Nematode infections are a major cause of morbidity and contribute significantly to the calculation of Disability Adjusted Life Years (DALYs), with the global disease burden associated with geohelminths being similar to that of malaria or tuberculosis (1, 2). More importantly, in many cases, such as filarial infection, chemotherapy against adult stages is either ineffective or limited to doxycycline that appears to target filarial Wolbachia symbionts and requires a treatment regime not generally applicable for use in the field. Perhaps less well-appreciated, but equally important for human nutrition and health, is the devastating economic impact of parasitic nematodes in agricultural settings. Parasitic nematodes infect livestock (sheep, cattle, horses) and major crops (corn and soybeans) and cause billions of dollars in economic losses yearly in the US alone. In the case of animal parasites, most commercially available anthelmintics are becoming ineffective because of growing resistance (benzimidazoles, levamisole, macrocyclic lactones and acetonitrile derivatives) or, in the case of plant parasitic nematodes, most nematocides have been banned recently because of toxicity to humans (3–5). The situation is reaching critical proportions and new drugs and drug targets are needed in all settings. This short review is designed to summarize the information on monoamine-dependent paralysis as a target for anthelmintic development, examine the conservation of monoamine receptors in the genomes of both free-living and parasitic nematodes, and highlight the utility of the Caenorhabditis elegans model system for dissecting the monoaminergic modulation of locomotory decision-making.
Most anthelmintics in use against nematode infections act as agonists at key receptors and cause paralysis by interfering with muscle contraction and/or locomotion. Since receptor activation is essential for the activity of most anthelmintics, receptor knockout may not necessarily be the “gold standard” for target validation (overexpression may be more diagnostic). For example, agonists at four distinct molecular targets have been exploited previously, including two cholinergic receptors (tetrahydropyrimidines/imidathiazoles and amino-acetonitriles) and glutamate (macrocyclic lactones)/GABA (piperazine)-gated Cl− channels. In addition, cyclooctadepsipeptides, such as emodepside, inhibit feeding and pumping and exhibit a novel action potentially involving G-protein coupled latrophilin-like receptors but, more probably Ca++-gated K+ channels (6–8). Importantly, each of these anthelmintics is active in C. elegans and our understanding of their modes of action has been greatly facilitated by the use of this free-living nematode model. As predicted based on the agonist hypothesis, the knockout of each of these validated targets is not lethal. In contrast to these general rules, the anthelmintic, derquantel (2-desoxoparahequamide), appears to function as a nicotinic antagonist and has marked activity in dissected C. elegans, but not intact worms, suggesting that the permeability of the C. elegans cuticle may in some cases be more limited than that of the parasites (69). With the exception of the amino-acetonitriles, resistance has begun to develop to all classes of anthelmintics, emphasizing the need for new drug targets. However, the identification of new targets within key signaling pathways has been limited by the lack of useful information about the identity, function and localization of the additional receptors regulating muscle contraction and locomotion, leading to the development of the “dual systems” approach outlined below, designed to take advantage of the well-developed and ever-expanding C. elegans analytical tool kit (9–14).
Although nematodes vary tremendously in size (about 1 mm for C. elegans compared with 30 cm for Ascaris suum), their body plans are remarkably conserved, with adults of both species exhibiting nearly identical neuronal wiring diagrams. This observation suggests that C. elegans, with its well-defined molecular genetics, numerous signaling mutants and cell-based assay systems might be a useful model to identify core signaling pathways in parasitic nematodes and could provide unique new insights compared with studies focused exclusively in parasites. In the past, some parasitologists have tended to minimize the use of C. elegans as a model for target identification. Certainly, observations from C. elegans need to be confirmed in individual parasites, but prejudice against C. elegans is largely without merit, particularly with regard to core signaling pathways and the locomotory machinery, as all commercially available anthelmintics have activity against C. elegans and recent studies have demonstrated the rescue of C. elegans null mutants with proteins from parasitic helminths (8, 63–66). For example, ivermectin and emodepside paralyze both C. elegans and parasitic nematodes through the activation of orthologous glutamate-gated Cl− and SLO-1 channels, respectively, and the expression of these receptors from parasitic nematodes can rescue the appropriate C. elegans null mutants, further validating the utility of a “dual systems” approach for target identification (8, 15). Indeed, the use of these chimeric C. elegans for screening may have advantages over molecular-based screens in that they include the nematode cuticle and appropriate nematode-specific accessory proteins, in addition to accounting for potential pharmacological differences among orthologous proteins from C. elegans and the parasites.
The recent expanded analysis of many nematode genomes has supported the hypothesis that many core signaling pathways are highly conserved in both free-living and parasitic nematodes, as discussed below for nematode monoamine receptors (16–20). However, nematodes exhibit significant diversity, so that there is no guarantee that processes in C. elegans will be exactly duplicated in parasitic nematodes. Indeed, physiological, biochemical and/or molecular differences between or among nematode species have been demonstrated. For example, the composition of gene families and individual splicing patterns can vary significantly within the phylum. In addition, other individual differences have been noted. For instance, the neuropeptide, AF1, appears to be differentially localized in C. elegans and A. suum, based on anti-peptide staining in A. suum and GFP expression driven by the af1(flp-8) promoter in C. elegans (21). However, recent work suggests that GFP expression can be promiscuous or, alternatively, some genes are functionally expressed in neurons not exhibiting GFP fluorescence using this approach, so that it would have been useful to use the same immuno-staining technique in C. elegans (22, 23). Certainly, observations from C. elegans need to be confirmed by direct assay in individual parasite species whenever possible. In contrast, although most core signaling pathways appear to be conserved among nematodes, C. elegans proteins themselves are most probably poor targets for high throughput screening, as their pharmacology and regulation can differ significantly among orthologues in different nematodes. However, all of the anthelmintics currently in use today appear to function similarly in both C. elegans and A. suum and our understanding of the mode of action of all four classes of anthelmintics has been greatly facilitated by genetic analyses in C. elegans.
