Abstract
Interferon (IFN)-α has been used to investigate pathways by which innate immune cytokines influence the brain and behavior. Accordingly, the impact of IFN-α on diurnal secretion of hypothalamic–pituitary–adrenal (HPA) axis hormones was assessed in 33 patients eligible for treatment with IFN-α plus ribavirin for hepatitis C. In addition, the relationship between IFN-α-induced HPA axis changes and proinflammatory cytokines and behavior was examined. Plasma ACTH and cortisol as well as tumor necrosis factor (TNF)-α, interleukin-6 and their soluble receptors, were measured hourly between 0900 and 2100 hours at baseline and following approximately 12 weeks of either no treatment (n = 13) or treatment with IFN-α/ribavirin (n = 20). Plasma IFN-α was also measured at each visit. Depression and fatigue were assessed using the Montgomery–Asberg depression rating scale and the multidimensional fatigue inventory. Compared to no treatment, IFN-α/ribavirin administration was associated with significant flattening of the diurnal ACTH and cortisol slope and increased evening plasma ACTH and cortisol concentrations. Flattening of the cortisol slope and increases in evening cortisol were correlated with increases in depression (r = 0.38, P < 0.05 and r = 0.36, P < 0.05, respectively) and fatigue (r = 0.43, P < 0.05 and r = 0.49, P < 0.01, respectively). No relationship was found between immune and HPA axis measures, although increases in plasma IFN-α, TNF-α and soluble TNF-α receptor2 were independently correlated with behavioral endpoints. These data indicate that chronic exposure to innate immune cytokines may contribute to the altered diurnal HPA axis activity and behavior found in medically ill individuals. However, given the lack of correlation between HPA axis and immune measures, the mechanism by which chronic cytokine exposure influences HPA axis function remains to be determined.
Keywords: cytokines, cortisol, adrenocorticotropic hormone, HPA axis, depression, fatigue
Introduction
Medically-ill patients are at a markedly increased risk for the development of an array of depressive symptoms, ranging from sadness and anhedonia to insomnia, anorexia and fatigue.1 Although depression in medically-ill patients often has been ascribed to the multiple psychological stressors that accompany serious illness, data increasingly suggest that these symptoms may also result from physiological processes inherent to sickness itself. One such process is activation of the body’s innate immune system with the subsequent production and release of innate immune cytokines, including interferon (IFN)-α, interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α.2 These cytokines can access the brain and exert profound effects on behavior.3–7 Indeed, administration or induction of innate immune cytokines in laboratory animals reliably induces a constellation of symptoms that resembles major depression and is reminiscent of behavioral changes seen in medically-ill patients.5 Conversely, cytokine antagonists have been found to block these behavioral changes in rodents and have been noted to reduce depression and fatigue in patients with autoimmune or inflammatory disorders.8,9 Consistent with these observations, innate immune cytokines can produce physiological changes frequently seen in the context of depression, including alterations in the activity of the hypothalamic–pituitary–adrenal axis (HPA)10–13 and reduced monoamine neurotransmitter availability.14,15
To identify specific alterations in neuroendocrine and immune pathways that may be involved in the link between the innate immune response and behavioral alterations, our group and others have studied patients receiving IFN-α. IFN-α is a cytokine of the early innate immune response to viral infection and has been shown to acutely induce the production and release of other innate immune cytokines, including IL-6, and to a lesser extent IL-β and TNF-α and their soluble receptors.11,16,17 Based on its anti-neoplastic and antiviral properties, IFN-α has emerged as a primary treatment for several malignancies and—in combination with ribavirin—for the treatment of chronic hepatitis C virus (HCV) infection. Although of therapeutic benefit for these conditions, chronic treatment with IFN-α is associated with a high rate of behavioral disturbance. Indeed, depending on dose and duration of treatment, 20–60% of subjects receiving INF-α will develop clinically relevant depression and up to 80% of subjects will develop significant fatigue.18–21
Like many other cytokines of the innate immune response, IFN-α also causes marked activation of the HPA axis when administered acutely.10,11 The magnitude of this activation has been associated with the risk of developing depression. For example, patients with malignant melanoma who developed major depression during IFN-α therapy exhibited a significantly higher production of ACTH and cortisol following the initial infusion of IFN-α compared to IFN-α-treated patients who did not become depressed. 11 Interestingly, however, based on a limited number of measurements taken in the morning after 4, 8 and 12 weeks of IFN-α therapy, plasma concentrations of ACTH and cortisol appeared to normalize following chronic IFN-α exposure and did not significantly differ between depressed and non-depressed patients.11 Furthermore, in a recent study by Wichers et al.17 no relationship was found between daily average salivary cortisol concentrations or the cortisol awakening response and depressive symptoms in a sample of patients treated with IFN-α for HCV infection. Nevertheless, the effects of chronic IFN-α administration on the diurnal rhythm of the HPA axis or relationships between potential changes in this rhythm and the development of behavioral symptoms have yet to be determined. In addition, given previously reported correlations between IFN-α-induced depressive symptoms and proinflammatory cytokines and their receptors (measured at baseline and/or during IFN-α therapy),17,22 the relationship between IFN-α-induced immune responses and potential changes in HPA axis function and behavior warrants further examination.
The potential for cytokines to derange normal daily patterns of ACTH and/or cortisol production is of special interest, because flattening of the diurnal cortisol rhythm has been associated with a variety of medical illnesses and has been found to predict reduced survival in patients with cancer.23–27 In addition, in two studies using a cross-sectional design, peripheral blood concentrations of IL-6 have been correlated with flattening of the diurnal cortisol rhythm in patients with metastatic colorectal cancer and increases in evening cortisol concentrations in patients with coronary artery disease.28,29 Given that diurnal cortisol rhythms are also associated with major depression30 and with behavioral symptoms in medically-ill subjects,31 especially fatigue;32 it is plausible that innate immune/inflammatory processes may contribute to behavioral disturbance in the context of sickness—at least in part—by disruption of the diurnal rhythm of HPA axis hormones. The present study was designed to test this hypothesis by prospectively examining diurnal rhythms of plasma ACTH and cortisol as well as IL-6 and TNF-α and their soluble receptors in patients before and after approximately 12 weeks of treatment with pegylated IFN-α plus ribavirin for infection with HCV.
Subjects and methods
Subjects
Thirty-three HCV-positive subjects (18 male subjects and 15 female subjects) were enrolled in the study. Subjects were required to be serum positive for anti-HCV antibodies or HCV-RNA by reverse transcription-polymerase chain reaction. Exclusion criteria included decompensated liver disease; liver disease from any cause other than HCV; unstable cardiovascular, endocrinologic, hematologic, renal or neurologic disease; a score < 24 on the Mini Mental State Exam (indicating more than mild cognitive impairment); 33 a history of schizophrenia or bipolar disorder and/or a diagnosis of major depression or substance abuse/dependence within 6 months of study entry (determined by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (SCID)).34 Patients were required to be off all psychotropic medications (antidepressants, antipsychotics, mood stabilizers, narcotics and benzodiazepines) for at least 2 weeks prior to study entry and at least 2 weeks prior to any assessments (8 weeks for fluoxetine). Use of antidepressant medications was not allowed between study entry and the week 12 assessment. Subjects included in this sample overlap with those included in a study on the effects of IFN-α on neurocognitive function.35
Study design
A prospective, longitudinal design was used to examine diurnal activity of the HPA axis and proinflammatory cytokines in patients with HCV prior to (visit 1), and following, 12 weeks (visit 2) of either no treatment (control group) or treatment with IFN-α plus ribavirin (treatment group). All subjects who underwent treatment with IFN-α received either pegylated IFN-α-2b (Pegintron, Schering Plough, Kenilworth, NJ, USA) or pegylated IFN-α-2a (PEGASYS, Roche, Nutley, NJ, USA). Participation in the control versus treatment group as well as type of IFN-α administered was determined by patients and their physicians and was not controlled by study protocol. At visits 1 and 2, all subjects were admitted to the Emory University General Clinical Research Center (GCRC) for evaluation. To allow for accommodation to the GCRC environment and to control for the effects of sleep and waking times on diurnal cortisol/ACTH rhythms, subjects were admitted to the GCRC at least 12 h prior to commencing HPA axis evaluation. Lights out occurred at 2200 hours, and all subjects were awakened at 0715 hours. and served breakfast prior to placement of an indwelling venous catheter at 0800 hours. During each GCRC admission, blood was withdrawn into EDTA-coated tubes hourly from 0900 to 2100 hours (for a total of 13 samples). During the sampling period, subjects were asked to rest quietly in a bed or chair. Lunch was served at 12:30 hours, and dinner was served at 1730 hours. Following each sampling, blood was immediately centrifuged at 1000 × g for 10 min at 4 °C. Plasma was then removed and frozen at −80 °C until assay. Because behavioral effects of pegylated IFN-α tend to be most pronounced immediately following the weekly injection and diminish thereafter, the visit 2 GCRC assessment was scheduled for all treatment subjects between 4 and 5 days following their last injection. Plasma concentrations of IFN-α were assessed at both visits 1 and 2 at 1600 hours to ensure treatment adherence and examine the relationship between IFN-α plasma concentrations and relevant biological and behavioral variables. Where appropriate, subjects who developed depressive symptom severity warranting psychiatric intervention prior to 12 weeks immediately underwent their second GCRC assessment (visit 2) and were then referred for psychiatric evaluation. Urine drug screens were conducted at each visit to rule out substance abuse.
