Abstract
BACKGROUND:
Chlamydia pneumoniae and human cytomegalovirus (HCMV) may be involved in the pathogenesis of atherosclerosis. Prospective studies indicate an increased risk for cardiovascular events in patients with evidence of multiple infections.
OBJECTIVE:
To determine whether there is a synergistic effect of coinfection with C pneumoniae and HCMV on expression of selected growth factors and cytokines.
METHODS:
The production of interleukin (IL)-6, IL-8, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and ‘regulated on activation normal T-cell expressed and secreted’ (RANTES) was measured in coinfected aortic smooth muscle cells (AoSMC).
RESULTS:
Using reverse transcription polymerase chain reaction and immunoassays, it was demonstrated that the expression of IL-6, IL-8, RANTES and bFGF was stimulated in a dose- and time-dependent fashion in C pneumoniae and also in HCMV-infected cultures. In contrast, the expression of PDGF-AA was only stimulated following HCMV infection. Coinfection with C pneumoniae and HCMV resulted in a supra-additive stimulation of IL-6 (30% increased expression, P≤0.05) at 48 h, IL-8 (137% increased expression, P≤0.001) at 24 h and bFGF (209% increased expression, P≤0.01) at 48 h following infection.
CONCLUSIONS:
The findings of the present study show that C pneumoniae and HCMV are able to act in synergy in coinfected AoSMC. The supra-additive induction of AoSMC growth factors and cytokines indicates a novel molecular link between infection and vascular disease development.
Keywords: Atherosclerosis, Chlamydia pneumoniae, Coinfection, Cytomegalovirus, Smooth muscle cells
Abstract
HISTORIQUE :
Le Chlamydia pneumoniae et le cytomégalovirus humain (CMVH) participent peut-être à la pathogenèse de l’athérosclérose. Selon des études prospectives, le risque d’événements cardiovasculaires est plus important chez les patients présentant des manifestations d’infections multiples.
OBJECTIF :
Déterminer si la co-infection par le C pneumoniae et le CMVH a un effet synergétique sur l’expression de facteurs de croissance et de cytokines précises.
MÉTHODOLOGIE :
Les chercheurs ont mesuré la production d’interleukine (IL)-6, d’IL-8, du facteur de croissance basique des fibroblastes (FCbF), du facteur de croissance dérivé des plaquettes (FCDP) et de la protéine RANTES régulée à l’activation, exprimée par les lymphocytes T normaux et sécrétée en présence de co-infection des cellules des muscles lisses de l’aorte (CMLA).
RÉSULTATS :
Au moyen de la réaction en chaîne de la polymérase à transcription inverse et du dosage immunologique, les chercheurs ont démontré que l’expression de l’IL-6, de l’IL-8, de la protéine RANTES et du FCbF étaient stimulés selon la dose et le délai dans les cellules infectées par le C pneumoniae ainsi que par le CMVH. Par contre, l’expression du FCDP-AA n’était stimulée qu’après une infection par le CMVH. Une co-infection par le C pneumoniae et le CMVH entraînait une stimulation supra-additive de l’IL-6 (expression accrue de 30 %, P≤0,05) 48 heures après l’infection, de l’IL-8 (expression accrue de 137 %, P≤0,001) 24 heures après l’infection et du FCbF (expression accrue de 209 %, P≤0,01) 48 heures après l’infection.
CONCLUSIONS :
Les résultats de la présente étude démontrent que le C pneumoniae et le CMVH peuvent agir en synergie en cas de co-infection des CMLA. L’induction supra-additive des facteurs de croissance des CMLA et des cytokines laisse supposer un nouveau lien moléculaire entre l’infection et l’apparition d’une maladie vasculaire.
Atherosclerosis is an inflammatory disease that may be caused or exacerbated by various factors (1). Conventional risk factors (eg, smoking, hypercholesterolemia and hypertension) cannot completely explain the pathogenesis of this disease (2). During the past two decades, several studies have reported an association between persistent viral and bacterial pathogens and atherosclerosis (3–5). Chlamydia pneumoniae and human cytomegalovirus (HCMV) are the most commonly implicated pathogens in this process (6). C pneumoniae, an obligate intracellular bacterium, is disseminated from the primary site of infection, the respiratory tract, within circulating monocytes that can transmit the infection to arterial cells, such as endothelial cells and smooth muscle cells (SMCs) (7). C pneumoniae may contribute to native atherosclerosis by inducing chronic inflammation during persistence in atherosclerotic plaques. In contrast, HCMV is mainly associated with accelerated graft atherosclerosis, which occurs in heart transplant patients and coronary restenosis following angioplasty. In immunocompetent persons, HCMV persists in a latent state within myeloid progenitor cells and endothelial cells. Virus reactivation and replication following angioplasty-induced injury or immunosuppression after transplantation may result in activation of endothelial cells and subsequent infection of SMCs in the vessel wall (8–10). However, HCMV-DNA was not only detected in atherosclerotic plaques but also in nonatherosclerotic tissues (6). Furthermore, large, prospective, clinical trials have failed to show benefits from antibiotic treatment in coronary artery disease (CAD) (11,12). On the other hand, antibiotics may not be capable of eradicating persistent C pneumoniae in atherosclerotic plaques because it is questionable whether antibiotics can penetrate the atheroma. Moreover, persistent C pneumoniae are resistant to antibiotic treatment because of their low metabolic activity (13). Recently, an increased risk for cardiovascular events in patients with serological evidence of previous infection, particularly in those with evidence of multiple infections has been reported (14). Because multiple pathogens in atheromatous tissue of CAD patients have been detected, attention has been broadened to include the burden of infection hypothesis – the concept that it is not one specific infection by itself, but the cumulative burden of infection that increases the risk of atherosclerosis (15).