Monoamines, including serotonin (5-HT), tyramine (TA), octopamine (OA) and dopamine (DA), modulate most key behaviors in nematodes, with monoaminergic signaling mediated by an array of G-protein coupled receptors (GPCRs) and unique monoamine-gated Cl− channels. Exogenous 5-HT, DA or TA independently paralyze both free-living and parasitic nematodes, i.e., the addition of monoamines can create uncoordinated, directionless movement, leading ultimately to immobilized worms. However, this paralysis often appears distinct from the classical, spastic paralysis initiated by cholinergic agonists, such as levamisole, or the flaccid paralysis associated with GABA-ergic agonists that result from the activation of receptors directly on body wall muscle. Indeed, paralysis in these monoamine-treated worms appears to result most often from the disruption of complex locomotory decision-making networks and the worms appear to be as much confused as paralyzed.
In C. elegans, 5-HT functions as a “food signal” and modulates feeding, pharyngeal pumping, egg-laying, and an array of locomotory behaviors through a limited number of serotonergic neurons (24–26). Monoamines are synthesized endogenously and presumably released from a limited number of neurons and, in some cases, non-neuronal tissues, in response to changing environmental conditions. Based on the expression of tph-1, that encodes tryptophan hydroxylase, the rate-limiting enzyme for 5-HT biosynthesis, 5-HT is synthesized in the two NSM neurosecretory motorneurons, ADF sensory neurons and adult HSN hermaphrodite-specific neurons, in addition to a limited number of male-specific neurons (27). In addition, some neurons, such as the two AIMs and the RIH do not appear to synthesize 5-HT, but instead accumulate the monoamine through a fluoxetine-sensitive 5-HT reuptake transporter, MOD-5, or may up-regulate 5-HT expression in response to changing environmental conditions, such as the hypoxia-induced expression of tph-1 in the ASG sensory neurons (28–30). Similarly, DA is synthesized in four pairs of mechanosensory neurons, TA in two RIM motorneurons, two RIC interneurons and four UV1 uterine cells and OA that is synthesized from TA by tyramine β-hydroxylase, TBH-1, in the two RIC interneurons and the gonadal sheath cells (31, 32). Much less is known about the sites of monoamine biosynthesis in parasitic nematodes; however, given the structural conservation of the nematode nervous system it is reasonable to assume that at least some of the sites of synthesis will be conserved. Indeed, based on immunostaining, 5-HT is also synthesized in the A. suum NSMs (33, 34). In contrast, although 5-HT and DA have been localized to neurons in Haemonchus contortus, the specific neurons have not yet been unequivocally identified (35).
C. elegans expresses at least five distinct 5-HT receptors, four G-protein coupled receptors (GPCRs), SER-1, SER-4, SER-5 and SER-7 and a unique 5-HT-gated Cl− channel that appear to be the major, if not the only 5-HT receptors in the genome, as quintuple null worms, lacking all five 5-HT receptors, fail to respond to 5-HT in all behavioral assays examined to date (36, 37). Most 5-HT dependent phenotypes involve a balance of both excitatory and inhibitory serotonergic inputs. For example, SER-1 and SER-7 are required for the 5-HT stimulation of egg-laying, but the addition of 5-HT to ser-7 ser-1 null worms on a food source actually inhibits egg-laying through SER-4 and MOD-1 (37). Similarly, 5-HT again stimulates egg-laying in ser-4;mod-1;ser-7 ser-1 null worms through SER-5, highlighting the complexity of serotonergic modulation (37). A similar balance has also been observed for other behaviors, such as pharyngeal pumping and locomotion and for other monoamines (38–40). Both the Gαo-coupled GPCR, SER-4, and the 5-HT-gated Cl− channel, MOD-1 are involved in 5-HT-dependent paralysis and modulate locomotion in wild-type worms moving on agar plates or swimming worms in liquid medium (37, 48, 49; Komuniecki, unpublished). For example, ser-4;mod-1 double mutants are completely resistant to 5-HT dependent paralysis and locomotion in quadruple null worms expressing only SER-4 or MOD-1 is dramatically inhibited by 5-HT, suggesting that agonists at either (or both) of these receptors have potential as anthelmintics (37). In addition, SER-1 is also involved in locomotory decision-making (26, 68). The DA-dependent inhibition of locomotion involves DOP-3 in the cholinergic motor neurons and the complex modulation of locomotion by TA involves the TA-gated Cl− channel, LGC-55, in head muscle and the AVB forward command interneurons and three GPCRs, SER-2/TYRA-2/TYRA-3 in as yet unidentified neurons (41–44; Komuniecki, unpublished). Together, studies from C. elegans suggest that 1) monoaminergic signaling is complex, 2) often involves antagonistic excitatory and inhibitory inputs into key behaviors, and, most importantly from the perspective of drug discovery, 3) individual monoamines can act throughout the sensory-mediated neuromuscular system to cause paralysis, including interneurons (SER-4, MOD-1, SER-2/TYRA-2), motorneurons (DOP-3) or head muscle (LGC-55). Finally, monoamines also have the capacity to activate more global peptidergic signaling cascades and, importantly, an array of different neuropeptides cause paralysis when injected into A. suum, suggesting that some peptide receptors may also be useful targets for drug discovery (45–47).