All subjects provided written informed consent, and study procedures received a priori approval by the Emory University Institutional Review Board.
Behavioral assessments
Depression was evaluated by trained clinician-raters using the mood disorders module of the SCID34 and the Montgomery–Asberg depression rating scale (MADRS). The MADRS is a 10-item, clinician-administered scale that assesses the severity of depressive symptoms, including sadness, inner tension, concentration difficulties, inability to feel, pessimistic thoughts, suicidal thoughts, reduced sleep, reduced appetite and lassitude.36 To evaluate the presence and severity of fatigue, subjects also completed the self-report, 20-item multidimensional fatigue inventory (MFI-20) at each assessment.37 Consistent with recent data regarding the structure of fatigue in medically-ill patients,38 the MFI assesses five dimensions of fatigue, including general fatigue, physical fatigue, mental fatigue, reduced activity and reduced motivation. In addition to scores for each subscale, a total score can be derived by summing the 5-subscale scores.39 Due to the profound nature of IFN-α/ribavirin effects on behavior, it was not considered feasible to uniformly blind clinician-raters to treatment assignment. Therefore, clinician-raters were not blinded to group assignment.
Assessment of HPA axis and immune variables
Commercially available immunoradiometric assay and radioimmunoassay kits were used for the assessment of plasma ACTH (ALPCO Diagnostics, Salem, NH, USA and Nichols Institute Diagnostics, San Juan Capistrano, CA, USA when available) and cortisol (DiaSorin Stillwater, MN, USA) respectively. Intra- and inter-αssay coefficients of variation respectively were 2.8 and 5.7% (ALPCO) or 4.5 and 6.3% (Nichols) for ACTH and 8.5 and 12.7% for cortisol. Concentrations of proinflammatory cytokines (TNF-α and IL-6) were measured by high sensitivity quantitative ELISA (enzyme-linked immunosorbent assays; R&D Systems, Minneapolis, MN, USA), and their soluble receptors (sTNF-R2 and IL6-sR) were determined by R&D Quantikine ELISA kits. Plasma concentrations of IFN-α were also measured by high sensitivity quantitative ELISA (Amersham Biosciences Corporation, Piscataway, NJ, USA). Assays were performed according to the manufacturer’s specifications, and were run in duplicate. Inter- and intra-αssay variability were reliably <12% for TNF-α and IL-6 and <10% for sTNF-R2, IL-6sR and IFN-α. All biological samples were analyzed by research staff blinded to the clinical status of study participants.
Statistical analysis
To evaluate the amplitude of the variation in ACTH and cortisol across the diurnal cycle, the slope for each hormone was calculated. As described previously, 26,27,31,32 slope is a measure of how well each subject fits a typical descending profile for HPA axis hormones across the diurnal period.40 Although alternate analytic strategies exist, slope has been one of the most widely used measures for the diurnal rhythm of cortisol. Consistent with prior reports, 26,27,31,32 we calculated the slope by log-transforming ACTH and cortisol values and using the β-value of the regression of all 13 values on the hour of sample collection. Larger β-values (that is, values closer to zero) reflect a flatter slope.26 Slope flattening, in turn, is thought to reflect various combinations of slower diurnal declines, abnormally timed/diminished peaks or increasing levels of hormones during the day.26 Given the diurnal variation in IL-6 secretion, 41 which unlike ACTH and cortisol exhibits an ascending profile during the day, we used a similar analytic strategy to calculate the IL-6 slope. To further explore diurnal hormonal variations that might contribute to changes in ACTH and cortisol slope, morning maximums and evening minimums of ACTH and cortisol were determined. The morning peak value of cortisol and ACTH was calculated as the highest value across the first three blood draws (that is, 0900 to 1100 hours), and the evening minimum was calculated as the lowest value across the last three blood draws (that is, 1900 to 2100 hours). Finally, overall HPA axis and immune activity was assessed by calculating the mean (±s.d.) value of ACTH, cortisol, IL-6, TNF-α, sIL-6R and sTNFR2 across all 13 blood draws. Because TNF-α, sTNFR2 and sIL-6R exhibited no diurnal rhythm, mean values for these immune biomarkers were the only variables considered in the statistical analyses. Because of marked differences in plasma IFN-α concentrations between patients treated with IFN-α 2a versus IFN-α 2b (IFN-α 2a concentrations were ~10 fold higher), patients receiving IFN-α 2a (n = 6) were excluded from analyses of relationships between plasma IFN-α and HPA axis, immune and behavioral measures.
Differences between groups at baseline (visit 1) or visit 2 were assessed using t-tests for continuous measures and χ2 or Fischer tests (as appropriate) for categorical variables. To evaluate effect of treatment with IFN-α/ribavirin on continuous behavioral and physiological variables, repeated measures analysis of variance was also conducted. Effect of group (IFN-α/ribavirin versus control), time (visit 1 versus visit 2) and group by time interactions were assessed. Post hoc comparisons between specific means of interest were conducted using the Student-Newman-Keuls method. Pearson correlation coefficients and the corresponding probabilities associated with these statistics were computed to evaluate associations among changes in HPA axis and immune variables and changes in MADRS and MFI scores between visit 1 and visit 2. Where appropriate, partial correlation coefficients were also computed controlling for relevant HPA axis or immune variables. Effect sizes were calculated using Hedges0 methodology. All tests of significance were two-tailed with the α-level set at 0.05.
Results
Twenty subjects received IFN-α/ribavirin during the study (pegylated IFN-α-2a, n = 6; pegylated IFN-α-2b, n = 14), and 13 subjects served as controls. Baseline demographic data for study participants are presented in Table 1. No differences between groups were observed in terms of age, sex, race, level of education, past history of major depression or substance abuse, body mass index or current tobacco use. Four subjects on IFN-α developed depressive symptoms severe enough to warrant GCRC evaluation followed by psychiatric referral before 12 weeks (2 subjects at 4 weeks, 1 subject at 7 weeks and 1 subject at 11 weeks). The average length of time from visit 1 to visit 2 was 12.1 weeks for the treatment group and 12.8 weeks for the control group (t = 1.12, df = 31, P = 0.27). The deviation from exactly 12 weeks between visits 1 and 2 was due to issues of scheduling convenience and planned GCRC closures over holidays, which randomly influenced the control group to a somewhat greater extent than the IFN-α/ribavirin-treated group (whose average time between visits 1 and 2 was shortened by subjects who were evaluated before 12 weeks).
