Abstract
Local anesthetics have an impressive history of efficacy and safety in medical and dental practice. Their use is so routine, and adverse effects are so infrequent, that providers may understandably overlook many of their pharmacotherapeutic principles. The purpose of this continuing education article is to provide a review and update of essential pharmacology for the various local anesthetic formulations in current use. Technical considerations will be addressed in a subsequent article.
Keywords: Local anesthetics, Pharmacology, Drug toxicity, Dentistry
Local anesthetics interrupt neural conduction by inhibiting the influx of sodium ions through channels or ionophores within neuronal membranes. Normally these channels exist in a resting state, during which sodium ions are denied entry. When the neuron is stimulated, the channel assumes an activated or open state, in which sodium ions diffuse into the cell, initiating depolarization. Following this sudden change in membrane voltage, the sodium channel assumes an inactivated state, during which further influx is denied while active transport mechanisms return sodium ions to the exterior. Following this repolarization, the channel assumes its normal resting state. An appreciation of these sodium channel states helps to explain the preferential sensitivity of local anesthetics for various classes of neuronal fibers.
Local anesthetics have greater affinity for receptors within sodium channels during their activated and inactivated states than when they are in their resting states.1,2 Therefore, neural fibers having more rapid firing rates are most susceptible to local anesthetic action. Also, smaller fibers are generally more susceptible, because a given volume of local anesthetic solution can more readily block the requisite number of sodium channels for impulse transmission to be entirely interrupted. For these reasons the tiny, rapid-firing autonomic fibers are most sensitive, followed by sensory fibers and finally somatic motor fibers.1,2 The anesthesiologist blocking mixed spinal nerves is acutely aware of these differential sensitivities. As patients recover from spinal anesthesia they first regain voluntary motor function, then sensation returns, and finally they can micturate (autonomic control). The dentist is generally spared this consideration because the trigeminal nerve branches anesthetized for dental procedures are comprised only of small, rapid-firing sensory fibers. However, the many classes of sensory fibers also vary in their diameters and firing rates. For example, pain fibers are more sensitive than those carrying pressure and proprioception. A patient may remain disturbed by a sense of pressure despite complete anesthesia of pain fibers.
GENERAL PROPERTIES OF LOCAL ANESTHETICS
The molecular structure of all local anesthetics consists of 3 components: (a) lipophilic aromatic ring, (b) intermediate ester or amide linkage, and (c) tertiary amine. Each of these components contributes distinct clinical properties to the molecule. (See Figure 1.)
Figure 1.
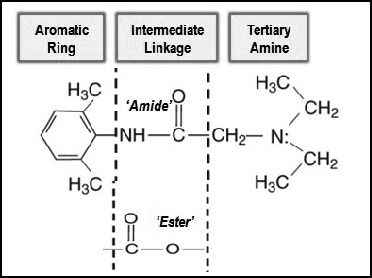
Local anesthetic structure.
Anesthetic Potency
Local anesthetics vary in their potency, allowing for concentrations that range typically from 0.5 to 4%. This is largely the result of differences in lipid solubility, which enhances diffusion through nerve sheaths and neural membranes. This property is determined by the aromatic ring and its substitutions, along with those added to the tertiary amine. For example, bupivacaine is more lipid soluble and potent than articaine, allowing it to be formulated as a 0.5% concentration (5 mg/mL) rather than a 4% concentration (40 mg/mL).
Time for Onset
Greater lipid solubility of a drug not only enhances potency but also enables more rapid diffusion through cell membranes. For local anesthetics, this hastens the onset for anesthesia in isolated fibers during in vitro studies, but it must be appreciated that other factors come into play clinically. For example, inherent vasodilating properties may promote systemic absorption before the anesthetic reaches the nerve membrane. High lipid solubility may impede dispersion throughout tissue fluids and also fosters sequestration in neighboring adipose tissues or myelin sheaths. In either case, fewer numbers of molecules reach the neuronal membrane and onset is delayed. Therefore, unlike in vitro studies of isolated fibers, greater lipid solubility generally slows the onset of anesthesia in the clinical setting. Injecting higher concentrations that allow a greater number of molecules to reach the membrane and hasten onset can offset this influence. Although bupivacaine and articaine are both highly lipid soluble, the 4% concentration of articaine provides for a much faster onset.
Despite myriad factors that influence the quantity of local anesthetic reaching the nerve fibers, the most important factor that determines the onset of anesthesia is the proportion of these molecules that exist in a lipid-soluble rather than a water-soluble state. The terminal amine illustrated in Figure 1 may exist in a tertiary form (3 bonds) that is lipid soluble, or as a quaternary form (4 bonds) that is positively charged and renders the molecule water soluble. For the local anesthetic base to be stable in solution, it is formulated as a hydrochloride salt. As such, the molecules exist in a quaternary, water-soluble state at the time of injection and are unable to penetrate the neuron. Therefore the time for onset of local anesthesia is directly related to the proportion of molecules that convert to the tertiary, lipid-soluble structure when exposed to physiologic pH (7.4). This proportion is determined by the ionization constant (pKa) for the anesthetic and is calculated using the Henderson-Hasselbalch equation:
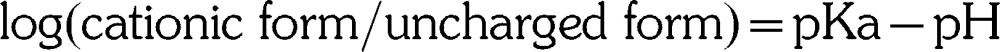 |
In simpler terms, if a local anesthetic were to have a pKa of 7.4 and to be injected into tissues having a physiologic pH of 7.4, 50% of the molecules would exist in the quaternary (cationic) form and 50% would exist in the tertiary (uncharged) form; only half the molecules would be lipid soluble and able to penetrate the neuron. Unfortunately, the pKa for all local anesthetics is greater than 7.4 (physiologic pH), and therefore a greater proportion of the molecules exist in the quaternary, water-soluble form when injected into normal tissue. The clinical caveat is that the higher the pKa for a local anesthetic, the fewer molecules are available in their lipid-soluble form. This will delay onset. Furthermore, the acidic environment associated with inflamed tissues lowers their pH well below 7.4 and favors the quaternary, water-soluble configuration even further. This has been suggested as one explanation for difficulty when attempting to anesthetize inflamed or infected tissues.1,2 In these situations, for example, bupivacaine (pKa 8.1) would be less desirable than mepivacaine (pKa 7.6).
It must be clarified, however, that once the tertiary molecules enter the neuron, they reionize to the quaternary form, which is credited with the actual blockade of the sodium channel. The sequence of events that leads to neural blockade is illustrated in Figure 2.
Figure 2.
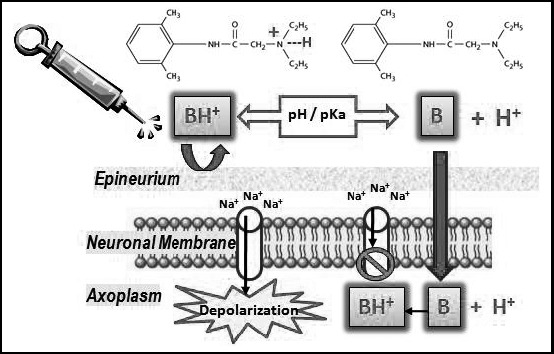
Local anesthetic action. An injected local anesthetic exists in equilibrium as a quaternary salt (BH+) and tertiary base (B). The proportion of each is determined by the pKa of the anesthetic and the pH of the tissue. The lipid-soluble base (B) is essential for penetration of both the epineurium and neuronal membrane. Once the molecule reaches the axoplasm of the neuron, the amine gains a hydrogen ion, and this ionized, quaternary form (BH+) is responsible for the actual blockade of the sodium channel. The equilibrium between (BH+) and (B) is determined by the pH of the tissues and the pKa of the anesthetic (pH/pKa).