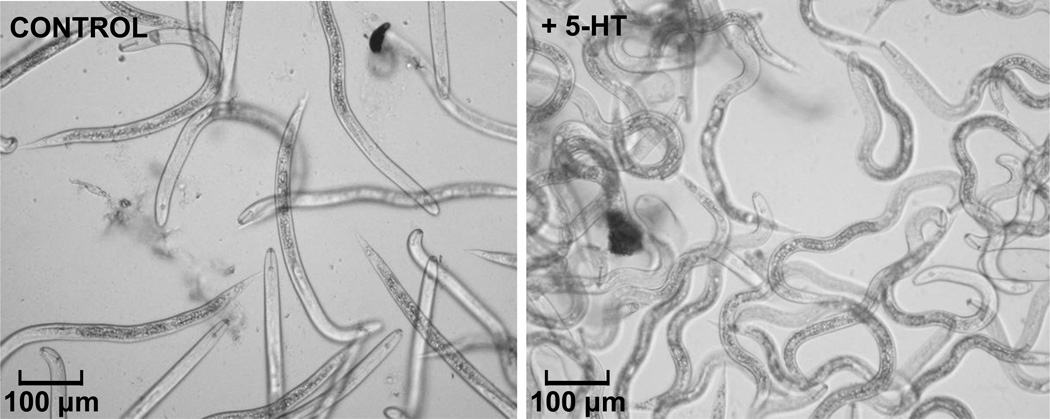
Monoamines also paralyze a variety of parasitic nematodes. For example, exogenous monoamines have locomotory effects in plant parasitic nematodes, such as Heterodera glycines and Meloidogyne incognita, with both 5-HT and DA differentially inhibiting locomotion and OA causing hyperactivity similar to the effects of OA in insects (50, 51). As described above, 5-HT paralyzes H. glycines J2s, again with a phenotype distinct from the classical patterns of flaccid or spastic paralysis with the animals assuming an unusual “kinked” phenotype (Figure 1). Similarly, the injection of 5-HT directly into pseudocoelom of A. suum causes immediate paralysis, increased body length and decreased propagating body waves (52). We have observed similar phenotypes after the incubation of A. suum fourth-stage larva (L4) in exogenous 5-HT. In addition, although both GABA and 5-HT increase body length, GABA induces a flaccid paralysis, whereas 5-HT-paralyzed A. suum are more rigid, in agreement with the “mixed” paralysis described above. 5-HT also inhibits ACh-induced muscle contractions in A. suum neuromuscular strips, but the time course of 5-HT inhibition of ACh-induced contractions (10–20 min) is much slower than the 5-HT dependent paralysis of locomotion (<1 min), suggesting that the major effects of 5-HT are upstream of the neuromuscular junction, most probably in interneurons in the nerve ring (52). Indeed, 5-HT decreases both the frequency of EPSPs in the DE2 motor neurons and the amplitude of slow oscillating potentials in VI motor neurons in A. suum (52). These observations are all in agreement with the neuronal localization of SER-4 and MOD-1 in C. elegans (49, 67). The monoamines, DA and OA, also altered locomotory waves when injected into A. suum, but TA did not; however, the lack of an effect for TA may reflect the localization of the TA-gated channel in head, but not body wall muscle (41, 50). The monoamines 5-HT and DA also uniquely inhibit locomotion in H. contortus with DA having a pronounced paralytic effect on the midbody of the worm (35). Together, these studies demonstrate that monoaminergic signaling has the potential to not only paralyze a wide variety of both free-living and parasitic nematodes, but also dramatically alter sensory-mediated locomotory behaviors that may be essential for host-finding, larval migration or site selection.
Figure 1. Exogenous serotonin (5-HT) causes an unusual “kinked” paralysis in J2s of the soybean cyst nematode, Heterodera glycines.
J2s were incubated for 20 min in liquid culture with 20 mM 5-HT. These high concentrations of exogenous 5-HT are necessary to overcome the relative impermeability of the nematode cuticle. However, as noted in the text, this paralysis appears to result exclusively from the actions of 5-HT on serotonergic signaling, as locomotion in C. elegans lacking key 5-HT receptors is completely unaffected by 5-HT at these concentrations. We would like to thank Julia Thissen for these unpublished pictures of H. glycines J2s.