Table 1.
Baseline demographic and psychiatric characteristics of study participants
| Characteristic | IFN-α (n = 20) |
Control (n = 13) |
P-value |
|---|---|---|---|
| Age (mean, s.d.) | 47.6 (6.3) | 46.8 (6.6) | 0.65 |
| Gender (n, %) | |||
| Males | 12 (60.0) | 6 (46.2) | 0.44 |
| Race (n, %) | |||
| Caucasian | 9 (45.0) | 7 (53.9) | 1.00 |
| Black | 10 (50.0) | 6 (46.1) | |
| Asian | 1 (5.0) | 0 (0.0) | |
| Education (n, %) | |||
| College (1 or more years) |
12 (60.0) | 11 (84.6) | 0.14 |
| Past MD (n, %) | 5 (25.0) | 0 (0) | 0.13 |
| Past substance abuse (n, %) |
12 (60) | 7 (53.9) | 0.73 |
| BMI (mean, s.d.) | 29.2 (5.1) | 28.7 (5.7) | 0.79 |
| Current tobacco use (n, %) |
5 (25.0) | 6 (46.0) | 0.27 |
Abbreviations: BMI, body mass index; MD, major depression; s.d., standard deviation.
Behavioral effects
For MADRS scores, there was a significant main effect of group assignment (IFN-α/ribavirin versus control) (F[1,31] = 6.06, P < 0.05), time (visit 1 versus visit 2) (F[1,31] = 11.18, P < 0.01) and a group by time interaction (F[1,31] = 14.32, P < 0.001), with MADRS scores in the IFN-α/ribavirin-treated group being significantly higher than control subjects at visit 2 (P < 0.05) (Table 2). In addition, whereas MADRS scores were unchanged from visit 1 to visit 2 in control subjects, patients treated with IFN-α/ribavirin exhibited significant increases in MADRS scores as a function of IFN-α/ribavirin treatment (P < 0.05). For MFI scores, there was no main effect of group (F[1,31] = 3.30, P = 0.08), but there was a significant effect of time (F[1,31] = 12.90, P < 0.005) and a significant group by time interaction (F[1,31] = 28.49, P < 0.0001). MFI scores were significantly higher in IFN-α/ribavirin-treated patients compared to control subjects at visit 2 (P < 0.05), and only IFN-α/ribavirin-treated patients exhibited a significant increase in MFI scores from visit 1 to visit 2 (Table 2). Of note, changes in MADRS and MFI scores were highly correlated (r = 0.75, P < 0.0001). Four subjects (20%) receiving IFN-α/ribavirin and no subjects in the control group met symptom criteria for major depression during the study. Of these subjects, three were receiving IFN-α 2a and one was receiving IFN-α 2b. No significant differences were found in the changes of either MADRS or MFI scores between patients treated with IFN-α 2a versus IFN-α 2b or in IFN-α-treated patients with or without a past history of major depression.
Table 2.
Mean (± s.d.) depression and fatigue scores in IFN-α-treated and control subjects
| IFN-α (n = 20) |
Control (n = 13) |
|||||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Delta | Visit 1 | Visit 2 | Delta | |
| MADRS | 3.3 (3.6) | 13.3 (10.4)*† | 10.0 (9.8) | 3.8 (5.0) | 3.2 (3.6) | −0.6 (2.7) |
| MFI | ||||||
| Total | 37.2 (10.3) | 61.2 (21.7)*,† | 24.0 (18.5) | 42.2 (14.9) | 37.5 (15.3) | −4.7 (6.8) |
| GF | 8.9 (3.2) | 14.2 (4.6)*† | 5.3 (4.3) | 10.3 (4.0) | 8.5 (3.9) | −1.8 (2.3) |
| PF | 8.3 (3.4) | 13.3 (4.5)*† | 5.0 (3.9) | 9.0 (3.3) | 8.8 (3.8) | −0.2 (1.7) |
| RA | 6.7 (2.6) | 12.4 (4.9)*† | 5.7 (4.2) | 8.1 (3.4) | 6.8 (3.4) | −1.2 (1.3) |
| RM | 6.7 (2.4) | 11.5 (4.6)*† | 5.7 (4.2) | 7.2 (3.0) | 6.5 (2.6) | −1.2 (1.3) |
| MF | 6.7 (2.5) | 9.9 (5.2)*† | 3.3 (4.7) | 7.6 (3.4) | 6.8 (3.7) | −0.8 (2.1) |
Abbreviations: GF, general fatigue; PF, physical fatigue; MADRS, Montgomery–Asberg depression rating scale; MF, mental fatigue; MFI, multidimensional fatigue inventory; RA, reduced activity; RM, reduced movement; s.d., standard deviation.
Significantly different from visit 1 (P < 0.05 using Student-Newman-Keuls method).
Significantly different from respective control value (P < 0.05 using Student-Newman-Keuls method).
HPA axis effects
Effects of IFN-α/ribavirin on measures of diurnal HPA axis activity are shown in Table 3 and Figure 1. There was no significant main effect of group (treatment versus control) or time (visit 1 versus visit 2) on the diurnal slopes of either ACTH or cortisol. Nevertheless, there was a significant group by time interaction for both hormones (ACTH: F[1,31] = 6.63, P < 0.05; cortisol: F[1,31] = 6.72, P < 0.05), with both hormones exhibiting significantly flatter diurnal slopes (higher β-values) at visit 2 in IFN-α/ribavirin-treated patients compared to controls (P < 0.05 for ACTH and cortisol). These between-group differences represent an effect size of 0.86 (95% confidence interval (CI) 0.13–1.59) for ACTH and 1.04 (95% CI 0.29–1.78) for cortisol. In addition, diurnal slopes for both ACTH and cortisol were significantly flatter at visit 2 compared to visit 1 within the IFN-α/ribavirin treatment group (P < 0.05). No significant differences were found across time for ACTH or cortisol slope in the control group, nor were there differences between groups in ACTH or cortisol slopes at baseline.
Table 3.
Diurnal ACTH and cortisol parameters in IFN-α-treated and control subjects
| IFN-α (n = 20) |
Control (n = 13) |
|||||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Delta | Visit 1 | Visit 2 | Delta | |
| ACTH (mean, s.d.) | ||||||
| Slope (Ne—6) | −16.7 (10.4) | −5.7 (10.0)*† | 10.3 (12.7) | −11.0 (9.7) | −14.0 (14.6) | −3.2 (17.5) |
| a.m. maximum | 33.6 (12.1) | 29.4 (16.0) | −4.2 (11.7) | 26.3 (12.1) | 25.3 (11.1) | 1.0 (6.6) |
| p.m. minimum | 12.7 (4.3) | 16.7 (9.0)* | 4.0 (7.0) | 13.6 (6.7) | 12.2 (6.0) | −1.4 (5.1) |
| Mean diurnal value | 23.6 (9.1) | 23.7 (10.9) | 0.1 (5.8) | 22.2 (12.5) | 20.1 (10.1) | −1.6 (9.5) |
| Cortisol (mean, s.d.) | ||||||
| Slope | −0.10 (0.03) | −0.07 (0.03)*† | 0.02 (0.04) | −0.09 (0.05) | −0.11 (0.06) | −0.02 (0.05) |
| a.m. maximum | 14.2 (3.9) | 12.8 (3.4) | −1.4 (3.2) | 12.4 (2.5) | 12.6 (1.9) | 0.2 (3.1) |
| p.m. minimum | 3.7 (1.5) | 4.4 (1.7)*† | 0.7 (1.6) | 3.5 (1.3) | 3.2 (1.5) | −0.3 (1.0) |
| Mean diurnal value | 8.4 (2.1) | 7.8 (2.2) | 0.6 (1.8) | 7.8 (2.0) | 7.7 (1.7) | −0.04 (2.3) |
Slope-β value of the regression of log transformed plasma ACTH and cortisol concentrations measured from 0900 to 2100 hours (larger β-values—closer to 0—reflect a flatter slope); a.m. maximum-highest hormone value between 0900 to 1100 hours; p.m. minimum-lowest value between 1900 to 2100 hours.