C pneumoniae and cytomegalovirus (CMV) are able to stimulate the expression of proatherogenic factors in SMCs (16,17). It has been demonstrated that superinfection with C pneumoniae significantly exacerbates the aortic inflammatory foci in murine CMV-infected, normocholesterolemic mice (18). The combined effect of CMV and C pneumoniae in normocholesterolemic mice indicates the involvement of interacting mechanisms in the development of chronic arterial diseases. Because of the high prevalence of coinfection with these pathogens, as well as the epidemiologically suggested concept of pathogen burden, we investigated whether these two pathogens could act in synergy in atherogenesis. Therefore, we studied the expression and the time course of secretion of interleukin-6 (IL-6), IL-8, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and ‘regulated on activation normal T cell expressed and secreted’ (RANTES) as markers of atherogenesis in aortic SMCs (AoSMC) following coinfection with C pneumoniae and CMV.
METHODS
C pneumoniae and HCMV strains
C pneumoniae strain TW-183 (obtained from the Institute of Ophthalmology, London, United Kingdom) was propagated in buffalo green monkey cells. Bacteria were inoculated onto cell monolayers in 25 cm2 flasks and centrifuged at 2000 g for 45 min at 37°C. The inoculum was removed and replaced with serum-free medium Panserin 401 (PAN Biotech, Germany). Infected cells were collected in phosphate-buffered saline (PBS) with 0.2 M sucrose and 2% fetal bovine serum (FBS) approximately 72 h after infection and lysed by sonication. The chlamydial suspension was centrifuged at 4000 g for 5 min to remove cellular debris. Stocks were stored at −70°C. To determine the titres of chlamydial stocks, serial dilutions were inoculated onto shell vial cultures of buffalo green monkey cells. At 48 h after infection, coverslips were fixed with methanol and stained with fluorescein isothiocyanate-conjugated antibody to chlamydial lipopolysaccharide (Imagen, Oxoid, Germany). Titres were calculated from determination of chlamydial inclusions per coverslip and expressed as inclusion-forming units per millilitre.
HCMV reference strain AD-169 was obtained from the Institute of Virology and Antiviral Chemotherapy (Jena, Germany). HCMV strain AD-169 was propagated in human embryonal fibroblasts. Virus-inoculated cell monolayers in 25 cm2 flasks were observed daily for cytopathic effects. When the cytopathic effects reached 90% to 100%, cells were collected in PBS with 0.2 M sucrose and 2% FBS. Virus suspensions were centrifuged at 4000 g to remove cell debris and stored at −70°C. For titration of HCMV stocks, serial dilutions were inoculated onto shell vial cultures of embryonal fibroblasts. At 48 h after infection, cells were fixed with methanol and sequentially incubated with antihuman CMV immediate early antigen (Paesel+Lorei, Germany) and R-Phycoerythrin-conjugated antimouse immunoglubulin (Ig) G (Caltag Laboratories, Germany). CMV-positive nuclei were counted and the number of immediate early forming units per millilitre was calculated.
Infection of AoSMC
Subcultures of human AoSMC (C-12533, PromoCell, Germany) were grown in 35 mm diameter culture wells. Mycoplasma contaminations were eradicated using the MycoDtect DNA array (Greiner Bio-One, Germany). Before infection, confluent monolayers were maintained in AoSMC basal medium (PromoCell, Germany) with 2% FBS but no antibiotics for 72 h. The cells were inoculated with C pneumoniae and/or HCMV at various multiplicities of infection (inclusion-forming units or immediate early forming unit per cell) and centrifuged at 4000 g at 37°C for 45 min, as described previously (16). After the inoculum was decanted, the cells were further incubated with AoSMC basal medium containing 2% FBS. To evaluate the infectivity of Chlamydia and HCMV strains for aortic SMCs, shell vial cultures were infected and stained as described above.