Although nematode monoamine-dependent GPCRs are similar to their mammalian counterparts, they have distinctly different pharmacologies. Certainly, ligands that specifically target nematode monoamine receptors and affect their downstream signaling can be developed, particularly since highly specific ligands have already been developed for each of the mammalian receptor subtypes. In addition, since mammals do not appear to express monoamine-gated Cl− channels, these targets have the added advantage of being invertebrate-specific. Indeed, monoaminergic receptors have already proven to be effective in the development of both anthelmintics and insecticides. For example, serotonergic agonists are effective in the treatment of H. contortus and Trichostrongylus colubriformis infections (55). PAPP (p-amino-phenethyl-m-trifluoromethylphenyl piperazine), an agonist of the H. contortus SER-4 orthologue, is highly active against the third-stage larvae (L3s) of both nematodes in a larval migration assay, with EC50 values comparable to those of levamisole (55). In addition, when applied orally, PAPP cleared over 99% of the H. contortus in infected jirds (Meriones unguiclatus), a clearance rate again comparable with that of levamisole (55). Similarly, the anthelmintic activity of a group of monoterpenoids, such as thymol and carvacrol, appears to target SER-2, a Gαi/o-coupled TA receptor (56). In addition to inhibiting locomotion, the excitatory side of this monoaminergic regulatory network also has been exploited pharmacologically through the development of the formamidine-based pesticides that activate Gαs-coupled OA receptors and stimulate hyperactivity/leaf walk-off (51). Similarly, cocaine, a naturally occurring insecticide, functions by blocking OA-reuptake to potentiate octopaminergic transmission and has similar insecticidal effects to the formamidines (57). Finally, in addition to the monoamine receptors and their downstream signaling, the agonism of additional sites in monoamine-mediated signaling cascades, including a variety of well-described or, as yet unidentified, ion channels and regulatory proteins involved in modulating neuronal excitability, synaptic plasticity and/or neurotransmitter release, could also be potentially useful targets for anthelmintic development.
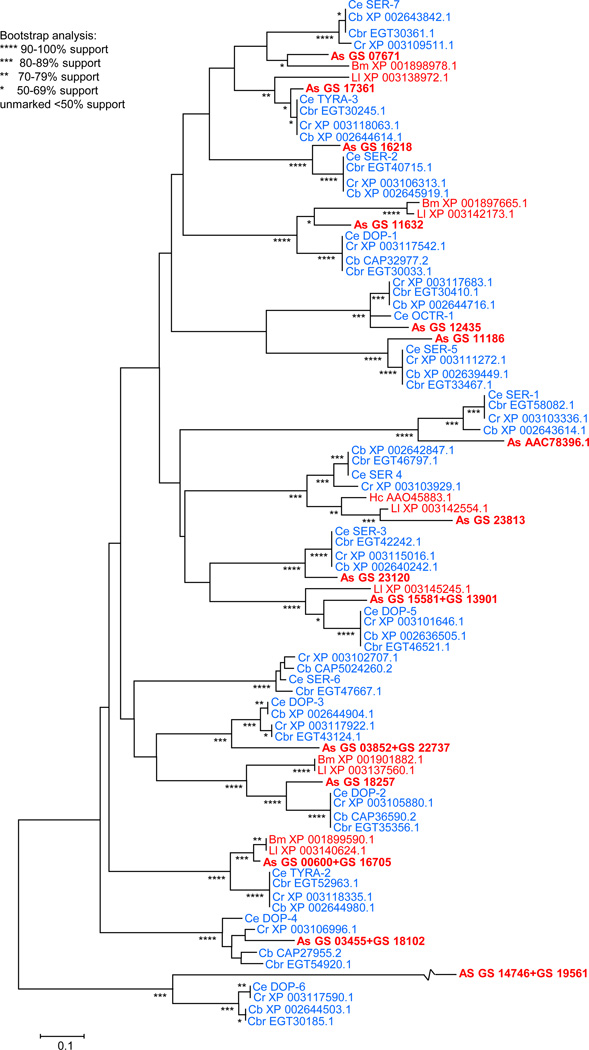
The ligand-specificity of most of the 16 predicted G-protein coupled monoamine receptors in the C. elegans genome has been, at least, partially characterized by heterologous expression and their physiological roles partially described in null animals (see 24 and 36 for reviews). A substantial amount of genomic and EST sequence data from parasitic nematodes data is now available for comparison with those for C. elegans and other free-living counterparts. Surprisingly, 15 of the 16 C. elegans monoamine receptors have clear orthologues in the recently completed A. suum genome, even though these animals diverged over hundreds of millions of years ago (20; Figure 2). In addition, most duplications of these genes in C. elegans (represented by multiple isoforms in the current annotation for this species) appear conserved in A. suum. Conspicuous examples include tyra-2 and tyra-3, both of which are represented by three isoforms in C. elegans, with distinct homologues detected for each isoform in A. suum (20). Putative orthologues for SER-1, TYRA-2 and SER-4 have been cloned from A. suum, Brugia malayi and H. contortus, respectively, and heterologously expressed; however, to date, little has been learned from comparing the pharmacologies of the receptors from free-living and parasitic nematodes, since they were expressed and assayed under substantially different conditions (58–61). In some cases the pharmacologies are quite similar (TYRA-2) and in others quite distinct (SER-4) (44, 48, 60, 61). Similarly, 5-HT, DA and TA-gated Cl− channels also appear to be conserved in parasitic nematodes. For example, a predicted MOD-1 orthologue has been identified in A. suum genome (ADY43724.1) and H. contortus cDNAs encoding DA and TA-gated Cl− channels similar to the C. elegans LGC-53 and LGC-55, respectively, have been characterized after heterologous expression (53, 54). These observations support the notion that monoamine-dependent signaling pathways might be conserved among nematodes.
Figure 2. The A. suum genome contains putative orthologues of most of the Caenorhabditid monoamine receptors, suggesting that the physiological roles of these receptors may be conserved and potentially characterized in the more genetically-tractable C. elegans model system.