Metabolism and Elimination
The intermediate chain or linkage provides a convenient basis for classification of local anesthetics, and also determines their pattern of elimination. Amides are biotransformed in the liver but esters are hydrolyzed in the bloodstream by plasma esterases. Ester local anesthetics are no longer packaged in dental cartridges and are used infrequently, with the exception of benzocaine, found in several topical anesthetic preparations. Articaine is unique in this regard. It is classified as an amide according to its intermediate linkage, but also contains an ester side chain on its aromatic ring. Hydrolysis of this side chain renders the molecule inactive, and it is therefore eliminated in a manner identical to ester anesthetics.
Duration of Action
Local anesthetics vary in their duration of action due primarily to differences in their affinity for protein. Like most drugs, local anesthetics reversibly bind to plasma proteins while circulating in the bloodstream. This property is expressed as the percentage of circulating drug that is protein bound and has been found to correlate with an anesthetic's affinity for protein within sodium channels as well. The greater the tendency for protein binding, the longer the anesthetic will sustain neural blockade. For example, bupivacaine exhibits 95% protein binding compared to 55% for mepivacaine, and this is credited for the difference in their duration of neural blockade.
Duration of anesthesia is also influenced by the time a local anesthetic remains in close proximity to neural fibers. Sequestration of highly lipid-soluble anesthetics locally may allow for continual release to the neuronal membranes, prolonging duration, but constriction of neighboring vasculature is more significant in this regard. For this reason, vasopressors are added to many formulations in order to delay absorption and prolong anesthesia. This is particularly important because local anesthetics themselves vary in their ability to produce vasodilation. For example, when used without vasopressors, lidocaine shortens its own duration by dilating local vasculature, whereas mepivacaine and bupivacaine do not. Plain lidocaine formulations may be useful for brief procedures following infiltration, but their efficacy for nerve block is poor.
LOCAL ANESTHETIC TOXICITY
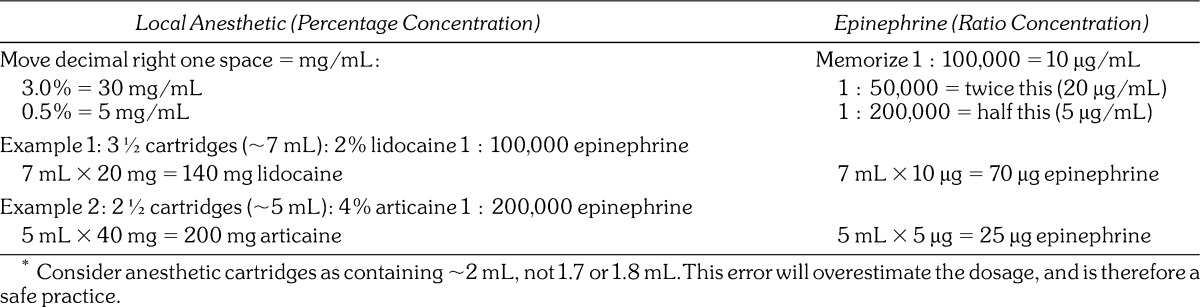
Systemic toxicity attributed to local anesthetics is dose dependent, but an understanding of these doses is not always a simple matter. The use of anesthetic cartridges in dentistry has unfortunately spawned carelessness in appreciating the actual amount of anesthetic we administer to our patients. Regrettably, this practice continues to be nurtured during undergraduate training and in many well-respected dental publications. A dental cartridge represents a volume, not a dose that is more properly expressed as milligrams or micrograms. Moreover, dental cartridges often contain 2 drugs: a local anesthetic and a vasopressor, each having a separate dose. Further complicating matters, dental cartridges contain peculiar volumes such as 1.7 or 1.8 mL. The sum of these issues makes actual dosage calculations trying and lends itself to memorization of amounts per cartridge rather than actual appreciation of proper doses. This practice becomes further complicated when cartridges contain various concentrations of local anesthetics and vasopressors. To simplify dosage calculations, it is wise to abort the concept of cartridges and consider each to contain 2 mL of volume. This will overestimate the amount administered to a patient, which is a safe practice. For example, when 4½ cartridges have been administered, estimate it as 9 mL. This unit of volume can be more easily converted to the approximate dose of each drug in milligrams or micrograms as illustrated in Table 1.
Table 1.
Approximation of Dosages*
As local anesthetics are absorbed from the injection site, their concentration in the bloodstream rises and the peripheral nervous system and central nervous system (CNS) are depressed in a dose-dependent manner. (See Figure 3.) Low serum concentrations are used clinically for suppressing cardiac arrhythmias and status seizures, but ironically, higher concentrations induce seizure activity. Convulsive seizures are the initial life-threatening consequence of local anesthetic overdose. Presumably this is due to selective depression of central inhibitory tracts, which allow excitatory tracts to run amuck. As serum concentrations continue to rise further, all pathways are inhibited, resulting in coma, respiratory arrest, and eventually cardiovascular collapse. Evidence of lidocaine toxicity may commence at concentrations >5 µg/mL, but convulsive seizures generally require concentrations >10 µg/mL.
Figure 3.
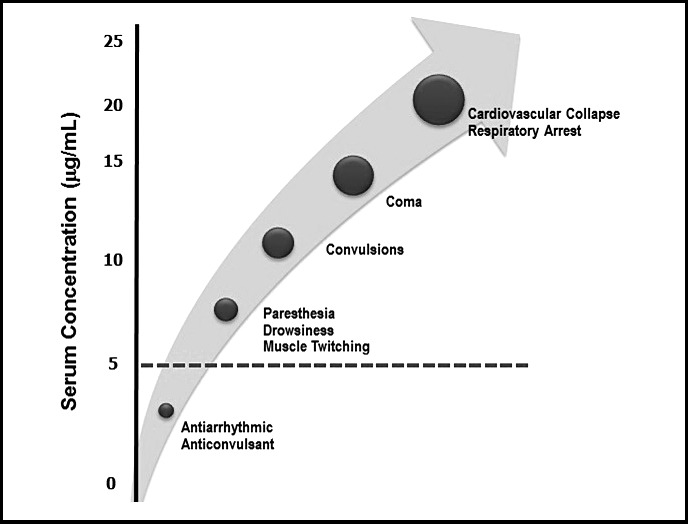
Approximate serum concentrations and systemic influences of lidocaine.