Reverse transcription polymerase chain reaction analysis
Total RNA was prepared using the RNeasy Mini Kit (Qiagen, Germany) and transcribed into complimentary DNA using the Promega reverse transcription system (Promega, Germany) according to the manufacturer’s protocols. Reverse transcription polymerase chain reaction (RT-PCR) was performed exactly as described in previous articles. The specific primers were as follows: 5′-AGCTCAGCTATGAACTCCTTCTC-3′ and 5′-GTCTCCTCATTGAATCCAGATTGG-3′ (338 bp product) for IL-6; 5′-CTTGGCAGCCTTCCTGATTT-3′ and 5′-CAGCCCTCTTCAAAAACTTC-3′ (263 bp product) for IL-8; 5′-TCCCCATATTCCTCGGAC-3′ and 5′-GATGTACTCCCGAACCCA-3′ (186 bp product) for RANTES; 5′-TCACCACGCTGCCCGCCTTGC-3′ and 5′-CAGTTCGTTTCAGTGCCACAT-3′ (342 bp product) for bFGF; 5′-CCTGCCCATTCGGAGGAAGAG-3′ and 5′-TTGGCCACCTTGACGCTGCG-3′ (225 bp product) for PDGF-A; 5′-GAAGGAGCCTGGGTTCCCTG-3′ and 5′-TTTCTCACCTGGACAGGTCG-3′ (217 bp product) for PDGF-B. The sequences of pyruvate dehydrogenase (PDH) primers used as control were 5′-GGTATGGATGAGGACCTGGA-3′ and 5′-CTTCCACAGCCCTCGACTAA-3′ (105 bp product). PCR products were electrophoresed on 1% agarose gels and visualized with SYBR green staining. The volumes (optical density × mm2) of the band images were quantitated with Multi Analyst software (Bio-Rad, Germany) and normalized against a housekeeping gene (PDH) signal from the same sample as reference against the expression level of the investigated gene (16).
Immunoassays for cytokines and growth factors
Supernatants of infected and mock-infected cultures were collected at 24 h, 48 h and 72 h and stored at −30°C until assayed. Levels of IL-6, IL-8 and RANTES were measured by CytoSet ELISA (Biosource, Germany). Levels of PDGF-AA and bFGF were determined by Quantikine ELISA (R&D Systems, Germany). All assays were performed according to the manufacturer’s protocols.
Statistical analysis
The data were expressed as means with SD. Student’s t test was used to assess the significance of observed differences. A P≤0.05 was considered to be statistically significant.
RESULTS
Growth of C pneumoniae and HCMV in AoSMC
Initial experiments confirmed that C pneumoniae strain TW-183 and HCMV strain AD-169 were capable of infecting AoSMC. HCMV strain AD-169 easily infected human AoSMC based on the expression of the immediate-early antigen. C pneumoniae TW-183 was less efficient in infecting AoSMC. For comparative experiments on the activation of AoSMC by C pneumoniae and HCMV, infectious doses of chlamydiae (multiplicity of infection [MOI] 1, 5 and 10) that were higher than the doses of HCMV (MOI 0.05, 0.2 and 1) but resulted in similar percentages of productively infected cells were used. The coinfection of AoSMC with C pneumoniae (MOI 5) and HCMV (MOI 0.2) resulted in intracellular growth, which was characterized by the development of typical inclusion bodies (Figure 1).
Figure 1).
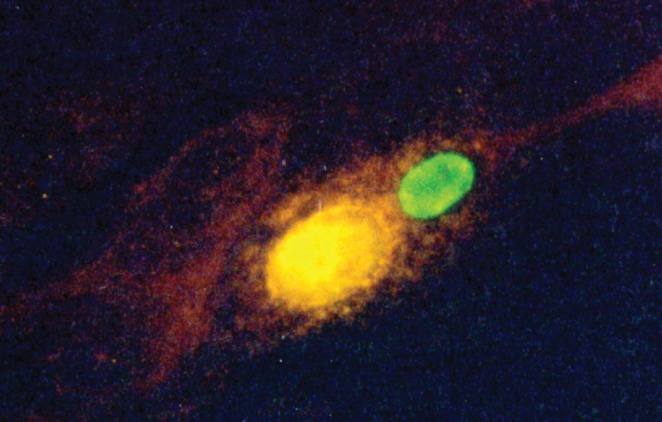
Morphology of Chlamydia pneumoniae TW-183 and human cytomegalovirus (CMV) AD-169 in coinfected aortic smooth muscle cells at 72 h postinfection. Monolayers were stained with fluorescein isothiocyanate (FITC)-conjugated antibody to chlamydial lipopolysaccharide (green) (Imagen, Dako Diagnostics, United Kingdom) and CMV monoclonal antibody against CMV immediate-early antigen (phycoerythrin, yellow) (Light Diagnostics, Millipore). Original magnification ×400
Comparison of SMC messenger RNA expression using RT-PCR in response to C pneumoniae and HCMV infection
To study the messenger RNA (mRNA) expression profiles in AoSMC, a panel of selected genes representing chemokines and cellular growth factors that have been shown to be regulated in SMCs by C pneumoniae (16,19,20) and partly by HCMV (17,21) were chosen. AoSMC were infected with C pneumoniae or CMV at various MOIs. mRNA levels were measured at 24 h after infection. Using RT-PCR, low levels of IL-6, IL-8, PDGF-A, bFGF and RANTES were found in mock-infected cultures. The mRNA expression of IL-6, IL-8, RANTES and bFGF was stimulated in C pneumoniae and also in CMV-infected cells in a dose-dependent manner. Because PDGF occurs in three dimeric isoforms (AA, BB, and AB) the β-receptor ligand PDGF-A mRNA and the β-receptor ligand PDGF-B were examined. The β-receptor ligand PDGF-A mRNA was only stimulated in HCMV-infected cultures. Neither mock-infected nor Chlamydia- or HCMV-infected AoSMC expressed mRNA of the β-receptor ligand PDGF-B (Figure 2). RNA isolated from endothelial cells was used as a positive PCR control (22).
