or not RB induced the generation of reactive oxygen species in ovarian carcinoma cells was determined using the OxiSelect™ Intracellular ROS Assay Kit (Cell Biolabs, Inc., San Diego, CA; catalogue #STA-342).
Cell lines
UWB1.289 ovarian carcinoma cells (hereafter referred to as UWB) were obtained from American Type Culture Collection (ATCC; Manassas, VA). This cell line was derived from a tumor of serous histology, which is one of the most common and lethal ovarian malignant tumors constituting 60% of the malignant tumors of the ovary and accounting for the majority of deaths from gynecologic malignancies. UWB carries a germline BRCA1 mutation within exon 11 and has a deletion of the wild-type allele. It is estrogen and progesterone receptor negative and has an acquired somatic mutation in p53. These cells were passaged in 50% RPMI-1640 medium (Life Technologies) and 50% Mammary Epithelial Growth Medium (MEGM, from Clonetics/Lonza, Basel, Switzerland), the latter of which was constituted from MEBM basal medium and SingleQuot additives supplemented with 3% fetal bovine serum (FBS).
The effects of RB were also determined in the following three cells lines: 1. UWB1.289+BRCA1 (referred to hereafter as BRCA1), which is a stable line derived from UWB1.289 in which wild-type BRCA1 was restored; 2. HEK-293, an embryonic kidney cell line transformed with adenovirus 5 DNA - this cell line served as a non-ovarian cancer control; and 3. Detroit 551, a normal human skin fibroblast cell line. The BRCA1 cell line was propagated in the same media as the UWB cell line, and the HEK-293 and Detroit 551 cell lines were propagated in Eagle's Minimum Essential Medium (EMEM) supplemented with 10% FBS.
Cell proliferation assay
Cells were harvested from near confluent tissue culture plates with 0.05% trypsin in EDTA (Invitrogen), washed in media, and resuspended to a final concentration of 2 x 105 cells/ml. 100 μL of each cell suspension were added to flatbottomed, 96-well plates (Costar), so that each well contained a total of 2 x 104 cells. In all of the experiments described below, eight wells were treated per group and in most cases, assays were run three times. After being incubated overnight at 37°C in 5% CO2, 0.16 to 2.5 mM carboplatin was added to wells containing UWB cells; these wells served as a positive control for UWB cell toxicity. Other plates containing UWB as well as BRCA1, HEK-293 and Detroit 551 cells were treated with up to 800 μM RB. Both test compounds were added to the wells in sterile PBS; control wells received sterile PBS alone. The cultures were then incubated for an additional four days after which the media were aspirated from the wells and replaced with 100 μL of phenol red-free high glucose-containing DMEM (Invitrogen); this media change was necessitated by the fact that phenol red can interfere with the MTT assay. After this media change, 10 μL of the 12 mM MTT stock solution was added to each well and the plates incubated for an additional four hours, after which time all but 25 μL of media in each well was aspirated and 50 μL of DMSO added to each well. The plates were then briefly shaken on a plate shaker and incubated for 10 minutes, after which the plates were again briefly shaken and the absorbance in the wells determined at 540 nm using a BioTek EL-808 plate reader. One plate was seeded with between 4 x 103 and 1 x 106 untreated UWB cells one hour prior to MTT treatment in order to generate an absorbance/cell density curve.
Apoptosis assay
UWB cells treated with RB were analyzed to determine whether they had undergone apoptosis using the Trevigen HT TiterTACS Assay Kit. Cells were incubated with 100 to 800 μM RB for four days as described above, after which they were washed and fixed in 10% formalin. The rest of the assay was carried out as described by the manufacturer, which included the quenching of endogenous peroxidase activity as well as the generation of a positive nuclease control. The final step involved the colorimetric detection of DNA fragmentation at an absorbance of 450 nm in the above-mentioned plate reader.