Significantly different from visit 1 (P < 0.05 using Student-Newman-Keuls method);
Significantly different from respective control value (P < 0.05 using Student-Newman-Keuls method). s.d., standard deviation.
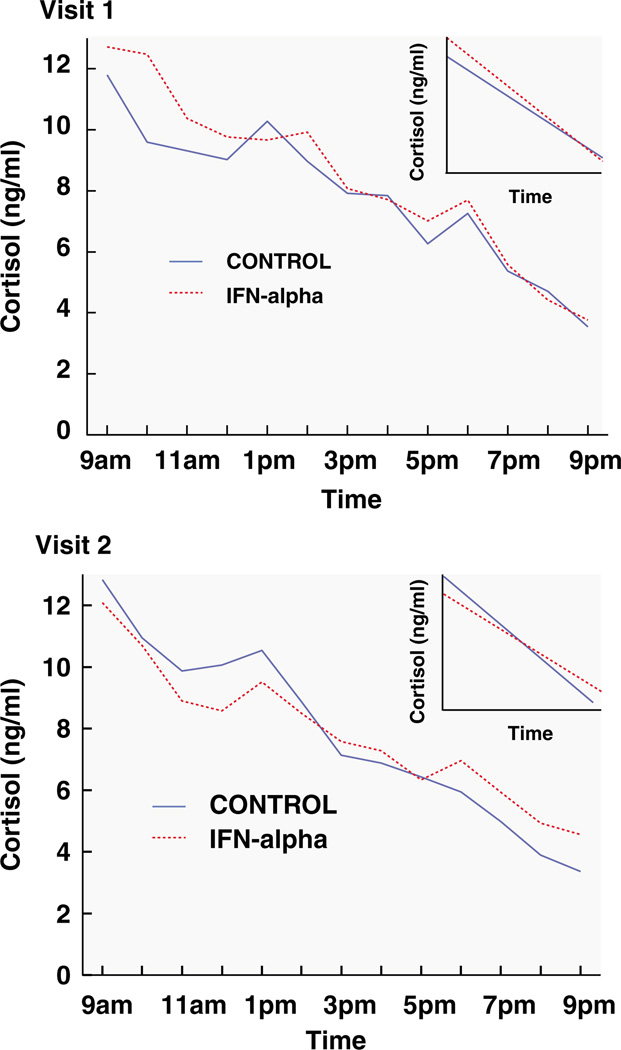
Figure 1.
Mean raw cortisol values from 0900 to 2100 hours in controls (blue line) versus subjects treated with interferon (IFN)-α plus ribavirin (red line) at visit 1 and 2. Cortisol slopes from 0900 to 2100 hours in controls (blue line) versus subjects treated with IFN-α plus ribavirin (red line) at visit 1 and 2 are also depicted in inserts in each graph. Compared to control subjects, IFN-α/ribavirin-treated patients exhibited a significantly flatter cortisol slope (P < 0.05) and significantly higher evening cortisol values (P < 0.05).
Because flattening of the diurnal slope might result from a reduced a.m. peak, an increased p.m. minimum or a combination of both, relative changes in morning peaks and evening troughs of ACTH and cortisol were examined (Table 3). No effect of group, time or their interaction was seen on maximum a.m. values for either ACTH or cortisol. Although there was no main effect of group or time on minimum p.m. values for ACTH or cortisol, a significant group by time interaction was found for both hormones (ACTH: F[1,31] = 5.16, P < 0.05; cortisol: F[1,31] = 5.66, P < 0.05). Post hoc testing revealed that minimum p.m. values for ACTH and cortisol were similar between groups at visit 1, but that patients receiving IFN-α had significantly higher minimum p.m. cortisol values than controls at visit 2 (P < 0.05). Moreover, whereas no change from visit 1 to visit 2 was observed in the hormones in control subjects, minimum p.m. concentrations of ACTH and cortisol significantly increased from visit 1 to visit 2 in patients receiving IFN-α/ribavirin (P < 0.05). Finally, there was no effect of group, time or their interaction on mean plasma concentrations of ACTH averaged over the 12-hour sampling period (Table 3), and only a significant effect of time was found for average plasma cortisol secretion (F[1,31] = 15.53, P < 0.001), with values at visit 2 being significantly lower than visit 1 in the group as a whole. No significant differences in any of the HPA axis measures were found between patients who received IFN-α 2a versus IFN-α 2b or in IFN-αtreated patients with or without a history of major depression.
HPA axis effects and behavior
Flattening of the cortisol slope (increased β-values) was significantly correlated with increased scores on the MADRS and MFI between visits 1 and 2 in the group as a whole (MADRS: r = 0.38, P < 0.05; MFI: r = 0.43, P < 0.05) (Figure 2). Of the five MFI subscales, flattening of the cortisol slope was significantly correlated with reduced activity (r = 0.55, P < 0.01), reduced motivation (r = 0.40, P < 0.05), physical fatigue (r = 0.37, P < 0.05) and mental fatigue (r = 0.36, P < 0.05) but not with general fatigue (r = 0.26, P = 0.14). In addition, whereas no correlations were observed between a.m. cortisol peak and any of the behavioral measures, increases in p.m. minimum cortisol concentrations were significantly correlated with increased scores on the MADRS (r = 0.36, P < 0.05) and the total MFI (r = 0.49, P < 0.01), as well as each of the MFI subscales (general fatigue: r = 0.41, P < 0.05; physical fatigue: r = 0.47, P < 0.01; reduced activity: r = 0.52, r < 0.01; reduced motivation: r = 0.37, P < 0.05; mental fatigue: r = 0.47, P < 0.01) (Figure 2). No correlations were observed between changes in behavioral endpoints and any measure of ACTH diurnal activity. Given the high correlation between changes in MADRS scores and MFI scores as well as the overlap of symptom domains measured by these scales (especially fatigue), correlations between the HPA axis parameters and MADRS scores were repeated with the fatigue item (lassitude) on the MADRS eliminated. Correlations between changes in the cortisol slope or increases in p.m. minimum cortisol concentrations and increases in MADRS scores minus the lassitude item were essentially the same as those obtained when the entire scale was used in the analyses (r = 0.37, P < 0.05 and r = 0.37, P < 0.05, respectively). Finally, when IFN-α-treated patients who developed major depression (n = 4) were compared to IFN-α/ribavirin-treated patients who did not develop major depression (n = 16), no significant differences were found between these two groups for any of the HPA axis measures.
Figure 2.
Correlation between changes in the diurnal rhythm of cortisol (δ-cortisol slope and δ-cortisol p.m. minimum) and the development of fatigue (δ-fatigue) in control and IFN-α-treated subjects. Change in multidimensional fatigue inventory (MFI) total scores between visit 1 and 2 were significantly correlated with the change in cortisol slope (r = 0.43, P < 0.05) (a). Change in MFI total scores between visit 1 and 2 were also correlated with the change in minimum p.m. plasma concentrations (r = 0.49, P < 0.01) (b). Increases in MFI scores were correlated with flattening of the cortisol slope and increases in minimum p.m. plasma cortisol concentrations. Similar relationships were found between changes in cortisol slope and p.m. cortisol minimum and depression as measured by the Montgomery–Asberg depression rating scale.