Figure 2).
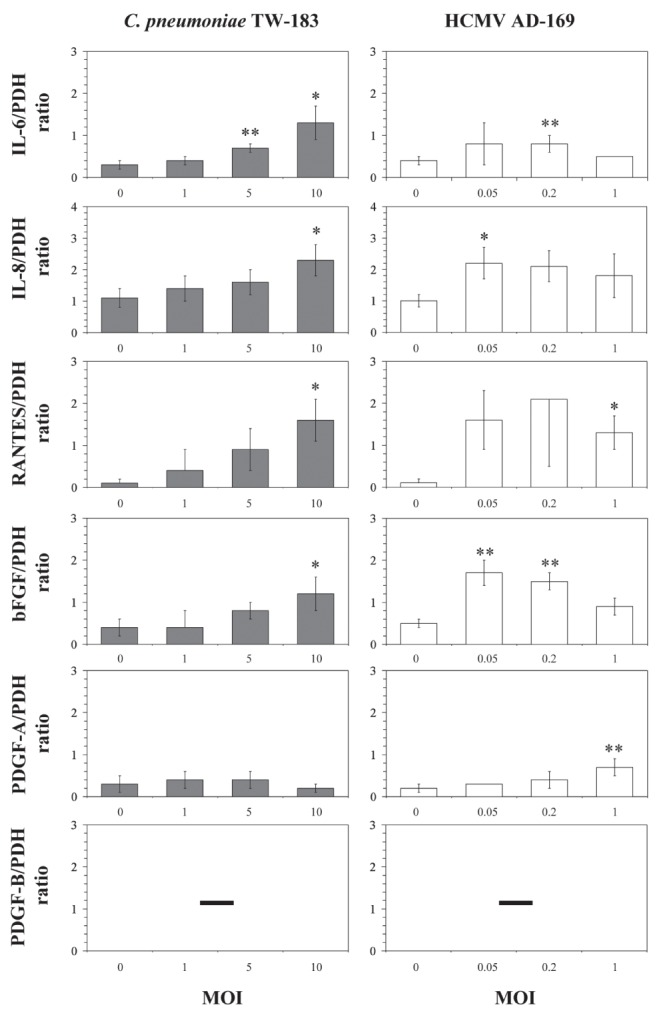
Comparative analysis of messenger RNA expression of interleukin (IL)-6 (338 bp), IL-8 (263 bp), basic fibroblast growth factor (bFGF) (342 bp), platelet-derived growth factor (PDGF)-A (225 bp), PDGF-B (217 bp) and ‘regulated on activation normal T-cell expressed and secreted’ (RANTES) (186 bp) in aortic smooth muscle cells (AoSMC) following Chlamydia pneumoniae or HCMV infection. AoSMC were infected with C pneumoniae and CMV at different multiplicities of infection (MOI). Levels of specific mRNAs following infection of AoSMC with C pneumoniae or HCMV were assessed at 24 h following infection. Reverse transcription polymerase chain reaction product ratios were normalized against the pyruvate dehydrogenase (PDH) (105 bp) signal from the same sample. Values are expressed as the mean±SD of four experiments, compared with values for mock-infected controls *P≤0.05; **P≤0.03 (Student’s t test)
Analysis of SMC protein secretion in response to C pneumoniae or HCMV infection
AoSMC were infected with C pneumoniae at an MOI of 5 and HCMV at an MOI of 0.2 for 24 h, 48 h and 72 h. Immunoassays were used to examine the expression of IL-6, IL-8, bFGF, RANTES and PDGF-AA in culture supernatants. The time points of maximal protein expression in the experiments differed between the bacterial pathogen C pneumoniae and the viral pathogen HCMV. IL-6 and IL-8 production peaked at 72 h after infection in both C pneumoniae (IL-6 5.9-fold increase, P≤0.01; IL-8 6.2-fold increase versus control, P≤0.01) and HCMV (IL-6 4.5 fold increase, P<0.01; IL-8 10.3-fold increase versus control, P≤0.01) infected cultures. bFGF expression peaked at 48 h after infection in C pneumoniae-infected cultures (sevenfold increase versus control, not significant [ns]) and at 72 h after infection in HCMV infected cultures (eightfold increase versus control, ns). Only a slight increase of RANTES in C pneumoniae infected cultures at 72 h following infection (2.5-fold increase versus control, P≤0.04) and a 29.8-fold (P≤0.04) increase in HCMV infected cultures at 72 h after infection were found. Because β-receptor ligand PDGF-B mRNA was not expressed in these cultures, the secretion of the dimeric glycoprotein PDGF-AA was analyzed. PDGF-AA was only stimulated following HCMV infection (12.5-fold increase versus control P≤0.01) at 24 h postinfection.