Measurement of the generation of reactive oxygen species (ROS)
Whether or not treatment with RB resulted in the generation of ROS in UWB cells was determined using the Cell Biolabs assay mentioned above. This assay involves the preloading of cells with dichlorodihydrofluorescein diacetate (DCFH-DA), which interacts with intracellular ROS to generate fluorescent dichlorofluorescein (DCF); fluorescence intensity is proportional to the ROS levels in the cytosol. Cells plated at a density of 1 × 104 cells per well were incubated for 24 hours, after which the media were discarded and the attached cells washed with PBS and then exposed to DCFH-DA for one hour at 37°C. After washing with PBS, the cells were treated with 10, 100, or 1000 μM H2O2 for 20 minutes, or 50 μM RB for 24 hours. The fluorescence emission spectrum of RB is close to that of DCF; a concentration of 50 μM RB was selected since it was empirically determined that it was the highest concentration that could be tested without residual RB in the wells resulting in the shifting and flattening of the standard curve. Following the 20 minute and 24 hour incubations, the cells were washed and the wells refilled with 100 μL of media and 100 μL of lysis buffer provided by the manufacturer. The plate was agitated and then incubated for five minutes, after which 150 μL from each well was transferred to wells of a black 96-well fluorometric plate. Fluorescent signal from the samples and standard curves were assayed at 480 nm/530 nm using a Fujifilm FLA-5100 (s/n: 4632022) laser scanner. Scanned images were analyzed, and values were exported to Excel using Fuji MultiGauge software, v3.0.
Statistical analyses
Data are expressed as the mean ± SEM and were analyzed using a one-way ANOVA allowing for unequal variances across the different treatment groups. When the overall ANOVA showed significance (p<0.05), a post-hoc Dunnett’s test was used to compare the treatment to the control groups.
Results
Effects of RB on cell growth and viability
UWB and BRCA1 ovarian cancer cells displayed dose-dependent suppression of growth in response to RB as indicated by the results of the MTT assays (Figures 1A and 1B). This suppression was similar to that seen in response to treatment with all doses of carboplatin tested (Figure 1C). Data from the control UWB absorbance/cell density growth curve (Figure 1D) suggested that at concentrations at and above 50 μM, RB completely inhibited the growth of the 2 x 104 cells that were seeded in the wells. RB-treated UWB cells appeared rounded, shrunken, and damaged, but were still attached to the plate (Figure 2). The data in Figure 3 show that the growth of UWB cells over four days was similarly suppressed when the cells were incubated with RB for only one hour.
Figure 1.

A and B. Effects of RB on UWB and BRCA1 cell growth; cells were cultured with RB for four days (*p<0.0001, **p<0.001, and †p=0.0002 relative to untreated control cells). C. UWB cells cultured with carboplatin showed significantly reduced cell growth compared to untreated cells at all tested concentrations (p<0.0001). D. Relationship between UWB cell number and absorbance in the MTT assay.
Figure 2.
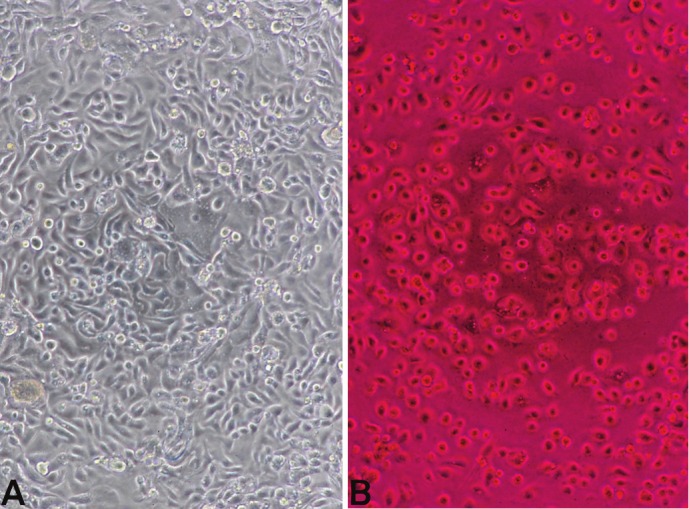
A. Untreated UWB cells after five days in culture, as viewed under low power (10X objective) with a Nikon TMS phase contrast microscope. B. UWB cells treated with 200 μM RB for four days, same magnification as A. Cells appeared rounded, shrunken, and damaged, but were still attached to the plate.