It is essential that local anesthetics be respected as CNS depressants, and they potentiate any respiratory depression associated with sedatives and opioids. Furthermore, serum concentrations required to produce seizures are lower if hypercarbia (elevated carbon dioxide) is present. This is the case when respiratory depression is produced by concurrent administration of sedatives and opioids. Goodson and Moore have documented catastrophic consequences of this drug interaction in pediatric patients receiving procedural sedation, along with excessive dosages of local anesthetics.3
Although all local anesthetics carry comparable risk for CNS toxicity, it should be noted that bupivacaine exhibits greater potential for direct cardiac toxicity than other agents.1,2 The explanation is not fully established, but is thought to be related to the fact that bupivacaine has greater affinity for the inactive and resting sodium channel configurations and dissociates from these channels more slowly. This delays recovery from action potentials, rendering cardiac tissues susceptible to arrhythmias. This concern is relevant for certain medical procedures, during which bupivacaine is administered in very high doses. It has never been found to occur with doses up to the maximum recommended in dental anesthesia.
The obvious question is what systemic serum concentration follows administration of a particular dose of local anesthetic. In 1972, Scott et al published one in a series of landmark clinical studies assessing variables that determine subsequent concentrations of lidocaine and prilocaine in serum.4 It is not surprising that serum concentrations were found to vary according to the relative vascularity of the tissues in which the anesthetic was injected. Using lidocaine 400 mg, the highest serum levels illustrated in Figure 4 followed infiltration of vaginal mucosa and the lowest followed subcutaneous abdominal infiltration. In each case, however, peak serum level occurred 20–30 minutes following injection of lidocaine alone. Regardless of the route of administration, peak levels were reduced and the rate of absorption was delayed by adding epinephrine 1 ∶ 200,000 to the local anesthetic solution. It is reasonable to assume that systemic concentrations following submucosal injection in the oral cavity would approximate those following injection into vaginal mucosa because of similar vascularity. Unfortunately, there are very few dental studies that address higher doses of local anesthetics. However, Hersh et al5 have published an impressive study that found comparable results following multiple intraoral injections totaling 7 cartridges (1.7 mL each = ∼480 mg) of articaine containing epinephrine 1 ∶ 200,000. (See Figure 4.) One can reasonably conclude that adhering to published maximum recommended dosages for local anesthetics will not result in systemic serum levels that approach those associated with toxicity.
Figure 4.
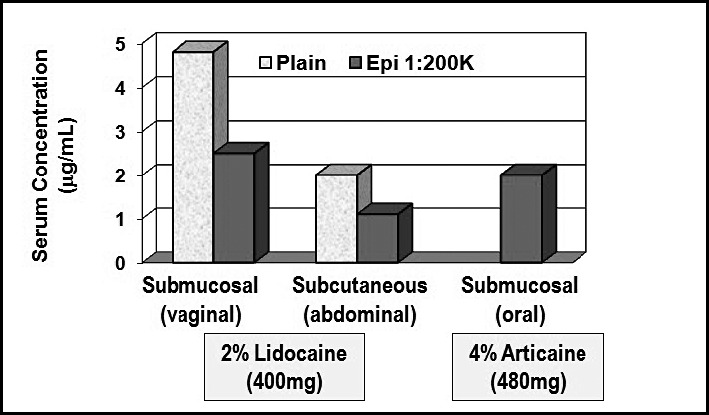
Local anesthetic serum concentrations. (See text for explanation. Adapted from Scott et al4 and Hersh et al.5)
Additional variables were also addressed by Scott et al.4 As expected, the dosage and speed of injection were directly related to serum concentration. A solution's concentration, eg, 2 versus 4%, was not relevant; serum concentrations were related to the total dosage. Administering 20 mL of 2% or 10 mL of 4% (400 mg) produced the same serum concentration. When using lidocaine or other anesthetics, regardless of their formulated concentration, one must consider the dosage (milligrams) administered, not the volume (milliliters or cartridges).
Contrary to conventional thought, the age or weight of a patient does not predict systemic serum concentration following doses calculated as milligrams per age (years) or milligrams per kilogram. However, when managing pediatric patients, maximum dosages are conventionally expressed in mg/kg, and this should be followed as a precaution. It is of little relevance for adults, however, and one should adhere to guidelines expressed as maximum dose in milligrams, regardless of weight or age. Obviously, this maximum amount should not be exceeded when calculating mg/kg doses for large children.
When considering the toxicity of any drug class, one should be mindful of metabolites, as well as the parent drug. A metabolite of prilocaine, o-toluidine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+). Hemes so altered do not bind oxygen and normal hemes on the same hemoglobin molecule do not readily release their oxygen. This form of hemoglobin is called methemoglobin, and when >1% of total hemoglobin is so altered, the condition is called methemoglobinemia. Patients appear cyanotic and become symptomatic when the proportion of methemoglobin exceeds 15%.6 Hemoglobin saturation by pulse oximetry (SpO2) will decline despite clinical evidence of effective oxygenation and ventilation. For example, pulse oximeter readings may be <90%, but actual arterial oxygen tension (PaO2) may be within normal range (>80 mm Hg). The condition becomes life threatening when methemoglobin levels exceed 50–60%, and it is managed using intravenous methylene blue, which reduces the hemes to their normal state. Methemoglobinemia attributed to prilocaine is unlikely to follow the administration of recommended doses. Rarely, one may encounter a patient with hereditary methemoglobinemia, which contraindicates the use of prilocaine.
Allergy to Local Anesthetics
It is not unusual for patients to claim they are allergic to local anesthetics. Upon careful questioning, however, one generally finds that what they experienced was either a syncopal episode associated with the injection, or cardiac palpitations attributed to epinephrine contained either in the solution or released endogenously. Allergic reactions following local anesthetic injections are more likely attributable to preservatives (methylparaben) or antioxidants (sulfites) contained in the solution.7 Methylparaben is included in multidose vials to prevent microbial growth. It is no longer found in single-dose vials or dental cartridges. Sulfites prevent the oxidation of vasopressors and are included only in those dental cartridges containing epinephrine or levonordefrin.
Allergic reactions are triggered by immune mechanisms whereby lymphocytes are sensitized to antigen and, upon subsequent exposure, mediate a series of pathophysiologic changes. Gell and Coombs first categorized hypersensitivity (allergic) reactions as Type I through IV, based on distinct immunologic mechanisms.8 Type I reactions occur within minutes of provocation and are mediated by antibodies or immunoglobulin E (IgE) produced by B lymphocytes. This is the type most commonly provoked by components of local anesthetic formulations. Type 4 reactions are delayed for several days following provocation and are mediated by sensitized T lymphocytes. This type of reaction to local anesthetics has been implicated only rarely.
For drugs to be immunogenic, they must be of large molecular weight and possess multiple valences to be recognized by the immune cells.9 Large proteins such as animal-derived insulin fulfill these requirements and are well established as immunogenic. Most drug molecules are too small and actually combine with other molecules that act as carriers to induce an allergic reaction. In the case of sulfonamide antibiotics, for example, the phenyl ring containing an amine substitution is the perpetrator in the formation of the immunogenic complex. This moiety is common to other derivatives of para-aminobenzoic acid (PABA) such as methylparaben and some, but not all, ester local anesthetics. In these cases there may be the potential for cross-allergenicity because they have this molecular structure in common, eg, sulfa antibiotics, methylparaben, and esters of PABA.