In principle, the profiles of mRNA expression were reflected in the levels of the factors in culture supernatants, showing a stimulated production of IL-6, IL-8, bFGF and RANTES following infection with C pneumoniae and HCMV. The expression of PDGF-AA was only stimulated following infection with HCMV (Figure 3).
Figure 3).
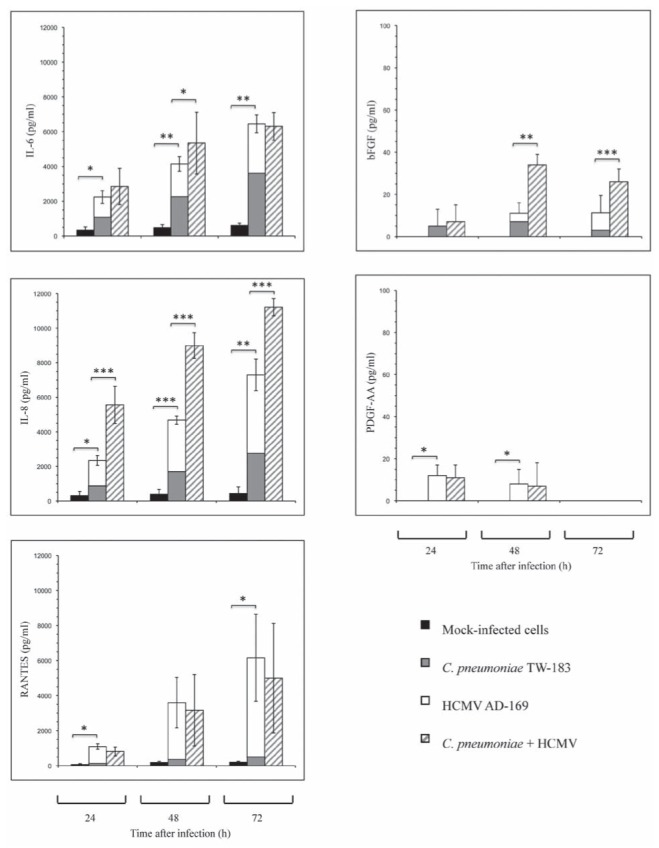
Release of interleukin (IL)-6, IL-8, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF)-AA, and ‘regulated on activation normal T-cell expressed and secreted’ (RANTES) at different time points in culture supernatants of Chlamydia pneumoniae-infected, HCMV-infected and C pneumoniae- and HCMV-coinfected aortic smooth muscle cells compared with mock-infected cells. Cells were cultured in 11 mm diameter tubes. Values are expressed as mean±SD of four experiments. *P≤0.05; **P≤0.01; ***P≤0.001 compared with mock-infected cells (Student’s t test). P in coinfected cultures are in comparison to C pneumoniae-infected/CMV-infected cells
Analysis of SMC protein secretion following coinfection with C pneumoniae and HCMV
Protein secretion in coinfected AoSMC was measured at different time points. Coinfection with HCMV and C pneumoniae resulted in a supra-additive stimulation of IL-6 (27.6% increased secretion compared with simple addition of IL-6 amount in HCMV and C pneumoniae-infected cultures, P≤0.05) at 48 h. Stimulation of IL-8 production peaked at 24 h following coinfection (137% increased secretion compared with simple addition of IL-8 amount in HCMV and C pneumoniae infected cultures, P≤0.001). This supra-additive effect was also significant at 48 h (92% increased secretion, P≤0.001) and 72 h following infection (53% increased secretion, P≤0.001). The secretion of bFGF in coinfected cultures peaked at 48 h following infection (209% increased secretion compared with simple addition of bFGF amount in HCMV and C pneumoniae-infected cultures, P≤0.01) and was also significant at 72 h following infection (136% increased secretion, P≤0.001). In contrast, no further stimulation of RANTES and PDGF-AA secretion was found.