Figure 3.
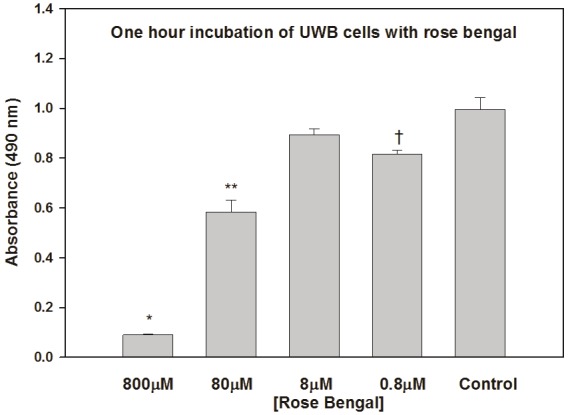
Effects of a one hour incubation of UWB cells with RB on their ability to proliferate; the MTT assay was carried out four days later. *p<0.0001, **p=0.0003, and †p=0.012 relative to control.
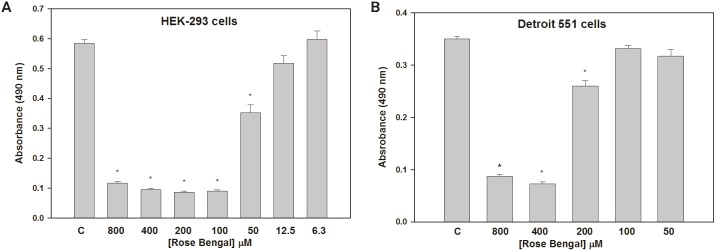
RB also inhibited the growth of HEK-293 tumor cells in a dose-dependent manner similar to that seen with UWB and BRCA1 cells (Figure 4A). On the other hand, only the highest doses of RB suppressed the growth of normal Detroit 551 cells (Figure 4B). These latter data suggest that RB-mediated growth suppression at these higher concentrations may be tumor cell specific.
Figure 4.
Suppression of HEK-293 (A) and Detroit 551 (B) cell growth by RB. The doses at which HEK-293 growth was suppressed were similar to those seen with UWB and BRCA1 cells, while only the highest doses suppressed growth of normal Detroit 551 cells. *p<0.001 relative to controls.
Evidence of apoptosis in RB-treated UWB cells
The degree to which RB induced apoptosis in UWB cells was determined colorometrically using the TiterTACS assay. Untreated cells exhibited a very low rate of apoptosis that was less than 10% of that seen in the nuclease positive controls. Cells treated with 200, 400, or 800 μM RB displayed a significantly increased degree of apoptosis that peaked at approximately four times the levels seen in untreated control cultures (Figure 5). Though the absolute number of apoptotic cells was not quantified, since wells treated with high doses of RB had close to the starting number of cells, it is reasonable to speculate that these cultures had a higher percentage of apoptotic cells relative to controls.
Figure 5.
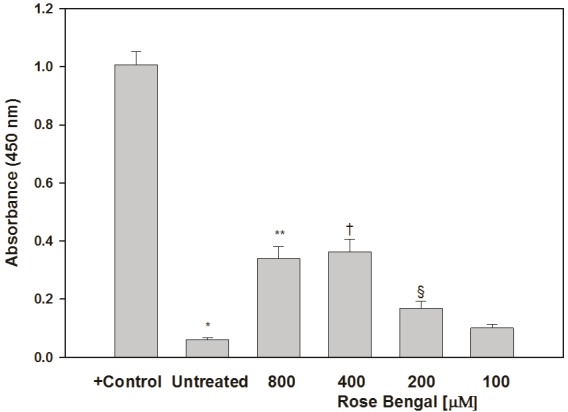
Quantitation of apoptosis in RB-treated UWB cells. The positive control consisted of nuclease-treated cells. *p<0.0001 relative to positive control. **p=0.0029, †p=0.0031, and §p=0.024 relative to untreated control cells.