Predicted protein sequences were aligned using ClustalW and an unrooted tree was constructed using a Neighbourhood-Joining method in MEGA5. Statistical support for tree branching was determined using bootstrap analysis with 5000 replicates. The level of bootstrap support for each branch is indicated with asterisks. A. suum sequences were based on the A. suum draft genome (20). Sequences for C. elegans, C. brenneri, C. briggsae, C. remanei, B. malayi, L. loa and H. contortus were obtained from GenBank. Accession numbers for C. elegans are: Ce DOP-1: NP_001024577.1, Ce DOP-2: NP_001024048.1, Ce DOP-3: NP_001024908.2, Ce DOP-4: NP_508238.2, Ce DOP-5: NP_505884.1, Ce DOP-6: NP_508739.3, CE OCTR-1: NP_001024569.1, Ce SER-1: NP_001024728.1, Ce SER-2: NP_001024339.1, Ce SER-3: NP_491954.1, Ce SER-4: NP_497452.1, Ce SER-5: NP_492273.2, Ce SER-6: NP_741350.1, Ce SER-7: NP_741730.1, Ce TYRA-2: NP_001033537.1, Ce TYRA-3: NP_001024805.1. Species abbreviations and accession numbers are color-coded: red for parasitic species and blue for free-living species. Abbreviations: Caenorhabditis elegans (Ce), Caenorhabditis brenneri (Cbr), Caenorhabditis briggsae (Cb), Caenorhabditis remanei (Cr), Ascaris suum (As), Brugia malayi (Bm), Loa loa (Ll), and Haemonchus contortus (Hc).
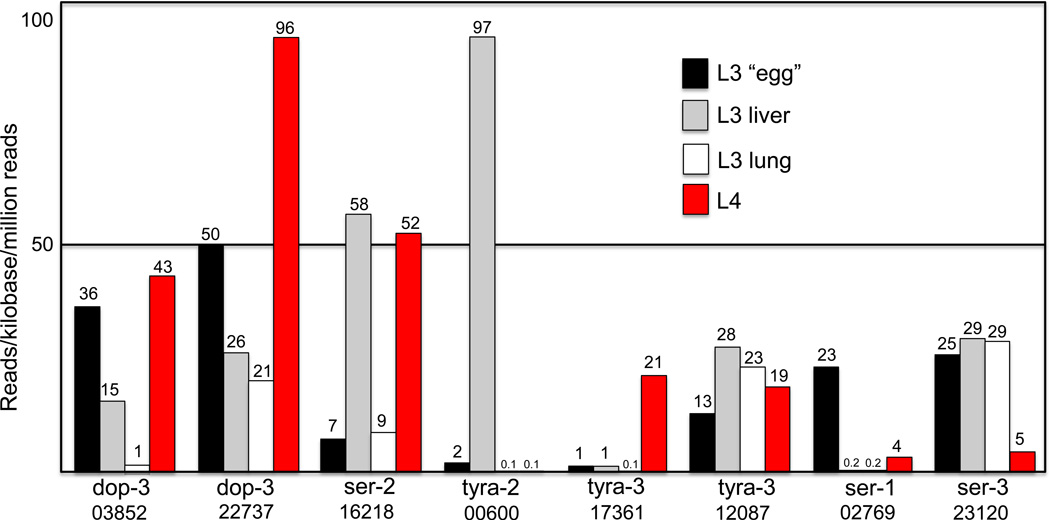
The conservation of C. elegans monoamine receptors in A. suum is also striking because one of the main functions of monoaminergic signaling appears to be the translation of nutritional status into the sensory-mediated modulation of most behaviors, including locomotion. A. suum has no motile, free-living stages, but the L3 that hatches in the gut must penetrate the intestinal epithelium, enter the bloodstream and migrate through liver and lungs to ultimately return to the gut and molt to the L4 and adult. Once in the small intestine, the adult most probably also responds to number of sensory cues to follow the transiting meal. It will be important to learn where these receptors are expressed and how their expression patterns compare with those of free-living nematodes. Indeed, transcriptional profiles from these migrating larval stages suggest that many of the monoamine receptors are differentially expressed (Figure 3; 20). For example, dop-3 and ser-3 orthologues appear to be highly expressed in most larval stages, including the egg, lung and liver L3 and L4s, whereas a ser-1 orthologue appears to be differentially expressed in the “egg.” In addition, some of these receptors may also play important roles in larval migration. For example, the expression of tyra-2 (GS_00600) increases almost 50-fold from the L3 in the embryonated “egg” to the liver L3 and then decreases over 1000-fold as the L3 migrates from the liver to the lung (Figure 3). Interestingly, like C. elegans, A. suum appears to encode multiple isoforms of some monoamine receptors (e.g., tyra-2 and tyra-3). Notably, some of these isoforms also differ in their transcriptional profile during larval migration, suggesting they may have differing, but as yet uncharacterized, roles (20). It is plausible that an additional layer of richness and diversity of monoamine receptors may exist in these species, and differ among them, as a result the alternative-splicing of transcripts as well. However, characterization of splicing events in C. elegans is incomplete and has not yet been developed to any depth in A. suum. Exploring these differences, particularly considering the complex migratory behavior of larval Ascaris, should be considered a high priority. Interestingly, the filarial nematode, B. malayi, with apparently a much smaller genome (11,500 protein coding genes in 71 Mb of the 90 Mb genome compared with 18,500 in A. suum and 20,470 in C. elegans) appears to also have a reduced monoamine receptor profile. The “absence” of these additional receptors may reflect the more sedentary life style of the adult filarid or simply result from the incomplete sequencing of the Brugia genome (62).