Coinfection with the bacterial pathogen C pneumoniae and the viral pathogen HCMV resulted in a supra-additive expression of IL-6, IL-8 and also bFGF (Figure 3).
DISCUSSION
Increased pathogen burden may be associated with an enhanced risk of atherogenesis (15,23). Coinfection with viral and bacterial pathogens may affect the gene expression in arterial wall cells. In the present study, we demonstrated for the first time that coinfection with C pneumoniae and HCMV was associated with a supra-additive expression of the atherogenic factors IL-6, IL-8 and bFGF in AoSMC.
More than 30 major members of the interleukin family have been proposed to play a critical role in atherogenesis (24,25). IL-6 and IL-8 are the cytokines with the most extensively studied proatherogenic profile (24). The important role of IL-6 was demonstrated through the exacerbation of early atherosclerosis by recombinant IL-6 in various atherosclerosis-prone murine models (24). Furthermore, IL-6 has been shown to increase platelet activity and fibrinogen levels leading to increased blood viscosity and endothelial damage (26). IL-8 is a strong factor for recruitment of inflammatory cells into the vascular wall, including T lymphocytes, neutrophils and SMCs (24). It has been found in atheromatous tissue, mostly derived from intimal macrophages (27). The novel finding of a supra-additive stimulation of IL-6 and IL-8 secretion in AoSMC following coinfection with C pneumoniae and HCMV supports the hypothesis that these two pathogens may be involved in the pathogenesis of atherosclerotic plaque formation. However, whether coinfection of SMCs with C pneumoniae and HCMV is associated with a supra-additive expression of other interleukins remains to be investigated.
RANTES is a basic 8 kDa polypeptide of the CC chemokine subfamily with strong chemotactic activity for eosinophils and lymphocytes (28), and is highly expressed within atheroma (29). Recently, it has been demonstrated that RANTES levels are elevated in patients with acute coronary syndromes while levels in stable CAD have been shown to be downregulated (30). It was shown that human SMCs express and release RANTES in response to T-helper 1 cytokines (31) and also C pneumoniae infection (32). In the present study, we found that HCMV was a stronger inducer of RANTES in AoSMC compared with expression of RANTES following C pneumoniae infection. However, coinfection of AoSMC with C pneumoniae and HCMV did not result in a further stimulation of RANTES expression.
The growth factors bFGF and PDGF have been associated with atherosclerosis through their mitogenic and angiogenic properties (33). Growth factor stimulation leads to SMC accumulation and increased secretion of extracellular matrix components that account for up to 60% of the volume in areas of intimal thickening (34). Previously, we have shown that bFGF production is stimulated in C pneumoniae-infected SMCs (19). In the present study, we demonstrated that bFGF production in AoSMC was also stimulated following HCMV infection. Furthermore, after coinfection with C pneumoniae and HCMV, the expression of bFGF was stimulated in a supra-additive manner compared with infection of AoSMC with either C pneumoniae or HCMV alone. bFGF is not only an important mediator for SMCs replication, but it is also able to upregulate the expression of interstitial collagenase, leading to an accelerated turnover of extracellular matrix (35). More recently, it was demonstrated that an increased expression of bFGF was associated with carotid atherosclerotic plaque instability via the nuclear factor kappa B pathway (36). The expression of the mitogen PDGF, a dimeric glycoprotein composed of two A (-AA) or two B (-BB) or a combination of the two (-AB) chains was also examined in our study. In our experiments, the mRNA expression of the a-receptor ligand PDGF-A was only stimulated following infection with HCMV. As we have previously shown, neither infected nor mock-infected human AoSMC express β-receptor ligand PDGF-B, which is expressed by monocytes and endothelial cells (20). Therefore, the expression of the PDGF-AA homodimer was examined in culture supernatants. As expected, PDGF-AA was only stimulated in HCMV-infected AoSMC but was not stimulated in C pneumoniae-infected cultures. We found no additional expression of PDGF-AA after coinfection with both pathogens. However, PDGF-AA is produced in SMCs and has been implicated in migration and proliferation of SMCs in atherosclerotic lesion formation (37). The importance of PDGF-AA in the arterial system has been recently strengthened since it was shown that proliferation of arterial SMCs was strongly stimulated by PDGF-AA, whereas venous SMCs showed a greater proliferation response to PDGF-BB (38).
CONCLUSIONS
The present study demonstrated for the first time that coinfection of AoSMC with C pneumoniae and HCMV stimulates the expression of IL-6, IL-8 and bFGF, which are known to be involved in the process of atherosclerosis. Therefore, our findings support the hypothesis that the cumulative burden of pathogens such as C pneumoniae and HCMV may contribute to the growth and destabilization of atherosclerotic plaques, and provide a possible molecular link between infection and vascular disease development.