Effects of RB on the generation of ROS in UWB cells
UWB cells were incubated with 10, 100, or 1000 μM H2O2 for 20 minutes, or 50 μM RB for 24 hours, after which the generation of ROS was analyzed by comparing their DCF levels to the levels found in untreated cells. Results are plotted as relative fluorescence units (RFUs) compared to baseline (see Figure 6). As expected, cells exposed to various concentrations of H2O2 generated ROS in a dose-dependent manner. Cells that were incubated with 50 μM RB generated ROS at levels that were significantly greater than untreated cells (p<0.001) and were similar to levels seen in cells treated with 1000 μM H2O2 for 20 minutes.
Figure 6.

Generation of ROS in UWB cells exposed to various concentrations of H2O2 or 50 μM RB. Cells generated ROS in a dose-dependent manner in response to a 20 minute incubation with H2O2. Cells that were incubated with RB for 24 hours similarly generated ROS at levels that were significantly greater than untreated cells (*p<0.001); these levels were statistically similar to those seen in cells treated with 1000 μM H2O2 for 20 minutes.
Discussion
The above data establish that RB inhibits the growth of UWB1.289 ovarian cancer cells, termed UWB in this paper, which carry a germline BRCA1 mutation, as well as the growth of UWB1.289+BRCA1 cells, termed BRCA1 in this paper, in which the wild-type BRCA1 allele was restored. Treatment with RB for one hour was sufficient to inhibit the growth of UWB cells. RB also blocked the growth of HEK-293 cells, which are adenovirus-transformed embryonic kidney cells. Interestingly, RB was not as effective at preventing the growth of the normal human fibroblast cell line Detroit 551; growth suppression in these cells only occurred at RB concentrations that were four-fold higher than those which inhibited the growth of the tumor cell lines. In support of a targeted effect of RB in tumor cells, it was previously reported that PV-10, the RB formulation developed by Provectus, freely entered cancer cells but was excluded from normal cells [13]. While much still needs to be done in the interim, this finding bodes well for the potential future directed use of this compound for the suppression of tumor cell growth in general, and ovarian cancer cell growth in particular, in humans. Since the MTT assay was reported to have been useful for in vitro chemosensitivity testing of fresh surgical specimens of gastric, colorectal, and hepatocellular carcinomas, and malignant lymphoma [16] as well as for directing the treatment of advanced breast cancer [17], our data showing suppression of ovarian cancer cell growth by RB using this assay suggest that RB may be toxic to these cells.
This premise is supported by phase contrast microscopy that revealed that RB-treated UWB cells were rounded not spindle-shaped, and shrunken in appearance, suggesting that RB not only inhibited their growth but damaged them as well. Further evidence came from the demonstration that cells treated with high concentrations of RB displayed a significant degree of apoptosis relative to untreated control cells. A host of chemotherapeutic and other compounds such as cisplatin, doxorubicin, taxol, PARP inhibitors, costunolide, progesterone, and proanthocyanidins were shown to induce apoptosis in ovarian cancer cells [18-24]. Clinically, the design of antagonists to anti-apoptotic proteins has led to the development of compounds currently being investigated for their activity against haematological and solid malignancies [25,26]. Drug-induced apoptosis in cancer cells was shown to correlate with the intracellular generation of ROS, high levels of which were reported to lead to necrotic cell death, while low levels induced apoptotic cell death [27-29]. The upregulation by RB of ROS in UWB cells to levels seen in cells treated short-term with high-dose H2O2 may similarly have contributed directly or indirectly to the induction of apoptosis. That said, it is important to remember that the role of ROS in cancer cells is complex, since they are also known to promote tumorigenesis by activating signaling pathways that regulate cellular proliferation, angiogenesis, and metastasis [30], in addition to simply causing genomic instability [31]. It is theorized that compounds such as those listed above, and potentially RB as well, that may cause mild oxidative stress in normal cells likely further enhance ROS levels in cancer cells to the point at which they trigger cell death [32].