Figure 3. Key A. suum monoamine receptor isoforms appear to be differentially transcribed during the major phases of larval hepato-pulmonary migration.
Relative expression is presented as reads per kilobase per million reads (statistically significant at P ≤ 0.001; see reference 20, for details).
In conclusion, monoamines appear to paralyze nematodes by interfering with the complex regulatory networks modulating locomotion, and may also play key roles in other parasite related decision-making, such as larval migration in the definitive host. Many of these monoamine receptors appear to be conserved in nematodes (e.g. between C. elegans in clade V and A. suum in clade III). Taken together, the present results and observations suggest that monoamine receptors and their associated signaling pathways might serve as useful targets for anthelmintic drug discovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bundy DA, de Silva NR. Can we deworm this wormy world? Br Med Bull. 1998;54:421–432. doi: 10.1093/oxfordjournals.bmb.a011698. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prichard R. Genetic variability following selection of Haemonchus contortus with anthelmintics. Trends Parasitol. 2001;17:445–453. doi: 10.1016/s1471-4922(01)01983-3. [DOI] [PubMed] [Google Scholar]
- 4.Sangster NC, Gill J. Pharmacology of anthelmintic resistance. Parasitol Today. 1999;15:141–146. doi: 10.1016/s0169-4758(99)01413-1. [DOI] [PubMed] [Google Scholar]
- 5.Waller PJ, Echevarria F, Eddi C, Maciel S, Nari A, Hansen JW. The prevalence of anthelmintic resistance in nematode parasites of sheep in southern Latin America: general overview. Vet Parasitol. 1996;62:181–187. doi: 10.1016/0304-4017(95)00909-4. [DOI] [PubMed] [Google Scholar]
- 6.Harder A, Schmitt-Wrede HP, Krücken J, Marinovski P, Wunderlich F, Willson J, et al. Cyclooctadepsipeptides: an anthelmintically active class of compounds exhibiting a novel mode of action. Int J Antimicrob Agents. 2003;22:318–331. doi: 10.1016/s0924-8579(03)00219-x. [DOI] [PubMed] [Google Scholar]
- 7.Martin RJ, Buxton SK, Neveu C, Charvet CL, Robertson AP. Emodepside and SL0-1 potassium channels: a review. Exp Parasitol. 2011 doi: 10.1016/j.exppara.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welz C, Krüger N, Schniederjans M, Miltsch SM, Krücken J, Guest M, et al. SLO-1- channels of parasitic nematodes reconstitute locomotor behaviour and emodepside sensitivity in Caenorhabditis elegans slo-1 loss of function mutants. PLoS Pathog. 2011;7(4):e1001330. doi: 10.1371/journal.ppat.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–731. doi: 10.1038/nmeth1075. [DOI] [PubMed] [Google Scholar]
- 10.Guo ZV, Hart AC, Ramanathan S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat Methods. 2009;6:891–896. doi: 10.1038/nmeth.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr RA, Schafer WR. Intracellular Ca2+ imaging in C. elegans. Methods Mol Biol. 2006;351:253–264. doi: 10.1385/1-59745-151-7:253. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay TH, Thiele TR, Lockery SR. Optogenetic analysis of synaptic transmission in the central nervous system of the nematode Caenorhabditis elegans. Nat Commun. 2011;2:306. doi: 10.1038/ncomms1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Kim SK. The early bird catches the worm: new technologies for the Caenorhabditis elegans toolkit. Nat Rev Genet. 2011 doi: 10.1038/nrg3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glendinning SK, Buckingham SD, Sattelle DB, Wonnacott S, Wolstenholme AJ. Glutamate-gated chloride channels of Haemonchus contortus restore drug sensitivity to ivermectin-resistant Caenorhabditis elegans. PLoS One. 2011;6:e22390. doi: 10.1371/journal.pone.0022390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown LA, Jones AK, Buckingham SD, Mee CJ, Sattelle DB. Contributions from Caenorhabditis elegans functional genetics to antiparasitic drug target identification and validation: nicotinic acetylcholine receptors, a case study. Int J Parasitol. 2006;36:617–624. doi: 10.1016/j.ijpara.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Geary TG, Thompson DP. Caenorhabditis elegans: how good a model for veterinary parasites? Vet Parasitol. 2001;101:371–386. doi: 10.1016/s0304-4017(01)00562-3. [DOI] [PubMed] [Google Scholar]
- 18.Gilleard JS, Woods DJ, Dow JA. Model-organism genomics in veterinary parasite drug-discovery. Trends Parasitol. 2005;21:302–305. doi: 10.1016/j.pt.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Ghedin E, Wang S, Spiro D, Caler E, Zhao Q, Crabtree J, et al. The genome of the filarial nematode parasite, Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jex AR, Liu S, Li B, Young ND, Hall RS, Li Y, et al. Ascaris suum draft genome. Nature. 2011;479:529–533. doi: 10.1038/nature10553. [DOI] [PubMed] [Google Scholar]