Immune effects
Mean (s.d.) concentrations of plasma cytokines and cytokine receptors in IFN-α-treated and control subjects during the study are indicated in Table 4. There was a main effect of time and a significant group by time interaction for sTNFR2 (F[1,31] = 18.52, P < 0.001 and F[1,31] = 13.30, P = 0.001, respectively), with patients receiving IFN-α/ribavirin treatment exhibiting significant increases in sTNFR2 from baseline to week 12 (P < 0.05). No differences were found between visits 1 and 2 in sTNFR2 in control subjects. For TNF-α, there was a significant main effect of time (F[1,31] = 8.83, P < 0.01) and a trend for a group by time interaction (F[1,31] = 3.86, P < 0.059). No significant main effects of group or time or their interaction were observed for IL-6 or sIL-6R, nor were there any main effects or interactions for IL-6 slope. There was a significant main effect of group assignment (F[1,31] = 6.75, P < 0.05), time (F[1,31] = 13.39, P < 0.001) and a group by time interaction (F[1,31] = 8.97, P < 0.01) for IFN-α, with IFN-α plasma concentrations being significantly higher in IFN-α-treated patients versus controls at visit 2 (P < 0.05). Moreover, IFN-α plasma concentrations were significantly higher on visit 2 compared to visit 1 within IFN-α/ribavirin-treated subjects (P < 0.05). Of note, there was a strong correlation between increases in plasma concentrations of IFN-α and sTNFR2 (r = 0.63, P < 0.001). No significant differences were found in the change of sTNFR2, TNF-α or IFN-α (or other proinflammatory cytokines and their receptors) over the course of the study in patients treated with IFN-α 2a versus IFN-α 2b or in IFN-α-treated patients with or without a history of major depression.
Table 4.
Mean (± s.d.) cytokines and cytokine receptors in IFN-α-treated and control subjects
| IFN-α (n = 20) |
Control (n = 13) |
|||||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Delta | Visit 1 | Visit 2 | Delta | |
| Cytokines (pg ml−1 | ||||||
| IFN-α‡ | 11.7 (11.3) | 44.2 (28.9)*† | 32.4 (29.7) | 9.6 (11.0) | 12.8 (25.7) | 3.2 (19.5) |
| TNF-α | 1.7 (0.85) | 2.1 (0.96) | 0.4 (0.6) | 1.7 (1.0) | 1.7 (1.0) | 0.0 (0.3) |
| IL-6 | 4.9 (3.9) | 4.6 (4.2) | −0.4 (4.8) | 4.0 (2.1) | 4.4 (2.0) | 0.4 (2.3) |
| Soluble receptors (ng ml−1) | ||||||
| sTNF-RII | 2.8 (1.7) | 3.6 (1.6)*,† | 0.8 (0.7) | 2.4 (1.2) | 2.5 (1.2) | 0.1 (0.4) |
| sIL-6R | 33.8 (8.3) | 33.4 (9.0) | −0.4 (4.8) | 32.4 (7.2) | 32.1 (7.4) | −0.3 (1.7) |
Abbreviations: IL-6, interleukin-6; sIL-6R, soluble interleukin-6 receptor; s.d., standard deviation; sTNF-RII, soluble tumor necrosis factor receptor; TNF, tumor necrosis factor.
Significantly different from visit 1 (P < 0.05 using Student-Newman-Keuls method).
Significantly different from respective control value (P < 0.05 using Student-Newman-Keuls method).
Only subjects taking IFN-α 2b were included in these analyses.
Immune effects and behavior
Increases in plasma concentrations of TNF-α and TNFR2 from visit 1 to visit 2 were significantly correlated with increases in scores on the MADRS (r = 0.47, P < 0.05 and r = 0.57, P < 0.001, respectively) and MFI (r = 0.50, P < 0.01, and r = 0.49, P < 0.01, respectively) (Figure 3). No correlations were observed between changes in either IL-6 or sIL-6R and the development of depressive symptoms (r = 0.12, P = 0.51 and r = 0.17, P = 0.34, respectively) or fatigue (r = 0.15, P = 0.40 and r = 0.05, P = 0.78, respectively). Moreover, there were no correlations between changes in IL-6 slope and changes in the behavioral variables. Because of the high inter-correlation between changes in MADRS and MFI scores (as noted above), correlations between the immune parameters and MADRS scores were repeated with the fatigue item (lassitude) eliminated. Correlations between increases in sTNF-R2 and increases in MADRS scores (with the lassitude item eliminated) remained largely unchanged (r = 0.56, P < 0.001), however, the correlation between increases in TNF-α and increases in MADRS scores minus the lassitude item was slightly reduced (r = 0.34, P = 0.06). No differences were found in the relationship between IL-6, sIL-6R or IL-6 slope and MADRS scores with the lassitude item removed. Finally, increases in plasma IFN-α were highly correlated with increases in both MADRS and MFI scores (r = 0.60, P < 0.001 and r = 0.64, P < 0.001, respectively) (Figure 3).
Figure 3.
Correlation between changes in plasma IFN-α, TNF-α and sTNFR2 (δ-plasma IFN-α, TNF-α and sTNFR2) and the development of fatigue (δ-fatigue) in control and IFN-α-treated subjects. Change in multidimensional fatigue inventory (MFI) total scores between visit 1 and 2 were significantly correlated with the change in plasma concentrations of IFN-α (r = 0.64, P < 0.001) (a), TNF-α (r = 0.50, P < 0.01) (b) and sTNFR2 (r = 0.49, P < 0.01) (c). Increases in IFN-α, TNF-α and sTNFR2 were associated with increases in fatigue. Similar relationships were found between changes in plasma IFN-α, TNF-α and sTNFR2 and depression as measured by the Montgomery–Asberg depression rating scale.
HPA axis effects and immune effects
The relationship between changes in immune parameters and HPA axis measures was examined. No correlations were found between change in cortisol slope and change in TNF-α (r = 0.0, P = 1.0), IL-6 (r = 0.10, P = 0.56), sTNFR2 (r = 0.15, P = 0.40), sIL-6R (r = −0.21, P = 0.24) or IL-6 slope (r = −0.19, P = 0.30). Moreover, no correlations were found between change in cortisol p.m. minimum and change in TNF-α (r = 0.14, P = 0.46), IL-6 (r = 0.22, P = 0.21), sTNFR2 (r = 0.08, P = 0.67), sIL-6R (r = −0.17, P = 0.34) or IL-6 slope (r = −0.11, P = 0.54). Similarly, no significant relationships were found between the cytokines and their receptors and measures of ACTH over the course of the study. Finally, no correlation was found between changes in plasma concentrations of IFN-α and either changes in cortisol slope or cortisol p.m. minimum (r = −0.09, P = 0.64 and r = 0.08, P = 0.68, respectively).
Effects of HPA axis and immune variables on behavior controlling for mutual influences
To determine the relative association of relevant HPA axis and immune variables to the development of depression and fatigue, correlational analyses were repeated while controlling for immune or HPA axis variables found to be predictive of MADRS and MFI scores. Correlations of changes in cortisol slope with changes in MADRS and MFI scores remained essentially the same when controlling for changes in plasma sTNFR2 (rp = 0.36, P < 0.05 and rp = 0.41, P < 0.05, respectively), TNF-α (rp = 0.39, P < 0.05 and rp = 0.42, P < 0.05, respectively) or IFN-α (rp = 0.39, P < 0.05 and rp = 0.48, P < 0.05, respectively). Similar findings were obtained for the correlations between changes in p.m. cortisol concentrations and MADRS and MFI scores, which remained largely the same after controlling for changes in plasma sTNFR2 (r = 0.39, P < 0.05 and r = 0.52, P < 0.01, respectively), TNF-α (rp = 0.34, P < 0.06 and rp = 0.47, P < 0.01, respectively) and IFN-α (rp = 0.36, P = 0.07 and rp = 0.48, P < 0.05, respectively). Conversely, correlations between changes in sTNF-R2 and MADRS and MFI scores remained largely the same when controlling for cortisol slope (rp = 0.56, P < 0.001 and rp = 0.48, P < 0.01, respectively) and cortisol p.m. minimum (r = 0.58, P < 0.001 and r = 0.52, P < 0.01, respectively) as did correlations between TNF-α and MADRS and MFI scores (controlling for cortisol slope: rp = 0.42, P < 0.05 and rp = 0.32, P = 0.07, respectively; controlling for cortisol p.m. minimum: rp = 0.39, P < 0.05 and rp = 0.29, P = 0.10, respectively) and IFN-α and MADRS and MFI scores (controlling for cortisol slope: rp = 0.65, P < 0.001 and rp = 0.70, P < 0.001, respectively; controlling for cortisol p.m. minimum: rp = 0.61, P < 0.01 and rp = 0.67, P < 0.001, respectively).