The data in this paper support the further examination of the effects of intralesional RB in an animal model of ovarian cancer. As mentioned above, a phase 2 study of the effects of PV-10 in melanoma patients showed that such treatment induced a complete or partial response, or stabilized disease in 49% of the 80 enrolled subjects following intralesional injection [12]. Of particular interest in that trial were data showing that 38 of the 80 subjects displayed immune bystander effects. The idea that intralesional injection may not only directly kill the targeted cells but also lead to beneficial vaccination against the future recurrence of malignancy dates back to the first use of Bacille Calmette-Guerin (BCG) in the mid-seventies [33]. Since then, there have been numerous reports of the potential benefits of intratumoral injection including those that showed that intratumoral injection of: a. low dose tumor necrosis factor (TNF)-α promoted antitumor and immunotherapeutic responses in a transgenic mouse model of pancreatic neuroendocrine tumors [34]; b. IL-12 suppressed the local and distant growth of Ewing’s sarcoma in a nude mouse model [35]; c. interferon (IFN)-α together with the systemic delivery of agonist anti-CD137 monoclonal antibodies showed activity against MC38 colon carcinoma cells in mice that were directly injected as well as distant tumors [36]; and d. α-gal glycolipids in B16 mouse melanoma cells induced a protective anti-tumor response which overcame the activity of regulatory T cells [37]. Peritoneal dissemination is common in ovarian cancer and carcinomatosis involving the peritoneal lining, the mesentery, and/or the intestinal wall often precludes optimal surgery. Thus, even when optimally debulked, many patients still exhibit tumor nodules too small to have been resected by even the most skilled surgeon that likely seed future growths. In light of the above data, it is theorized that injection of these nodules with RB at the time of surgery may prove to be an effective strategy for not only their elimination, but for vaccinating patients against the future regrowth of gross tumors. This notion is supported by data that showed that PV-10 treatment increased the levels of tumor infiltrating lymphocytes [38] and that the overall survival of ovarian cancer patients was greater in patients whose tumors contained T cells [39-43].
In summary, our data showed that RB inhibited the growth of ovarian and adenovirus-transformed embryonic kidney cancer cells but was less effective at preventing the growth of normal human fibroblasts. Phase contrast microscopy suggested that RB not only inhibited ovarian cancer cell growth but also damaged these cells, a conclusion supported by the demonstration of RB-induced apoptosis and enhanced ROS accumulation in these cells. These results justify the further investigation of the effects of RB in an ovarian cancer animal model.
Acknowledgements
The author thanks Andre Kopoyan of the University of Massachusetts Proteomics & Mass Spectrometry Facility for his technical assistance in running the ROS assays.
References
- 1.Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–119. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan-Lim D, Berry M. Still confused about rose bengal? Curr Eye Res. 2004;29:311–317. doi: 10.1080/02713680490516864. [DOI] [PubMed] [Google Scholar]
- 3.Kishen A, Upadya M, Tegos GP, Hamblin MR. Efflux pump inhibitor potentiates antimicrobial photodynamic inactivation of Enterococcus faecalis biofilm. Photochem Photobiol. 2010;86:1343–1349. doi: 10.1111/j.1751-1097.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato H, Komagoe K, Inoue T, Katsu T. In situ monitoring of photodynamic inactivation of the membrane functions of bacteria using electrochemical sensors. Anal Sci. 2010;26:1019–1021. doi: 10.2116/analsci.26.1019. [DOI] [PubMed] [Google Scholar]