- 21.Sithigorngul P, Jarecki JL, Stretton AO. A specific antibody to neuropeptide AF1 (KNEFIRFamide) recognizes a small subset of neurons in Ascaris suum: differences from Caenorhabditis elegans. J Comp Neurol. 2011;519:1546–1561. doi: 10.1002/cne.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezak MJ, Ferkey DM. The C. elegans D2-like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS One. 2010;5(2):e9487. doi: 10.1371/journal.pone.0009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezcurra M, Tanizawa Y, Swoboda P, Schafer WR. Food sensitizes C. elegans avoidance behaviors through acute dopamine signaling. EMBO J. 2011;30:1110–1122. doi: 10.1038/emboj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 25.Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. WormBook. 2007 doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris G, Hapiak V, Wragg R, Miller S, Smith K, Hughes L, et al. Three distinct amine receptors operating a different levels within the locomotory circuit are each essential for the serotonergic modulation of chemosensation in Caenorhabditis elegans. J Neurosci. 2009;29:1446–1456. doi: 10.1523/JNEUROSCI.4585-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signaling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- 28.Jafari G, Xie Y, Kullyev A, Liang B, Sze JY. Regulation of extrasynaptic 5-HT by serotonin reuptake transporter function in 5-HT-absorbing neurons underscores adaptation behavior in Caenorhabditis elegans. J Neurosci. 2011;31:8948–8957. doi: 10.1523/JNEUROSCI.1692-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullyev A, Dempsey CM, Miller S, Kuan CJ, Hapiak VM, Komuniecki RW, et al. A genetic survey of fluoxetine action on synaptic transmission in Caenorhabditis elegans. Genetics. 2010;8186:929–941. doi: 10.1534/genetics.110.118877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pocock R, Hobert O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat Neurosci. 2010;13:610–614. doi: 10.1038/nn.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulston J, Dew M, Brenner S. Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 1975;163(2):215–226. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- 32.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Johnson CD, Reinitz CA, Sithigorngul P, Stretton AO. Neuronal localization of serotonin in the nematode Ascaris suum. J Comp Neurol. 1996;367:352–360. doi: 10.1002/(SICI)1096-9861(19960408)367:3<352::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Brownlee DJ, Fairweather I, Johnston CF, Shaw C. Immunocytochemical demonstration of peptidergic and serotoninergic components in the enteric nervous system of the roundworm, Ascaris suum (Nematoda, Ascaroidea) Parasitology. 1994;108:89–103. doi: 10.1017/s0031182000078562. [DOI] [PubMed] [Google Scholar]
- 35.Rao VT, Forrester SG, Keller K, Prichard RK. Localization of serotonin and dopamine in Haemonchus contortus. Int J Parasitol. 2011;41:249–254. doi: 10.1016/j.ijpara.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Komuniecki R, Harris G, Hapiak V, Wragg R, Bamber B. Monoamines activate neuropeptide signaling cascades to modulate nociception in C. elegans: a useful model for the modulation of chronic pain? Invert Neurosci. 2011 doi: 10.1007/s10158-011-0127-0. [DOI] [PubMed] [Google Scholar]
- 37.Hapiak V, Hobson R, Hughes L, Smith K, Harris G, Condon C, et al. Dual excitatory and inhibitory serotonergic inputs modulate egg-laying in Caenorhabditis elegans. Genetics. 2009;181:153–163. doi: 10.1534/genetics.108.096891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- 39.Allen AT, Maher KN, Wani KA, Betts KE, Chase DL. Coexpressed D1- and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans. Genetics. 2011;188:579–590. doi: 10.1534/genetics.111.128512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobson RJ, Hapiak VM, Xiao H, Buehrer KL, Komuniecki R. SER-7: a Caenorhabditis elegans 5-HT7-like receptor is essential for the 5-HT stimulation of pharyngeal pumping and egg-laying. Genetics. 2006;172:159–169. doi: 10.1534/genetics.105.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron. 2009;62:526–538. doi: 10.1016/j.neuron.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. J Neurochem. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- 43.Rex E, Molitor SC, Hapiak V, Xiao H, Henderson M, Komuniecki R. Tyramine receptor (SER-2) isoforms are involved in the regulation of pharyngeal pumping and foraging behavior in Caenorhabditis elegans. J Neurochem. 2004;91:1104–1115. doi: 10.1111/j.1471-4159.2004.02787.x. [DOI] [PubMed] [Google Scholar]
- 44.Rex E, Hapiak V, Hobson R, Smith K, Xiao H, Komuniecki R. TYRA-2 (F01E11.5): a Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J Neurochem. 2005;94:181–191. doi: 10.1111/j.1471-4159.2005.03180.x. [DOI] [PubMed] [Google Scholar]
- 45.Mills H, Wragg R, Hapiak V, Castelletto M, Zahratka J, Harris G, et al. Monoamines and neuropeptides interact to inhibit aversive behavior in Caenorhabditis elegans. EMBO J. 2011 doi: 10.1038/emboj.2011.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinitz CA, Herfel HG, Messinger LA, Stretton AO. Changes in locomotory behavior and cAMP produced in Ascaris suum by neuropeptides from Ascaris suum or Caenorhabditis elegans. Mol Biochem Parasitol. 2000;111:185–197. doi: 10.1016/s0166-6851(00)00317-0. [DOI] [PubMed] [Google Scholar]