Discussion
IFN-α administration was associated with significant alterations in diurnal HPA axis activity including flattening of the ACTH and cortisol slope and increases in evening ACTH and cortisol concentrations. Both flattening of the cortisol slope and increases in the evening cortisol were in turn significantly correlated with IFN-α-induced increases in depression and fatigue. IFN-α-induced increases in plasma concentrations of IFN-α as well as TNF-α and sTNFR2 were also significantly correlated with behavioral changes, however, no relationship was found between these or other immune variables and changes in HPA axis parameters. Given the prospective, longitudinal design of the study, these data provide some of the first evidence that chronic exposure to innate immune cytokines such as IFN-α may be a relevant pathophysiologic pathway by which HPA axis dysregulation (especially flattening of the cortisol slope) occurs in patients with various medical illnesses. Nevertheless, given the lack of a direct relationship between HPA axis and immune measures, the mechanism by which chronic cytokine exposure influences HPA axis function remains to be determined.
A number of studies suggest that dysregulation of HPA axis diurnal activity represents an important link between illness and behavioral disturbance. Flattening of the cortisol rhythm has been observed in patients with—or at risk for—a number of medical disorders, including cancer, type 2 diabetes and cardiovascular disease.23–26 In addition, flattening of the cortisol slope has been associated with depression, fatigue, anxiety and maladaptive coping styles in both medically ill and medically healthy individuals. 30–32,42–44 The data reported here replicate the association between flattening of the cortisol slope and behavioral alterations including depression and fatigue. Moreover, the changes in cortisol slope as a function of IFN-α treatment are very much in line with previous reports in other clinical populations that have exhibited alterations in diurnal cortisol rhythm. These similarities were apparent despite the fact that in the majority of previous studies, cortisol slope was derived from diurnal sampling of cortisol in saliva over several (typically 3) days in the subject’s home environment. For example, patients with meta-static breast cancer in the study by Abercrombie et al.26 exhibited a slope of −0.092 log ug per 100ml per hour (s.d. 0.033) compared to −0.113 (s.d. 0.030) in healthy control subjects. These results are similar to the cortisol slopes in IFN-α/ribavirin treated patients (−0.07 (s.d. 0.03)) versus controls (−0.11 (s.d. 0.06)) at visit 2 in the current study. Likewise, in the study by Giese-Davis et al.31 slopes for metastatic breast cancer patients labeled as ‘high anxious’ or ‘repressor’ (slope = −0.07 for both groups (s.d. 0.08 and 0.04, respectively)) were exactly the same as IFN-α-treated patients in the current study, whereas patients labeled as self-αssured or non-extreme exhibited slopes of −0.10 (s.d. 0.05) and 0.11 (s.d. 0.05), respectively; virtually the same as our control subjects. In the Matthews CARDIA study, individuals with cardiac calcifications exhibited a slope of −0.061 (s.d. 0.064) and those without calcifications exhibited a slope of −0.084 (s.d. 0.054), a smaller effect size than the one observed between IFN-α-treated and control groups.23 Finally, in the study by Bower et al.32 cortisol slope was −0.21 (s.d. 0.13) in controls versus −0.14 (s.d. 0.06) in breast cancer survivors with fatigue.32 Although the slope values are to an extent ‘steeper’ in the study by Bower and co-workers than those observed in the current study (and the studies by Abercrombie et al.,26 Giese-Davis et al.31 and Matthews et al.23), the 67% difference in slopes between fatigued patients versus controls is similar to the 64% difference observed between IFN-α-treated and control patients at visit 2. Based on these data, it is reasonable to conclude that IFN-α had a significant effect on the HPA axis and cortisol slope that is very much in line with the magnitude of effects that have been observed in other clinical populations in previous studies.
It is of great interest that the association between flattening of the cortisol slope and the development of depression and fatigue as a function of cytokine exposure appeared to be primarily related to increased cortisol production late in the day. Less attention has been paid to cortisol activity near the circadian nadir than to either the overall diurnal slope or the morning peak of cortisol release. However, increased pulsatile release of cortisol late in the day has emerged as a primary HPA axis abnormality in major depression.30 In addition, cortisol elevation late in the day correlates with age-related disruption of sleep integrity and loss of slow-wave sleep and has been shown to have more adverse metabolic effects than elevation of cortisol in the morning.45,46 For example, in subjects given metyrapone to suppress endogenous cortisol production, administration of hydrocortisone in the afternoon produced significantly greater impairments in glucose metabolism/insulin sensitivity when compared to an identical dose of hydrocortisone administered in the morning.46 Thus, increased cortisol activity late in the day may be an important mechanism by which activation of innate immune responses contribute to both behavioral alterations and vulnerability to medical illnesses.
Correlations between IFN-α-induced HPA axis changes and behavior were found using scales of both depression and fatigue. Nevertheless, the MADRS (depression) and MFI (fatigue) were highly inter-correlated. However, even when the fatigue item (lassitude) was removed from the MADRS, correlations between HPA axis changes and depression largely persisted. These data indicate that the MADRS and MFI are measuring overlapping constructs, which are both overexpressed as a function of IFN-α exposure and cannot easily be disentangled.
It is important to note that HPA axis changes as a function of chronic IFN-α exposure are in contrast to the changes seen following acute IFN-α administration, where plasma ACTH and cortisol levels as well as IL-6, were dramatically elevated for up to 3 hours.10–13 These data suggest that significant adaptation of the HPA axis occurs following chronic IFN-α treatment and emphasize the distinction between acute and chronic cytokine effects on HPA axis function. Moreover, given the similarity to HPA axis changes following acute versus chronic stress (where acute stress is typically associated with marked HPA axis activation and chronic stress is associated with flattening of the cortisol slope), the data suggest that chronic IFN-α administration acts on the HPA axis much like a chronic stressor. Moreover, the data highlight the relevance of chronic cytokine (IFN-α) exposure as a model for individuals exposed to chronic immune activation as a function of either medical illness and/or chronic stress.
The fact that many of the diseases associated with flattening of the diurnal cortisol rhythm are characterized by activation of the body’s innate immune/inflammatory response,47–53 further supports the notion that chronic exposure to innate immune cytokines can disrupt normal HPA axis function. Specific mechanisms by which cytokines, such as IFN-α, might disrupt normal HPA axis rhythms are currently unknown; however, several possibilities warrant consideration including effects of cytokines on factors that regulate HPA axis function as well as cytokine effects on sleep-wake cycles.