It is careless to describe esters as more allergenic than amides when discussing local anesthetics. An ester is merely a chemical linkage and imparts no immunogenicity to a compound. Rather, it is a molecular component joined by this linkage that is the culprit. This misconception has caused several agents to be inaccurately labeled as cross-allergenic with sulfonamide antibiotics. Articaine is classified as an amide local anesthetic because of the linkage between its lipid-soluble ring and terminal amine. Its thiophene ring contains a sulfur atom, which has no immunogenic property, and an ester side chain that renders the compound inactive following hydrolysis. However, articaine does not liberate a metabolite resembling PABA and does not introduce concern regarding cross-immunogenicity with sulfonamides. In contrast, procaine is representative of esters derived from PABA and hydrolysis liberates a moiety that is potentially immunogenic (Figure 5).
Figure 5.
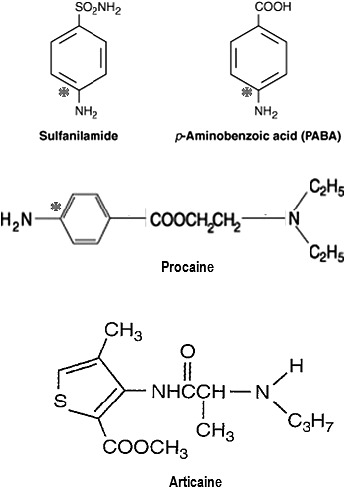
Molecular structures and allergenicity. Immunogenicity is attributable to medications having a phenyl ring with a para-amine substitution. This is found in sulfonamide antibiotics and compounds containing para-aminobenzoic acid (PABA) such as certain sunscreens and cosmetics. It is also found in methylparaben preservatives and ester local anesthetics such as procaine. Ester linkages (procaine) or side chains (articaine) are not immunogenic, nor is the sulfur atom of a thiophene ring (articaine). * indicates immunogenic moiety.
A final misconception pertains to sulfites. These are included in local anesthetic solutions containing vasopressors to prevent their oxidation. They are inorganic compounds (−SO3) that have been implicated in allergic reactions, but they have no relation to immunogenicity attributed to PABA-related compounds. These agents are also used as antioxidants in fresh fruits and vegetables to preserve their color and overall appearance. It is significant that patients claiming allergy to such foods may experience cross-reactions with local anesthetic solutions containing vasopressors because they contain these same sulfites.
Reports of allergic reactions to local anesthetics have appeared in the scientific literature with some frequency.10,11 However, it is difficult to comprehend the accuracy or actual frequency because of inconsistency in methods of confirmation that include skin prick testing, intradermal injections, and drug provocative challenges. In many cases there has been no confirmation of the actual culprit, preservative or actual local anesthetic. Furthermore, only a very few have actually confirmed the presence of IgE to the offending drug by immunoblot testing. An extensive analysis of this literature has recently been provided by Speca et al.12
In virtually all case reports, patients did indeed experience signs and symptoms consistent with an allergic reaction. Whether the actual pathogenesis was truly immune mediated (allergy) is probably more academic than pragmatic. The final event in these reactions is attributed to the synthesis and release of mediators referred to collectively as autacoids, of which histamine and leukotrienes are most significant. These autacoids not only produce direct effects on tissues but may also recruit various inflammatory cells that contribute to a so-called late-phase response that may not appear for days following provocation. Indeed, it is not uncommon for drugs to generate these autacoids by actions that are not immune mediated and therefore are not correctly classified as allergy. Meperidine stimulates release of histamine from mast cells, and nonsteroidal anti-inflammatory drugs may promote synthesis of leukotrienes. In such cases the patient's response has been conventionally labeled as pseudoallergic, to distinguish it from true allergy, which is immune mediated.
If a patient describes a reaction that is at least clinically consistent with allergy, the dentist should avoid using the offending agent until evaluated by an allergist. In the event an anesthetic is required before medical clearance can be obtained, the wisest choice would be either mepivacaine or prilocaine without vasopressors. Conventional wisdom holds that, if local anesthetics do indeed produce allergies, esters of PABA would be more likely than amide local anesthetics. Furthermore, by avoiding those solutions containing vasopressors, one avoids any bisulfites that are included as antioxidants. Sensitivity to various sulfites is possible, especially among asthmatic or atopic patients. These principles are the basis for the flowchart presented in Figure 6. A patient should never be denied the benefit of local anesthesia based on flawed assumptions regarding allergy.
Figure 6.
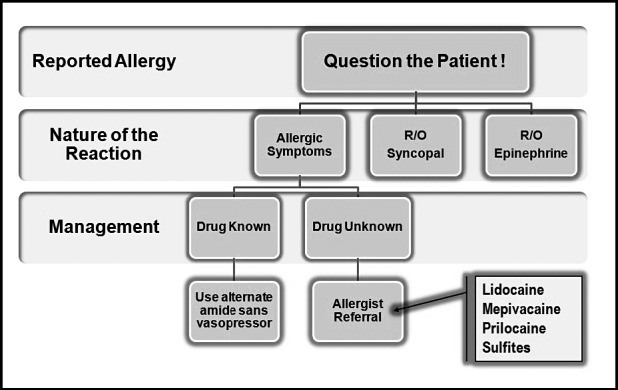
Managing patients allergic to local anesthetics. Rule out common reactions misinterpreted as allergy, eg, syncope and tachycardia. Then establish that the nature of their reaction at least resembled a hypersensitivity reaction, eg, rash, pruritus, urticaria, or dyspnea. If the drug is known, choose another amide, free of vasopressor so no sulfites are present. Otherwise refer the patient to an allergist, for testing of sulfites and exemplary local anesthetics such as lidocaine, mepivacaine, and prilocaine. (Adapted from deShazo and Kemp.13)
Local Toxicity
Ischemic necrosis of tissues may follow injections of local anesthetics. This can be due to the irritating nature of a solution, pressure from large volumes, or constriction of the vasculature by vasopressors. This concern is greatest when injecting into attached mucosa such as the hard palate. There is also mounting concern regarding direct neurotoxicity related to formulations containing high concentrations such as 4% articaine and prilocaine.
Haas and Lennon reported an increased incidence of paresthesias in Canada following the introduction of articaine in the mid-1980s.14 In 1993 alone, 14 cases of paresthesia were reported, and all were attributed to articaine or prilocaine. When articaine was first submitted for approval to the Food and Drug Administration in the United States, it was identified as having a higher risk for paresthesia than lidocaine.
More recently, Garisto et al15 reviewed claims of paresthesia in the United States during the period of November 1997 through August 2008 and found 248 cases of paresthesia following dental procedures. Most cases (∼95%) involved mandibular nerve blocks, and in 89% of these the lingual nerve was affected. Compared to other local anesthetics, paresthesia was found to be 7.3 times more likely with 4% articaine and 3.6 times more likely with 4% prilocaine. Similar findings from reports of paresthesia in Denmark were published by Hillerup et al.16 This data may be even more significant when one considers the number of cases that may very well go unreported.