- 5.Panzarini E, Tenuzzo B, Dini L. Photodynamic therapy-induced apoptosis of HeLa cells. Ann N Y Acad Sci. 2009;1171:617–626. doi: 10.1111/j.1749-6632.2009.04908.x. [DOI] [PubMed] [Google Scholar]
- 6.Dini L, Inguscio V, Tenuzzo B, Panzarini E. Rose bengal acetate photodynamic therapy-induced autophagy. Cancer Biol Ther. 2010;10:1048–1055. doi: 10.4161/cbt.10.10.13371. [DOI] [PubMed] [Google Scholar]
- 7.Nonaka M, Yamamoto M, Yoshino S, Umemura S, Sasaki K, Fukushima T. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res. 2009;29:943–950. [PubMed] [Google Scholar]
- 8.Sugita N, Iwase Y, Yumita N, Ikeda T, Umemura S. Sonodynamically induced cell damage using rose bengal derivative. Anticancer Res. 2010;30:3361–3366. [PubMed] [Google Scholar]
- 9.Sugita N, Kawabata K, Sasaki K, Sakata I, Umemura S. Synthesis of amphiphilic derivatives of rose bengal and their tumor accumulation. Bioconjug Chem. 2007;18:866–873. doi: 10.1021/bc060189p. [DOI] [PubMed] [Google Scholar]
- 10.Foote MC, Burmeister BH, Thomas J, Mark Smithers B. A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series. Melanoma Res. 2010;20:48–51. doi: 10.1097/CMR.0b013e328331caa2. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18:405–411. doi: 10.1097/CMR.0b013e32831328c7. [DOI] [PubMed] [Google Scholar]
- 12.Agarwala S, Thompson J, Smithers BM, Ross MI, Coventry BJ, Minor DR, Scoggins C, Wachter EA. Chemoablation of metastatic melanoma with intralesional PV-10. Pigment Cell Melanoma Res. 2010;23:891. [Google Scholar]
- 13.Agarwala S. PV-10, aka Rose Bengal: Intralesional therapy for metastatic melanoma. The Melanoma Letter. 2012;30:6–9. [Google Scholar]
- 14.American Cancer Society: Cancer facts & figures 2008. American Cancer Society Atlanta, Ga; 2008. [Google Scholar]
- 15.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2004. Bethesda, MD: National Cancer Institute; 2007. http://seer.cancer.gov/csr/1975_2004/ posted to the SEER web site. [Google Scholar]
- 16.Suto A, Kubota T, Shimoyama Y, Ishibiki K, Abe O. MTT assay with reference to the clinical effect of chemotherapy. J Surg Oncol. 1989;42:28–32. doi: 10.1002/jso.2930420108. [DOI] [PubMed] [Google Scholar]
- 17.Xu JM, Song ST, Tang ZM, Jiang ZF, Liu XQ, Zhou L, Zhang J, Liu XW. Predictive chemotherapy of advanced breast cancer directed by MTT assay in vitro. Breast Cancer Res Treat. 1999;53:77–85. doi: 10.1023/a:1006122912146. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol. 2001;59:657–663. doi: 10.1124/mol.59.4.657. [DOI] [PubMed] [Google Scholar]
- 19.Rogalska A, Gajek A, Szwed M, Jozwiak Z, Marczak A. The role of reactive oxygen species in WP 631-induced death of human ovarian cancer cells: a comparison with the effect of doxorubicin. Toxicol In Vitro. 2011;25:1712–1720. doi: 10.1016/j.tiv.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Ahn HJ, Kim YS, Kim JU, Han SM, Shin JW, Yang HO. Mechanism of taxol-induced apoptosis in human SKOV3 ovarian carcinoma cells. J Cell Biochem. 2004;91:1043–1052. doi: 10.1002/jcb.20006. [DOI] [PubMed] [Google Scholar]
- 21.Ye K. PARP inhibitor tilts cell death from necrosis to apoptosis in cancer cells. Cancer Biol Ther. 2008;7:942–944. doi: 10.4161/cbt.7.6.6198. [DOI] [PubMed] [Google Scholar]
- 22.Yang YI, Kim JH, Lee KT, Choi JH. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol Oncol. 2011;123:588–596. doi: 10.1016/j.ygyno.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen H, Syed V. Progesterone inhibits growth and induces apoptosis in cancer cells through modulation of reactive oxygen species. Gynecol Endocrinol. 2011;27:830–836. doi: 10.3109/09513590.2010.538100. [DOI] [PubMed] [Google Scholar]