Innate immune cytokines have been shown to activate HPA axis pathways, in part, through their induction of corticotropin releasing hormone.54,55 Moreover, acting through several intracellular signaling pathways, cytokines of the innate immune response have been found to inhibit glucocorticoid receptor function,56,57 which, in turn can disrupt negative feedback regulation of HPA axis function, potentially leading to increased concentrations of ACTH and cortisol late in the day. These possibilities are consistent with recent studies in which IL-6 was found to correlate with a dampened cortisol rhythm in patients with metastatic colon cancer and increased p.m. cortisol in patients with coronary artery disease.28,29 Nevertheless, the current study found no significant correlations between immune and HPA axis variables. Although the sample size for this study was relatively small (in part related to the intensive sampling demands and strict entry criteria), correlation coefficients between immune variables and measures of diurnal cortisol secretion, all of which were non-significant, ranged from r = 0.0 to r = 0.22, thereby accounting for less than 5% of the variance. These correlation coefficients are in contrast to the much stronger (and statistically significant) relationships that were observed between measures of diurnal cortisol secretion and behavior as well as the immune parameters and behavior, where correlation coefficients ranged from r = 0.36 to r = 0.64, accounting for 13–41% of the variance in measures of depression and fatigue. Taken together, these data suggest that either innate immune cytokines and HPA axis alterations are independent contributors to changes in behavior, or there are additional, yet to be identified, pathways or factors which serve to link HPA axis and immune measures. One possibility in this regard includes additional cytokines that were not measured as part of the study design. For example, in a study by Wichers et al.17 IL-8 (which was not assessed in the current study) was found to significantly correlate with daily average cortisol over time in a longitudinal study of IFN-α-treated patients with hepatitis C. Interestingly, Wichers et al.17 also found positive correlations between the awakening cortisol response and IL-6 as well as IL-8, IL-10 and sIL-2R. These data suggest that while correlations between HPA axis and immune parameters may exist, they may be, in part, a function of the HPA axis or immune parameter assessed.
Another potential pathway by which innate immune cytokines such as IFN-α might influence diurnal HPA axis activity is by affecting sleep. Complex bi-directional relationships exist between inflammation and the sleep-wake cycle.58,59 For example, innate immune cytokines including IL-6 and IFN-α have been found to disrupt sleep efficiency and architecture in humans.7,60 Sleep disruption, in turn, has been shown to promote further cytokine production and release.7,58,61,62 Sleep disruption has also been shown to disrupt the diurnal cortisol rhythm,63 and as noted above, is especially likely to increase cortisol levels late in the day63—a pattern of changes identical to those observed in the current study following chronic IFN-α exposure. Interestingly, in a recent case report of two patients with subacute sclerosing panencephalitis, intracerebroventricular administration of IFN-α was found to markedly inhibit the production of orexin, a neuropeptide involved in the maintenance of sleep-wake cycles.64 Thus, the effects of innate immune cytokines (for example, IFN-α) on neuropeptides and/or neurotransmitters that regulate sleep may in turn influence diurnal cortisol secretion. Finally, IFN-α has been shown to influence daily rhythms of locomotor activity and body temperature in association with effects on the expression of relevant clock-genes in the suprachiasmatic nucleus, a hypothalamic brain region intimately involved in the regulation of circadian rhythms.65 Such effects of cytokines on daily rhythms, sleep and other relevant behaviors that have been associated with altered diurnal cortisol secretion (for example, depression)30 may explain, in part, the lack of a direct correlation between cytokines and HPA axis function.
Regarding the relationship between cytokines and behavior, to our knowledge, this is the first study to report a significant correlation between plasma concentrations of IFN-α and depression and fatigue. These data indicate that there is a dose–response relationship between IFN-α and behavior, and thus IFN-α dosage reduction during IFN-α therapy for hepatitis C (or other conditions) appears to be an appropriate strategy for the initial management of IFN-α-induced behavioral change. A similar relationship was found between behavioral changes and plasma concentrations of both TNF-α and sTNF-R2. Of note, mean plasma concentrations of TNF-α and sTNFR2 in HCV control patients in the current study were similar to those reported previously for healthy control subjects, indicating that HCV infection itself was not associated with elevated plasma concentrations of these immune parameters in this study population.66–68 Furthermore, the magnitude of elevation in sTNFR2 from visit 1 to visit 2 in IFN-α-treated patients (0.8 ng ml−1) corresponds closely with differences that have been observed between control subjects and either patients with type 2 diabetes or cancer survivors with fatigue (~0.7 ng ml−1), supporting the clinical relevance of the observed IFN-α-induced increases in sTNFr2.66,67 Soluble TNFRs remain elevated for long periods of time after TNF-α administration and are believed to reflect previous TNF-α effects.69,70 Thus, elevations in TNFR2 in the current study, along with significant correlations between TNF-α and depression and fatigue, provide evidence that activation of TNF-α and its signaling pathways may be an important component of the effects of IFN-α on behavior.
Several strengths and limitations of the current study should be noted. The use of a prospective, longitudinal design allowed for a ‘within-subjects’ design that reduced the potential effects of baseline variables such as age and body mass index that have been cross-sectionally associated with diurnal rhythm of the HPA axis in prior studies.71,72 Environmental factors such as sleep and wake times and the timing of meals can also influence the pattern of diurnal cortisol activity and are typically not controlled in studies of cortisol slope conducted in outpatients.32 The use of an inpatient environment in the current study may have strengthened findings by controlling these variables.
In terms of study limitations, several issues warrant comment. First, the sample size was relatively small, and therefore the inability to detect significant correlations between HPA axis parameters and proinflammatory cytokines (and their receptors) may have been secondary to a lack of power. Nevertheless, as noted above, the correlations that were obtained between HPA axis and immune parameters were relatively small, and even if found statistically significant with a larger sample size would only account for a relatively small amount of the variance in diurnal HPA axis activity compared to what was found with the behavioral measures. Another limitation is that relatively few cytokine parameters were assessed, and as noted above there could be other cytokines that may correlate with HPA axis function. Similarly, immune parameters were only measured at one time point during IFN-α treatment, and it is possible that cytokines, such as IL-6, which were not elevated following 12 weeks of treatment may have been increased at an earlier time point and may have contributed to the initial development of neuroendocrine and/or behavioral changes, with these changes being subsequently maintained by other cytokine, neurotransmitter or neuroendocrine systems. Another limitation of the study is that group assignment was not randomized. The lack of randomization resulted in the occurrence of five patients with a history of past major depression in the IFN-α/ribavirin treatment grou and no patients with a major depression history in the control group. Nevertheless, further evaluation of IFN-α-treated patients with and without a past history of major depression revealed no significant differences in terms of the effects of IFN-α/ribavirin on any of the HPA axis, immune or behavioral variables.
It should be noted that all subjects included in this study were infected with HCV, and analyses of behavioral and neurobiological parameters were not controlled for viral load. Therefore, it remains possible that different levels of HCV infection may have variably augmented the effect of chronic IFN-α exposure on diurnal HPA axis activity and immunologic parameters. Nevertheless, based on the similarity of cortisol slope values in HCV control subjects in the current study versus cortisol slope values in healthy controls from the published literature (see above),26 there is no clear evidence that chronic viral infection itself was associated with alterations in diurnal HPA axis activity in the absence of IFN-α administration. As per standard of care, all subjects who received IFN-α were also treated with the antiviral agent, ribavirin. Although there is no evidence that ribavirin affects the HPA axis, the possibility that ribavirin influenced the results cannot be ruled out. Nevertheless, individuals treated with IFN-α monotherapy for cancer show similar behavioral changes to those reported here.73
A final limitation of the study is that HPA axis hormones were only assessed hourly for 12h during the day, and no information is available on the 24-h circadian rhythm of these hormones. The 12-h protocol was used to limit the potential influence of overnight blood sampling on sleep (which in turn may influence cortisol rhythm) and to coincide with previous studies that have used daytime salivary sampling strategies to examine the relationship between diurnal cortisol rhythm and both medical and psychiatric/psychological outcomes. Nevertheless, more frequent blood sampling over a 24h period as conducted by Licinio et al.74 might have revealed more nuanced changes in hormone secretion including changes in hormonal spike frequencies and amplitude as well as phase shifting in the circadian rhythm.
In summary, results of the current study indicate that administration of the innate immune cytokine IFN-α leads to profound behavioral changes that are associated with flattening of the diurnal cortisol slope, with the primary change being an increase in evening cortisol plasma concentrations. Potential mechanisms for these effects may include cytokine-induced disruption of the sleep-wake cycle as well as inhibitory effects of cytokines on glucocorticoid receptor functioning. Further studies clarifying these potential mechanisms may provide novel insights into the pathogenesis of behavioral disturbances in the context of sickness as well as provide clues to novel treatment targets for addressing the consequences of cytokines on the brain and behavior.