Although the dental community has been slow to reach consensus regarding this issue, it should be appreciated that the medical anesthesia literature is emphatic in claiming that greater concentration of local anesthetic solutions increases risk for direct neurotoxicity to nerve trunks: “All the clinically used local anesthetics can produce direct toxicity to nerves if they achieve sufficiently high intraneural concentrations. Clinicians should be aware that the concentrations of formulated local anesthetic solutions are neurotoxic per se and that their dilution, in situ or in tissue, is essential for safe use.”1
This fact is further supported by Hillerup et al, who demonstrated greater neural toxicity of 4 compared to 2% articaine in sciatic nerve preparations.17 As with all drugs, each practitioner needs to perform a risk-benefit analysis before using a medication. Only if the benefit of using articaine outweighs the risk for this practitioner in this patient should it be considered for use. It might be wise to limit the use of 4% concentrations for infiltration and avoid their use for nerve blocks, opting instead for agents formulated in lower concentrations.15,16
LOCAL ANESTHETIC COMPARISONS
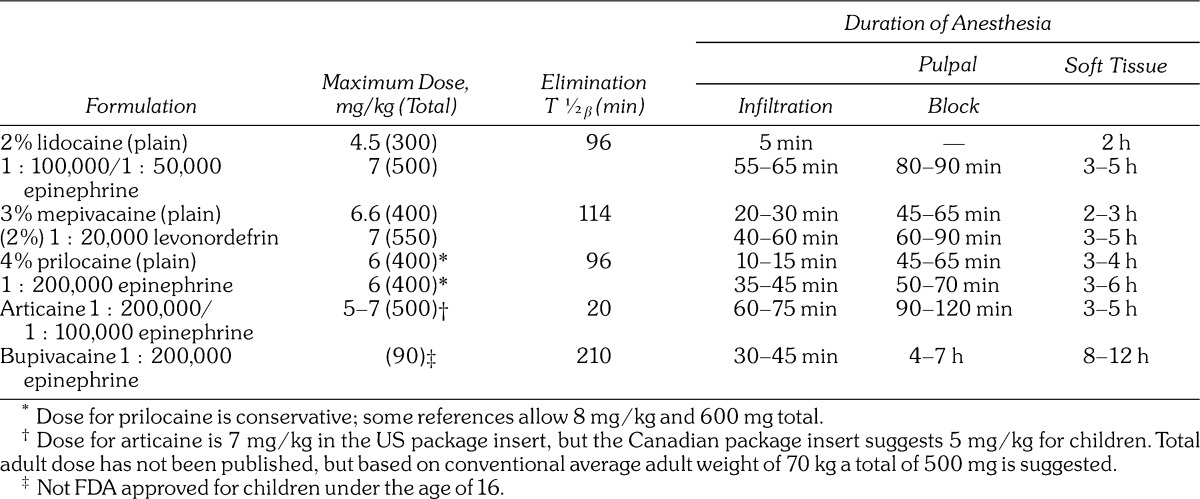
Lidocaine continues its prominence as the most widely used local anesthetic in the United States, but all of these agents have comparable efficacy. They differ in potency and several pharmacokinetic parameters that account for differences in the onset and duration of anesthesia. Selection of a particular agent must take into account the duration of the procedure planned and issues regarding vasopressor concentrations. For lengthy procedures, bupivacaine is the logical choice, but it has been implicated as one of the more painful agents during injection according to studies that have compared various anesthetics.18–20 One strategy is to provide the initial 60–90 minutes of anesthesia using a less irritating agent (lidocaine or prilocaine) and then reinject the anesthetized tissue with bupivacaine to provide analgesia well into the postoperative period. Such a strategy is most effective following nerve blocks; shorter duration for pulpal anesthesia should be anticipated following infiltration. (See Table 2.)
Table 2.
Despite anecdotal claims regarding the superiority of articaine over lidocaine for inferior alveolar block, published studies have found little if any difference, especially when teeth are symptomatic.22–24 Any slight advantage of articaine is offset by its greater risk for paresthesia addressed above. However, for infiltration of the mandible, articaine is clearly superior and carries no risk for neural toxicity unless injected near the mental nerve.22,25,26 The superiority of articaine can be explained by its high lipid solubility and the concentration of its formulations. Unlike other anesthetics having benzene as their aromatic ring, articaine has a thiophene ring and substitutions that confer greater lipid solubility than other local anesthetics with the exception of bupivacaine. This property should have allowed its formulation in a lower concentration, but in fact it was formulated as a 4% solution. Not only is articaine more lipid soluble, but its formulation provides a greater number of molecules than an equal volume of 2% lidocaine, for example. To date there have been no published studies comparing articaine to 4% lidocaine solutions for mandibular infiltration. Lidocaine in this concentration would present an unacceptable risk for systemic toxicity, which introduces another attractive property of articaine: pattern of clearance.
Although articaine is classified as an amide, because of linkage of its intermediate chain, the thiophene ring also contains an ester side chain. This chain is hydrolyzed by plasma esterases rendering the molecule inactive. The result is that articaine has an elimination half-life of only 20–40 minutes compared to >90 minutes for lidocaine and other amides that require hepatic clearance. For this reason, articaine presents less risk for systemic toxicity during lengthy appointments when additional doses of anesthetic are administered. Be reminded, however, that a 4% concentration articaine contains twice the dose of 2% lidocaine per volume administered, and their maximum recommended doses are identical.
Maximum Doses for Local Anesthetics
Based on the data originally presented by Scott et al,4 lidocaine 400 mg injected submucosally produces systemic serum concentrations well below toxic levels. This is approximately the amount found in 10 dental anesthetic cartridges, and this number has been cited historically as the limit per dental appointment. Notwithstanding the fact that somewhat higher amounts can be used when formulated with vasopressors, this suggestion is obviously a safe guideline for lidocaine.
The elimination half-life (T1/2β) of the various local anesthetics ranges from 90 minutes for conventional agents such as lidocaine to >200 minutes for agents such as bupivacaine. This decline commences after peak serum concentration is achieved: approximately 20 minutes with anesthetics alone4 and ∼20–30 minutes for those combined with vasopressors.5,27 Once the peak concentration is achieved, additional doses will become absorbed as original doses are in decline. This is a perilous time because one cannot accurately predict the serum concentration at any period. Furthermore, patient responses follow a bell-shaped pattern of distribution and render these theoretical calculations even more problematic. Keep in mind that both liver and renal functions decline 50% by age 6528 and beta blockers reduce hepatic blood flow. Articaine is the exception because it has an ester side chain and is inactivated in serum by plasma cholinesterases.
Frequently the dentist administers a combination of local anesthetic formulations, and it must be appreciated that systemic effects of these combinations follow principles of summation.1 When adhering to maximum dosage guidelines, systemic effects of various agents should be regarded as additive. For example, if you have administered half the maximum dose for lidocaine and wish to add bupivacaine, reduce its maximum dose by half.
VASOPRESSORS
Vasopressors are drugs that provide constriction of blood vessels by activating alpha-1 adrenergic receptors. They are combined with local anesthetics to provide hemostasis in the operative field and to delay anesthetic absorption. Delayed absorption of local anesthetics not only reduces the risk for systemic toxicity, but also prolongs the duration of anesthesia. Epinephrine is the most common agent used for this purpose, despite the fact that it exhibits considerable cardiac stimulation because of its additional action as a beta-1 adrenergic agonist.