- 24.Kim KK, Singh AP, Singh RK, Demartino A, Brard L, Vorsa N, Lange TS, Moore RG. Anti-angiogenic activity of cranberry proanthocyanidins and cytotoxic properties in ovarian cancer cells. Int J Oncol. 2012;40:227–235. doi: 10.3892/ijo.2011.1198. [DOI] [PubMed] [Google Scholar]
- 25.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 26.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 27.Izeradjene K, Douglas L, Tillman DM, Delaney AB, Houghton JA. Reactive oxygen species regulate caspase activation in tumor necrosis factor-related apoptosis-inducing ligand-resistant human colon carcinoma cell lines. Cancer Res. 2005;65:7436–7445. doi: 10.1158/0008-5472.CAN-04-2628. [DOI] [PubMed] [Google Scholar]
- 28.Kannan K, Holcombe RF, Jain SK, Alvarez-Hernandez X, Chervenak R, Wolf RE, Glass J. Evidence for the induction of apoptosis by endosulfan in a human T-cell leukemic line. Mol Cell Biochem. 2000;205:53–66. doi: 10.1023/a:1007080910396. [DOI] [PubMed] [Google Scholar]
- 29.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 30.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 31.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mastrangelo MJ, Bellet RE, Berkelhammer J, Clark WH Jr. Regression of pulmonary metastatic disease associated with intralesional BCG therapy of intracutaneous melanoma metastases. Cancer. 1975;36:1305–1308. doi: 10.1002/1097-0142(197510)36:4<1305::aid-cncr2820360417>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci USA. 2012;109:7841–7846. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia SF, Duan X, Worth LL, Guan H, Kleinerman ES. Intratumor murine interleukin-12 gene therapy suppressed the growth of local and distant Ewing's sarcoma. Cancer Gene Ther. 2006;13:948–957. doi: 10.1038/sj.cgt.7700968. [DOI] [PubMed] [Google Scholar]
- 36.Dubrot J, Palazon A, Alfaro C, Azpilikueta A, Ochoa MC, Rouzaut A, Martinez-Forero I, Teijeira A, Berraondo P, Le Bon A, Hervas-Stubbs S, Melero I. Intratumoral injection of interferon-alpha and systemic delivery of agonist anti-CD137 monoclonal antibodies synergize for immunotherapy. Int J Cancer. 2011;128:105–118. doi: 10.1002/ijc.25333. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Motal UM, Wigglesworth K, Galili U. Intratumoral injection of alpha-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity. Cancer Immunol Immunother. 2009;58:1545–1556. doi: 10.1007/s00262-009-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mousavi H, Zhang X, Gillespie S, Wachter E, Hersey P. Rose Bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. Melanoma Res. 2006;16:S8. [Google Scholar]
- 39.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 40.Marth C, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Doppler W, Daxenbichler G. Interferon-gamma expression is an independent prognostic factor in ovarian cancer. Am J Obstet Gynecol. 2004;191:1598–1605. doi: 10.1016/j.ajog.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Dranoff G. The therapeutic implications of intratumoral regulatory T cells. Clin Cancer Res. 2005;11:8226–8229. doi: 10.1158/1078-0432.CCR-05-2035. [DOI] [PubMed] [Google Scholar]
- 42.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, De Geest K, Gershenson DM, Lutgendorf SK, Ferrone S, Sood AK. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14:3372–3379. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosmuller H, Haitchi-Petnehazy S, Webersinke G, Marschon R, Roithmeier F, Stummvoll W, Fehm T, Klier-Richter M, Bonzheim I, Staebler A, Fend F. Intratumoral lymphocyte density in serous ovarian carcinoma is superior to ERCC1 expression for predicting response to platinum-based therapy. Virchows Arch. 2011;459:183–191. doi: 10.1007/s00428-011-1110-1. [DOI] [PubMed] [Google Scholar]