Acknowledgments
This study was funded by grants from the National Institute of Mental Health (K05 MH069124, K23 MH064619, R01 MH070553 and R01 HL073921) an NIH/NCRR General Clinical Research Center grant (M01 RR00039) and the Centers for Disease Control and Prevention.
Footnotes
Disclosures
CL Raison has served as a speaker for Lilly and Wyeth and as a consultant or an advisory board member for Schering-Plough, Wyeth, Lilly and Centocor; AS Borisov, BJ Woolwine, B Massung and G Vogt have nothing to declare; AH Miller has served as a consultant or an advisory board member for Schering-Plough and Centocor, and has received research funding from Janssen/Johnson and Johnson, GlaxoSmithKline and Schering-Plough.
References
- 1.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of major depression. Trend Immun. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of proinflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- 4.Van Dam AM, Bol JG, Gaykema RP, Goehler LE, Maier SF, Watkins LR, et al. Vagotomy does not inhibit high dose lipopolysaccharideinduced interleukin-1beta immunoreactivity in rat brain and pituitary gland. Neurosci Lett. 2000;285:169–172. doi: 10.1016/s0304-3940(00)01031-4. [DOI] [PubMed] [Google Scholar]
- 5.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 6.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 7.Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- 8.Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- 9.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 10.Gisslinger H, Svoboda T, Clodi M, Gilly B, Ludwig H, Havelec L, et al. Interferon-alpha stimulates the hypothalamic-pituitary-adrenal axis in vivo and in vitro. Neuroendocrinol. 1993;57:489–495. doi: 10.1159/000126396. [DOI] [PubMed] [Google Scholar]
- 11.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 12.Muller H, Hammes E, Hiemke C, Hess G. Interferon-alpha-2-induced stimulation of ACTH and cortisol secretion in man. Neuroendocrinol. 1991;54:499–503. doi: 10.1159/000125944. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu H, Ohtani K, Sato N, Nagamine T, Mori M. Increase in serum interleukin-6, plasma ACTH and serum cortisol levels after systemic interferon-alpha administration. Endocr J. 1995;42:551–556. doi: 10.1507/endocrj.42.551. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Scharpe S, Meltzer HY, Okayli G, Bosmans E, D'Hondt P, et al. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response. Psychiatry Res. 1994;54:143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 15.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JL, Grossberg SE. The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol. 1998;25:23–29. [PubMed] [Google Scholar]
- 17.Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric side effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:1–19. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacol. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 20.Maddock C, Landau S, Barry K, Maulayah P, Hotopf M, Cleare AJ, et al. Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry. 2005;10:332–333. doi: 10.1038/sj.mp.4001634. [DOI] [PubMed] [Google Scholar]
- 21.Maddock C, Baita A, Orru MG, Sitzia R, Costa A, Muntoni E, et al. Psychopharmacological treatment of depression, anxiety, irritability and insomnia in patients receiving interferon-alpha: a prospective case series and a discussion of biological mechanisms. J Psychopharmacol. 2004;18:41–46. doi: 10.1177/0269881104040230. [DOI] [PubMed] [Google Scholar]
- 22.Friebe A, Schwarz MJ, Schmid-Wendtner M, Volkenandt M, Schmidt F, Horn M, et al. Pretreatment levels of sTNF-R1 and sIL-6R are associated with a higher vulnerability for IFN-alpha-induced depressive symptoms in patients with malignant melanoma. J Immunother. 2007;30:333–337. doi: 10.1097/01.cji.0000211346.19330.c9. [DOI] [PubMed] [Google Scholar]
- 23.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 24.Ticher A, Haus E, Ron IG, Sackett-Lundeen L, Ashkenazi IE. The pattern of hormonal circadian time structure (acrophase) as an assessor of breast-cancer risk. Int J Cancer. 1996;65:591–593. doi: 10.1002/(SICI)1097-0215(19960301)65:5<591::AID-IJC6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 26.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinol. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Nat Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 28.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 29.Nijm J, Kristenson M, Olsson AG, Jonasson L. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J Intern Med. 2007;262:375–384. doi: 10.1111/j.1365-2796.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- 30.Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, et al. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 31.Giese-Davis J, Sephton SE, Abercrombie HC, Duran RE, Spiegel D. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol. 2004;23:645–650. doi: 10.1037/0278-6133.23.6.645. [DOI] [PubMed] [Google Scholar]
- 32.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinol. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- 35.Majer M, Wellberg LAM, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2007.12.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 38.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Sympt Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes M. Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psychol Med. 2005;35:433–441. doi: 10.1017/s0033291704003526. [DOI] [PubMed] [Google Scholar]
- 40.Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, et al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinol. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- 41.Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 42.Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinol. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 43.Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinol. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Barnett RC, Steptoe A, Gareis KC. Marital-role quality and stressrelated psychobiological indicators. Ann Behav Med. 2005;30:36–43. doi: 10.1207/s15324796abm3001_5. [DOI] [PubMed] [Google Scholar]
- 45.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 46.Plat L, Leproult R, L0Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84:3082–3092. doi: 10.1210/jcem.84.9.5978. [DOI] [PubMed] [Google Scholar]
- 47.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–261. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 48.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–I10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 49.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 51.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 52.Pitsavos C, Tampourlou M, Panagiotakos DB, Skoumas Y, Chrysohoou C. Association between low-grade system inflammation and type 2 diabetes mellitus among men and women from the ATTICA Study. Rev Diab Stud. 2007;4:98–104. doi: 10.1900/RDS.2007.4.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 54.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 55.Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 56.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, et al. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinol. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- 58.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 59.Opp MR, Obal F, Jr, Krueger JM. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am J Physiol. 1991;260:R52–R58. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- 60.Spath-Schwalbe E, Lange T, Perras B, Fehm HL, Born J. Interferon-alpha acutely impairs sleep in healthy humans. Cytokine. 2000;12:518–521. doi: 10.1006/cyto.1999.0587. [DOI] [PubMed] [Google Scholar]
- 61.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 62.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 63.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa Y, Kanbyashi T, Yano T, Sawaishi Y, Saito Y, Shimizu T. Cerebrospinal fluid-orexin decreases during intraventricular a-interferon therapy of patients with subacute sclerosing panencephalitis. Sleep Biol Rhyth. 2003;1:143–145. [Google Scholar]
- 65.Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nature Med. 2001;7:356–360. doi: 10.1038/85507. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Real JM, Lainez B, Vendrell J, Rigla M, Castro A, Penarroja G, et al. Shedding of TNF-alpha receptors, blood pressure, and insulin sensitivity in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;282:E952–E959. doi: 10.1152/ajpendo.00444.2001. [DOI] [PubMed] [Google Scholar]
- 67.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Dome P, Teleki Z, Rihmer Z, Peter L, Dobos J, Kenessey I, et al. Circulating endothelial progenitor cells and depression: a possible novel link between heart and soul. Mol Psychiatry. 2008:1–9. doi: 10.1038/sj.mp.4002138. epub. [DOI] [PubMed] [Google Scholar]
- 69.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schroder J, Stuber F, Gallati H, Schade FU, Kremer B. Pattern of soluble TNF receptors I and II in sepsis. Infection. 1995;23:143–148. doi: 10.1007/BF01793854. [DOI] [PubMed] [Google Scholar]
- 71.Ice GH. Factors influencing cortisol level and slope among community dwelling older adults in Minnesota. J Cross Cult Gerontol. 2005;20:91–108. doi: 10.1007/s10823-005-9085-5. [DOI] [PubMed] [Google Scholar]
- 72.Daniel M, Moore DS, Decker S, Belton L, DeVellis B, Doolen A, et al. Associations among education, cortisol rhythm, and BMI in blue-collar women. Obesity. 2006;14:327–335. doi: 10.1038/oby.2006.42. [DOI] [PubMed] [Google Scholar]
- 73.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alpha. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 74.Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nature Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]