Despite the popularity of epinephrine 1 ∶ 100,000, concentrations greater than 1 ∶ 200,000 (5 µg/mL) offer little if any advantage. Greater concentrations do not provide better onset or duration for inferior alveolar nerve block.29,30 Nor do higher concentrations reduce local anesthetic serum concentrations.4,5 However, greater concentrations, eg, 1 ∶ 100,000 (10 µg/mL) and 1 ∶ 50,000 (20 µg/mL), may provide better hemostasis when infiltrated at the surgical site when this influence is desired.
Cardiovascular Influences
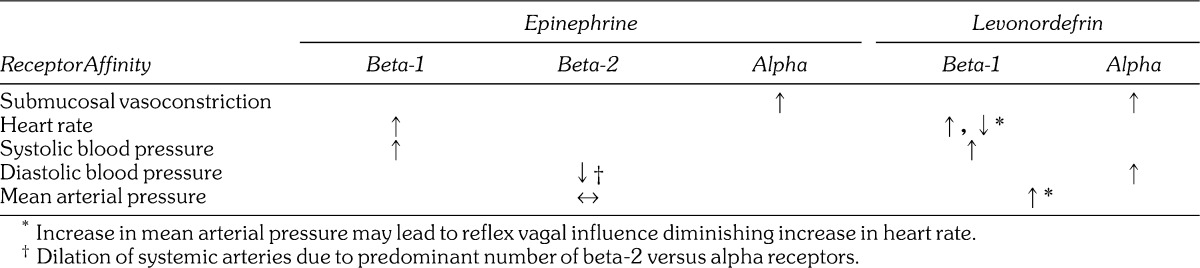
To properly address safety issues, one must first appreciate principles of dosage calculations that were presented in Table 1 of this article. There is continued debate regarding deleterious influences of epinephrine on patients having cardiovascular disease. Often this dispute continues without fully appreciating the actual action and effects of this commonly used drug. Epinephrine acts as an agonist on alpha, beta-1, and beta-2 receptors. These actions account for its effects on the cardiovascular system, as illustrated in Figure 7. Before analyzing this figure, it is important to clarify a common misconception. The dentist regards epinephrine as a vasoconstrictor based on its effects when administered into submucosal tissues. This is because the tiny vessels in this location contain only alpha receptors. Larger systemic arteries that determine arterial resistance and diastolic blood pressure contain far more beta-2 than alpha receptors, and following absorption, low doses of epinephrine produce dilation of these vessels.
Figure 7.
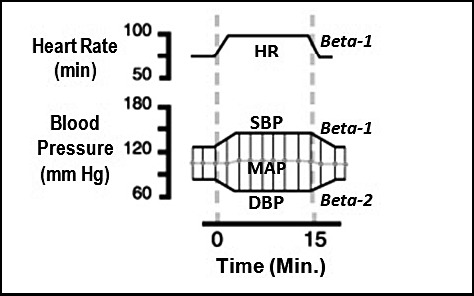
Cardiovascular effects of epinephrine.31 The following graph illustrates the typical cardiovascular response to epinephrine administered as a continuous intravenous infusion of 10 µg/min. (This is the amount contained in 1 mL of a 1 ∶ 100,000 concentration.) Epinephrine increases heart rate (HR) by activating beta-1 receptors in the sinoatrial node, the heart's normal pacemaker. It also activates beta-1 receptors on myocardial cells, increasing their contractility and increasing systolic blood pressure (SBP). However, it activates beta-2 receptors on systemic arteries producing vasodilation. This decline in arterial resistance produces a reduction in diastolic pressure (DBP). These effects result in little change of mean arterial pressure (MAP).
Clinical trials have confirmed unequivocally that even small dosages of epinephrine in local anesthetic solutions have an influence on cardiovascular function. Dionne et al32 studied the influence of 3 cartridges of 2% lidocaine with epinephrine 1 ∶ 100,000 (∼60 µg epinephrine). Submucosal injection of this dosage increased cardiac output, heart rate, and stroke volume. Systemic arterial resistance was reduced and mean arterial pressure remained essentially unchanged. Likewise, Hersh et al5 observed similar results following the administration of articaine containing 1 ∶ 100,000 (∼120 µg) and 1 ∶ 200,000 (∼60 µg) epinephrine, with a greater response from the higher dosage. These findings are consistent with well-established influences as illustrated in Figure 7.
The results of studies such as those just mentioned must be viewed in perspective. For example the influence of ∼120 µg epinephrine published by Hersh et al5 was minor: heart rate increases of ∼8–10 beats per minute and blood pressure changes of ∼5 mm Hg on average. However, the 14 participants were in perfect health, with resting vital signs that averaged 68 for heart rate, 125 mm Hg for systolic pressure, and 73 mm Hg for diastolic pressure. Furthermore, they were taking no significant medications. Obviously, such individuals can easily tolerate the dosages administered, but it should be noted that 2 of these healthy participants actually reported palpitations.
Even small doses of epinephrine produce cardiovascular effects; this is unequivocal. At issue is whether or not the cardiovascular influences of epinephrine pose a significant risk to patients having varying degrees of compromise. Standards and guidelines continue to be promoted, but in fact are all anecdotal. To suggest that a “2-cartridge” limit be imposed for patients with cardiovascular disease is naïve. Ultimately, the decision requires the dentist to exercise sound clinical judgment based on a thorough analysis of each patient under consideration. If consultation with the patient's physician is indicated, discuss the anticipated dosage range in terms of micrograms, not concentrations or cartridges. For example, if 2–4 cartridges of local anesthetic are planned, explain that you will be using 40–80 µg of epinephrine infiltrated submucosally, not 2–4 cartridges of epinephrine 1 ∶ 100,000. The physician is unfamiliar with a dosage expressed as cartridges or concentrations. As reference, consider the conventional epinephrine dose for managing an allergic reaction is 0.3 mg or 300 µg. A physician will generally be concerned with doses of 100 µg or greater.
Levonordefrin (NeoCobefrin) is the vasopressor combined with 2% mepivacaine formulations in the United States. It more closely resembles norepinephrine than epinephrine, lacking activity at beta-2 receptors. For this reason it elevates not only systolic blood pressure like epinephrine, but diastolic and mean arterial pressures as well. In some patients this can trigger a reflex vagal influence on heart rate that may offset some of its direct beta-1 receptor stimulation of heart rate. However, studies that assess cardiovascular influences following intraosseous injections have found little difference between epinephrine and levonordefrin.33,34 This likely is explained by the rapid absorption, which allows for direct beta-1 stimulation before reflex responses to mean arterial pressure intervene. A comparison of epinephrine and levonordefrin is presented in Table 3.
Table 3.