- 47.Reinitz CA, Pleva AE, Stretton AO. Changes in cyclic nucleotides, locomotory behavior, and body length produced by novel endogenous neuropeptides in the parasitic nematode Ascaris suum. Mol Biochem Parasitol. 2011;80:27–34. doi: 10.1016/j.molbiopara.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olde B, McCombie WR. Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J Mol Neurosci. 1997;8:53–62. doi: 10.1007/BF02736863. [DOI] [PubMed] [Google Scholar]
- 49.Ranganathan R, Cannon SC, Horvitz HR. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 2000;408:470–475. doi: 10.1038/35044083. [DOI] [PubMed] [Google Scholar]
- 50.Masler EP. Responses of Heterodera glycines and Meloidogyne incognita to exogenously applied neuromodulators. J Helminthol. 2007;81:421–427. doi: 10.1017/S0022149X07850243. [DOI] [PubMed] [Google Scholar]
- 51.Dudai Y, Buxbaum J, Corfas G, Ofarim M. Formamidines interact with Drosophila octopamine receptors, alter the flies' behavior and reduce their learning ability. J Comp Physiol A. 1987;161:739–746. [Google Scholar]
- 52.Reinitz CA, Stretton AO. Behavioral and cellular effects of serotonin on locomotion and male mating posture in Ascaris suum(Nematoda) J Comp Physiol A. 1996;178(5):655–667. doi: 10.1007/BF00227378. [DOI] [PubMed] [Google Scholar]
- 53.Rao VT, Accardi MV, Siddiqui SZ, Beech RN, Prichard RK, Forrester SG. Characterization of a novel tyramine-gated chloride channel from Haemonchus contortus. Mol Biochem Parasitol. 2010;173:64–68. doi: 10.1016/j.molbiopara.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Rao VT, Siddiqui SZ, Prichard RK, Forrester SG. A dopamine-gated ion channel (HcGGR3*) from Haemonchus contortus is expressed in the cervical papillae and is associated with macrocyclic lactone resistance. Mol Biochem Parasitol. 2009;166:54–61. doi: 10.1016/j.molbiopara.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 55.White WH, Gutierrez JA, Naylor SA, Cook CA, Gonzalez IC, Wisehart MA, et al. In vitro and in vivo characterization of p-amino-phenethyl-m-trifluoromethylphenyl piperazine (PAPP), a novel serotonergic agonist with anthelmintic activity against Haemonchus contortus, Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet Parasitol. 2007;146:58–65. doi: 10.1016/j.vetpar.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Lei J, Leser M, Enan E. Nematicidal activity of two monoterpenoids and SER-2 tyramine receptor of Caenorhabditis elegans. Biochem Pharmacol. 2010;79:1062–1071. doi: 10.1016/j.bcp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Nathanson JA, Hunnicutt EJ, Kantham L, Scavone C. Cocaine as a naturally occurring insecticide. Proc Natl Acad Sci. 1993;90:9645–9648. doi: 10.1073/pnas.90.20.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang X, Duran E, Diaz F, Hong X, Komuniecki R. Alternative-splicing of putative serotonin receptors in the pharynx of the parasitic nematode, Ascaris suum. Mol Biochem Parsitol. 1999;101:95–106. doi: 10.1016/s0166-6851(99)00059-6. [DOI] [PubMed] [Google Scholar]
- 59.Huang X, Xiao H, Rex E, Hobson R, Messer W, Komuniecki P, et al. Functional characterization of alternatively-spliced 5-HT2 receptor isoforms from the pharynx and muscle of the parasitic nematode, Ascaris suum. J Neurochem. 2002;83:249–258. doi: 10.1046/j.1471-4159.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 60.Smith K, Rex E, Komuniecki R. Are C. elegans receptors useful targets for drug discovery: pharmacological comparison of tyramine receptors with high identity from Caenorhabditis elegans (TYRA-2) and Brugia malayi (Bm4) Mol Biochem Parasitol. 2007;154:52–61. doi: 10.1016/j.molbiopara.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith MW, Borts TL, Emkey R, Cook CA, Wiggins CJ, Gutierrez JA. Characterization of a novel G-protein coupled receptor from the parasitic nematode H. contortus with high affinity for serotonin. J Neurochem. 2003;86:255–266. doi: 10.1046/j.1471-4159.2003.01849.x. [DOI] [PubMed] [Google Scholar]
- 62.Smith K, Komuniecki R, Ghedin E, Spiro D, Gray J. Genes encoding putative biogenic amine receptors in the parasitic nematode, Brugia malayi. Invert Neurosci. 2007;7:227–244. doi: 10.1007/s10158-007-0058-y. [DOI] [PubMed] [Google Scholar]
- 63.Blaxter ML. Nematoda: genes, genomes and the evolution of parasitism. Adv Parasitol. 2003;54:101–195. doi: 10.1016/s0065-308x(03)54003-9. [DOI] [PubMed] [Google Scholar]
- 64.Dent JA, Smith MM, Vassilatis DK, Avery L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc Natl Acad Sci. 2000;97(6):2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- 66.Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau JL. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dernovici S, Starc T, Dent JA, Ribeiro P. The serotonin receptor SER-1 (5HT2ce) contributes to the regulation of locomotion in Caenorhabditis elegans. Dev Neurobiol. 2007;67:189–204. doi: 10.1002/dneu.20340. [DOI] [PubMed] [Google Scholar]
- 69.Ruis-Lancheros E, Viau C, Walter TN, Geary T. Activity of novel anthelmintics in cut preparations of Caenorhabditis elegans. Int J Parasitol. 2011;41(3–4):455–461. doi: 10.1016/j.ijpara.2010.11.009. [DOI] [PubMed] [Google Scholar]