Acknowledgments
This work was supported by grant B307-04004 from the Interdisciplinary Centre for Clinical Research of Jena, Germany.
REFERENCES
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Markus HS, Carrington MD, Steinmetz MA. Chlamydia pneumoniae infection and early asymptomatic carotid atherosclerosis. Circulation. 1999;100:832–7. doi: 10.1161/01.cir.100.8.832. [DOI] [PubMed] [Google Scholar]
- 3.Ngeh J, Anand V, Gupta S. Chlamydia pneumoniae and atherosclerosis – what we know and what we don’t. Clin Microbiol Infect. 2002;8:2–13. doi: 10.1046/j.1469-0691.2002.00382.x. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson AC, Hajjar DP. Herpesvirus in atherosclerosis and thrombosis: Etiologic agents or ubiquitous bystanders? Arterioscler Thromb Vasc Biol. 1998;18:339–48. doi: 10.1161/01.atv.18.3.339. [DOI] [PubMed] [Google Scholar]
- 5.Vercellotti GM. Effects of viral activation of the vessel wall on inflammation and thrombosis. Blood Coagul Fibrinolysis. 1998;9:3–6. [PubMed] [Google Scholar]
- 6.Xenaki E, Hassoulas J, Apostolakis S, Sourvinos G, Spandidos DA. Detection of cytomegalovirus in atherosclerotic plaques and nonatherosclerotic arteries. Angiology. 2009;60:504–8. doi: 10.1177/0003319708322390. [DOI] [PubMed] [Google Scholar]
- 7.Rupp J, Koch M, van Zandbergen G, Solbach W, Brandt E, Maass M. Transmission of Chlamydia pneumoniae infection from blood monocytes to vascular cells in a novel transendothelial migration model. FEMS Microbiol Lett. 2005;242:203–8. doi: 10.1016/j.femsle.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Gerna G, Baldanti F, Revello MG. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol. 2004;65:381–6. doi: 10.1016/j.humimm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Reeves MB, Coleman H, Chadderton J, Goddard M, Sissons JG, Sinclair JH. Vascular endothelial and smooth muscle cells are unlikely to be major sites of latency of human cytomegalovirus in vivo. J Gen Virol. 2004;85:3337–41. doi: 10.1099/vir.0.80285-0. [DOI] [PubMed] [Google Scholar]
- 10.Evers DL, Wang X, Huang ES. Cellular stress and signal transduction responses to human cytomegalovirus infection. Microb Infect. 2004;6:1084–93. doi: 10.1016/j.micinf.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor CM, Dunne MW, Pfeffer MA. Investigators in the WIZARD study, azithromycin for the secondary prevention of coronary heart disease events: The WIZARD study: A randomised controlled trial. JAMA. 2003;290:1459–66. doi: 10.1001/jama.290.11.1459. [DOI] [PubMed] [Google Scholar]
- 12.Cleland JG, Huan LP, Freemantle N, Clark AL, Coletta AP. Clinical trials update from the European Society of Cardiology: SENIORS, ACES, PROVE-IT, ACTION, and the HF-ACTION trial. Eur J Heart Fail. 2004;6:787–91. doi: 10.1016/j.ejheart.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP. Chlamydia pneumoniae and coronary artery disease: The antibiotic trials. Mayo Clin Proc. 2003;78:321–32. doi: 10.4065/78.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Pesonen E, El-Segaier M, Persson K, et al. Infections as a stimulus for coronary occlusion, obstruction, or acute coronary syndromes. Ther Adv Cardiovasc Dis. 2009;3:447–54. doi: 10.1177/1753944709345598. [DOI] [PubMed] [Google Scholar]
- 15.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections: Role of endothelial dysfunction. Circulation. 2002;106:184–90. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 16.Rödel J, Prochnau D, Prager K, Baumert J, Schmidt KH, Straube E. Chlamydia pneumoniae decreases smooth muscle cell proliferation through induction of prostaglandin E2 synthesis. Infect Immun. 2004;72:4900–4. doi: 10.1128/IAI.72.8.4900-4904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streblow DN, Dumortier J, Moses AV, Orloff SL, Nelson JA. Mechanisms of cytomegalovirus-accelerated vascular disease: Induction of paracrine factors that promote angiogenesis and wound healing. Curr Top Microbiol Immunol. 2008;325:397–415. doi: 10.1007/978-3-540-77349-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burian K, Berencsi K, Endresz V, et al. Chlamydia pneumoniae exacerbates aortic inflammatory foci caused by murine cytomegalovirus infection in normocholesterolemic mice. Clin Diagn Lab Immunol. 2001;8:1263–6. doi: 10.1128/CDLI.8.6.1263-1266.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rödel J, Woytas M, Groh A, et al. Production of basic fibroblast growth factor and interleukin 6 by human smooth muscle cells following infection with Chlamydia pneumoniae. Infect Immun. 2000;68:3635–41. doi: 10.1128/iai.68.6.3635-3641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rödel J, Lehmann M, Vogelsang H, Straube E. Chlamydia pneumoniae infection of aortic smooth muscle cells reduces platelet-derived growth factor receptor-beta expression. FEMS Immunol Med Microbiol. 2007;51:363–71. doi: 10.1111/j.1574-695X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 21.Gredmark-Russ S, Dzabic M, Rahbar A, et al. Active cytomegalovirus infection in aortic smooth muscle cells from patients with abdominal aortic aneurysm. J Mol Med. 2009;87:347–56. doi: 10.1007/s00109-008-0413-4. [DOI] [PubMed] [Google Scholar]