Actions of Epinephrine Versus Levonordefrin
Maximum permissible doses of vasopressors have not been established. To express limits in terms of appointments is impractical; time of treatment may be as brief as 30 minutes or as long as 3–4 hours. Furthermore, the influence of a given dose of vasopressor among patients is highly variable. Peak influences of epinephrine are generally observed within 5–10 minutes following injection5 and they decline rapidly; epinephrine and levonordefrin are catecholamines and rapidly metabolized by catechol-o-methyltransferase. In fact, the elimination half-life for most catecholamines is only 1–3 minutes. Generally, the hemodynamic influences are witnessed within minutes of injection and have completely subsided in 10–15 minutes. An epinephrine dose of 40 µg (approximately 2 cartridges containing epinephrine 1 ∶ 100,000) is the most conservative and frequently cited dose limitation for patients having significant cardiovascular disease. It should be clarified that this guideline more appropriately reflects 30-minute time periods, not appointments. A more rational suggestion is to base the dosage on patient assessment, not maximal amounts. For example, if for any reason the medical status of a patient is in question, a sensible protocol is to record baseline heart rate and blood pressure preoperatively and again following every 20–40 µg administered. This would equate to 1–2 cartridges containing a 1 ∶ 100,000 epinephrine concentration. Virtually any patient can tolerate the cardiovascular influences of this amount. If the patient remains stable, additional doses may be administered and followed by a similar pattern of reassessing vital signs.
Drug Interactions
Potential drug interactions have been thoroughly addressed in a previous continuing education article in this journal.35 The most important of these relate to possible enhanced cardiovascular stimulation. Vasopressors found in local anesthetic formations have cardiotonic effects, and this may become more significant when patients are medicated with any drug having similar influences. These include tricyclic and monoamine oxidase inhibitor antidepressants, digoxin, thyroid hormone, or any of the sympathomimetics used for weight control or attention deficit disorders. Vasopressors are not contraindicated in these patients, but they should be administered with caution in a manner addressed above for medically compromised patients. For patients suspected of stimulant drug abuse, eg, cocaine, it may be wise to avoid vasopressors altogether.
Cautious use of vasopressors is also advised for patients medicated with nonselective beta blockers. Unlike selective agents that only block beta-1 receptors on the heart, nonselective agents also block vascular beta-2 receptors. In this case the alpha agonist action of vasopressors becomes more pronounced and both diastolic and mean arterial pressures can become dangerously elevated. This is generally accompanied by a sudden reflex slowing of heart rate. Significant consequences of this interaction are well documented.36–38 The interaction with beta blockers follows a time course identical to that observed for normal cardiovascular responses to epinephrine. It commences following absorption from the injection site, which generally peaks within 5 minutes and declines over the subsequent 10–15 minutes. Vasopressors are not contraindicated in patients taking nonselective beta blockers, but doses should be conservative and blood pressure monitored periodically during administration as described above. Gingival retraction cords impregnated with racemic epinephrine should be avoided. These products contain epinephrine in amounts far exceeding those contained in local anesthetic formulations.
LOCAL ANESTHETIC REVERSAL
In closing, it should be mentioned that a local anesthetic reversal agent has been introduced that effectively reverses the influence of vasopressors on submucosal vessels. Phentolamine (OraVerse) is an alpha receptor blocker formulated in dental cartridges. When it is injected into the identical site where anesthetic was administered, vessels dilate, leading to enhanced absorption of local anesthetic, which shortens the duration of anesthesia.27 It will likely receive limited use because of its expense and the fact that sustained anesthesia is generally a benefit during the postoperative period for pain management. However, it may be useful in the management of small children or patients with special needs who may be prone to self-inflected injury while tissues remain numb. A consideration may also be given to the fragile diabetic or elderly patient for whom adequate nutritional intake may be hindered by prolonged numbness. Reversal may also be offered to the busy patient who must return to work and communicate effectively.
CONTINUING EDUCATION QUESTIONS
1. All of the following influence duration of local anesthesia EXCEPT:
A. Addition of vasopressor to the formulation
B. Dissociation constant (pKa) of the local anesthetic
C. Relative protein binding affinity of the local anesthetic
D. Relative vasodilating property of the local anesthetic
2. The maximum recommended dose for lidocaine with epinephrine is 500 mg, and 90 mg for bupivacaine. Anesthesia is difficult to obtain and you have administered 6 cartridges of 2% lidocaine with epinephrine to remove 4 third molars. The lower molars remain sensitive and you elect to reinject using 0.5% bupivacaine 1 ∶ 200,000 epinephrine. Which of the following number of cartridges would be the maximum number you can safely administer? (Assume 2 mL per cartridge.)
A. 2–3
B. 4–5
C. 8–9
D. 10–11
3. The risk for direct neurotoxicity from local anesthetics is most closely associated with which of the following characteristics?
A. Greater concentration
B. Greater lipid solubility
C. Higher pH
D. Lower pKa
4. Which of the following is the most common initial life-threatening consequence of local anesthetic overdose?
A. Anaphylactic reactions
B. Convulsive seizure
C. Respiratory arrest
D. Ventricular fibrillation
References
- 1.Berde CB, Strichartz GR. Local anesthetics. In: Miller RD, Eriksson LI, Fleisher LA, et al., editors. Miller's Anesthesia. 7th ed. Philadelphia, Pa: Elsevier, Churchill Livingstone; 2009. [Google Scholar]
- 2.Katzung BG, White PF. Local anesthetics. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw-Hill Companies Inc; 2009. [Google Scholar]
- 3.Goodson JM, Moore PA. Life-threatening reactions after pedodontic sedation: an assessment of narcotic, local anesthetic and antiemetic drug interactions. J Am Dent Assoc. 1983;107:239–245. doi: 10.14219/jada.archive.1983.0225. [DOI] [PubMed] [Google Scholar]
- 4.Scott DB, Jebson PJR, Braid DP, et al. Factors affecting plasma levels of lignocaine and prilocaine. Brit J Anaesth. 1972;44:1040–1049. doi: 10.1093/bja/44.10.1040. [DOI] [PubMed] [Google Scholar]
- 5.Hersh EV, Giannakopoulos H, Levin LM, et al. The pharmacokinetics and cardiovascular effects of high-dose articaine with 1 ∶ 100,000 and 1 ∶ 200,000 epinephrine. J Am Dent Assoc. 2006;137:1562–1571. doi: 10.14219/jada.archive.2006.0092. [DOI] [PubMed] [Google Scholar]
- 6.Benz EJ. Disorders of hemoglobin. In: Longo DL, Kasper DL, Jameson JL, et al., editors. Harrison's Principles of Internal Medicine. 18th ed. New York, NY: McGraw Hill; 2012. [Google Scholar]
- 7.Schatz M. Adverse reactions to local anesthetics. Immunol Allergy Clin North Am. 1992;12:585–609. [Google Scholar]
- 8.Gell PGH, Coombs RRA. Classification of allergic reactions responsible for clinical hypersensitivity and disease. In: Gell PGH, Coombs RRA, Hachmann PJ, editors. Clinical Aspects of Immunology. 3rd ed. Oxford, England: Blackwell Scientific; 1975. [Google Scholar]
- 9.Adkinson NF., Jr . Drug allergy. In: Adkinson NF Jr, Yunginger JW, Busse WW, et al., editors. Middleton's Allergy: Principles and Practice. 6th ed. Philadelphia, Pa: Mosby Inc; 2003. [Google Scholar]
- 10.Gall H, Kaufmann R, Kalveram CM. Adverse reactions to local anesthetics: analysis of 197 cases. J Allergy Clin Immunol. 1996;97:933–937. doi: 10.1016/s0091-6749(96)80067-4. [DOI] [PubMed] [Google Scholar]
- 11.Berkun Y, Ben-Zvi A, Levy Y, Galili D, Shalit M. Evaluation of adverse reactions to local anesthetics: experience with 236 patients. Ann Allergy Asthma Immunol. 2003;91:342–345. doi: 10.1016/S1081-1206(10)61680-8. [DOI] [PubMed] [Google Scholar]
- 12.Speca SJ, Boynes SG, Cuddy MA. Allergic reactions to local anesthetic formulations. Dent Clin North Am. 2010;54:655–664. doi: 10.1016/j.cden.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 13.deShazo RD, Kemp SF. Allergic reactions to drugs and biologic agents. JAMA. 1997;278:1895–1906. [PubMed] [Google Scholar]
- 14.Haas DA, Lennon D. A 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995;61:319–330. [PubMed] [Google Scholar]
- 15.Garisto GA, Gaffen AS, Lawrence HP, Tenenbaum HC, Haas DA. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010;141:836–844. doi: 10.14219/jada.archive.2010.0281. [DOI] [PubMed] [Google Scholar]
- 16.Hillerup S, Jensen RH, Ersboll BK. Trigeminal nerve injury associated with injection of local anesthetics: needle lesion or neurotoxicity. J Am Dent Assoc. 2011;142:531–539. doi: 10.14219/jada.archive.2011.0223. [DOI] [PubMed] [Google Scholar]
- 17.Hillerup S, Bakke M, Larsen JO, Thomsen CE, Gerds TA. Concentration-dependent neurotoxicity of articaine: an electrophysiological and stereological study of the rat sciatic nerve. Anesth Analg. 2011;112:1330–1338. doi: 10.1213/ANE.0b013e3182172a2e. [DOI] [PubMed] [Google Scholar]
- 18.Morris R, McKay W, Mushlin P. Comparison of pain associated with intradermal and subcutaneous infiltration with various local anesthetic solutions. Anesth Analg. 1987;66:1180–1182. [PubMed] [Google Scholar]
- 19.Wahl MJ, Overton D, Howell J, Siegel E, Schmitt MM, Muldoon M. Pain on injection of prilocaine plain vs. lidocaine with epinephrine. A prospective double-blind study. J Am Dent Assoc. 2001;132:1396–1401. doi: 10.14219/jada.archive.2001.0054. [DOI] [PubMed] [Google Scholar]
- 20.Wahl MJ, Schmitt MM, Overton DA, Gordon MK. Injection pain of bupivacaine with epinephrine vs. prilocaine plain. J Am Dent Assoc. 2002;133:1652–1656. doi: 10.14219/jada.archive.2002.0115. [DOI] [PubMed] [Google Scholar]
- 21.Yagiela JA. Local anesthetics. In: Dionne RA, Phero JP, Becker DE, editors. Management of Pain & Anxiety in the Dental Office. St Louis, Mo: WB Saunders/Elsevier Science; 2002. [Google Scholar]
- 22.Brandt RG, Anderson PF, McDonald NJ, Sohn W, Peters MC. The pulpal anesthetic efficacy of articaine versus lidocaine in dentistry: a meta-analysis. J Am Dent Assoc. 2011;142:493–504. doi: 10.14219/jada.archive.2011.0219. [DOI] [PubMed] [Google Scholar]
- 23.Malamed SF, Gagnon S, Leblanc D. Efficacy of articaine: a new amide local anesthetic. J Am Dent Assoc. 2000;131:635–642. doi: 10.14219/jada.archive.2000.0237. [DOI] [PubMed] [Google Scholar]
- 24.Mikesell P, Nusstein J, Reader A, Beck M, Weaver J. A comparison of articaine and lidocaine for inferior alveolar nerve blocks. J Endod. 2005;31:265–270. doi: 10.1097/01.don.0000140576.36513.cb. [DOI] [PubMed] [Google Scholar]
- 25.Robertson D, Nusstein J, Reader A, Beck M, McCartney M. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007;138:1104–1112. doi: 10.14219/jada.archive.2007.0324. [DOI] [PubMed] [Google Scholar]
- 26.Abdulwahab M, Boynes S, Moore P, et al. The efficacy of six local anesthetic formulations used for posterior mandibular buccal infiltration anesthesia. J Am Dent Assoc. 2009;140:1018–1024. doi: 10.14219/jada.archive.2009.0313. [DOI] [PubMed] [Google Scholar]
- 27.Moore PA, Hersh EV, Papas AS, et al. Pharmacokinetics of lidocaine with epinephrine following local anesthesia reversal with phentolamine mesylate. Anesth Prog. 2008;55:40–48. doi: 10.2344/0003-3006(2008)55[40:POLWEF]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montamat SC, Cusack BJ, Vestal RE. Management of drug therapy in the elderly. N Engl J Med. 1989;321:303–309. doi: 10.1056/NEJM198908033210507. [DOI] [PubMed] [Google Scholar]
- 29.Dagher FB, Yared GM, Machtou P. An evaluation of 2% lidocaine with different concentrations of epinephrine for inferior alveolar nerve block. J Endod. 1997;23:178–180. doi: 10.1016/S0099-2399(97)80271-3. [DOI] [PubMed] [Google Scholar]
- 30.Tofoli GR, Ramacciato JC, de Oliveira PC, et al. Comparison of effectiveness of 4% articaine associated with 1 ∶ 100,000 or 1 ∶ 200,000 epinephrine in inferior alveolar nerve block. Anesth Prog. 2003;50:164–168. [PMC free article] [PubMed] [Google Scholar]
- 31.Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill Companies Inc; 2011. [Google Scholar]
- 32.Dionne RA, Goldstein DS, Wirdzek PR. Effects of diazepam premedication and epinephrine-containing local anesthetic on cardiovascular and catecholamine responses to oral surgery. Anesth Analg. 1984;63:640–646. [PubMed] [Google Scholar]
- 33.Guglielmo A, Reader A, Nist R, Beck M, Weaver J. Anesthetic efficacy and heart rate effects of the supplemental intraosseous injection of 2% mepivacaine with 1 ∶ 20,000 levonordefrin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:284–293. doi: 10.1016/s1079-2104(99)70210-6. [DOI] [PubMed] [Google Scholar]
- 34.Lawaty I, Drum M, Reader A, Nusstein J. A prospective, randomized, double-blind comparison of 2% mepivacaine with 1 ∶ 20,000 levonordefrin versus 2% lidocaine with 1 ∶ 100,000 epinephrine for maxillary infiltrations. Anesth Prog. 2010;57:139–144. doi: 10.2344/0003-3006-57.4.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker DE. Adverse drug interactions. Anesth Prog. 2011;58:31–41. doi: 10.2344/0003-3006-58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster CA. Aston SJ. Propranolol-epinephrine interaction: a potential disaster. Plast Reconstr Surg. 1983;72:74–78. doi: 10.1097/00006534-198307000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Gandy W. Severe epinephrine-propranolol interaction. Ann Emerg Med. 1989;18:98–99. doi: 10.1016/s0196-0644(89)80324-5. [DOI] [PubMed] [Google Scholar]
- 38.Mito RS, Yagiela JA. Hypertensive response to levonordefrin in a patient receiving propranolol: report of a case. J Am Dent Assoc. 1988;116:55–57. doi: 10.14219/jada.archive.1988.0155. [DOI] [PubMed] [Google Scholar]