- 22.Prochnau D, Rödel J, Hartmann M, Straube E, Figulla HR. Growth factor production in human endothelial cells after Chlamydia pneumoniae infection. Int J Med Microbiol. 2004;294:53–7. doi: 10.1016/j.ijmm.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Espinola-Klein C, Rupprecht HJ, Blankenberg S, et al. Are morphological or functional changes in the carotid artery wall associated with Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, or herpes simplex virus infection? Stroke. 2000;31:2127–33. doi: 10.1161/01.str.31.9.2127. [DOI] [PubMed] [Google Scholar]
- 24.von der Thüsen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–66. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 25.Kern JM, Maass V, Maass M. Chlamydia pneumoniae adversely modulates vascular cell properties by direct interaction with signalling cascades. Thromb Haemost. 2009;102:1064–70. doi: 10.1160/TH09-06-0348. [DOI] [PubMed] [Google Scholar]
- 26.Pai J, Knoop FC, Hunter WJ, III, et al. Chlamydia pneumoniae and occlusive vascular disease: Identification and characterization. J Pharmacol Toxicol Methods. 1998;39:51–61. doi: 10.1016/s1056-8719(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 27.Yue TL, Wang X, Sung CP, et al. Interleukin-8: A mitogen and chemotractant for vascular smooth muscle cells. Circ Res. 1994;75:1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: Linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–50. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 29.Veillard NR, Kwak B, Pelli G, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–61. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 30.Rothenbacher D, Muller-Scholze S, Herder C, Koenig W, Kolb H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler Thromb Vasc Biol. 2006;26:194–9. doi: 10.1161/01.ATV.0000191633.52585.14. [DOI] [PubMed] [Google Scholar]
- 31.John M, Hirst SJ, Jose PJ, et al. Human airway smooth muscle cells express and release RANTES in response to T helper 1 cytokines: Regulation by T helper 2 cytokines and corticosteroids. J Immunol. 1997;158:1841–7. [PubMed] [Google Scholar]
- 32.Dechend R, Gieffers J, Dietz R, et al. Hydroxymethylglutaryl coenzyme A reductase inhibition reduces Chlamydia pneumoniae-induced cell interaction and activation. Circulation. 2003;108:261–5. doi: 10.1161/01.CIR.0000083367.93022.78. [DOI] [PubMed] [Google Scholar]
- 33.Reidy MA. Growth factors and arterial smooth muscle cell proliferation. Ann N Y Acad Sci. 1994;714:225–30. doi: 10.1111/j.1749-6632.1994.tb12047.x. [DOI] [PubMed] [Google Scholar]
- 34.Stary HC, Blankenhorn DH, Chandler AB, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1992;12:120–34. doi: 10.1161/01.atv.12.1.120. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy SH, Ronda S, Qin H, Aho S, Selber J, Tan EM. Basic FGF regulates intersitial collagenase gene expression in human smooth muscle cells. J Cell Biochem. 1997;65:32–41. [PubMed] [Google Scholar]
- 36.Sigala F, Savvari P, Liontos M, et al. Increased expression of bFGF is associated with carotid atherosclerotic plaques instability engaging the NF-kappaB pathway. J Cell Mol Med. 2010;3 doi: 10.1111/j.1582-4934.2010.01082.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cucina A, Pagliei S, Borreli V, et al. Oxidized LDL (OxLDL) induces production of platelet derived growth factor AA (PDGF AA) from aortic smooth muscle cells. Eur J Vasc Endovasc Surg. 1998;16:197–202. doi: 10.1016/s1078-5884(98)80220-7. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Blumenthal DK, Terry CM, He Y, Carlson ML, Cheung AK. PDGF-induced proliferation in human arterial and venous smooth muscle cells: Molecular basis for differential effects of PDGF isoforms. J Cell Biochem. 2011;112:289–98. doi: 10.1002/jcb.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]


